Abstract
The cell cycle-regulated DNA methyltransferase CcrM is conserved in most Alphaproteobacteria, but its role in bacteria with complex or multicentric genomes remains unexplored. Here, we compare the methylome, the transcriptome and the phenotypes of wild-type and CcrM-depleted Agrobacterium tumefaciens cells with a dicentric chromosome with two essential replication origins. We find that DNA methylation has a pleiotropic impact on motility, biofilm formation and viability. Remarkably, CcrM promotes the expression of the repABCCh2 operon, encoding proteins required for replication initiation/partitioning at ori2, and represses gcrA, encoding a conserved global cell cycle regulator. Imaging ori1 and ori2 in live cells, we show that replication from ori2 is often delayed in cells with a hypo-methylated genome, while ori2 over-initiates in cells with a hyper-methylated genome. Further analyses show that GcrA promotes the expression of the RepCCh2 initiator, most likely through the repression of a RepECh2 anti-sense RNA. Altogether, we propose that replication at ori1 leads to a transient hemi-methylation and activation of the gcrA promoter, allowing repCCh2 activation by GcrA and contributing to initiation at ori2. This study then uncovers a novel and original connection between CcrM-dependent DNA methylation, a conserved epigenetic regulator and genome maintenance in an Alphaproteobacterial pathogen.
Graphical Abstract
Graphical Abstract.
Introduction
Approximately 10% of the sequenced bacterial strains display complex genomes with more than one essential replicon and/or more than one essential replication origin. These include many Alphaproteobacteria that act as human, animal or plant pathogens, such as Brucella abortus or Agrobacterium tumefaciens, and that have very diverse modes of life (1,2). Multipartite or multicentric genomes pose significant challenges for genome stability and survival over generations, as the replication and the partitioning of multiple essential origins need to be coordinated with one another and with other events of the cell cycle. Even if ∼26% of the sequenced bacterial strains with multipartite genomes correspond to Alphaproteobacteria (3), mechanisms that these bacteria use to ensure the maintenance of such multipartite/multicentric genomes are still largely underexplored compared to Gammaproteobacteria (4–6). In Alphaproteobacteria, replication of the main chromosome is supposedly always dependent on the very conserved initiator of DNA replication DnaA that usually binds to multiple sites on bacterial origins of replication (7). Instead, replication of secondary chromosomes (carrying essential genes) or mega-plasmids is usually dependent on repABC operons (4,5). In such cases, RepC acts as the initiator of DNA replication by binding to an origin that is usually directly located inside the repC gene (4,8). Then, RepA and RepB (homologs of ParA and ParB) contribute to replicon partitioning through their binding to parS sequences located inside and/or next to the cognate repABC operon (9). Interestingly, repABC loci usually carry a higher-than-expected number of 5′-GANTC-3′ motifs that are often located in the promoter region driving the transcription of repABC operons, in putative origins located inside repC genes and in putative repE promoter regions driving the transcription of RepE small regulatory RNAs that can down-regulate repC transcription and translation (4,10). In nearly all Alphaproteobacteria except Rickettsiales and Magnetococcales, adenines located in such GANTC motifs are methylated (m6A) by the cell cycle-regulated DNA methyltransferase (MTase) CcrM (11). This solitary MTase, which is not associated with a cognate endonuclease, was initially discovered in the Caulobacter crescentus Alphaproteobacterium that has a single circular chromosome. In this bacterium, CcrM was shown to be present and active only at the very end of the S-phase of the cell cycle in pre-divisional cells (12,13). As a consequence, newly replicated GANTC motifs spread throughout the genome stay hemi-methylated for a significant period of the cell cycle after the passage of the replication fork, especially if these motifs are located closer to the origin of replication than to the terminus of replication of its unique chromosome (14). In C. crescentus, the methylation of hundreds of promoter regions by CcrM has a major impact on its transcriptome (11), notably because the co-conserved global cell cycle regulator GcrA can sense such epigenetic signals to modulate gene expression (15–18). One such gene strongly activated through methylation is the ftsZ gene required for cell division (11,19). Molecular genetics and experimental evolution analyses demonstrated that the essentiality of ccrM in fast-growing C. crescentus is specifically dependent on this key epigenetic activation of ftsZ expression (19,20). In Brevundimonas subvibrioides, where ccrM is not essential, the CcrM and GcrA regulons are significantly different compared to those of C. crescentus (21), demonstrating a relatively fast evolution of epigenetic regulatory pathways even in closely related Alphaproteobacteria. However, so far, studies did not focus on the impact of CcrM-dependent methylation in Alphaproteobacteria with more complex or multipartite genomes, despite observations indicating that CcrM is also essential in B. abortus (22) and A. tumefaciens (23,24). In the study described here, we aimed at filling this gap using the A. tumefaciens plant pathogen, which is the causal agent of the crown gall disease and a live biotechnological tool used for the genetic manipulation of plants (25). Earlier findings indicated that CcrM is also a cell cycle-regulated DNA MTase in this Alphaproteobacterium (23). A. tumefaciens displays a complex genome with two essential (ori1 and ori2) and two dispensible (oripAt and oripTi) origins (24,26,27). In the original C58 strain sequenced in 2001, it was shown that its genome consists in one circular chromosome (Ch1 with a DnaA-dependent ori1), one linear chromosome (Ch2 with a RepCCh2-dependent ori2) and two mega-plasmids encoding important virulence factors (pTi and pAt, with RepCpTi/RepCpAt-dependent oripTi and oripAt, respectively) (26,27). However, a very recent study from 2022 showed that many of the C58 strains used in laboratories over the world carry a unique dicentric linear chromosome instead of two distinct chromosomes (Figure 1a, left side) (28). Still, even in such strains, the repABCCh2 module (including ori2) appears to remain essential for survival since it could not be disrupted during Tn-seq experiments and since the repBCh2 gene could not be deleted (28), showing that initiation at ori2 and/or ori2 partitioning are essential in the two described A. tumefaciens C58 strains.
Figure 1.
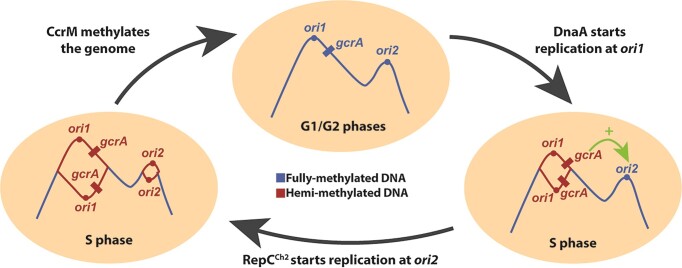
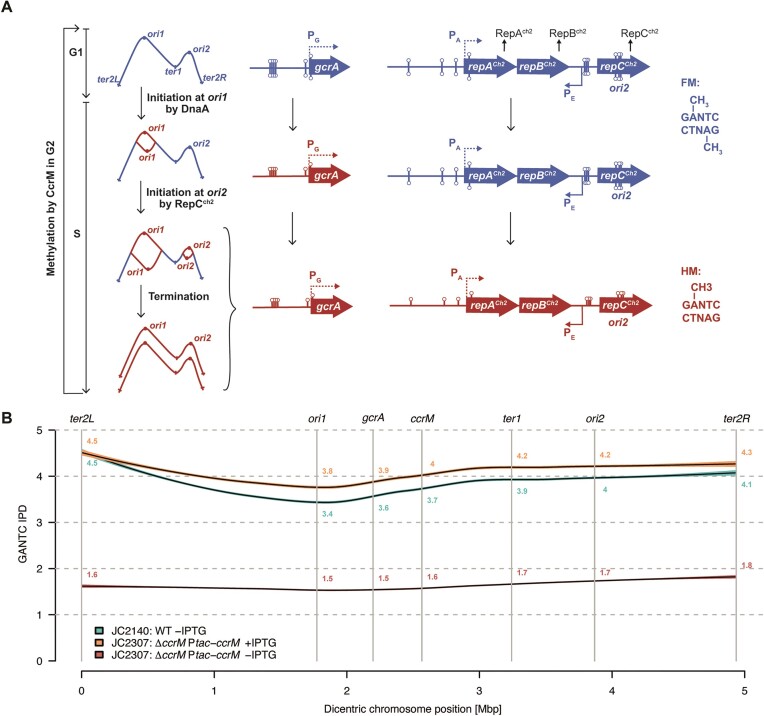
Methylation of GANTC motifs on the dicentric chromosome of A. tumefaciens. (A) Schematic showing the predicted methylation state of GANTC motifs depending on their chromosomal location and on cell cycle progression. ‘FM’ stands for fully-methylated DNA (blue color) with m6A on both GANTC strands and ‘HM’ stands for hemi-methylated DNA (red color) with m6A on only one GANTC strand (the newly-replicated strand is not yet methylated by the CcrM MTase). The right side of this schematic focuses on the predicted methylation state of the gcrA and repABCCh2 loci as a function of cell cycle progression, which depends on their chromosomal position. Lollipops indicate m6A in GANTC motifs (including 200 bp upstream of ORFs). (B) CcrM-dependent methylation of GANTC motifs on the A. tumefaciens dicentric chromosome. gDNA samples from WT and JC2307 (ΔccrM Ptac-ccrM) cells that grew exponentially in ATGN ± IPTG for 7 h were analyzed by SMRT-seq. GANTC IPD ratios were directly generated by the PacBio software. A smooth curve was then fitted between these ratios and the dicentric chromosome position using the LOESS function of R with default parameters. The 95% confidence interval was then plotted to predict average GANTC IPD ratios depending on their position on the dicentric chromosome from ter2L (position ‘0 Mbp’) to ter2R (position ‘4.936 Mbp’).
Here, we constructed a conditional ccrM mutant and combined methylome and transcriptome analyses, together with single cell reporter assays, to test the impact of CcrM-dependent methylation on the expression and the maintenance of the A. tumefaciens complex genome. Our results demonstrate that CcrM-dependent methylation is an essential process for fast-growing A. tumefaciens cells and that it has a major impact on the global transcriptome including on the expression of its three repABC operons. We also found that the timing or the frequency of ori2 firing/partitioning are affected when the A. tumefaciens genome becomes hypo- or hyper-methylated due to genetic perturbations. Altogether, we uncovered an original connection between CcrM-dependent methylation and genome maintenance in a pathogenic Alphaproteobacterium with an essential RepABC-dependent replicon.
Materials and methods
Bacterial growth conditions
Growth conditions are described in Supplementary Information.
Bacterial strains, plasmids and oligonucleotides
Bacterial strains, plasmids and primers used in this study are listed in Supplementary Tables S1, S2 and S3, respectively. The genomes of the A. tumefaciens C58 derivatives (from (29)) used in this study were analyzed by PCR as described before (28) and this analysis showed that these strains are so-called ‘C58 fusion strains’ with a unique dicentric chromosome (Supplementary Figure S1). This feature was subsequently verified when re-assembling the genomes of the WT/JC2307 strains from whole-genome SMRT-seq data.
Construction of plasmids and strains
Construction of plasmids and strains is described in Supplementary Information.
