Abstract
Case series
Patients: Male, 30-year-old • Male, 33-year-old
Final Diagnosis: Atrial fibrillation • Brugada syndrome
Symptoms: Palpitation
Clinical Procedure: Echocardiography • electrocardiogram • electrophysiological study
Specialty: Cardiology
Objective:
Rare disease
Background:
Brugada syndrome (BrS) is a cardiac arrhythmia disorder characterized by ventricular arrhythmias, which can lead to sudden cardiac death (SCD). BrS is also associated with atrial arrhythmias, particularly atrial fibrillation (AF). There is ongoing debate regarding whether treated AF can still precipitate ventricular arrhythmias in patients with BrS. This case series aims to elucidate the prognostic significance of treated AF in BrS patients who experienced SCD.
Case Reports:
We report on 2 patients diagnosed with Brugada syndrome (BrS) who presented with atrial fibrillation (AF). Both patients exhibited type I Brugada electrocardiographic patterns, and echocardiographic assessments revealed normal cardiac structure and function. Thyroid function tests and electrolyte levels were within normal ranges. An electrophysiology study (EPS) performed on the first patient demonstrated the induction and termination of AF, but no inducible ventricular arrhythmia was observed. Both patients declined the ablation procedure for AF treatment, opting instead for pharmacologic rhythm control with amiodarone. During follow-up visits every 3 months, neither patient reported palpitations or syncope, and electrocardiography consistently indicated sinus rhythm. Despite this, sudden cardiac death (SCD) occurred in the first patient during the first year of follow-up and in the second patient during the second year of follow-up.
Conclusions:
Patients with BrS who have treated AF remain at a high risk of SCD. The presence of AF in BrS patients may indicate a specific variant of the SCN5A mutation, which can heighten the risk of ventricular arrhythmias and consequent SCD.
Key words: Atrial Fibrillation, Brugada Syndrome, Mortality
Introduction
Brugada syndrome (BrS) is a cardiac arrhythmia disorder characterized by a heightened risk of ventricular arrhythmias and sudden cardiac death (SCD) [1,2]. Despite its relatively low incidence, BrS is implicated in nearly 20% of cases of SCD in individuals with structurally normal hearts [2]. Therefore, primary prevention through implantable cardioverter-defibrillator (ICD) implantation remains paramount in managing BrS.
While the arrhythmic substrate in BrS traditionally resides in the ventricles, recent evidence suggests that BrS can also manifest with atrial fibrillation (AF), with reported incidences ranging from 6% to 38% [3]. A meta-analysis by Kewcharoen et al found that AF significantly elevates the risk of major arrhythmic events (MAE) in BrS patients [4]. Theoretically, AF may diminish ventricular myocyte refractoriness and precipitate proarrhythmic effects, thereby promoting ventricular arrhythmias. However, findings from a case series by Letsas et al demonstrated that polymorphic ventricular tachycardia persisted in BrS patients despite pharmacological rhythm control for AF, suggesting additional underlying mechanisms [5]. Given the uncertainty surrounding the prognosis of AF in BrS patients managed with rhythm control strategies, this report of 2 cases assessed the utility of treated AF in predicting SCD events among BrS patients lacking other high-risk markers for MAE, apart from a spontaneous type I Brugada electrocardiographic (ECG) pattern.
