Abstract
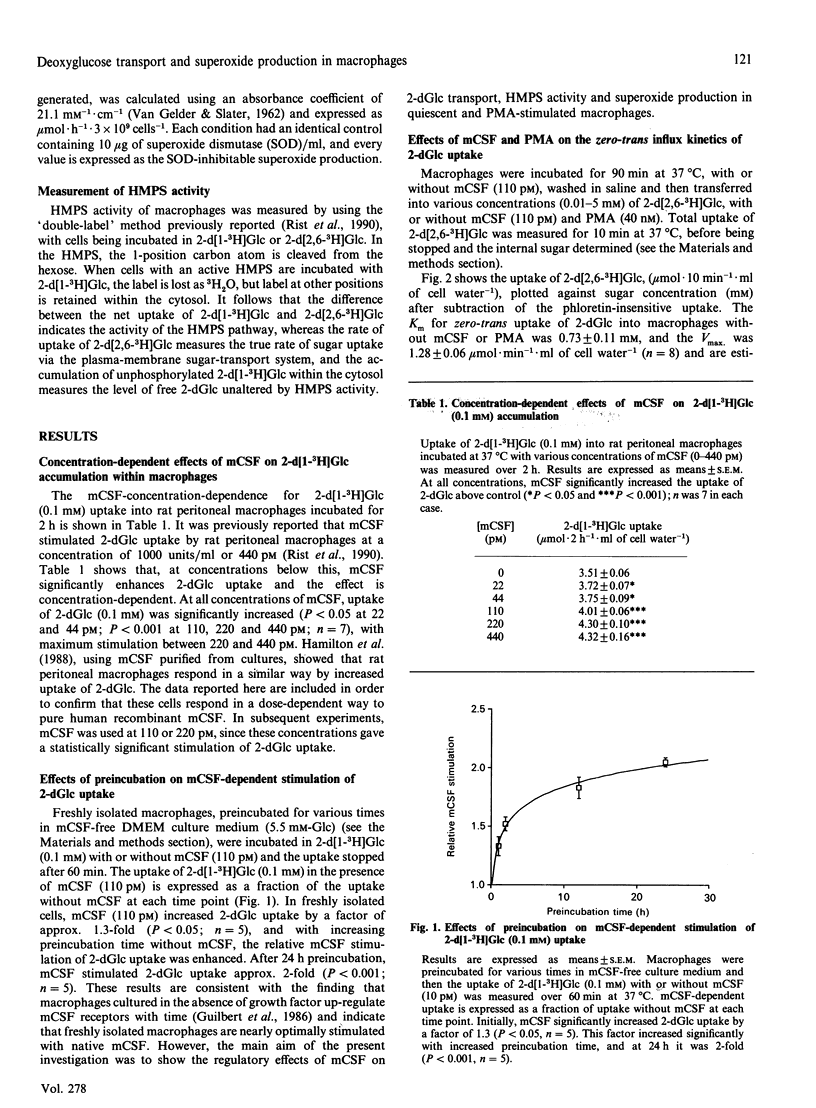
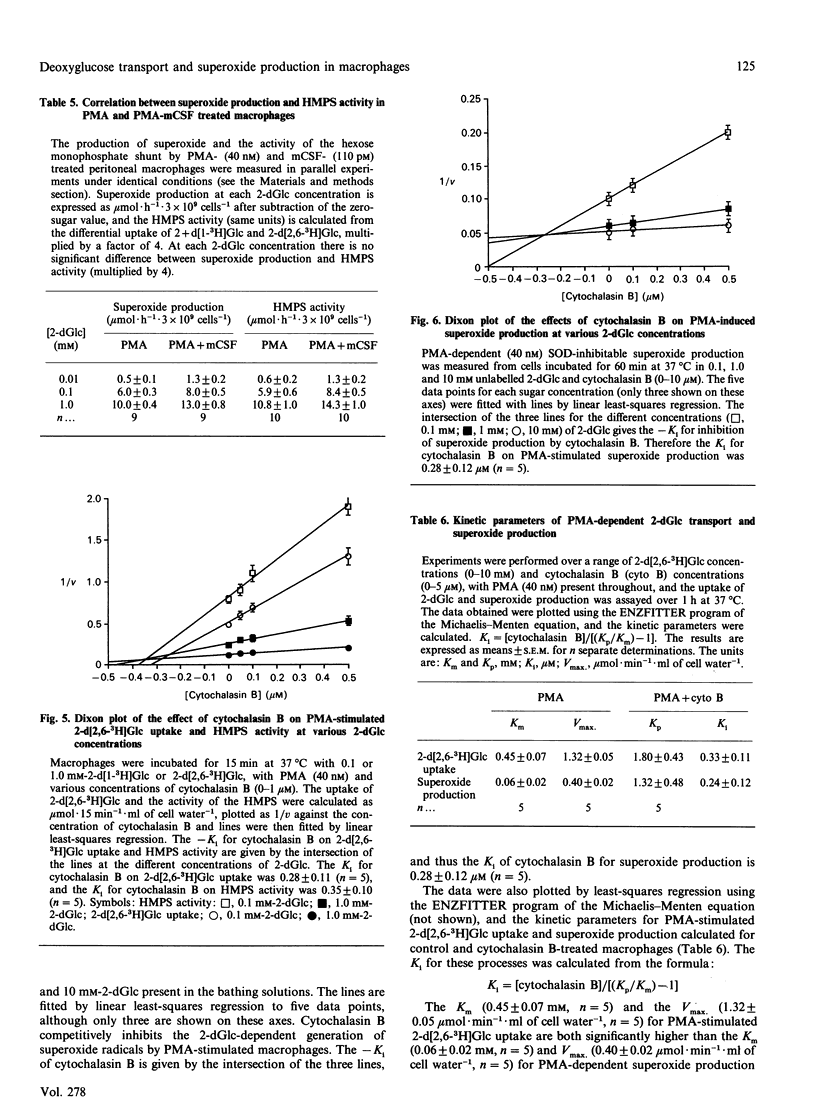
2-D-Deoxyglucose (2-dGlc) uptake and accumulation into rat peritoneal macrophages was increased by colony-stimulating factor (mCSF) by stimulating the coupling between endofacial hexokinase activity and the sugar transporter. The evidence for this is as follows: (1) mCSF significantly decreased the Km for zero-trans uptake (P less than 0.05), without altering Vmax.; (2) the accumulation of free 2-dGlc was increased by mCSF (P less than 0.05); (3) mCSF retarded the rate of exit of accumulated free 2-dGlc. The mCSF-dependent increase in 2-dGlc uptake by macrophages was enhanced by preincubation of the cells in mCSF-free solution. The activity of the hexose monophosphate shunt (HMPS) measured by the differential uptake of 2-d[1-3H]Glc and 2-d[2,6-3H]Glc was not stimulated by mCSF. Also, in quiescent cells, superoxide production, as determined by cytochrome c reduction, was unaffected by mCSF. Phorbol myristate acetate (PMA; 40 nM) stimulated both the HMPS activity and superoxide production. Both these effects were dependent on the uptake of external sugar (2-dGlc). Incubation of the macrophages with mCSF enhanced the sugar transport and PMA-dependent stimulation of HMPS activity and superoxide production, indicating a role for mCSF in the 'priming' of macrophage functions. Both HMPS activity and superoxide production are entirely dependent on uptake of exogenous sugar, since the potent sugar-transport inhibitor cytochalasin B competitively inhibited 2-dGlc uptake, HMPS activity and superoxide generation in PMA-activated cells (Ki approximately 0.3 microM for all three processes). Over a wide range of 2-dGlc concentrations, 4 mol of superoxide were generated/mol of 2-dGlc metabolized in the HMPS pathway, indicating coupling between these processes. The Km of 2-d[2,6-3H]Glc