Abstract
Human parainfluenza virus type 3 (HPIV-3) is an airborne pathogen that infects the epithelial cells of the respiratory tract. In the present study we investigated the interaction of HPIV-3 with the type II alveolar human lung polarized epithelial A549 cells. Although HPIV-3 entry and budding were bidirectional from both the apical and the basolateral domains, HPIV-3 exhibited preferential entry and release from the apical pole. While disruption of the cellular actin microfilament and microtubule by cytochalasin D and nocodazole, respectively, had no effect on virus entry, disruption of the microtubule but not the microfilament inhibited HPIV-3 release.
Human parainfluenza virus type 3 (HPIV-3) is an enveloped, single-stranded, negative-sense RNA virus within the family of Paramyxoviridae that infects lower respiratory tract epithelial cells of children to cause diseases such as bronchiolitis, pneumonia, and croup (4). Since HPIV-3 is an airborne pathogen, the lung epithelial cells of the respiratory tract are the initial target of HPIV-3 infection (4). Epithelial cells line the major cavities of the body, functioning in selective secretion and adsorption and providing a barrier to the external environment (5, 21). These cells are highly polarized since the plasma membranes of these cells are divided into two discrete domains, namely, the apical domain (facing the luminal side) and the basolateral domain (facing the systemic circulation). Moreover, these two separate plasma membrane domains have distinct protein and lipid compositions. Specialized complexes termed tight junctions serve to preserve these domains and prevent the mixing of components between them. The tight junctions are also involved in the formation of a monolayer of tightly adherent cells.
Many viruses bind, internalize, and bud from polarized epithelial cells in culture either asymmetrically or symmetrically, with potential implications for viral pathogenesis. Among the Paramyxoviridae family, while Sendai (23) and respiratory syncytial viruses (20) are exclusively internalized and released from the apical pole, measles virus entering the epithelial cells from the apical domain is released bidirectionally from both the apical and basolateral domains (2).
Since HPIV-3 is a pathogen of major clinical importance which naturally infects the respiratory epithelium, we have examined HPIV-3 infection in cultured human lung polarized epithelial A549 cells to determine the polarity of virus binding, internalization, and budding and the role of intact actin microfilament and microtubule networks during these events.
HPIV-3-susceptible human respiratory epithelial A549 cells are lung adenocarcinoma cells that have been routinely used as a model of type II alveolar epithelial cells (26). Moreover, when these cells are grown on porous membrane filter supports, they form polarized monolayers with high transepithelial resistance, a measure of the tightness of cell monolayers (1). The use of a filter insert allows selective inoculation or isolation of viruses either from the apical (upper chamber) or the basolateral (lower chamber) domain.
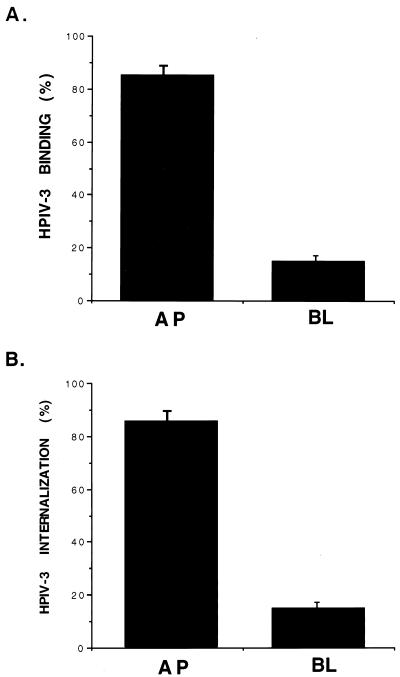