Case Reports
Case 1
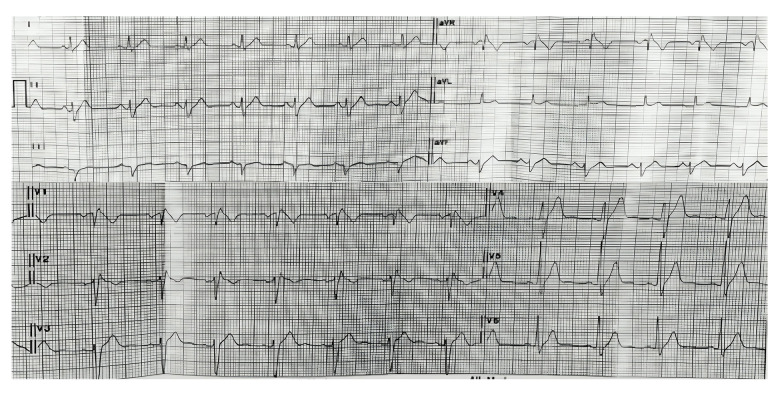
A 30-year-old man presented to the clinic with a chief concern of intermittent palpitations over the past 3 months, accompanied by an irregular heartbeat occurring 1–2 times per week. The patient reported no episodes of syncope, near syncope, dyspnea, breathlessness, or chest pain. He had no history of hypertension, diabetes mellitus, or dyslipidemia. Notably, his father had died suddenly at the age of 40 while at rest. The patient’s vital signs were stable. Electrocardiography (ECG) revealed a sinus rhythm with a type I Brugada ECG pattern (Figure 1).
Figure 1.
Electrocardiography showed a type I Brugada ECG pattern on V1–V2.
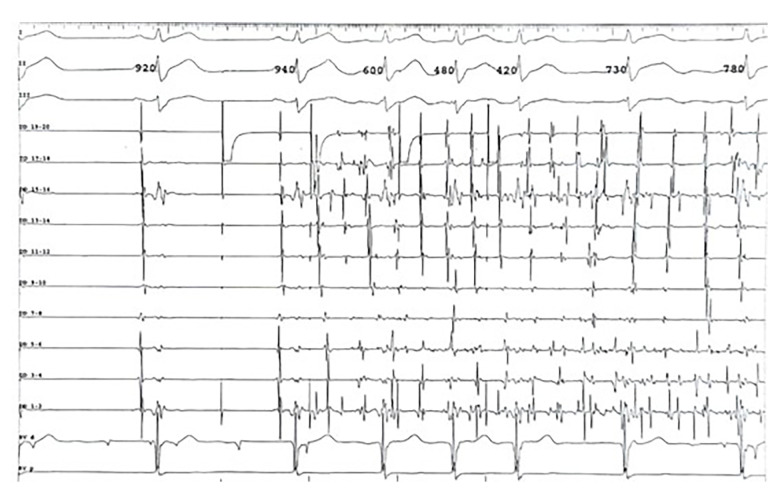
Echocardiography showed normal chamber dimensions, normal left ventricular diastolic and systolic function with an ejection fraction of 60%, normal valvular anatomy and function, low probability of pulmonary hypertension, and normal right ventricular systolic function. Laboratory results, including thyroid function and electrolytes, were within normal limits. Continuous monitoring with a Holter device for 3 days did not document any arrhythmias. An electrophysiology study (EPS) was performed using programmed atrial and ventricular stimulation with incremental atrial (from the right atrium) and ventricular (right ventricular apex) pacing from S1 500 milliseconds (ms) down to 260 ms, and single-double extra stimuli from 400 ms to 200 ms. No inducible ventricular arrhythmia was observed. Atrial fibrillation (AF) was induced during S1 500 ms and terminated spontaneously after 5 minutes (Figure 2).
Figure 2.
The EPS: Duodecapolar was inserted along the right atrial crista terminalis to the coronary sinus (DD 19–20 to DD 1–2). Pacing S1500 was performed on DD 19–20. The rapid, irregular, and fractionated electrogram on DD confirmed AF diagnosis. The AF was induced spontaneously despite non-capture pacing. ECG speed was displayed on 100 ms. DD – duodecapolar; RV – right ventricular.
The patient reported that the palpitations experienced during AF were similar to previous episodes, suggesting that AF was the clinical arrhythmia. No inducible ventricular arrhythmias were observed with ventricular pacing up to the third extra stimulus. The patient was diagnosed with type I Brugada syndrome with AF manifestation, and was scheduled for ablation but declined the procedure. Hence, he was prescribed amiodarone 200 mg orally 3 times daily for 1 month, followed by 200 mg orally once daily as rhythm control therapy. The patient reported no palpitations after discharge. During routine follow-up every 3 months, no ventricular arrhythmias or syncope were documented, and the ECG persistently showed a sinus rhythm. However, the patient suffered a sudden death 1 year later while at rest.