uptake in PMA-treated cells was 0.45 +/- 0.07 mM, and Vmax. was 1.32 +/- 0.05 mumol.min-1.ml of cell water-1. It is evident that there is a large degree of slippage between HMPS activity and membrane-associated hexokinase activity, since the Km for HMPS activity was 0.06 +/- 0.02 mM and the Vmax. was 0.10 +/- 0.03 mumol.min-1.ml of cell water-1.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arthur M. J., Kowalski-Saunders P., Wright R. Corynebacterium parvum-elicited hepatic macrophages demonstrate enhanced respiratory burst activity compared with resident Kupffer cells in the rat. Gastroenterology. 1986 Jul;91(1):174–181. doi: 10.1016/0016-5085(86)90455-5. [DOI] [PubMed] [Google Scholar]
- Arthur M. J., Kowalski-Saunders P., Wright R. Effect of endotoxin on release of reactive oxygen intermediates by rat hepatic macrophages. Gastroenterology. 1988 Dec;95(6):1588–1594. doi: 10.1016/s0016-5085(88)80082-9. [DOI] [PubMed] [Google Scholar]
- Babior B. M., Peters W. A. The O2--producing enzyme of human neutrophils. Further properties. J Biol Chem. 1981 Mar 10;256(5):2321–2323. [PubMed] [Google Scholar]
- Babior B. M. The respiratory burst of phagocytes. J Clin Invest. 1984 Mar;73(3):599–601. doi: 10.1172/JCI111249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior G. L., Rosin R. E., McMurrich B. J., Peters W. A., Babior B. M. Arrangement of the respiratory burst oxidase in the plasma membrane of the neutrophil. J Clin Invest. 1981 Jun;67(6):1724–1728. doi: 10.1172/JCI110210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basketter D. A., Widdas W. F. Asymmetry of the hexose transfer system in human erythrocytes. Comparison of the effects of cytochalasin B, phloretin and maltose as competitive inhibitors. J Physiol. 1978 May;278:389–401. doi: 10.1113/jphysiol.1978.sp012311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonventre P. F., Straus D., Baughn R. E., Imhoff J. Enhancement of carrier-mediated transport after immunologic activation of peritoneal macrophages. J Immunol. 1977 May;118(5):1827–1835. [PubMed] [Google Scholar]