In order to test the polarity of HPIV-3 infection, [35S]methionine-labeled HPIV-3 (35S-HPIV-3) (multiplicity of infection [MOI], 1) was added either to the apical or the basolateral chamber of A549 cells grown on filter inserts (Millipore; MCE membrane, 0.45-μM pore size) that were preincubated at 4°C for 1 h. For the binding experiment, the chilled cells were incubated with the virus for 1.5 h at 4°C. This temperature allows virus binding to the cell surface in the absence of internalization. For the virus internalization assay, following cell surface binding of the virus at 4°C for 1.5 h, the cells were washed with phosphate-buffered saline (PBS) and were further incubated with fresh media at 37°C for 3 h to allow internalization of cell surface-bound virus. Following 3 h of incubation, cells were washed twice with PBS and trypsinized for 15 min at 37°C to remove cell surface-attached virus. The protease activity was neutralized with complete Dulbecco modified Eagle medium, and the cells were washed twice with PBS. Washed cells were then lysed, and the lysate radioactivity representing the internalized 35S-HPIV-3 was counted. The results from these experiments (Fig. 1) revealed that HPIV-3 infection proceeded much more efficiently following inoculation of the apical surface of polarized A549 cells. Binding (Fig. 1A) and internalization (Fig. 1B) of HPIV-3 from the apical domain of polarized cells were sixfold higher than those from the basolateral domain. However, it has been reported that the total surface area of the apical plasma membrane of polarized epithelial MDCK cells is fourfold lower than that of the basolateral cell membrane (25). Assuming this is true for A549 cells, the efficiency of virus entry from the apical surface would be 24-fold higher than that from the basolateral domain. Nevertheless, it is interesting to note that, although HPIV-3 infection in humans occurs from the luminal (apical) surface, our results suggest that basolaterally presented virus is also capable of binding and internalization from that domain, although at a lower efficiency. To further confirm the accessibility of the basolateral membrane to HPIV-3, A549 cells were seeded onto inverted filter inserts and, following overnight attachment of the cells, the filters were placed upright in 12-well culture plates, thus orienting the basolateral side of the monolayer upward as described previously (9). In this orientation, the binding and internalization of HPIV-3 added either to the upper (basolateral) or the lower (apical) chamber were similar to the basolateral and apical binding and internalization of HPIV-3 to the cells that were grown normally on the filters, with upper and lower chambers representing apical and basolateral sides, respectively. Under normal cell seeding conditions, where the upper chamber represents the apical side, binding and internalization of 35S-labeled vesicular stomatitis virus (VSV) were restricted to the basolateral domains of A549 cells (data not shown) as previously reported for other polarized epithelial cells (13, 15, 24). Thus, from the above results it appears that HPIV-3 binds and internalizes preferentially through the apical-membrane domains of polarized A549 cells.
FIG. 1.
Polarity of HPIV-3 entry into A549 cells. Polarized A549 cells grown on filter inserts were inoculated through either the apical (AP) or basolateral (BL) surface with 35S-HPIV-3 (MOI, 1). (A) Polarized cell surface binding of 35S-HPIV-3 was determined following incubation of virus with chilled A549 cells at 4°C for 1.5 h. The cell surface-associated radioactivity (counts per minute) present in the cell lysate was measured by a liquid scintillation counter. The percent binding was calculated from the total amount of radioactivity bound to the AP and BL surfaces. (B) Polarized internalization of 35S-HPIV-3 was determined following incubation of cell surface-bound virus (4°C, 1.5 h) at 37°C for 3 h. The amount of radioactivity present in the cell lysate was measured as described above. The percent internalization was calculated from the total amount of radioactivity present in the apically or basolaterally infected cells. The results represent the averages of three separately replicated experiments.
