Abstract
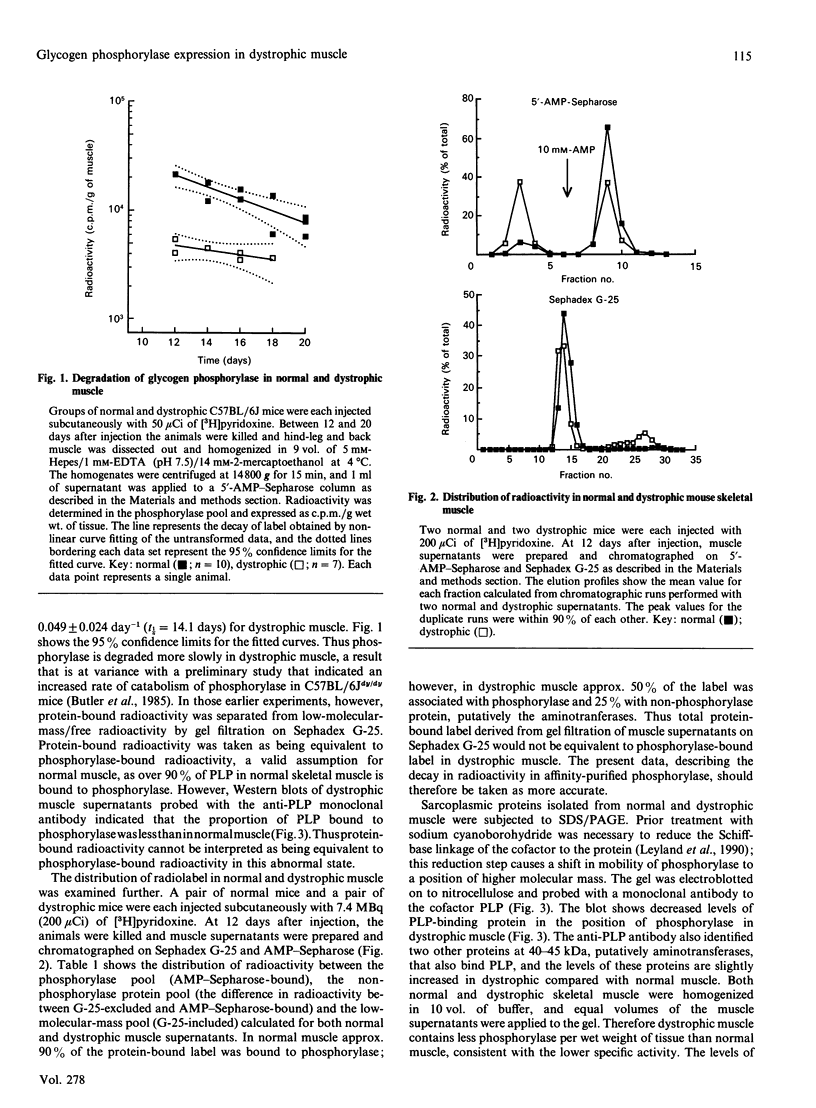
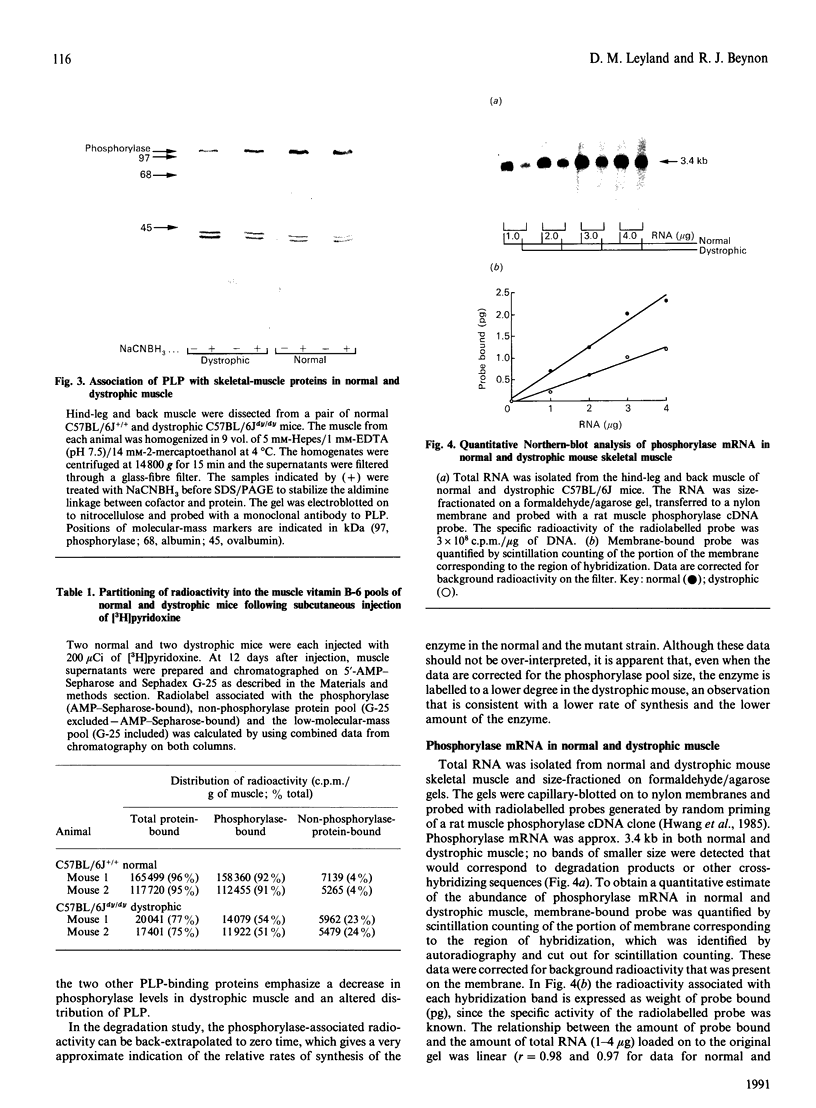
Specific cofactor labelling was employed to determine the degradation rate of glycogen phosphorylase in normal adult C57BL/6J mice and their dystrophic counterparts (C57BL/6Jdy/dy). The rate constant for the decay of phosphorylase-bound label was 0.125 day-1 in normal muscle and 0.49 day-1 in dystrophic muscle, i.e. a lower rate of catabolism of phosphorylase in dystrophic muscle. Quantitative Northern-blot analyses of total RNA isolated from normal and dystrophic muscle indicated that the abundance of phosphorylase mRNA as a percentage of total RNA was approx. 40% lower in dystrophic muscle. The specific activity of phosphorylase in dystrophic muscle is approx. 60% lower than in normal muscle, and is elicited by a lower rate of turnover of the enzyme, i.e. both synthesis and degradation are decreased.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butler P. E., Cookson E. J., Beynon R. J. Accelerated degradation of glycogen phosphorylase in denervated and dystrophic mouse skeletal muscle. Biosci Rep. 1985 Jul;5(7):567–572. doi: 10.1007/BF01117069. [DOI] [PubMed] [Google Scholar]
- Carney I. T., Beynon R. J., Kay J., Birket N. A semicontinuous assay for glycogen phosphorylase. Anal Biochem. 1978 Mar;85(1):321–324. doi: 10.1016/0003-2697(78)90309-3. [DOI] [PubMed] [Google Scholar]
- Cookson E. J., Beynon R. J. Further evaluation of cofactor as a turnover label for glycogen phosphorylase. Int J Biochem. 1989;21(9):975–982. doi: 10.1016/0020-711x(89)90229-2. [DOI] [PubMed] [Google Scholar]
- David E. S., Crerar M. M. Quantitation of muscle glycogen phosphorylase mRNA and enzyme amounts in adult rat tissues. Biochim Biophys Acta. 1986 Jan 15;880(1):78–90. doi: 10.1016/0304-4165(86)90122-4. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Garber A. J., Schwartz R. J., Seidel C. L., Silvers A., Entman M. L. Skeletal muscle protein and amino acid metabolism in hereditary mouse muscular dystrophy. Accelerated protein turnover and increased alanine and glutamine formation and release. J Biol Chem. 1980 Sep 10;255(17):8315–8324. [PubMed] [Google Scholar]