- Boocock C. A., Jones G. E., Stanley E. R., Pollard J. W. Colony-stimulating factor-1 induces rapid behavioural responses in the mouse macrophage cell line, BAC1.2F5. J Cell Sci. 1989 Jul;93(Pt 3):447–456. doi: 10.1242/jcs.93.3.447. [DOI] [PubMed] [Google Scholar]
- Christopher C. W., Kohlbacher M. S., Amos H. Transport of sugars in chick-embryo fibroblasts. Evidence for a low-affinity system and a high-affinity system for glucose transport. Biochem J. 1976 Aug 15;158(2):439–450. doi: 10.1042/bj1580439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devés R., Krupka R. M. Cytochalasin B and the kinetics of inhibition of biological transport: a case of asymmetric binding to the glucose carrier. Biochim Biophys Acta. 1978 Jul 4;510(2):339–348. doi: 10.1016/0005-2736(78)90034-2. [DOI] [PubMed] [Google Scholar]
- Edwards S. W., Hallett M. B., Campbell A. K. Oxygen-radical production during inflammation may be limited by oxygen concentration. Biochem J. 1984 Feb 1;217(3):851–854. doi: 10.1042/bj2170851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faik P., Morgan M., Naftalin R. J., Rist R. J. Transport and accumulation of 2-deoxy-D-glucose in wild-type and hexokinase-deficient cultured Chinese-hamster ovary (CHO) cells. Biochem J. 1989 May 15;260(1):153–155. doi: 10.1042/bj2600153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantone J. C., Ward P. A. Role of oxygen-derived free radicals and metabolites in leukocyte-dependent inflammatory reactions. Am J Pathol. 1982 Jun;107(3):395–418. [PMC free article] [PubMed] [Google Scholar]
- Forman H. J., Nelson J., Fisher A. B. Rat alveolar macrophages require NADPH for superoxide production in the respiratory burst. Effect of NADPH depletion by paraquat. J Biol Chem. 1980 Oct 25;255(20):9879–9883. [PubMed] [Google Scholar]