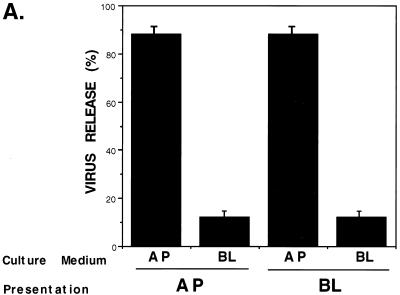
In order to examine the polarity of HPIV-3 budding, virus (MOI, 1) was added either to the apical or basolateral surfaces. At 24 h postinfection, the culture supernatants collected either from the apical or basolateral chamber were analyzed for infectious progeny virus by a plaque assay as described earlier (7, 14). At 24 h postinfection and during extended postinfection time periods (24 to 72 h), there was no change in the transepithelial resistance of virus-infected cells compared to that of the mock-infected cells, suggesting that HPIV-3 infection does not disturb the integrity of polarized monolayers. Moreover, the apical-to-basolateral permeability and diffusion rate of fluid-phase markers added to the apical (upper) chamber remained unaltered in HPIV-3-infected cells (at 24, 48, and 72 h postinfection) compared to those in the mock-infected cells (data not shown). This result suggests that HPIV-3 infection of A549 cells does not lead to the disruption of the integrity of the polarized monolayer. In addition, infection of an A549 monolayer with VSV from the basolateral side resulted in preferential release of the virus from the basolateral domain (data not shown) as previously described (13, 24). In contrast to what was found for VSV, our results (Fig. 2A) demonstrate that newly replicated HPIV-3 particles are released during apical or basolateral infection of polarized A549 cells almost exclusively through the apical surface. The number of HPIV-3 progeny virions released from the apical surface (virus presented either apically or basolaterally) was sevenfold higher than the number released from the basolateral surface. Moreover, the ratios of apical to basolateral HPIV-3 release at 24, 48, and 72 h postinfection were the same. We conclude from these experiments that the apical plasma membrane is the major budding site for progeny HPIV-3 virions. Interestingly, a small amount of virus is also released from the basolateral surface, providing interesting implications for viral pathogenesis.
FIG. 2.
Polarity of HPIV-3 budding in A549 cells. (A) A549 cells grown on filter inserts were inoculated with HPIV-3 (MOI, 1) through either the apical (AP) or basolateral (BL) surface. At 24 h postinfection, the culture supernatants collected from the AP and BL chambers were added to CV-1 cells for a plaque assay as described previously (7, 14). The percent virus release was calculated as a ratio of the number of plaques observed from either the AP or the BL medium/total number of plaques observed, where the total number of plaques was derived by adding the number of plaques observed in both the AP and BL culture supernatants. The results represent the averages of three separately replicated experiments. (B) A549 cells grown on filter inserts were either mock infected (left) or infected with HPIV-3 (MOI, 1) from the apical surface (right). At 24 h postinfection cells labeled with anti-HPIV-3 RNP antibody and goat anti-rabbit fluorescein isothiocyanate were examined by indirect immunofluorescence confocal microscopy. Shown is the Z-section image of the polarized epithelial A549 cells. Dashed line, basement membrane of the monolayer.
Although the preferential release of virus in the apical (luminal) medium may be associated with localized infection of the respiratory tract, the small proportion of basolaterally (systemic circulation) released virus may represent the mechanism involved in the development of systemic infection and viremia associated with immunosupressed children. In fact, such immunodeficient children undergo fatal systemic infection with HPIV-3, and virus was shown to spread systemically to the liver, myocardium, and cerebrospinal fluid (6, 11, 12). These observations indicate that HPIV-3 can occasionally disseminate from the respiratory tract in humans and infect other visceral organs. In that case our results (Fig. 2A) suggest that a small proportion of basolaterally released virus, in the absence of innate immune defense, can propagate itself in the systemic circulation to infect non-respiratory-tract cells. Moreover, infection of T lymphocytes with HPIV-3 was shown to be important for immunoregulation of these cells that leads to the failure to induce lifelong immunity against the virus (22). Since T lymphocytes are selectively localized in the basolateral surfaces of epithelial cells following infiltration from the systemic circulation (3), the basolaterally derived HPIV-3 could also infect T lymphocytes at this site to modulate the immunoregulation mechanism of these cells.