- Gorin F., Ignacio P., Gelinas R., Carlsen R. Abnormal expression of glycogen phosphorylase genes in regenerated muscle. Am J Physiol. 1989 Sep;257(3 Pt 1):C495–C503. doi: 10.1152/ajpcell.1989.257.3.C495. [DOI] [PubMed] [Google Scholar]
- Hwang P. K., See Y. P., Vincentini A. M., Powers M. A., Fletterick R. J., Crerar M. M. Comparative sequence analysis of rat, rabbit, and human muscle glycogen phosphorylase cDNAs. Eur J Biochem. 1985 Oct 15;152(2):267–274. doi: 10.1111/j.1432-1033.1985.tb09193.x. [DOI] [PubMed] [Google Scholar]
- Kitahara A., Imai F., Takaya S., Sato K. Enzyme alteration in skeletal muscle of mice with muscular dystrophy. Biochim Biophys Acta. 1977 Dec 22;500(2):256–266. doi: 10.1016/0304-4165(77)90018-6. [DOI] [PubMed] [Google Scholar]
- Kitchin S. E., Watts D. C. Comparison of the turnover patterns of total and individual muscle proteins in normal mice and those with hereditary muscular dystrophy. Biochem J. 1973 Dec;136(4):1017–1028. doi: 10.1042/bj1361017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebherz H. G. Content and synthesis of glycolytic enzymes in normal, denervated, and dystrophic skeletal muscle fibers. Int J Biochem. 1984;16(12):1201–1205. doi: 10.1016/0020-711x(84)90217-9. [DOI] [PubMed] [Google Scholar]
- Leyland D. M., Turner P. C., Beynon R. J. Effect of denervation on the expression of glycogen phosphorylase in mouse skeletal muscle. Biochem J. 1990 Nov 15;272(1):231–237. doi: 10.1042/bj2720231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little B. W., Meyer W. L. Ribonuclease-inhibitor system abnormality in dystrophic mouse skeletal muscle. Science. 1970 Nov 13;170(3959):747–749. doi: 10.1126/science.170.3959.747. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizobuchi M., Inoue R., Miyaka M., Kakimoto Y. Accelerated protein turnover in the skeletal muscle of dystrophic mice. Biochim Biophys Acta. 1985 Nov 22;843(1-2):78–82. doi: 10.1016/0304-4165(85)90052-2. [DOI] [PubMed] [Google Scholar]