Microscopy
To visualize and analyze cell morphology, cells were fixed using a 5X-fix solution (150 mM NaPO4, 12.5% formaldehyde at pH7.5) and stored at 4°C before being imaged on 0.5× PBS (phosphate-buffered saline) and 1% agarose pads using a Plan-Apochromat 100×/1.45 oil phase contrast (Ph3) objective on an AxioImager M1 microscope (Zeiss) with a cascade 1K EMCCD camera (Photometrics) controlled by the VisiView 7.5 software. For phase contrast and fluorescence microscopy, live cells were immobilized onto 0.5× PBS + 1% agarose pads and imaged using the same microscope system as described above. To analyze the subcellular localization and the number of fluorescent origin foci, and to construct demographs, image analyses were performed using the Fiji 2.3.0 software with the MicrobeJ plugin (default parameters) (30).
gDNA preparation
Genomic DNA samples were prepared from 1 to 2 ml of bacterial cultures. Cells were pelleted and immediately frozen in liquid nitrogen prior to conservation at -80°C. gDNA was extracted using an isopropanol-ethanol purification kit (Puregene Yeast/Bact. Kit B from Qiagen) and following the manufacturer's protocol including a 60-minute RNase A treatment. gDNA was rehydrated in H2O. gDNA sample quality and quantity were assessed using a Nanodrop spectrophotometer before storage at −20°C.
SMRT-sequencing and analyses
High molecular weight gDNA was sheared with the Megaruptor 3 (Diagenode) to obtain 10–15 kb fragments. After shearing, the DNA size distribution was checked using a Fragment Analyzer (Agilent Technologies). Multiplexed SMRTbell libraries were prepared from 365 ng of sheared DNA with the PacBio SMRTbell Express Template Prep Kit 2.0 (Pacific Biosciences) according to the manufacturer's recommendations (protocol 101-696-100, v07). A final size selection step was performed with Pacific Biosciences Ampure beads to remove libraries smaller than 3 kb in size. The resulting libraries were pooled and sequenced over a 15H movie length on a single PacBio Sequel II SMRT cell 8M using the Binding kit version 1.0 and the Sequencing kit version 2.0 (Pacific Biosciences). To analyze the methylome of our strains, the raw data were processed using the SMRTlink version 10.2.0.133434 (Pacific Biosciences, USA). The tool named ‘Base Modification Analysis Application’ was then used to detect modified DNA bases and to identify methylated DNA motifs. IPD values of each GANTC motif reported by the PacBio software along with their genomic position (on the assembled genome sequence of the dicentric C58 strain) were extracted by the Perl script named ‘grabAllMotifs’ and deposited on Zenodo [https://zenodo.org/doi/10.5281/zenodo.12517805]. Additional GANTC motifs present on the genome but absent from the list returned by the PacBio software, were added using the script named ‘completeGANTC’, that also attributed an IPD value of 1.0 to the 3717 (out of 12 680) GANTC motifs from sample JC2307_neg_ipd for which the PacBio software did not return any value or reported ‘NA’. The final resulting IPD file has also been deposited on Zenodo as ‘all_GANTC_fused_WG.txt’, along with the script used to generate Figure 1B.
RNA extraction
RNA samples were prepared from 4 to 5 ml of bacterial cultures. Cells were pelleted and immediately frozen in liquid nitrogen prior to conservation at −80°C. RNA were extracted using the RNeasy Mini-Kit from Qiagen following the manufacturer's protocol and including a DNase I (RNase-free DNases set from Qiagen) treatment. Samples were additionally treated with a TURBO DNA-free kit from Invitrogen following the manufacturer's protocol. RNA samples were purified again and eluted in water. Absence of DNA contaminations was verified by standard PCR. Quality and quantity of RNA samples were verified on an agarose gel and using a Nanodrop spectrophotometer before storage at −80°C.
RNA-sequencing and analyses
RNA quality was assessed using a Fragment Analyzer (Agilent Technologies) and all RNAs had RQNs between 9.7 and 10. RNA-seq libraries were prepared from 800 ng of total RNA with the TruSeq Stranded mRNA Prep reagents (Illumina) using a unique dual indexing strategy, and following the official protocol automated on the Sciclone liquid handling robot (PerkinElmer). The polyA selection step was replaced by an rRNA depletion step with the QIAseq FastSelect - 5S/16S/23S bacterial rRNA removal kit (Qiagen). Libraries were quantified by a fluorometric method (QubIT, Life Technologies) and their quality assessed using the Fragment Analyzer. Sequencing was performed on the Illumina HiSeq 4000 v4 SR flow cell with the v4 HiSeq 3000/4000 SBS Kit reagents for 150 cycles. Sequencing data were demultiplexed using the bcl2fastq2 Conversion Software (version 2.20, Illumina). Sequences matching to ribosomal RNA sequences were removed with fastq_screen (v. 0.11.1) (31). Remaining reads were further filtered for low complexity with reaper (v. 15–065) (32). Reads were aligned against the Agrobacterium fabrum C58 ASM9202v1 genome using STAR (33) (v. 2.5.3a). The number of read counts per gene locus was summarized with htseq-count (34) (v. 0.9.1) using a custom Agrobacterium fabrum C58 ASM9202v1 gene annotation. Quality of the RNA-seq data alignment was assessed using RSeQC (35) (v. 2.3.7). Counts per gene table was used for statistical analysis in R (R version 4.1.0). Genes with low counts were filtered out according to the rule of 1 count per million (cpm) in at least 1 sample. Library sizes were scaled using TMM normalization (EdgeR package (36) version 3.34.0) and log-transformed with limma cpm function (Limma package (37) version 3.48.0). Differential expression was computed with limma by fitting the samples into a linear model and performing comparisons with moderated t-test. Global p-value adjustment with Bonferroni–Hotchberg method was used for all comparisons. Genes with an adjusted P-value < 0.01 and a minimum fold-change of 2 were considered as significantly mis-regulated. Volcano plots showing differentially expressed genes were made in R (v4.2.1) by plotting the log2(fold change) against the –log2(adjusted P-value) for this comparison.
qRT-PCR experiments and analyses
Primers were chosen based on secondary structure predictions using the Mfold web server for nucleic acid folding and hybridization prediction (default parameters, except ‘folding temperature’ set to 60°C) (38). Primer pairs were then checked by standard PCR on A. tumefaciens C58 gDNA (1 ng/μl) to verify that they gave a single amplification product of the expected size. Primer pairs were then checked again by qPCR (4 times 4-fold serial dilutions) to check efficiency. 700 ng of RNA samples were retrotranscribed using the Verso cDNA synthesis kit (Thermoscientific) for 30 min at 42°C into a final volume of 20 μl and following the manufacturer's protocol. The Verso reverse transcriptase was then inactivated by a 2-min incubation at 95°C before cDNA samples were stored at –20°C. For each qPCR assay, 2.4 μl of cDNA samples diluted 1:20 in Tris 10mM (pH 8.5) were used as templates. The qPCR reaction mix contained 0.3 μM of each primer into 10 μl of Power SYBR Green PCR Master Mix (Applied Biosystems). The 384-well plate was filled using an Evo® TECAN robot and transferred into a qPCR machine (Thermofisher - QuantStudio6) with automated threshold calculations (Software QuantStudio Q6_v1.6). Cycling: 10 min at 95°C once, 15 s at 95°C and 1 min at 60°C 40 times, and then 15 s at 95°C once. For RNA samples from the JC2307 strain, differences in gene expression were evaluated based on stable internal gene controls chosen from the RNAseq data: purH (Atu_2823, bifunctional purine biosynthesis protein) for ATGN ± IPTG 7 h conditions or yidC (Atu_0384, membrane protein insertase YidC) for YEB ± IPTG 5.5 h conditions. For RNA samples from the JC2899 strain, differences in gene expression were evaluated based on the stable hemF (Atu_2247, oxygen-dependent coproporphyrinogen oxidase) internal gene control. The Delta-Delta Ct method (39) was used to estimate relative mRNA levels from averages calculated from three technical replicates. Three biological replicates were used for each strain/condition tested.
Conjugation assays for functional analysis of the RepABCCh2/ori2 module
pSW25T-repABCCh2 derivatives were transferred from MFDpir E. coli donor cells (DAP auxotrophs in which the R6K origin is functional) to recipient S. meliloti Rm1021 cells (StrepR cells in which the R6K origin is not functional) by conjugation to determine if these plasmids were able to replicate in this Alphaproteobacterium. S. meliloti Rm1021 cells were grown overnight in LB supplemented with streptomycin, while MFDpir E. coli cells containing the pSW25T-repABCCh2 derivative were grown overnight in LB supplemented with DAP and kanamycin. The two overnight cultures (250mL) were then centrifuged at 8000 rpm for 3 min, washed and resuspended into 50 mL of LB supplemented with DAP. Next, donor and recipient cells were mixed 50/50 and spotted onto a nitrocellulose filter membrane placed on top of an LB agar plate supplemented with DAP. Plates were incubated overnight at 30°C. The next morning, cells were resuspended into 2ml of LB. To determine the proportion of S. meliloti Rm1021 cells that acquired the pSW25T-repABCCh2 derivative (trans-conjugants), 100 μl aliquots from serial 10-fold dilutions were plated on selective LB plates supplemented with kanamycin and streptomycin and on non-selective LB plates supplemented with streptomycin alone. After three days of growth at 30°C, conjugation efficiencies were estimated by calculating the ratio between the number of trans-conjugants (colonies that grew on selective media) and the total number of potential recipient cells (colonies that grew on non-selective media).
Promoter activity measurements by fluorometry
pOT1e derivatives (with WT or mutant PA-egfp or PE-egfp fusions) were inserted into C. crescentus NA1000 (JC450) cells by transformation. The resulting transformants were first grown overnight at 28°C in PYE supplemented with gentamycin and then diluted back into M2G supplemented with gentamycin. Overnight cultures were then diluted to an OD600∼0.05 into the same medium and transferred into 96-well-plates. Fluorescence intensities and OD600 were measured after ∼2 generations of growth (OD600∼ 0.2 ± 0.05) using a microplate reader (Biotek Synergy H1) using the following parameters: excitation at 488 nm and emission at 510 nm. Relative fluorescence units (RFU) were calculated as the fluorescence intensities (AU)/OD600. Promoter activities were then estimated by calculating the ratio between the RFU of test strains (carrying pOT1e derivatives with cloned promoters) and the RFU of the control strain with the empty pOT1e.
Statistics and reproducibility
Statistical methods and sample sizes (n) are indicated in figure legends for each experiment. Statistical analyses were done using the Excel, GraphPad-PRISM or R softwares.