Case 2
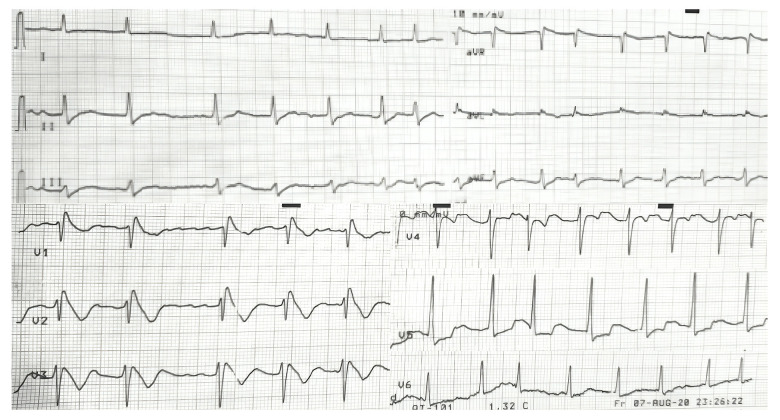
A 33-year-old man presented with intermittent palpitations and an irregular heartbeat over the previous 2 months. Neither the patient nor his family had a history of syncope, near syncope, aborted sudden cardiac death (SCD), or SCD. He had no notable risk factors such as hypertension, diabetes, or dyslipidemia. ECG revealed AF with ST-segment elevation in the right precordial leads, indicative of a type I Brugada ECG pattern (Figure 3).
Figure 3.
Electrocardiography revealed atrial fibrillation with type I Brugada Pattern on V1–V3.
Echocardiography revealed normal chamber dimensions, preserved left ventricular diastolic and systolic function with an ejection fraction of 65%, intact valvular structure and function, low likelihood of pulmonary hypertension, and normal right ventricular systolic function. Laboratory results, including electrolytes and thyroid function, were within normal limits. The patient was scheduled to undergo an ablation procedure but ultimately declined. Therefore, pharmacological cardioversion was chosen with amiodarone 200 mg orally 3 times daily for 1 month, followed by 200 mg orally once daily. During routine follow-ups every 3 months, the patient reported no palpitations, syncope, or near syncope. The sinus rhythm consistently observed on ECG during the follow-up period. However, the patient died suddenly while sleeping 2 years later.
Discussion
This case series shows that despite controlling atrial fibrillation (AF) with antiarrhythmic medication, patients with Brugada syndrome (BrS) remain at increased risk for sudden cardiac death (SCD). This observation suggests an underlying pathway linking AF to ventricular arrhythmias in BrS patients.
BrS primarily results from mutations in the SCN5A gene, a key member of the human voltage-gated sodium channel gene family crucial for cardiac electrophysiological function. SCN5A mutations are implicated not only in BrS but also in various cardiac arrhythmias, such as sick sinus syndrome, atrial arrhythmias (including AF), ventricular arrhythmias, long QT syndrome type 3 (LQTS3), and progressive cardiac conduction defects [1,2]. Atrial arrhythmias associated with SCN5A mutations encompass atrial standstill and AF. These mutations promote ectopic activity, prolong atrial action potential duration, and increase excitability, thereby predisposing individuals to AF [6].