- Nwagwu M. Activity of polyribosomes from the muscle of normal and dystrophic mice in cell-free amino-acid incorporation. Eur J Biochem. 1975 Aug 1;56(1):123–127. doi: 10.1111/j.1432-1033.1975.tb02214.x. [DOI] [PubMed] [Google Scholar]
- Petell J. K., Marshall N. A., Lebherz H. G. Content and synthesis of several abundant glycolytic enzymes in skeletal muscles of normal and dystrophic mice. Int J Biochem. 1984;16(1):61–67. doi: 10.1016/0020-711x(84)90051-x. [DOI] [PubMed] [Google Scholar]
- SIMON E. J., GROSS C. S., LESSELL I. M. Turnover of muscle and liver proteins in mice with hereditary muscular dystrophy. Arch Biochem Biophys. 1962 Jan;96:41–46. doi: 10.1016/0003-9861(62)90447-2. [DOI] [PubMed] [Google Scholar]
- Srivastava U. Biochemical changes in progressive muscular dystrophy. 8. Protein synthesis in skeletal muscle of mouse as a function of muscular dystrophy. Arch Biochem Biophys. 1969 Dec;135(1):237–243. doi: 10.1016/0003-9861(69)90535-9. [DOI] [PubMed] [Google Scholar]
- Viceps-Madore D., Cidlowski J. A., Kittler J. M., Thanassi J. W. Preparation, characterization, and use of monoclonal antibodies to vitamin B6. J Biol Chem. 1983 Feb 25;258(4):2689–2696. [PubMed] [Google Scholar]
- Watts D. C., Reid J. D. Comparison of the protein-synthesizing machinery in the skeletal muscle of normal and dystrophic Bar Harbor mice. Biochem J. 1969 Nov;115(3):377–382. doi: 10.1042/bj1150377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster C., Silberstein L., Hays A. P., Blau H. M. Fast muscle fibers are preferentially affected in Duchenne muscular dystrophy. Cell. 1988 Feb 26;52(4):503–513. doi: 10.1016/0092-8674(88)90463-1. [DOI] [PubMed] [Google Scholar]