- Guilbert L. J., Tynan P. W., Stanley E. R. Uptake and destruction of 125I-CSF-1 by peritoneal exudate macrophages. J Cell Biochem. 1986;31(3):203–216. doi: 10.1002/jcb.240310303. [DOI] [PubMed] [Google Scholar]
- Hamilton J. A., Vairo G., Lingelbach S. R. Activation and proliferation signals in murine macrophages: stimulation of glucose uptake by hemopoietic growth factors and other agents. J Cell Physiol. 1988 Mar;134(3):405–412. doi: 10.1002/jcp.1041340311. [DOI] [PubMed] [Google Scholar]
- Hamilton J. A., Vairo G., Lingelbach S. R. CSF-1 stimulates glucose uptake in murine bone marrow-derived macrophages. Biochem Biophys Res Commun. 1986 Jul 16;138(1):445–454. doi: 10.1016/0006-291x(86)90301-3. [DOI] [PubMed] [Google Scholar]
- Kiyotaki C., Peisach J., Bloom B. R. Oxygen metabolism in cloned macrophage cell lines: glucose dependence of superoxide production, metabolic and spectral analysis. J Immunol. 1984 Feb;132(2):857–866. [PubMed] [Google Scholar]
- Klebanoff S. J. Oxygen metabolism and the toxic properties of phagocytes. Ann Intern Med. 1980 Sep;93(3):480–489. doi: 10.7326/0003-4819-93-3-480. [DOI] [PubMed] [Google Scholar]
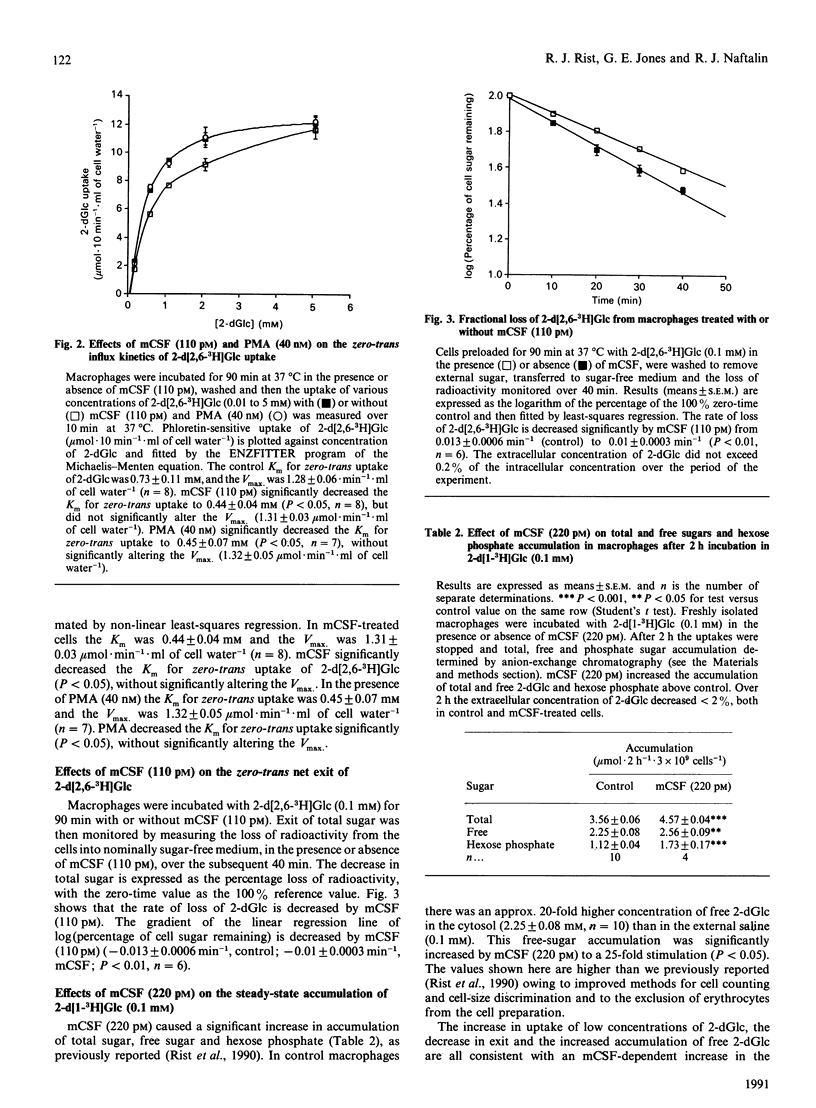
- Kuroda M., Honnor R. C., Cushman S. W., Londos C., Simpson I. A. Regulation of insulin-stimulated glucose transport in the isolated rat adipocyte. cAMP-independent effects of lipolytic and antilipolytic agents. J Biol Chem. 1987 Jan 5;262(1):245–253. [PubMed] [Google Scholar]
- Lee M. T., Warren M. K. CSF-1-induced resistance to viral infection in murine macrophages. J Immunol. 1987 May 1;138(9):3019–3022. [PubMed] [Google Scholar]
- Mohsenin V., Latifpour J. Respiratory burst in alveolar macrophages of diabetic rats. J Appl Physiol (1985) 1990 Jun;68(6):2384–2390. doi: 10.1152/jappl.1990.68.6.2384. [DOI] [PubMed] [Google Scholar]