Atrial fibrillation (AF) is hypothesized to share a mechanism with ventricular arrhythmia, suggesting that these conditions can coexist in a single patient. Theoretically, AF can decrease refractoriness in ventricular muscle, induce a rapid ventricular rate, and ultimately lead to ventricular tachyarrhythmia. Additionally, due to its irregular rhythm, AF can exert proarrhythmic effects, resulting in a short-long-short pattern of electrical activity [7,8]. A case series by Letsas et al demonstrated that 1 out of 3 symptomatic Brugada syndrome (BrS) patients with treated AF experienced polymorphic ventricular tachycardia during follow-up [5]. Similarly, in this series, sudden cardiac death (SCD) occurred in BrS patients with a spontaneous type I Brugada ECG pattern and treated AF, indicating there is another underlying pathophysiology linking AF and ventricular arrhythmias. In 2019, Micaglio et al reported a case where a specific SCN5A variant mutation (c.4700_4701del, p.Phe1567Cysfs*221) was implicated in both ventricular tachycardia and atrial fibrillation in a BrS patient [9]. This finding suggests that specific SCN5A gene mutations at the atrial level are also associated with subclinical electrical disorders at the ventricular level, potentially leading to SCD, as observed in our cases. However, the correlation between AF and ventricular arrhythmia in this case series remains inconclusive due to insufficient data.
Currently, the management of Brugada syndrome (BrS) primarily focuses on preventing sudden cardiac death (SCD) due to ventricular tachyarrhythmias, with implantable cardioverter-defibrillator (ICD) implantation and quinidine administration being the main strategies [10,11]. In this case series, we utilized pharmacological cardioversion to convert atrial fibrillation (AF) to sinus rhythm. Throughout the follow-up period, neither patient reported palpitations, and electrocardiograms consistently indicated sinus rhythm. However, both patients experienced ventricular tachyarrhythmia, resulting in sudden cardiac death. Although amiodarone is effective in reducing the risk of malignant ventricular arrhythmias, incidents of sudden cardiac death due to malignant arrhythmia still occurred in the amiodarone group [12,13]. This underscores the necessity for implantable cardioverter-defibrillator (ICD) implantation to fully prevent arrhythmic sudden cardiac death in patients with Brugada syndrome (BrS).
According to the American Heart Association and European Society of Cardiology guidelines, ICD implantation is strongly recommended (Class I) for symptomatic BrS patients, including those with a history of resuscitated cardiac arrest or sustained ventricular arrhythmia (VA), as well as patients with episodes of non-vagal syncope or nocturnal agonal respiration [10,11]. Additionally, ICD implantation may be considered (Class IIb recommendation) for asymptomatic BrS patients with a spontaneous type I Brugada ECG pattern and inducible ventricular arrhythmias during an electrophysiological study (EPS) [10,11]. In this case series, since no inducible ventricular arrhythmias were observed during the electrophysiological study, and due to the absence of specific guidelines for managing Brugada syndrome (BrS) presenting with atrial fibrillation (AF) in the absence of prior cardiac arrest, syncope, or documented ventricular arrhythmias (VA), we did not proceed with implantable cardioverter-defibrillator (ICD) placement in these patients. Therefore, there is a critical need for prospective cohort studies to elucidate the role of ICDs in BrS patients who exhibit a type I Brugada ECG pattern alongside AF. These studies should carefully evaluate the correlation between AF and major arrhythmic events (MAE) in BrS patients, adjusting for potential confounding variables and conducting subgroup analyses of patients with treated and untreated AF. Such investigations are crucial for establishing a definitive association between BrS, AF, and the risk of sudden cardiac death (SCD).
There are 2 primary limitations in this case series. First, we cannot definitively ascertain whether the sudden deaths in these 2 patients were due to malignant arrhythmias, as there was no available ECG data at the time of death. Consequently, the relationship between atrial fibrillation (AF) and ventricular arrhythmias in these Brugada syndrome (BrS) patients remains inconclusive. Continuous Holter monitoring may be necessary in future studies to evaluate the association between AF and the risk of sudden cardiac death (SCD) in BrS patients. Second, genetic testing to identify the Brugada phenotype was not conducted due to the lack of advanced laboratory facilities. This limitation leaves the pathogenesis of AF in these patients unclear. Future studies should incorporate genetic testing to elucidate the underlying mechanisms of AF in BrS patients and to clarify the pathway by which AF may induce ventricular arrhythmias in this population.