Results
CcrM is essential in A. tumefaciens cells cultivated in complex media
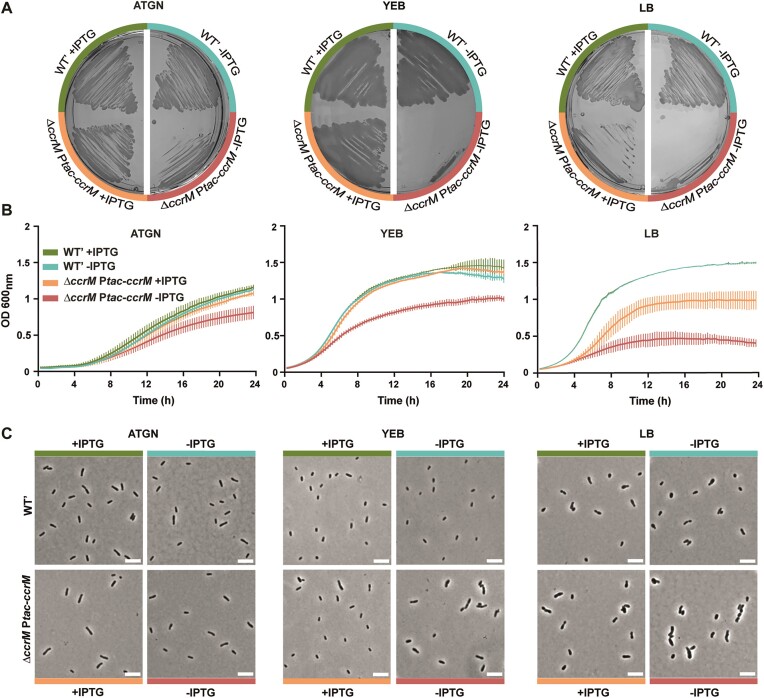
Considering the predicted essentiality of ccrM in A. tumefaciens (23,24), we engineered a conditional ccrM mutant to be able to analyze the methylome, the transcriptome and the phenotypes of cells as CcrM gets depleted. This enabled us to obtain information on the impact of DNA methylation in A. tumefaciens. In practice, a second copy of ccrM (Atu0794) under the control of the IPTG-inducible Ptac promoter was introduced at the tetRA locus of the dicentric chromosome of an A. tumefaciens C58 derivative strain, and then the native ccrM gene was deleted following a standard double recombination procedure (29), generating strain JC2307 (ΔccrM Ptac-ccrM) on ATGN minimal medium containing IPTG. Even if colonies grew very slowly on ATGN lacking IPTG compared to ATGN with IPTG (Figure 2A), colonies were still largely detectable, indicating either that CcrM was not efficiently depleted in the absence of the IPTG inducer, or that ccrM is not strictly essential under such growth conditions. Consistent with this first observation, we also found that JC2307 cells could grow relatively well in liquid ATGN medium, even if their growth rate progressively decreased over time after the removal of the IPTG inducer (Figure 2B). Colony forming unit (CFU) assays also showed that cell viability remained normal for extended periods of time (Supplementary Figure S2b, c), even if CcrM levels already became very hard to detect from cell extracts using immunoblotting experiments after 7 h of growth in ATGN without IPTG (Supplementary Figure S3). The morphology of CcrM-depleted cells (cultivated in ATGN without IPTG) appeared as relatively similar to control cells (Figure 2C), even if quantitative analyses showed that such cells were slightly shorter than control cells expressing ccrM (median length of 1.64 μm compared to 1.94–1.99 μm) (Supplementary Figure S4, ATGN).
Figure 2.
Depletion of CcrM results in slow growing, elongated and swollen cells when cultivated in complex media. (A) JC2141 (WT’) and JC2307 (ΔccrM Ptac-ccrM) cells were plated onto ATGN, YEB or LB agarose plates containing or not the IPTG inducer and colonies were then cultivated for ∼3 days prior to imaging. (B) JC2141 and JC2307 strains were pre-cultured into liquid ATGN, YEB or LB media + IPTG. Cells from over-night cultures were then washed and diluted to an OD600∼0.1 into the indicated medium and placed into a plate reader to record the OD600 every 15 min. Error bars represent means ± standard deviations from three biologically independent replicates. Note that the two WT’ curves overlap for the LB growth condition. (C) JC2141 and JC2307 strains were pre-cultured into liquid ATGN + IPTG and then diluted into ATGN or YEB or LB with IPTG. Once cultures reached exponential phase again, cultures were washed and cells were resuspended to an OD600∼0.2 into ATGN, YEB or LB ± IPTG at time T0. Cells were then fixed and imaged by phase-contrast microscopy after 24 h of growth. White scale bars inside images are 5 μm.
Strikingly, the impact of CcrM depletion was much stronger in cells cultivated in complex media. Indeed, JC2307 cells could hardly form colonies on YEB or LB plates lacking IPTG (Figure 2A), grew very slowly after a few generations in liquid YEB or LB media without IPTG (Figure 2B) and appeared as significantly elongated and/or enlarged (Figure 2C and Supplementary Figure S4) before they started to lyse/die (Supplementary Figure S2b, c). Time-lapse microscopy experiments also showed that most JC2307 cells cultivated onto YEB agarose pads lacking IPTG lysed or developed abnormal morphologies (swollen, elongated and multi-polar cells) over time (Supplementary Figure S5 and Supplementary Movies S1-S3). Importantly, the presence of IPTG in these media to induce Ptac-ccrM led to a complete (in YEB) or partial (in LB) complementation of these phenotypes (Figure 2 and Supplementary Figure S2 and S4), showing that they were, indeed, connected to insufficient levels of CcrM and not to polar effects.
Altogether, the use of this conditional mutant for phenotypic analyses demonstrated that CcrM is essential for the survival of A. tumefaciens cells cultivated in or on complex media, but that it may be dispensable, or at least not required at similar levels, in cells cultivated in or on minimal media.
CcrM-depleted cells cultivated in minimal medium have a hypo-methylated genome and display motility and adhesion defects
To test if CcrM levels become too limiting to ensure an efficient methylation of genomic GANTC motifs in the conditional mutant cultivated in minimal medium (when cells remain viable), we performed two assays. First, we compared the efficiency of digestion by HinfI of gDNA samples prepared from WT or JC2307 cells cultivated in ATGN with or without IPTG. HinfI is a methylation-dependent endonuclease that can only cut non-methylated GANTC motifs (23). Consistent with the known efficient activity of CcrM in A. tumefaciens, we confirmed that restriction of the WT genome by HinfI was essentially undetectable (Supplementary Figure S6). In contrast, the genome of JC2307 cells cultivated in ATGN without IPTG for 7 h became highly sensitive to HinfI digestion (Supplementary Figure S6), indicating that a majority of its double-stranded GANTC motifs become un-methylated when CcrM is depleted. To get a more quantitative evaluation of the methylation state of the A.tumefaciens genome upon CcrM depletion, we next analyzed the methylome of cells cultivated in these same growth conditions using single molecule real-time sequencing (SMRT-Seq), a method that is now commonly used to distinguish methylated (m6A) from non-methylated adenines on bacterial genomes based on measures of interpulse duration (IPD) (40,41). Using gDNA extracted from WT cells, we found that the average IPD ratio of adenines located in GANTC motifs located near ori1 (IPD ∼ 3.4) was lower than that of GANTC motifs located next to ter2L (IPD ∼ 4.5) on the dicentric chromosome (Figure 1B), which is fully consistent with the known cell cycle regulation of CcrM-dependent methylation in A. tumefaciens cells (Figure 1A) (23). Indeed, if CcrM was present and active during the whole S-phase of the cell cycle in A. tumefaciens, newly replicated double-stranded GANTC motifs located next to ori1 would not stay in a hemi-methylated state for a long enough period to be detectable. Interestingly, the methylome of JC2307 (ΔccrM Ptac-ccrM) cells cultivated in ATGN with the IPTG inducer was very similar to the methylome of WT cells (Figure 1B and Supplementary Figure S7), indicating that CcrM is probably regulated by post-transcriptional mechanisms of regulation in A. tumefaciens, similarly to what happens in C. crescentus (42,43). In contrast, using gDNA from JC2307 cells cultivated in ATGN without IPTG for 7 h, the average IPD ratio of adenines located in GANTC motifs throughout the dicentric chromosome dropped to an average value of ∼1.6 (compared to an average of ∼3.8 for WT cells and ∼4.1 for ΔccrM Ptac-ccrM cells cultivated in ATGN with IPTG) (Figure 1B and Supplementary Figure S7). This observation confirmed that a vast majority of the 5166 double-stranded GANTC motifs found on the dicentric chromosome of JC2307 cells are in a non-methylated state after 7 hours of CcrM depletion. Importantly, finding viable conditions (Figure 2 and Supplementary Figure S2) when A. tumefaciens cells display a largely hypo-methylated genome (Figure 1B and Figures S6 and S7) made it possible to test the impact of DNA methylation by CcrM on the transcriptome of A. tumefaciens cells with a complex multicentric genome.
Interestingly, even if such mutant cells did not display striking viability or cell morphology defects in ATGN medium (Figure 2 and Figures S2 and S4), we still noticed two interesting phenotypes. First, cells displayed a significant swarming defect as could be seen with the ∼35% decrease in swarming area when placed onto semi-solid ATGN medium without IPTG compared to semi-solid ATGN with IPTG (Supplementary Figure S8a). Second, we observed a ∼35% decrease in adhesion/biofilm formation in ATGN without IPTG (Supplementary Figure S8b) as could be measured using static coverslip assays with crystal violet (29). These phenotypes may relate to the slower growth of CcrM-depleted cells or result from changes in gene expression.
Altogether, these methylome and phenotypic assays showed that A. tumefaciens cells with hypo-methylated GANTC motifs on their genome are viable in minimal medium, but still display detectable phenotypes suggesting that some genes may be mis-expressed as a result of their hypo-methylation.
DNA methylation by CcrM has a major impact on the A. tumefaciens transcriptome
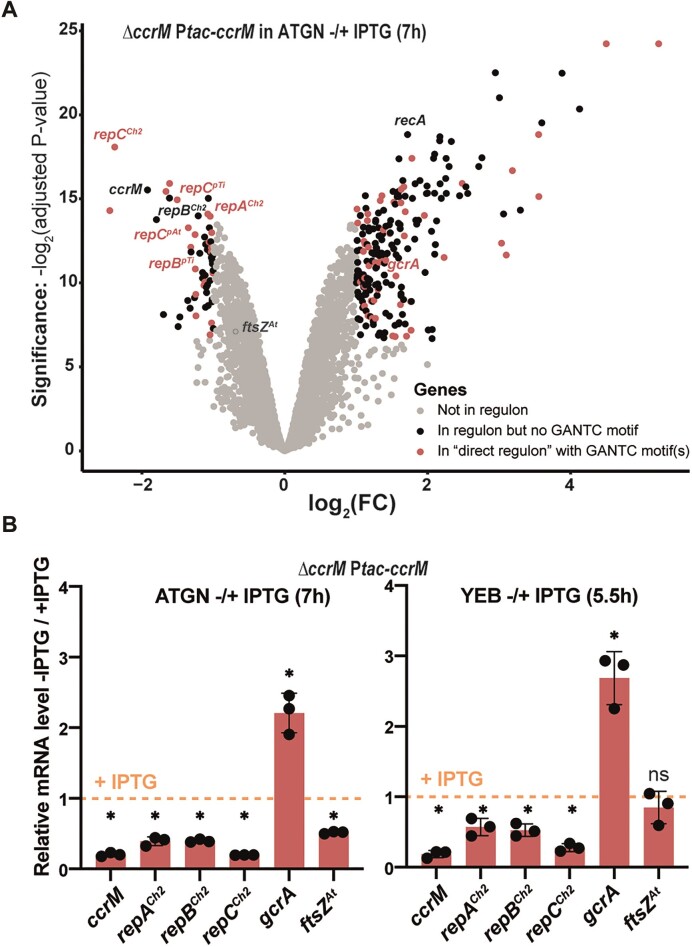
To collect information on the origin of the observed phenotypes (Figure 2 and Supplementary Figure S2, S4 and S8) and gather potential cues on why DNA methylation by CcrM becomes essential in cells cultivated in complex media (Figure 2 and Supplementary Figure S2), we next decided to compare the transcriptome of viable A. tumefaciens JC2307 cells after the drop in detection of methylated GANTC motifs (7 h in ATGN without IPTG) with that of mutant cells cultivated in ATGN with IPTG or of WT cells (Figure 1B). RNA-Seq experiments revealed a significant (adjusted P-value < 0.01) and strong (fold change > 2) impact on the expression of 273 genes (Figure 3A, Supplementary Table S4 and Supplementary Figure S9): 59 genes were down-regulated, while 214 genes were up-regulated in response to CcrM depletion (comparing JC2307 cells cultivated with or without IPTG for 7 h). Instead, the transcriptome of CcrM-repleted cells (JC2307 cells cultivated with IPTG), appeared as extremely similar to the transcriptome of WT cells (Supplementary Figure S9 and Supplementary Table S4), showing that expression of ccrM from the Ptac promoter instead of from its native promoter does not lead to significant gene mis-regulation. Among the 273 genes that were significantly mis-regulated upon CcrM depletion, 75 genes (27%) displayed at least one GANTC motif in their putative promoter region (200 bp upstream of each ORF), including 22 of the 59 (37%) down-regulated genes and 53 of the 214 (25%) up-regulated genes (Figure 3A and Supplementary Table S5). Thus, genes with putative promoters carrying GANTC motif(s) are significantly enriched among the genes that appear as (directly or indirectly) activated by CcrM compared to random A. tumefaciens genes (24% have GANTC motif(s) in their putative promoter region).