Since our results demonstrated that the apical surface is the primary plasma membrane domain in polarized A549 cells for HPIV-3 binding, internalization, and budding, it was reasonable to assume that the HPIV-3 RNP are confined mainly to the apical surface. To address this issue, we examined mock-infected and HPIV-3-infected A549 cells grown on a filter support by confocal microscopy (Fig. 2B). HPIV-3-infected A549 cells grown on filter inserts were processed for confocal microscopy 24 h postinfection. Following fixation of the filters with 3.7% formaldehyde and permeabilization with PBS containing 5% fetal calf serum, 5% glycine, and 0.1% Triton X-100, the cells were incubated with rabbit anti-HPIV-3 RNP (1:100 [vol/vol]) prepared as described previously (14) followed by staining with goat anti-rabbit immunoglobulin G conjugated to fluorescein isothiocyanate (1:1,000 [vol/vol]). In order to distinguish between the apical (top) and the basolateral (bottom and lateral) domains of the A549 cell monolayer, Z-section imaging representing the longitudinal cross section of the cell monolayer was carried out by confocal microscopy. Figure 2B shows a Z-section image of mock-infected (left) and HPIV-3-infected (right) A549 cell monolayers labeled with anti-HPIV-3 RNP antibody (dashed line, basement membrane). No staining was detected in mock-infected cells (Fig. 2B, left) and intense staining for RNP detected in the infected cells (Fig. 2B, right) was primarily restricted to the apical surface in the presence of a very diffuse RNP staining at the lateral domain. Therefore, the polarized accumulation of HPIV-3 RNP at the apical pole is consistent with the preferential budding of HPIV-3 from the apical plasma membranes (Fig. 2A) of A549 cells.
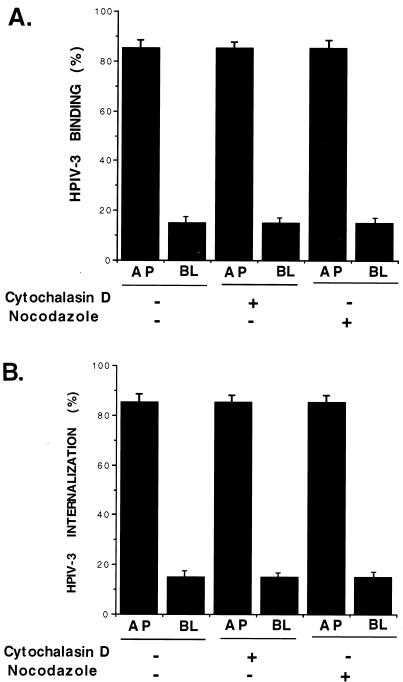
Several studies have reported the importance of two cytoskeletal networks, the actin microfilament and the microtubule, in the binding and internalization of several viruses (10, 17). In order to examine the role of an intact microfilament and microtubule in the binding and internalization of HPIV-3, A549 cells grown on filter inserts were pretreated with actin microfilament-depolymerizing drug cytochalasin D (15 μg/ml) and microtubule-depolymerizing drug nocodazole (20 μg/ml) for 12 h before 35S-HPIV-3 binding (Fig. 3A) and internalization (Fig. 3B) assays were performed as described for Fig. 1A and B, respectively. Our results indicate that an intact actin microfilament and microtubule are not necessary for HPIV-3 binding and internalization in A549 cells.
FIG. 3.
Role of the actin microfilament and microtubule in polarized HPIV-3 entry into A549 cells. (A) 35S-HPIV-3 binding assays with A549 cells pretreated with either cytochalasin D (15 μg/ml) or nocodazole (20 μg/ml) for 12 h were performed essentially as described for Fig. 1A. (B) 35S-HPIV-3 internalization assays with A549 cells pretreated with cytochalasin D (15 μg/ml) or nocodazole (20 μg/ml) for 12 h were performed essentially as described for Fig. 1B.