Conclusions
The presence of atrial fibrillation (AF) in patients with Brugada syndrome (BrS) warrants increased clinical vigilance. Atrial electrical disorders in this population may signify underlying genetic mutations that predispose BrS patients to a heightened risk of sudden cardiac death (SCD). Recognizing AF as a potential marker for these genetic abnormalities is crucial for early identification and management of patients at elevated risk for life-threatening ventricular arrhythmias.
Footnotes
Publisher’s note: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher
Ethics Approval
This study was approved by the Medical Research Ethics Committee of Dr. Hasan Sadikin General Hospital, West Java, Indonesia.
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
- 1.Vutthikraivit W, Rattanawong P, Putthapiban P, et al. Worldwide prevalence of Brugada syndrome: A systematic review and meta-analysis. Acta Cardiol Sin. 2018;34(3):267–77. doi: 10.6515/ACS.201805_34(3).20180302B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kabra N, Gupta R, Aronow WS, Frishman WH. Sudden cardiac death in Brugada syndrome. Cardiol Rev. 2020;28(4):203–7. doi: 10.1097/CRD.0000000000000259. [DOI] [PubMed] [Google Scholar]
- 3.Francis J, Antzelevitch C. Atrial fibrillation and Brugada syndrome. J Am Coll Cardiol. 2008;51(12):1149–53. doi: 10.1016/j.jacc.2007.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kewcharoen J, Rattanawong P, Kanitsoraphan C, et al. Atrial fibrillation and risk of major arrhythmic events in Brugada syndrome: A meta-analysis. Ann Noninvasive Electrocardiol. 2019;24(6):e12676. doi: 10.1111/anec.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Letsas KP, Sideris A, Efremidis M, et al. Prevalence of paroxysmal atrial fibrillation in Brugada syndrome: A case series and a review of the literature. J Cardiovasc Med (Hagerstown) 2007;8(10):803–6. doi: 10.2459/JCM.0b013e3280112b21. [DOI] [PubMed] [Google Scholar]
- 6.Li W, Yin L, Shen C, et al. SCN5A variants: Association with cardiac disorders. Front Physiol. 2018;9:01372. doi: 10.3389/fphys.2018.01372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen LY, Benditt DG, Alonso A. Atrial fibrillation and its association with sudden cardiac death. Circ J. 2014;78(11):2588–93. doi: 10.1253/circj.cj-14-0814. [DOI] [PubMed] [Google Scholar]
- 8.Chugh SS, Reinier K, Teodorescu C, et al. Epidemiology of sudden cardiac death: Clinical and research implications. Prog Cardiovasc Dis. 2008;51(3):213–28. doi: 10.1016/j.pcad.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Micaglio E, Monasky MM, Ciconte G, et al. Novel SCN5A frameshift mutation in Brugada syndrome associated with complex arrhythmic phenotype. Front Genet. 2019;10:547. doi: 10.3389/fgene.2019.00547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeppenfeld K, Tfelt-Hansen J, de Riva M, et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Developed by the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC) Endorsed by the Association for European Paediatric and Congenital Cardiology (AEPC) Eur Heart J. 2022;43(40):3997–4126. doi: 10.1093/eurheartj/ehac262. [DOI] [PubMed] [Google Scholar]
- 11.Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS Guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Circulation. 2018;138(13):e272–391. doi: 10.1161/CIR.0000000000000549. [DOI] [PubMed] [Google Scholar]
- 12.Sim I, McDonald KM, Lavori PW, et al. Quantitative overview of randomized trials of amiodarone to prevent sudden cardiac death. Circulation. 1997;96(9):2823–29. doi: 10.1161/01.cir.96.9.2823. [DOI] [PubMed] [Google Scholar]
- 13.Boutitie F, Boissel JP, Connolly SJ, et al. Amiodarone interaction with β-blockers. Circulation. 1999;99(17):2268. doi: 10.1161/01.cir.99.17.2268. [DOI] [PubMed] [Google Scholar]