Figure 3.
Depletion of CcrM has a major impact on the A. tumefaciens transcriptome in cells cultivated for 7 hours in minimal medium. (A) Volcano plot showing RNA-Seq results and analyses comparing the transcriptome of exponentially growing JC2307 (ΔccrM Ptac-ccrM) cells cultivated in ATGN ± IPTG for 7 h (same cultures as those used for methylome experiments described in Figure 1B). FC indicates the fold-change when comparing the - IPTG condition with the + IPTG condition. Each dot corresponds to a gene. Grey dots correspond to genes that are not considered as differentially regulated (= FC < 2 or adjusted P-value > 0.01). Black dots correspond to 198 genes that are differentially regulated (FC > 2 and adjusted P-value < 0.01) but that do not contain a GANTC motif in the 200 bp region upstream of the ORF. Red dots correspond to the 75 genes that are differentially regulated (FC > 2 and adjusted P-value < 0.01) and that do contain minimum one GANTC motif in the 200 bp region upstream of the ORF (‘direct regulon’). The precise identity/annotation/COG category/GANTC motif locations for all genes can be found in Supplementary Tables S4 and S5. A Glimma Volcano Plot (interactive HTML graphic) is also available in the supplementary information. Adjusted P-values were calculated based on three independent biological replicates for each strain/growth condition. (B) qRT-PCR results confirming the impact of CcrM on the expression of a selection of genes in JC2307 (ΔccrM Ptac-ccrM) cells cultivated in minimal (left) and complex (right) media. The graphs show relative mRNA levels in cells cultivated without the IPTG inducer, compared to cells cultivated with the IPTG inducer (then set to an arbitrary value of 1 for each gene = orange dotted line). The same RNA samples as those used in (A) were used for the ‘ATGN ± IPTG (7 h)’ panel. RNA samples used for the ‘YEB ± IPTG (5.5 h)’ panel were prepared from JC2307 cells pre-cultured in ATGN + IPTG and then diluted into YEB + IPTG for growth until exponential phase. Cells were then washed and resuspended into YEB ± IPTG at Time 0. RNA samples were prepared after 5.5 h of growth. For both panels, three biological replicates with three technical replicates each were used. Significant differences (Wilcoxon rank sum test) when comparing – IPTG / + IPTG are indicated by * (P< 0.0001). ns: not significant (P-value > 0.05).
As a first point of interest, we compared this potential ‘direct regulon’ of CcrM (75 genes) in A. tumefaciens (Supplementary Table S5) with previously published ‘direct regulons’ of CcrM in the distantly related C. crescentus (152 genes) and B. subvibrioides (129 genes) Alphaproteobacteria. This analysis showed that only six genes of the A. tumefaciens CcrM ‘direct regulon’ had orthologs that also belonged to the C. crescentus or B. subvibriodes ‘direct regulons’ (Supplementary Table S5). Even if the precise transcriptional start site (TSS) of each gene/operon is not yet known in A. tumefaciens, we concluded that epigenetic mechanisms of regulation apparently evolved significantly between Alphaproteobacteria with different genome architectures and lifestyles.
Another striking new finding was that the A. tumefaciens CcrM regulon included six genes belonging to the three repABC operons (essential for the replication of the dicentric chromosome and of the two mega-plasmids) among the genes that were significantly down-regulated in CcrM depleted cells (Figure 3a and Supplementary Table S5). In addition, many genes encoding proteins potentially involved in SOS-related responses to DNA damage (44) or replication defects (examples: RecA, RecQ, three orthologs of DNA Pol Y and three orthologs of ImuA) were significantly up-regulated. Noteworthy, the gcrA homolog of A. tumefaciens (Atu0426), encoding a putative methylation-sensitive global regulator, was strongly activated (2.8-fold induction) upon CcrM depletion, while the ftsZAt (Atu2086/ftsZ2) gene required for A. tumefaciens cell division was hardly repressed (less than 2-fold) in ATGN medium (Figure 3 and Supplementary Table S4) confirming strong differences with what was previously observed in C. crescentusΔccrM mutant cells (11,19). The impact of CcrM depletion on the expression of this interesting selection of genes was also verified by qRT-PCR (Figure 3B and Supplementary Figure S10) using RNA samples prepared not only from mutant cells cultivated in ATGN ± IPTG for 7 h (same conditions as the RNA-Seq experiments), but also from mutant cells cultivated in YEB ± IPTG for 5.5 h (prior to the detection of cell death without the IPTG inducer of Ptac-ccrM as shown in Supplementary Figure S2). These experiments showed that a lack of DNA methylation by CcrM also reduces repCCh2/repCpTi/RepCpAt expression and promotes gcrA expression in cells cultivated in complex YEB medium, while it does not have a significant impact on the expression of the essential ftsZAtgene under such growth conditions (Figure 3B, right panel).
These discoveries led us to hypothesize that CcrM-depleted cells may express sub-optimal levels of the essential RepCCh2ori2 initiator and of the RepABCh2ori2 partitioning proteins, leading to potential replication delays from ori2. This may also relate to the essentiality of ccrM in fast-growing A. tumefaciens cells (in YEB and LB media) (Figure 2 and Supplementary Figure S2) since initiation at ori2 is apparently essential in A. tumefaciens (28).
Replication/partitioning of ori2 is decoupled from replication/partitioning of ori1 in CcrM-depleted cells
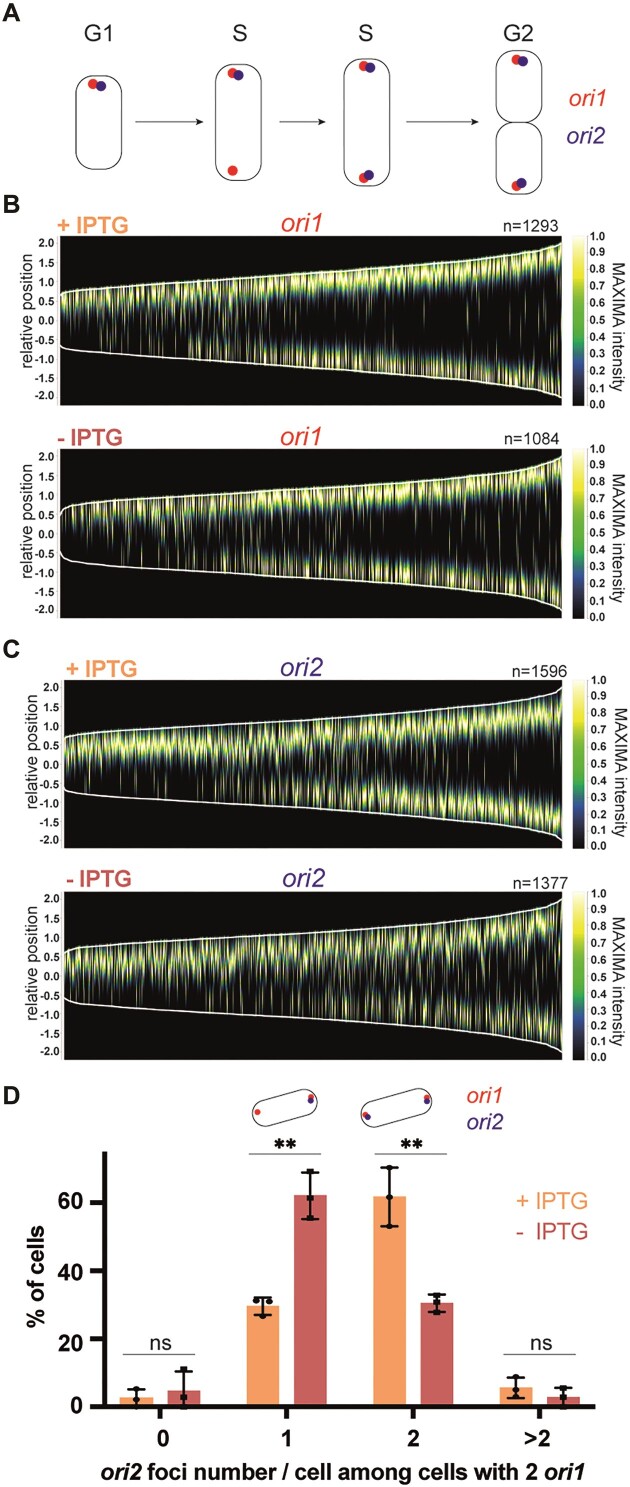
A few recent studies used live cell fluorescence microscopy experiments to visualize the number and the sub-cellular localization of ori1 and ori2 as a function of cell cycle progression (cell length). These studies showed that ori1 and ori2 colocalize at the old pole of newborn G1 phase cells (45) (Figure 4A). At the onset of the S-phase of the cell cycle, replication first starts at ori1 and then at ori2 following a significant delay. Once duplicated, one copy of each origin (ori1 and then ori2) moves towards the new cell pole (46) (Figure 4A). Importantly, the timing of origin firing and the localization of origins are strikingly similar in C58 strains with two chromosomes compared with strains with one dicentric chromosome (28). We therefore decided to use these same fluorescent reporters to visualize the impact of CcrM depletion on the timing of ori1 and ori2 replication and on their partitioning during the A. tumefaciens cell cycle.
Figure 4.
Depletion of CcrM results in ori2 replication/partitioning delays in cells cultivated in minimal medium for prolonged periods of time. (A) Schematic representing the subcellular localization of ori1 and ori2 during the A. tumefaciens cell cycle. (B and C) Demographs showing the subcellular localization of ori1 (B) or ori2 (C) foci as a function of cell size (from images as shown in Supplementary Figure S8). JC2660 (ΔccrM Ptac-ccrM with ori1/ygfp reporter in (B)) or JC2661 (ΔccrM Ptac-ccrM with ori2/ygfp reporter in (C)) cells were cultivated over-night in ATGN ± IPTG. Cultures were then diluted into ATGN ± IPTG and grown exponentially for ∼6.5 h (same medium at all steps). Relative position = 0 corresponds to mid-cell; only cells measuring from 1 to 4 μm-long were included into these demographs; n = number of cells used to construct each demograph. (D) Quantification of ori2 number per cell among cells that are in S-phase (with two ori1). JC2836 (ΔccrM Ptac-ccrM with ori1/mcherry and ori2/ygfp reporters) cells were cultivated over-night in ATGN ± IPTG. Cultures were then diluted into ATGN ± IPTG and grown exponentially for ∼6.5 h (>15 h into ATGN ± IPTG). Phase contrast/YGFP/mCherry images were acquired as shown in Supplementary Figure S9a. Among cells that displayed minimum two ori1 foci (S-phase cells from Supplementary Figure S10), the number of ori2/cell was measured (from minimum 100 cells/condition) and means from three independent experiments were plotted for each condition. Error bars correspond to standard deviations. Student's t-test: ns = P-value > 0.01, ** = P-value < 0.01.