The involvement of the cytoskeleton network, namely, the microtubule and actin microfilament, in the budding and morphogenesis of several viruses has been reported (18, 19). In that context, we wanted to study the role of these networks in the budding of HPIV-3. Previous studies have demonstrated that a polymerized actin microfilament is required for viral transcription and replication (8) and that treatment of cells with actin microfilament-disrupting drug cytochalasin D results in intracellular inhibition of viral transcription and replication (14). Since the simultaneous addition of HPIV-3 and cytochalasin D to cells results in the loss of intracellular viral proteins, it was difficult to study the role of the actin microfilament in HPIV-3 budding. In order to circumvent this problem, we developed an experimental system to study the role of the microfilament in virus budding. A549 cells grown on filter supports infected with HPIV-3 (MOI, 1) from either the apical or basolateral surface for 24 h were treated with cycloheximide (30 μg/ml) for 3 h to accumulate an intracellular viral protein pool in the absence of de novo protein synthesis. Following 3 h of incubation with cycloheximide, cells were washed twice with PBS to remove unbound virus and then fresh medium containing cycloheximide in the absence or presence of cytochalasin D (15 μg/ml) was added to the cells to examine the assembly and budding of the accumulated viral proteins as infectious virions in the presence of a disrupted actin microfilament network. Following 12 h of treatment with these drugs, both the apical and basolateral culture supernatants were assessed for viral titer by a plaque assay.
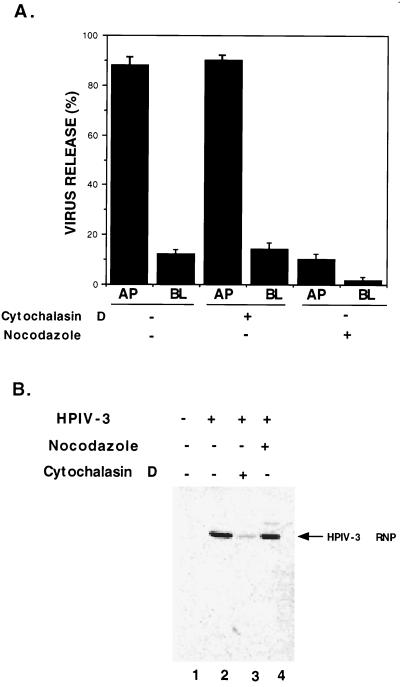
To study the role of the microtubule in the budding of HPIV-3, we carried out the experiment in a similar fashion but in the presence of nocodazole (20 μg/ml). As shown in Fig. 4A, disruption of the microfilament had no effect on the budding of HPIV-3. Moreover, the polarity of virus budding in cytochalasin-treated cells was intact. In contrast, disruption of the microtubule network by nocodazole led to drastic inhibition of virus release. Moreover, in these experiments, the addition of HPIV-3 from either the apical or basolateral surface rendered similar results with regard to virus budding in the absence or presence of cytochalasin D or nocodazole. During the 15-h incubation period and under identical experimental conditions, cycloheximide treatment alone led to a sixfold reduction in HPIV-3 release compared to that from untreated cells. Thus, these results demonstrated that an intact microtubule, but not a microfilament, is required for HPIV-3 budding to the apical surface.
FIG. 4.
Role of the actin microfilament and microtubule in polarized budding of HPIV-3. (A) A549 cells grown on filter inserts were infected with HPIV-3 (MOI, 1) from either the apical (AP) or basolateral (BL) surface. At 24 h postinfection, the washed cells were incubated with cycloheximide (30 μg/ml) for 3 h. Following cycloheximide treatment, the cells were incubated with either cytochalasin D (15 μg/ml) or nocodazole (20 μg/ml) along with cycloheximide (30 μg/ml) for 12 h. Following these drug treatments, the culture supernatants collected from the AP and BL chambers were analyzed for virus titer by a plaque assay of CV-1 cells as described earlier. The percent virus release was calculated essentially as described for Fig. 2A. During the course of this experiment, the drugs were present both in the AP and BL media. (B) A549 cell lysates obtained from mock-infected (lane 1) and HPIV-3-infected cells in the absence (lane 2) or presence of either cytochalasin D (lane 3) or nocodazole (lane 4) were subjected to Western blot analysis with the HPIV-3 RNP antibody as described previously (14).