As a first test, we visualized ori1 or ori2 localization using a ygfp-parBpMT1-parSpMT1 reporter integrated next to ori1 or ori2 (46), respectively, in the dicentric chromosome of JC2307 (ΔccrM Ptac-ccrM) cells. Cells were cultivated exponentially in ATGN ± IPTG and then imaged by fluorescence microscopy. Analyses of images (Supplementary Figure S11a and b) and demographs (Figure 4B, C) revealed that the duplication/partitioning of ori2 tends to take place in CcrM-depleted (ATGN - IPTG) cells that have reached a longer length compared to CcrM-repleted (ATGN + IPTG) cells (Figure 4C), while no obvious difference could be seen when looking at ori1 duplication/partitioning (Figure 4B). Moreover, a lower proportion of CcrM-depleted cells (∼18%) displayed two ori2 foci compared to CcrM-repleted cells (∼38%) (Supplementary Figure S11b), while CcrM depletion had an insignificant impact on the overall proportion of S-phase cells (∼39% instead of 43% of cells displayed 2 ori1 foci) (Supplementary Figure S11a). These first observations suggested that ori2 duplication or partitioning is/are specifically delayed in cells that display a hypo-methylated genome.
As a second test, we also co-visualized ori1 and ori2 in JC2307 cells using a first ygfp-parBpMT1-parSpMT1reporter near ori2 and a second mcherry-parBP1-parSP1 reporter near ori1 (45). Fluorescence imaging of cells growing exponentially in ATGN ± IPTG (Supplementary Figure S12a) and analyses of demographs (Supplementary Figure S12b) confirmed that duplication/partitioning of ori2 tends to take place in CcrM-depleted (ATGN - IPTG) cells that have reached a longer length compared to CcrM-repleted (ATGN + IPTG) cells. Quantitative analyses using this double-labelled strain further showed that the proportion of S-phase cells (with two ori1 foci) also displaying two ori2 foci dropped ∼2-fold (from ∼62% to ∼30%) when mutant cells were cultivated in ATGN-IPTG compared to ATGN + IPTG (Figure 4D), while the overall proportion of S-phase cells (with two ori1 foci) remained essentially similar (from ∼58% to ∼52%) (Supplementary Figure S13). Altogether, these results indicate that replication initiation at ori2 and/or ori2 partitioning are/is specifically delayed in mutant A. tumefaciens cells displaying a hypo-methylated genome, coinciding with a lower expression of the repABCCh2 operon (Figure 3).
Chromosome replication over-initiates from ori2 in CcrM-overexpressing cells
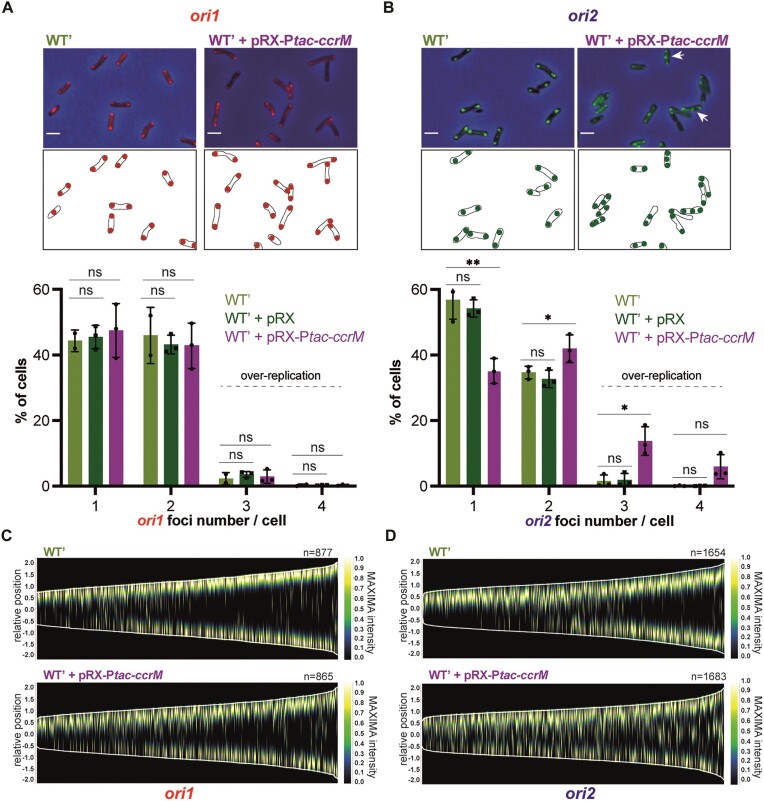
The results described above suggest that DNA methylation by CcrM promotes DNA replication from ori2 in A. tumefaciens cells. To confirm this hypothesis, we also looked at the impact of CcrM over-expression on the number of ori1 and ori2 foci per cell. We expect that CcrM over-expression from the pRX-Ptac-ccrM vector leads to a significant hyper-methylation of the A. tumefaciens genome with most GANTC motifs being fully-methylated throughout the cell cycle as previously shown using related vectors (23). In ATGN medium containing IPTG, only ∼35% of the cells over-expressing CcrM displayed a single ori2 focus, compared to ∼54% of the cells carrying the empty control vector, while differences concerning ori1 appeared as non-significant (47% compared to 45% with P-value > 0.05) (Figure 5A, B). This observation suggested that replication from ori2 can start sooner after (or even before) replication from ori1 in cells with a hyper-methylated genome compared to WT cells, which is supported by demographs comparing the timing of ori1/ori2 duplication as a function of cell size/cell cycle progression (Figure 5C, D). Consistent with such a boost of replication from ori2, we also observed that ∼20% of the CcrM-overexpressing cells displayed more than two ori2 foci per cell, something that only very rarely (∼2% of cells) happened in WT cells (Figure 5A, B). Thus, A. tumefaciens cells with a hyper-methylated genome regularly over-initiate DNA replication from ori2.
Figure 5.
Overexpression of CcrM results in hyper-initiation events at ori2 in cells cultivated in minimal medium. (A) and (B) selected microscopy images of JC2656 (WT' with ori1/ygfp reporter) cells (panel A) or JC2657 (WT' with ori2/ygfp reporter) carrying or not the pRX-Ptac-ccrM vector. Cells were cultivated over-night in ATGN + IPTG. Cultures were then diluted into ATGN + IPTG and grown exponentially for ∼6.5 h. Upper panels: overlays of phase contrast and YGFP images. Lower panels: schematics showing ori1 (red color in (A)) or ori2 (green color in (B)) subcellular localization in the cells imaged above. Under these microscopy images, plots show the proportion of cells with the indicated number of ori1 (in (A)) or ori2 (in (B)) foci for each strain (pRX is the empty control vector). Minimum two independent experiments (with a total of minimum 1000 cells) were done for each strain with each dot corresponding to an independent experiment and mean values were plotted with error bars corresponding to standard deviations. Student's t-test: ns = P-value > 0.05, * = P-value < 0.05, ** = P-value < 0.01. (C, D) Demographs showing the subcellular localization of ori1 (panel c) or ori2 (panel d) foci as a function of cell size (from images as shown in panels (A) and (B)). Relative position = 0 corresponds to mid-cell; only cells measuring from 1 to 4 μm-long were included into these demographs; n = number of cells used to construct each demograph.
To test if this over-initiation at ori2 may be linked with a transient over-expression of the repABCCh2 operon in these cells, we next compared the repACh2/repBCh2/repCCh2 mRNA levels by qRT-PCR analyses on RNA samples extracted from WT cells carrying pRX (the repABCCh2/ori2 module is then transiently hemi-methylated during the second half of the S-phase of the cell cycle as shown in Figure 1A) or pRX-Ptac-ccrM (the repABCCh2/ori2 module is then probably fully-methylated throughout the whole cell cycle) and cultivated in the same ATGN + IPTG conditions. We found that the expression of these three genes was not significantly affected in such a population of A. tumefaciens cells with a hyper-methylated genome, compared to control cells (Supplementary Figure S14). This result was not particularly surprising since ccrM overexpression is expected to change the methylation state of the repABCCh2 operon during an only very limited period of the cell cycle of each cell in the population (only during the second part of the S-phase of the cell cycle, estimated as less than 20% of the complete cell cycle). Then, the observed over-initiation at ori2 when ccrM is over-expressed cannot be attributed to a simple excess of repCCh2 expression. Instead, we believe that preventing the transient hemi-methylation of the GANTC motifs found in the predicted ori2 (Figure 6A) in these cells may disturb the frequency of replication initiation at ori2 during the S-phase of the cell cycle as initiation events can easily take place within minutes if conditions are favorable.
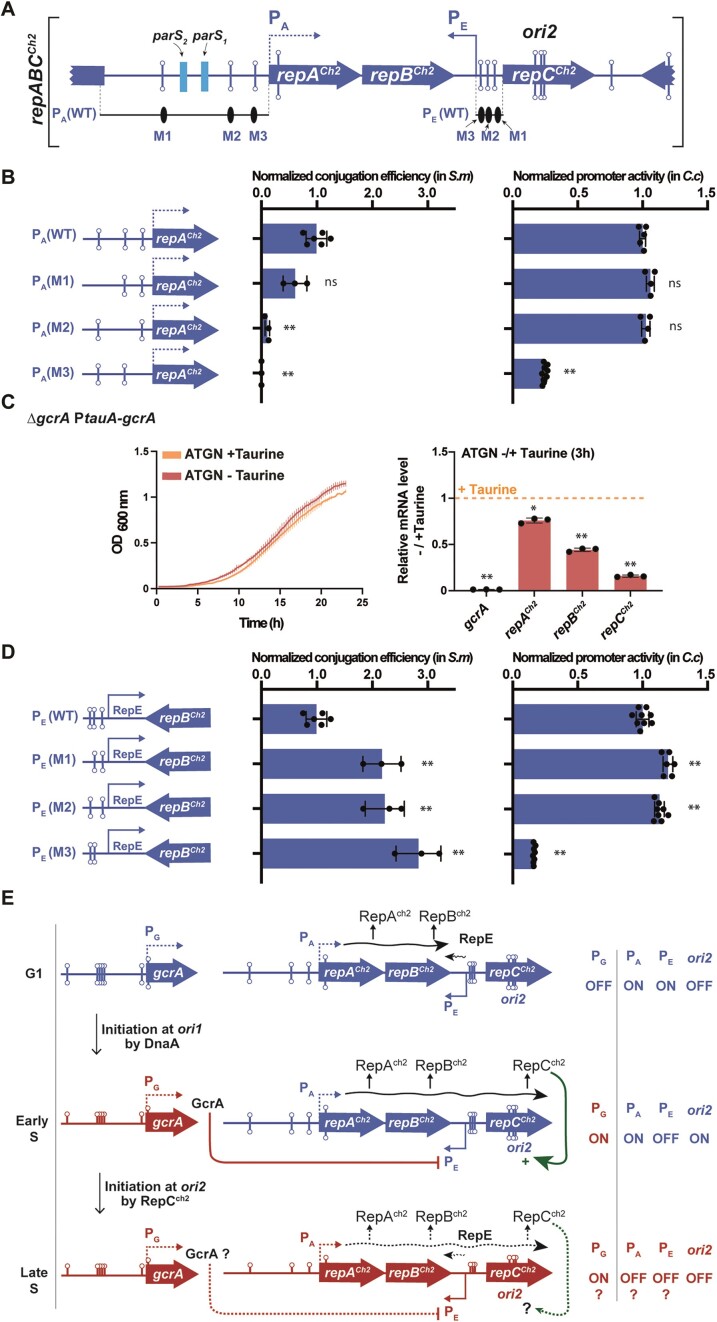
Figure 6.
The RepABCCh2/ori2 module is sufficient for autonomous replication and the expression of its RepCCh2 initiator is activated by GcrA. (A) Schematic of the repABCCh2/ori2 module showing where ORFs (blue arrows), GANTC motifs (lollipops) and predicted parS sites (light blue rectangles) (9) are located. Note that the three ORFs are not drawn to scale to better visualize the organization of IG regions. This schematic shows the exact WT module cloned into pSW25T for the conjugation assays shown in the left panels of (B) and (D). It also shows the PA(WT) and PE(WT) promoter regions cloned into pOT1e for the promoter activity assays shown in the right panels of (b) and (d), respectively. (B) Impact of the three GANTC motifs found in the PA(WT) promoter on plasmid replication/maintenance in S. meliloti cells (left) and on promoter activity in C. crescentus cells (right). For the PA(M1), PA(M2) and PA(M3) derivatives of PA(WT), single methylatable GANTC motifs were mutated into non-methylatable GTNTC motifs. Means from minimum 3 independent experiments were plotted for each construct. (C) The impact of GcrA on repABCCh2 expression in A. tumefaciens. Left panel: JC2899 (ΔgcrA PtauA-gcrA) cells were pre-cultured into ATGN + Taurine. Cells were then washed and diluted to an OD600∼0.05 into ATGN ± Taurine and placed into a plate reader to record OD600 every 15 min. Error bars represent means ± standard deviations from three biologically independent replicates. Right panel: JC2899 cells were pre-cultured in ATGN + Taurine. Once cells reached exponential phase, they were then washed and resuspended to an OD600∼0.2 into ATGN ± Taurine. RNA samples were prepared after 3h of growth for qRT-PCR analyses. The graph shows relative mRNA levels in cells cultivated without the Taurine inducer, compared to cells cultivated with the Taurine inducer (then set to an arbitrary value of 1 for each gene = orange dotted line). Three biological replicates with three technical replicates were used for each condition. Significant differences (Wilcoxon rank sum test) when comparing -Taurine/+Taurine are indicated by * (P< 0.05) and ** (P< 0.01). (D) Impact of the three GANTC motifs found in the PE (WT) promoter on plasmid replication/maintenance in S. meliloti cells (left) and on promoter activity in C. crescentus cells (right). For the PE(M1), PE(M2) and PE(M3) derivatives of PE(WT), single methylatable GANTC motifs were mutated into non-methylatable GTNTC motifs. For (B) and (D), minimum 3 independent samples (dots) were used for each construct. Conjugation efficiencies and promoter activities were normalized so that the mean value of the WT construct equals one in each case. Mean values were plotted with error bars corresponding to standard deviations. Student's t-tests comparing mutant constructs with WT constructs: ns = not significant (P-value > 0.05), ** = P-value < 0.001.(E) Model for the cell cycle-dependent regulation of the repABCCh2/ori2 module by GcrA. Dashed arrows or ‘?’ indicate still hypothetical regulatory pathways. Wavy lines indicate when transcription from PA (promoter region upstream of the repABCCh2operon) or PE (promoter driving RepECh2 expression) is expected to take place based on their methylation states. When the TSS could not be predicted (for PA and PG), it was arbitrarily positioned at the beginning of the ORF.
Dissecting the impact of GANTC motifs and GcrA on the expression and the function of the repABCCh2/ori2 module
Looking more carefully at the repABCCh2/ori2 module (Figure 6A), we notably found that it carries three GANTC motifs in the promoter region controlling the transcription of the repABCCh2 operon (PA), three GANTC motifs in the intergenic (IG) region located between repBCh2 and repCCh2, and three last ones overlapping the putative ori2 region inside repCCh2. We thus hypothesized that the methylation of some of these motifs by CcrM may affect the expression and/or the function of the different elements of the repABCCh2/ori2 module.
As a first step to get mechanistic insight into the reasons why CcrM can promote ori2 replication/partitioning, we cloned the entire repABCCh2/ori2 module into a vector (pSW25T) that carries a unique conditional R6K origin that can only function in pir+ bacterial cells (Supplementary Figure S15a). We then tried to move this recombinant plasmid from pir+ E. coli donor cells (MFDpir) to pir- E. coli recipient cells (DH5α) by conjugation. Interestingly, trans-conjugants were not obtained (trans-conjugants/recipient cells < 10−7), showing that the repABCCh2/ori2 module is not sufficient for the autonomous replication/maintenance of this recombinant RepABCCh2-dependent plasmid in pir- E. coli cells. This observation supports the notion that specific regulators present in Alphaproteobacteria but not Gammaproteobacteria, such as CcrM and/or GcrA, may play an essential role in allowing ori2-dependent replication. We next tried to transfer this RepABCCh2-dependent plasmid into Sinorhizobium meliloti cells that do have CcrM and GcrA homologs (note here that we avoided making these tests in A. tumefaciens cells as we felt that it would most likely interfere with the replication/partitioning of the ori2-dependent chromosome that is essential for cell viability) (Supplementary Figure S15b). Using S. meliloti as recipient cells, we obtained many trans-conjugants with the pSW25T-repABCCh2(WT) plasmid compared to essentially none with the empty pSW25T’ control (Supplementary Figure S15c), demonstrating that the RepABCCh2/ori2 module of A. tumefaciens contains a functional origin and that it is encoding or finding all the necessary proteins for autonomous replication/partitioning once it enters S. meliloti cells by conjugation. We next tested whether any of the three methylatable A bases present in the PA promoter region controlling repABCCh2transcription may be required for RepABCCh2-dependent replication. Indeed, site-directed mutagenesis of each of these three methylatable A into non-methylatable T bases (GANTC motifs mutated into GTNTC mutations), showed that two of these A (in M2 and M3 motifs) were critical for RepABCCh2-dependent replication, as trans-conjugants were now only very rarely obtained (Figure 6B, left panel). Transcriptional fusions between the WT or the mutated PA promoters (479 bp region upstream of repA) and an egfp reporter further showed that the A present in the methylatable M3 GANTC motif was critical for transcription from PA in Caulobacter crescentus cells (also naturally expressing ccrM and gcrA homologs) (Figure 6B, right panel). Altogether, these results indicate that M1 has no significant impact on repABCCh2/ori2 expression or function, while M2 and M3 do play an important role in the ability of repABCCh2/ori2 to replicate/maintain the recombinant plasmid. The A present in the M3 motif is apparently required for repABCCh2transcription and plasmid replication, while the A present in the M2 motif may instead be required for repABC/ori2-dependent plasmid partitioning since it is located closer to the two predicted parS sites (9) (Figure 6A).
Knowing that GcrA is usually the methylation-sensitive transcriptional regulator working together with CcrM in Alphaproteobacteria (15,18,21,47), we next hypothesized that GcrA may also be part of this potential epigenetic mechanism controlling repABCCh2 expression in A. tumefaciens. To test this possibility, we constructed a conditional gcrA mutant where the only copy of gcrA is under the control of the taurine-inducible PtauA promoter. This mutant grew relatively well into ATGN minimal medium complemented or not with the taurine inducer (Figure 6C, left panel), showing that GcrA is not essential for the survival of A. tumefaciens in these tested conditions. We then used these same growth conditions to test if GcrA affects repACh2, repBCh2 and/or repCCh2 expression by qRT-PCR (Figure 6C, right panel). As expected, mutant cells cultivated for 3 h without the taurine inducer expressed negligible amounts of gcrA compared to cells cultivated with the inducer. This analysis then showed that GcrA has a limited impact on repACh2 expression, while it apparently acts as a very significant (direct or indirect) inducer of repBCh2 and repCCh2 expression (Figure 6C, right panel).
Since the impact of GcrA on repCCh2 was much stronger than on repACh2, we hypothesized that the main role of GcrA during repABCCh2 regulation may be to repress a putative RepECh2 anti-sense RNA transcribed from the IG region located between repBCh2 and repCCh2, rather than to activate the PA promoter. A closer look at this IG region (Supplementary Figure S16) revealed a striking resemblance with the known PE core promoter of pTi driving the synthesis of the RepEpTi anti-sense RNA that represses repCpTitranscription and translation (10). To verify that this IG region in repABCCh2 also includes an active antisense PE promoter, we constructed a transcriptional reporter with egfp under the control of this putative PE promoter (96 bp region upstream of the predicted TSS of repECh2) and introduced it into C. crescentus cells. Fluorescence measurements showed that this region does contain an active PE promoter (Figure 6D, right panel), confirming the existence of a RepECh2 antisense RNA that is then expected to repress repCCh2 expression (Figure 6E). Then, it is likely that GcrA represses this PE promoter to promote repCCh2 (and repBCh2) expression. Since GcrA usually binds to methylated promoters, some of the GANTC motifs found on this PE promoter may have a negative impact on repECh2 expression. To test this assumption, we mutated each of the three GANTC motifs found in the PE promoter into non-methylatable GTNTC motifs and tested the impact of these mutations on PE activity in C. crescentus cells. This assay showed a limited, but still significant, increase in PE activity when the M1 and M2 motifs of the PE promoter were mutated, while the mutation introduced into the M3 motif apparently blocked most of the activity the promoter (Figure 6D, right panel). This last observation was not particularly surprising since the M3 motif overlaps the predicted -10 region of the PE core promoter (Supplementary Figure S16). This M3 mutation also improved the replication/maintenance of our engineered RepABCCh2-dependent plasmids in S. meliloti (Figure 6D, left panel), consistent with the negative impact of RepECh2 on RepABCCh2-dependent replication. Intriguingly, we observed that the M1 and M2 mutations improved the replication/maintenance of our engineered RepABCCh2-dependent plasmids in S. meliloti (Figure 6D, left panel) even if these mutations did not repress PE activity (Figure 6D, right panel). These mutations may, instead, promote the stability or the translation of the polycistronic repABCCh2 mRNA.
Dissecting the impact of DNA methylation by CcrM on gcrA expression
Knowing that GcrA promotes repCCh2 expression (Figure 6C), we wished to gather more information on how CcrM-dependent methylation may repress gcrA expression (Figure 3). Strikingly, we observed that the 250bp region located upstream of the gcrA ORF, which is expected to include the gcrA promoter (PG), carries 6 GANTC motifs (Supplementary Figure S17a). This is a strong over-representation compared to the average distribution of GANTC motifs on the A. tumefaciens chromosome (Supplementary Figure S7). To confirm that PG is in this region and to test whether the methylation of these motifs may repress the activity of PG (consistent with our previous observation that gcrA is activated in CcrM-depleted cells shown in Figure 3), we cloned it into the placZ290 vector to create a PG-lacZ transcriptional reporter. This reporter was then introduced into WT and JC2307 A. tumefaciens cells or into E. coli cells that expressed or not a catalytically active CcrM MTase to compare the activity of PG by β-galactosidase assays. These assays showed that PG was significantly less active in WT or CcrM-repleted than in CcrM-depleted A. tumefaciens cells (Supplementary Figure S17b) and in Escherichia coli cells expressing an active CcrM MTase than in control cells expressing a catalytically inactive variant (Supplementary Figure S17c). These results suggest that the gcrA promoter is less active when it is methylated than when it is hypo- or non-methylated, even in the absence of Alphaproteobacterial-specific transcriptional regulators since the effect is already detectable in engineered E. coli cells that methylate GANTC motifs constitutively. It is then possible that the full methylation of this PG promoter in G1 phase A. tumefaciens cells may limit the recruitment of the Sigma factor (or of another conserved transcriptional activator) required for the initiation of gcrA transcription. Consistent with this proposition, we also found that gcrA mRNA levels were significantly lower in A. tumefaciens cells that over-expressed ccrM from pRX-Ptac-ccrM than in control cells carrying the empty pRX control vector (Supplementary Figure S14). Thus, preventing the transient hemi-methylation of PG through CcrM overproduction may reduce PG activity in S-phase cells.