Although cytochalasin D was shown to inhibit the transcription of HPIV-3 (14), the effect of nocodazole on HPIV-3 protein synthesis in vivo is not known. In order to examine the effect of nocodazole on HPIV-3 RNP levels in A549 cells, cell lysates from mock-infected A549 cells (Fig. 4B, lane 1) and HPIV-3-infected A549 cells in the absence (lane 2) or presence of either cytochalasin D (lane 3) or nocodazole (lane 4) were subjected to Western blot analysis with an anti-HPIV-3 RNP antibody as described previously (14). As expected cytochalasin D treatment led to a dramatic decrease in RNP levels, whereas nocodazole treatment had no effect on RNP levels. These results suggest that, although an intact microtubule is not required for viral transcription or replication, it is required for budding and morphogenesis of HPIV-3. Interestingly, vectorial targeting of the plasma membrane and secretory proteins to the apical surfaces of polarized epithelial cells was indeed shown to be dependent on an intact microtubule network (16).
Future studies will dissect the molecular mechanism(s) involved in the polarized infection of epithelial cells by HPIV-3.
Acknowledgments
We thank Judy Drazba (Imaging Facility, The Cleveland Clinic Foundation) for help with the confocal microscopy and Bishnu P. De for critically reading the manuscript.
S.B. was supported by a fellowship from the Morgenthaler Foundation. This work was supported by U.S. Public Health Service grant AI32027 (to A.K.B.).
REFERENCES
- 1.Birkness K A, Deslauriers M, Bartlett J H, White E H, King C H, Quinn F D. An in vitro tissue culture bilayer model to examine early events in Mycobacterium tuberculosis infection. Infect Immun. 1999;67:653–658. doi: 10.1128/iai.67.2.653-658.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blau D M, Compans R W. Entry and release of Measles virus are polarized in epithelial cells. Virology. 1995;210:91–99. doi: 10.1006/viro.1995.1320. [DOI] [PubMed] [Google Scholar]
- 3.Cepek K L, Shaw S K, Parker C M, Russell G J, Morrow J S, Rimm D L, Brenner M B. Adhesion between epithelial cells and T-lymphocytes mediated by E-cadherin and the alpha E beta 7 integrin. Nature. 1994;372:190–193. doi: 10.1038/372190a0. [DOI] [PubMed] [Google Scholar]
- 4.Collins P L, Chanock R M, Mackintosh K. The paramyxovirus. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 1205–1241. [Google Scholar]
- 5.Compans R W, Srinivas R V. Protein sorting in polarized epithelial cells. Curr Top Microbiol Immunol. 1991;170:141–181. doi: 10.1007/978-3-642-76389-2_5. [DOI] [PubMed] [Google Scholar]
- 6.Craver R D, Gohd R S, Sundin D R, Hierholzer J C. Isolation of parainfluenza virus type 3 from cerebrospinal fluid associated with aseptic meningitis. Am J Clin Pathol. 1993;99:705–707. doi: 10.1093/ajcp/99.6.705. [DOI] [PubMed] [Google Scholar]
- 7.De B P, Gupta S, Banerjee A K. Cellular protein kinase C ζ regulates HPIV-3 replication. Proc Natl Acad Sci USA. 1995;92:5204–5208. doi: 10.1073/pnas.92.11.5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De B P, Lesoon A, Banerjee A K. Human parainfluenza virus type 3 transcription in vitro: role of cellular actin in mRNA synthesis. J Virol. 1991;65:3268–3275. doi: 10.1128/jvi.65.6.3268-3275.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esclatine A, Lemullous M, Servin A L, Quero A, Geniteau-Legendre M. Human cytomegalovirus infects Caco-2 intestinal epithelial cells basolaterally regardless of the differentiation state. J Virol. 2000;74:513–517. doi: 10.1128/jvi.74.1.513-517.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan D P, Sefton B M. The entry into host cells of Sindbis virus, vesicular stomatitis virus and Sendai virus. Cell. 1978;15:985–992. doi: 10.1016/0092-8674(78)90282-9. [DOI] [PubMed] [Google Scholar]