Discussion
In this study, we discovered that the CcrM DNA MTase, which is conserved in all known Alphaproteobacteria with multipartite genomes (11), can play a role in controlling the timing of ori2 replication/partitioning and the frequency at which ori2 is replicated in A. tumefaciens cells. Our observations indicate that ori2 initiates/segregates with a significant delay during the A. tumefaciens cell cycle (Figure 4 and Figures S11 and S12) when its dicentric chromosome is hypo-methylated due to a depletion of CcrM (Figure 1B and Figures S6 and 7). This replication delay could be due to insufficient levels of the RepCCh2 initiator since repCCh2 expression is strongly reduced in such mutant cells (Figure 3). Conversely, ori2 appears to over-initiate when CcrM is over-expressed, leading to cells with unusually high ori2 copy numbers (Figure 5). We believe that these over-initiation events may result from the constitutive full-methylation of the three GANTC motifs found in the putative ori2 (Figure 6E), rather than from a very transient mis-regulation of the repABCCh2 operon during the second half of the S-phase, since we could not detect a noticeable boost in repABCCh2 expression from mixed cell populations under such conditions (Supplementary Figure S14). In this case, the hemi-methylation of the ori2 after the onset of replication from ori2 may be involved in preventing new ori2 firing before the very end of the S-phase of the cell cycle when CcrM re-methylates the whole genome (model shown in Figure 6E). This would be somewhat reminiscent of the control of DnaA-dependent origins by the Dam/SeqA couple in Gammaproteobacteria, where the replication origin is sequestered in an poorly active hemi-methylated state after the initiation of replication to reduce risks of over-initiation events (48). Altogether, we therefore envision that the cell cycle-dependent methylation of the A. tumefaciens genome may play a dual role in controlling initiation at ori2: first, a role in regulating the timing of the first initiation event at ori2 through repCCh2 regulation and, second, a role in regulating ori2 initiation frequency through the transient hemi-methylation of the ori2 after initiation. This impact of CcrM-dependent methylation on ori2 control may also provide an explanation for the apparent essentiality of ccrM in cells cultivated in complex media (Figure 2 and Supplementary Figure S2) since initiation at ori2 is essential in A. tumefaciens (28). ori2 and/or PA/PE methylation by CcrM may be needed to reset ori2 activity and/or repCCh2 expression once cells have reached the G2 phase of the cell cycle and need to get ready to restart a new cell cycle (model in Figure 6E). Why this process becomes more critical in complex media may, for example, relate to higher coordination requirements during fast growth or to shorter half-lives of critical regulators (CcrM, RepABCCh2, …) leading to more critical depletions of such elements. Interestingly, such CcrM-dependent control of secondary chromosomal origins may be conserved far beyond A. tumefaciens, as a former study showed that CcrM over-expression leads to abnormally high intra-cellular DNA contents in the B. abortus human pathogen (22).
How DNA methylation by CcrM promotes initiation from ori2 in A. tumefaciens is however not yet fully understood. Still, our data indicates that the A present in several GANTC motifs located in the PA promoter controlling the transcription of the repABCCh2 operon (M2 and M3 in PA) and in the PE promoter controlling the synthesis of the RepECh2 anti-sense RNA (M1, M2 and M3 in PE), can decrease or increase, respectively, the activity of ori2 in cis on a plasmid (Figure 6b,d). While a few of these methylatable A may simply be part of the core promoters required for Sigma factor binding (M3 motifs in PA and PE), others have a more minor impact on promoter activity while they still influence ori2 activity (M2 in PA and M1/M2 in PE). These may be regulatory GANTC motifs influencing repECh2transcription (M1/M2 in PE) and/or other events required for ori2 activity/partitioning (M1/M2 in PE and PA). Considering that the three genes of the repABCCh2operon are down-regulated when the A. tumefaciens genome becomes hypo-methylated (Figure 3A, B), we envision that methylation of the A in the M3 motif of the PA promoter by CcrM may promote repABCCh2 transcription (model in Figure 6E). If this is the case, the GcrA epigenetic regulator would not play a major role, as its depletion had an only minor impact on repACh2 expression in A. tumefaciens (Figure 6C). In contrast, GcrA showed a strong positive impact of repCCh2 expression, suggesting that it can repress the RepECh2 sRNA (model in Figure 6E). This newly detected antisense RNA (Figure 6D) shows striking resemblances with the RepEpTi sRNA (Supplementary Figure S16) that has been shown to repress not only repCpTi transcription (through premature transcriptional termination of the repABCpTimulticistronic transcript upstream of the repCpTi ORF), but most likely also repCpTitranslation (10). Since we observed that RepABCCh2/ori2-dependent plasmids carrying GANTC mutations repressing repECh2 expression enter and are maintained more efficiently into cells than plasmids carrying the WT module (Figure 6D), we believe that RepECh2 is similarly a potent inhibitor of repCCh2 expression (model in Figure 6E). Considering that GcrA is usually a methylation-sensitive regulator, its impact on PE may be modulated by the methylation state of its three GANTC motifs. Altogether, it is then possible that DNA methylation by CcrM has a dual impact on repCCh2 expression, by promoting its transcription from the PA promoter (M3 motif) and through an inhibition of the PE promoter via the GcrA epigenetic regulator (model in Figure 1E).
Another important question is why should this RepABCCh2/ori2 module be regulated by such complex epigenetic mechanisms of regulation? An attractive answer may be that such a system could be used as a sophisticated timer to activate ori2 only once the DnaA-dependent ori1 has already started the replication of the chromosome as observed (Figure 4). This would then play a role in coordinating the initiation of replication at the two chromosomal origins, which is important for genome maintenance. Indeed, our data suggests that the gcrA promoter (PG), which is located at a distance of ∼0.43 Mbp from the ori1 (Figure 1B), may be activated when it switches from a fully- to a hemi-methylated state once the replication fork reaches that locus following initiation at ori1. A burst of GcrA synthesis at that early time of the S-phase of the cell cycle may then repress the fully-methylated RepECh2 anti-sense RNA, leading to a strong activation of repCCh2 expression (model in Figure 6E). RepCCh2 would then accumulate at sufficiently high levels to start replication at ori2 significantly after ori1, but in a coordinated manner. Once replication has started at ori2, over-initiation from ori2 may then be prevented through the hemi-methylation of ori2 (model in Figure 6E), as seen for DnaA-dependent origins in some Gammaproteobacteria (48). To complete this working model, future studies should notably focus on testing the impact of DNA methylation on the cell cycle-regulation of GcrA levels and on testing whether GcrA and/or RepC are methylation-sensitive DNA binding proteins in A. tumefaciens. Such studies can shed interesting light on the complex (epigenetic) regulatory networks that primitive organisms with complex genomes can use to coordinate genome replication from multiple origins the way more complex organisms also do.
Beyond the observed impact of CcrM on repABCCh2 expression, our transcriptome analysis revealed that DNA methylation by CcrM modulates the expression of many other A. tumefaciens genes. Gene Set Enrichment Analysis (GSEA) tests showed enrichments for COG categories J (translation/biogenesis), L (replication/recombination/repair) and N (cell motility) when comparing the transcriptome of CcrM-depleted cells with that of WT or CcrM-repleted cells (Supplementary Figure S18). The COG category N correlates with the motility/adhesion defects observed for CcrM-depleted cells (Supplementary Figure S8), while the COG category L may correlate with up-coming replication issues, even if initiation from ori2 still appeared as normal in most CcrM-depleted cells after 7h of growth in ATGN - IPTG (data not shown). Among the genes that were significantly mis-regulated upon CcrM depletion, up to 75 may be directly regulated by CcrM-dependent methylation (Figure 3A and Supplementary Table S5) and this ‘direct regulon’ is apparently very different from previously described CcrM regulons in distantly-related bacteria (11,21) (Supplementary Table S5). Moreover, the essential ftsZAt gene of A. tumefaciens (that has one GANTC motif in the 200 bp upstream of the ORF) was not significantly down-regulated in CcrM-depleted cells cultivated in complex media (Figure 3B), contrarily to what was previously observed in C. crescentus ΔccrM mutant cells where FtsZCc levels can become too limiting to sustain cell division and viability (19,20). We then propose that the essentiality of ccrM in A. tumefaciens is instead connected with its impact on repCCh2 expression and/or ori2 methylation/activity to ensure genome maintenance since ori2 is apparently essential in A. tumefaciens (replication forks may stall at the ter1 region located between ori1 and ori2) (28). The elongated cell phenotype observed for CcrM-depleted cells cultivated in complex media before they die (Figure 2C and Supplementary Figure S2 and S4) may also be connected with this replication defect as cell division is often delayed when chromosome replication/segregation is stuck in bacteria. Overall, even if ccrM is often (conditionally) essential in Alphaproteobacteria (19,22,23,49), the reasons why ccrM can become essential appears to vary from one species to another, confirming the interest of studying the biological function of conserved DNA MTases in a variety of organisms even if their molecular function appears as identical.
Supplementary Material
Acknowledgements
We thank Pamela Brown, Xindan Wang, Patrick Viollier, Gaël Panis, Albert Jeltsch and Didier Mazel for gifts of strains/plasmids/antibodies. We also thank Hannes Richter, Jordan Vacheron, Clara Heiman, Garance Sarton, Jessica Burnier and Gaël Close for technical help during statistical/bioinformatic/microscopy analyses. We thank Jacqueline Masternak, Laurent Casini and Jakub Czarnecki for several plasmid constructions, together with Noémie Matthey and Giorgia Wennubst for discussions during the project and feedback on the manuscript.
Contributor Information
Sandra Martin, Department of Fundamental Microbiology, Faculty of Biology and Medicine, University of Lausanne, Lausanne CH-1015, Switzerland.
Florian Fournes, Department of Fundamental Microbiology, Faculty of Biology and Medicine, University of Lausanne, Lausanne CH-1015, Switzerland.
Giovanna Ambrosini, Bioinformatics Competence Center, University of Lausanne, Lausanne CH-1015, Switzerland; Bioinformatics Competence Center, Ecole Polytechnique Fédérale de Lausanne, Lausanne CH-1015, Switzerland.
Christian Iseli, Bioinformatics Competence Center, University of Lausanne, Lausanne CH-1015, Switzerland; Bioinformatics Competence Center, Ecole Polytechnique Fédérale de Lausanne, Lausanne CH-1015, Switzerland.
Karolina Bojkowska, Lausanne Genomic Technologies Facility, Faculty of Biology and Medicine, University of Lausanne, Lausanne CH-1015, Switzerland.
Julien Marquis, Lausanne Genomic Technologies Facility, Faculty of Biology and Medicine, University of Lausanne, Lausanne CH-1015, Switzerland.
Nicolas Guex, Bioinformatics Competence Center, University of Lausanne, Lausanne CH-1015, Switzerland; Bioinformatics Competence Center, Ecole Polytechnique Fédérale de Lausanne, Lausanne CH-1015, Switzerland.
Justine Collier, Department of Fundamental Microbiology, Faculty of Biology and Medicine, University of Lausanne, Lausanne CH-1015, Switzerland.
Data availability
Metadata and RNASeq/SMRT-Seq data are available in the NCBI BioProject PRJNA1064157: https://www.ncbi.nlm.nih.gov/search/all/?term=PRJNA1064157. Other data that support the findings of this study are available from the corresponding author upon request.
Supplementary data
Supplementary Data are available at NAR Online.
Funding
Swiss National Science Foundation [31003A_173075, 310030_204822 to J.C.]. Funding for open access charge: Swiss National Science Foundation.
Conflict of interest statement. None declared.
References
- 1. diCenzo G.C., Finan T.M.. The divided bacterial genome: structure, function, and evolution. Microbiol. Mol. Biol. Rev. 2017; 81:e00019-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. diCenzo G.C., MacLean A.M., Milunovic B., Golding G.B., Finan T.M.. Examination of prokaryotic multipartite genome evolution through experimental genome reduction. PLoS Genet. 2014; 10:e1004742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Almalki F., Choudhary M., Azad R.K.. Analysis of multipartite bacterial genomes using alignment free and alignment-based pipelines. Arch. Microbiol. 2022; 205:25. [DOI] [PubMed] [Google Scholar]
- 4. Pinto U.M., Pappas K.M., Winans S.C.. The ABCs of plasmid replication and segregation. Nat. Rev. Micro. 2012; 10:755–765. [DOI] [PubMed] [Google Scholar]
- 5. Fournes F., Val M.E., Skovgaard O., Mazel D. Replicate once per cell cycle: replication control of secondary chromosomes. Front. Microbiol. 2018; 9:1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Val M.E., Soler-Bistue A., Bland M.J., Mazel D. Management of multipartite genomes: the vibrio cholerae model. Curr. Opin. Microbiol. 2014; 22:120–126. [DOI] [PubMed] [Google Scholar]
- 7. Frandi A., Collier J.. Multilayered control of chromosome replication in Caulobacter crescentus. Biochem. Soc. Trans. 2019; 47:187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pinto U.M., Flores-Mireles A.L., Costa E.D., Winans S.C.. RepC protein of the octopine-type Ti plasmid binds to the probable origin of replication within repC and functions only in cis. Mol. Microbiol. 2011; 81:1593–1606. [DOI] [PubMed] [Google Scholar]
- 9. Czarnecki J., Chapkauskaitse E., Bos J., Sentkowska D., Wawrzyniak P., Wyszynska A., Szuplewska M., Bartosik D. Differential localization and functional specialization of parS centromere-like sites in repABC replicons of Alphaproteobacteria. Appl. Environ. Microb. 2022; 88:e0020722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chai Y., Winans S.C.. A small antisense RNA downregulates expression of an essential replicase protein of an Agrobacterium tumefaciens Ti plasmid. Mol. Microbiol. 2005; 56:1574–1585. [DOI] [PubMed] [Google Scholar]
- 11. Gonzalez D., Kozdon J.B., McAdams H.H., Shapiro L., Collier J.. The functions of DNA methylation by CcrM in Caulobacter crescentus: a global approach. Nucleic Acids Res. 2014; 42:3720–3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zweiger G., Marczynski G., Shapiro L.. A Caulobacter DNA methyltransferase that functions only in the predivisional cell. J. Mol. Biol. 1994; 235:472–485. [DOI] [PubMed] [Google Scholar]
- 13. Stephens C., Reisenauer A., Wright R., Shapiro L.. A cell cycle-regulated bacterial DNA methyltransferase is essential for viability. Proc. Natl. Acad. Sci. U.S.A. 1996; 93:1210–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kozdon J.B., Melfi M.D., Luong K., Clark T.A., Boitano M., Wang S., Zhou B., Gonzalez D., Collier J., Turner S.W.et al.. Global methylation state at base-pair resolution of the Caulobacter genome throughout the cell cycle. Proc. Natl. Acad. Sci. U.S.A. 2013; 110:E4658–E4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fioravanti A., Fumeaux C., Mohapatra S.S., Bompard C., Brilli M., Frandi A., Castric V., Villeret V., Viollier P.H., Biondi E.G.. DNA binding of the cell cycle transcriptional regulator GcrA depends on N6-adenosine methylation in caulobacter crescentus and other Alphaproteobacteria. PLoS Genet. 2013; 9:e1003541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Haakonsen D.L., Yuan A.H., Laub M.T.. The bacterial cell cycle regulator GcrA is a sigma70 cofactor that drives gene expression from a subset of methylated promoters. Genes Dev. 2015; 29:2272–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mohapatra S.S., Fioravanti A., Biondi E.G.. DNA methylation in Caulobacter and other Alphaproteobacteria during cell cycle progression. Trends Microbiol. 2014; 22:528–535. [DOI] [PubMed] [Google Scholar]
- 18. Adhikari S., Curtis P.D.. DNA methyltransferases and epigenetic regulation in bacteria. FEMS Microbiol. Rev. 2016; 40:575–591. [DOI] [PubMed] [Google Scholar]
- 19. Gonzalez D., Collier J.. DNA methylation by CcrM activates the transcription of two genes required for the division of Caulobacter crescentus. Mol. Microbiol. 2013; 88:203–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gonzalez D., Collier J.. Genomic adaptations to the loss of a conserved bacterial DNA methyltransferase. mBio. 2015; 6:e00952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Adhikari S., Erill I., Curtis P.D.. Transcriptional rewiring of the GcrA/CcrM bacterial epigenetic regulatory system in closely related bacteria. PLoS Genet. 2021; 17:e1009433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Robertson G.T., Reisenauer A., Wright R., Jensen R.B., Jensen A., Shapiro L., Roop R.M. 2nd. The Brucella abortus CcrM DNA methyltransferase is essential for viability, and its overexpression attenuates intracellular replication in murine macrophages. J. Bacteriol. 2000; 182:3482–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kahng L.S., Shapiro L.. The CcrM DNA methyltransferase of Agrobacterium tumefaciens is essential, and its activity is cell cycle regulated. J. Bacteriol. 2001; 183:3065–3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Curtis P.D., Brun Y.V.. Identification of essential alphaproteobacterial genes reveals operational variability in conserved developmental and cell cycle systems. Mol. Microbiol. 2014; 93:713–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brown P.J.B., Chang J.H., Fuqua C.. Agrobacterium tumefaciens: a transformative agent for fundamental insights into host-microbe interactions, genome biology, chemical signaling, and cell biology. J. Bacteriol. 2023; 205:e0000523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Goodner B., Hinkle G., Gattung S., Miller N., Blanchard M., Qurollo B., Goldman B.S., Cao Y., Askenazi M., Halling C.et al.. Genome sequence of the plant pathogen and biotechnology agent agrobacterium tumefaciens C58. Science. 2001; 294:2323–2328. [DOI] [PubMed] [Google Scholar]
- 27. Wood D.W., Setubal J.C., Kaul R., Monks D.E., Kitajima J.P., Okura V.K., Zhou Y., Chen L., Wood G.E., Almeida N.F. Jret al.. The genome of the natural genetic engineer Agrobacterium tumefaciens C58. Science. 2001; 294:2317–2323. [DOI] [PubMed] [Google Scholar]
- 28. Liao Q., Ren Z., Wiesler E.E., Fuqua C., Wang X.. A dicentric bacterial chromosome requires XerC/D site-specific recombinases for resolution. Curr. Biol. 2022; 32:3609–3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Figueroa-Cuilan W., Daniel J.J., Howell M., Sulaiman A., Brown P.J.. Mini-Tn7 insertion in an artificial attTn7 site enables depletion of the essential master regulator CtrA in the phytopathogen agrobacterium tumefaciens. Appl. Environ. Microb. 2016; 82:5015–5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ducret A., Quardokus E.M., Brun Y.V.. MicrobeJ, a tool for high throughput bacterial cell detection and quantitative analysis. Nat. Microbiol. 2016; 1:16077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wingett S.W., Andrews S.. FastQ screen: a tool for multi-genome mapping and quality control. F1000Res. 2018; 7:1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Davis M.P., van Dongen S., Abreu-Goodger C., Bartonicek N., Enright A.J.. Kraken: a set of tools for quality control and analysis of high-throughput sequence data. Methods. 2013; 63:41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dobin A., Davis C.A., Schlesinger F., Drenkow J., Zaleski C., Jha S., Batut P., Chaisson M., Gingeras T.R.. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013; 29:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Anders S., Pyl P.T., Huber W.. HTSeq–a Python framework to work with high-throughput sequencing data. Bioinformatics. 2015; 31:166–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang L., Wang S., Li W.. RSeQC: quality control of RNA-seq experiments. Bioinformatics. 2012; 28:2184–2185. [DOI] [PubMed] [Google Scholar]
- 36. Robinson M.D., McCarthy D.J., Smyth G.K.. edgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010; 26:139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ritchie M.E., Phipson B., Wu D., Hu Y., Law C.W., Shi W., Smyth G.K.. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015; 43:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003; 31:3406–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Livak K.J., Schmittgen T.D.. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001; 25:402–408. [DOI] [PubMed] [Google Scholar]
- 40. Sanchez-Romero M.A., Casadesus J.. The bacterial epigenome. Nat. Rev. Micro. 2020; 18:7–20. [DOI] [PubMed] [Google Scholar]
- 41. Flusberg B.A., Webster D.R., Lee J.H., Travers K.J., Olivares E.C., Clark T.A., Korlach J., Turner S.W.. Direct detection of DNA methylation during single-molecule, real-time sequencing. Nat. Methods. 2010; 7:461–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wright R., Stephens C., Zweiger G., Shapiro L., Alley M.R.. Caulobacter lon protease has a critical role in cell-cycle control of DNA methylation. Genes Dev. 1996; 10:1532–1542. [DOI] [PubMed] [Google Scholar]
- 43. Zhou X., Wang J., Herrmann J., Moerner W.E., Shapiro L.. Asymmetric division yields progeny cells with distinct modes of regulating cell cycle-dependent chromosome methylation. Proc. Natl. Acad. Sci. U.S.A. 2019; 116:15661–15670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Baharoglu Z., Mazel D. SOS, the formidable strategy of bacteria against aggressions. FEMS Microbiol. Rev. 2014; 38:1126–1145. [DOI] [PubMed] [Google Scholar]
- 45. Ren Z., Liao Q., Barton I.S., Wiesler E.E., Fuqua C., Wang X.. Centromere interactions promote the maintenance of the multipartite genome in agrobacterium tumefaciens. mBio. 2022; 13:e0050822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ren Z., Liao Q., Karaboja X., Barton I.S., Schantz E.G., Mejia-Santana A., Fuqua C., Wang X.. Conformation and dynamic interactions of the multipartite genome in Agrobacterium tumefaciens. Proc. Natl. Acad. Sci. U.S.A. 2022; 119:e2115854119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mouammine A., Collier J.. The impact of DNA methylation in Alphaproteobacteria. Mol Microbiol. 2018; 110:1–10. [DOI] [PubMed] [Google Scholar]
- 48. Katayama T., Ozaki S., Keyamura K., Fujimitsu K.. Regulation of the replication cycle: conserved and diverse regulatory systems for DnaA and oriC. Nat. Rev. Micro. 2010; 8:163–170. [DOI] [PubMed] [Google Scholar]
- 49. Wright R., Stephens C., Shapiro L.. The CcrM DNA methyltransferase is widespread in the alpha subdivision of proteobacteria, and its essential functions are conserved in Rhizobium meliloti and Caulobacter crescentus. J. Bacteriol. 1997; 179:5869–5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Metadata and RNASeq/SMRT-Seq data are available in the NCBI BioProject PRJNA1064157: https://www.ncbi.nlm.nih.gov/search/all/?term=PRJNA1064157. Other data that support the findings of this study are available from the corresponding author upon request.