- 11.Fishaut M, Tubergen D, McIntosh K. Cellular response to respiratory viruses with particular reference to children with disorders of cell-mediated immunity. J Pediatr. 1980;96:179–186. doi: 10.1016/s0022-3476(80)80799-2. [DOI] [PubMed] [Google Scholar]
- 12.Frank J A, Warren R W, Tucker J A, Zeller J, Wilfert C M. Disseminated parainfluenza infection in a child with severe combined immunodeficiency. Am J Dis Child. 1983;137:1172–1174. doi: 10.1001/archpedi.1983.02140380032010. [DOI] [PubMed] [Google Scholar]
- 13.Fuller S, von Bonsdorff C H, Simons K. Vesicular stomatitis virus infects and matures only through the basolateral surface of the polarized epithelial cell line, MDCK. Cell. 1984;38:65–77. doi: 10.1016/0092-8674(84)90527-0. [DOI] [PubMed] [Google Scholar]
- 14.Gupta S, De B P, Drazba J A, Banerjee A K. Involvement of actin microfilament in the replication of human parainfluenza virus type 3. J Virol. 1998;72:2655–2662. doi: 10.1128/jvi.72.4.2655-2662.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson L G, Olsen J C, Naldini L, Boucher R C. Pseudotyped human lentiviral vector-mediated gene transfer to airway epithelia in vivo. Gene Ther. 2000;7:568–574. doi: 10.1038/sj.gt.3301138. [DOI] [PubMed] [Google Scholar]
- 16.Lafont F, Burkhardt J K, Simons K. Involvement of microtubule motors in basolateral and apical transport in kidney cells. Nature. 1994;372:801–803. doi: 10.1038/372801a0. [DOI] [PubMed] [Google Scholar]
- 17.Nauwynck H J, Duan X, Favoreel H W, van Oostveldt P, Pensaert M B. Entry of porcine reproductive and respiratory syndrome virus into porcine alveolar macrophages via receptor mediated endocytosis. J Gen Virol. 1999;80:297–305. doi: 10.1099/0022-1317-80-2-297. [DOI] [PubMed] [Google Scholar]
- 18.Ravkov E V, Nichol S T, Peters C J, Compans R W. Role of actin microfilament in Black Creek Canal virus morphogenesis. J Virol. 1998;72:2865–2870. doi: 10.1128/jvi.72.4.2865-2870.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberts P C, Compans R W. Host cell dependence of viral morphology. Proc Natl Acad Sci USA. 1998;95:5746–5751. doi: 10.1073/pnas.95.10.5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts S R, Compans R W, Wertz G W. Respiratory syncytial virus matures at the apical surfaces of polarized epithelial cells. J Virol. 1995;69:2667–2673. doi: 10.1128/jvi.69.4.2667-2673.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodriguez-Boulan E, Nelson W J. Morphogenesis of the polarized epithelial cell phenotype. Science. 1989;245:718–725. doi: 10.1126/science.2672330. [DOI] [PubMed] [Google Scholar]
- 22.Sieg S, Muro-Cacho C, Robertson S, Huang Y, Kaplan D. Infection and immunoregulation of T lymphocytes by parainfluenza virus type 3. Proc Natl Acad Sci USA. 1994;91:6293–6297. doi: 10.1073/pnas.91.14.6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tashiro M, Yamakawa M, Tobita K, Seto J T, Klenk H D, Rott R. Altered budding site of a pantropic mutant of Sendai virus, F1-R, in polarized epithelial cells. J Virol. 1990;64:4672–4677. doi: 10.1128/jvi.64.10.4672-4677.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Meer G, Simons K. Viruses budding from either the apical or the basolateral plasma membrane domain of MDCK cells have unique phospholipid compositions. EMBO J. 1982;1:847–852. doi: 10.1002/j.1460-2075.1982.tb01258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.von Bonsdorff C H, Fuller S D, Simons K. Apical and basolateral endocytosis in Madin-Darby canine kidney (MDCK) cells grown on nitrocellulose filters. EMBO J. 1985;4:2781–2792. doi: 10.1002/j.1460-2075.1985.tb04004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao H B, De B P, Das T, Banerjee A K. Inhibition of human parainfluenza virus-3 replication by interferon and human MxA. Virology. 1996;220:330–338. doi: 10.1006/viro.1996.0321. [DOI] [PubMed] [Google Scholar]