- Naftalin R. J., Rist R. J. Effects of phorbol, dexamethasone and starvation on 3-O-methyl-D-glucose transport by rat thymocytes. Modulation of transport by altered trans effects. Biochem J. 1990 Jan 1;265(1):251–259. doi: 10.1042/bj2650251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naftalin R. J., Rist R. J. Evidence that activation of 2-deoxy-D-glucose transport in rat thymocyte suspensions results from enhanced coupling between transport and hexokinase activity. Biochem J. 1989 May 15;260(1):143–152. doi: 10.1042/bj2600143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naftalin R. J., Smith P. M. A model for accelerated uptake and accumulation of sugars arising from phosphorylation at the inner surface of the cell membrane. Biochim Biophys Acta. 1987 Feb 12;897(1):93–111. doi: 10.1016/0005-2736(87)90318-x. [DOI] [PubMed] [Google Scholar]
- Phillips W. A., Hamilton J. A. Colony stimulating factor-1 is a negative regulator of the macrophage respiratory burst. J Cell Physiol. 1990 Aug;144(2):190–196. doi: 10.1002/jcp.1041440203. [DOI] [PubMed] [Google Scholar]
- Rist R. J., Jones G. E., Naftalin R. J. Synergistic activation of 2-deoxy-D-glucose uptake in rat and murine peritoneal macrophages by human macrophage colony-stimulating factor-stimulated coupling between transport and hexokinase activity and phorbol-dependent stimulation of pentose phosphate-shunt activity. Biochem J. 1990 Jan 1;265(1):243–249. doi: 10.1042/bj2650243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root R. K., Metcalf J. A. H2O2 release from human granulocytes during phagocytosis. Relationship to superoxide anion formation and cellular catabolism of H2O2: studies with normal and cytochalasin B-treated cells. J Clin Invest. 1977 Dec;60(6):1266–1279. doi: 10.1172/JCI108886. [DOI] [PMC free article] [PubMed] [Google Scholar]
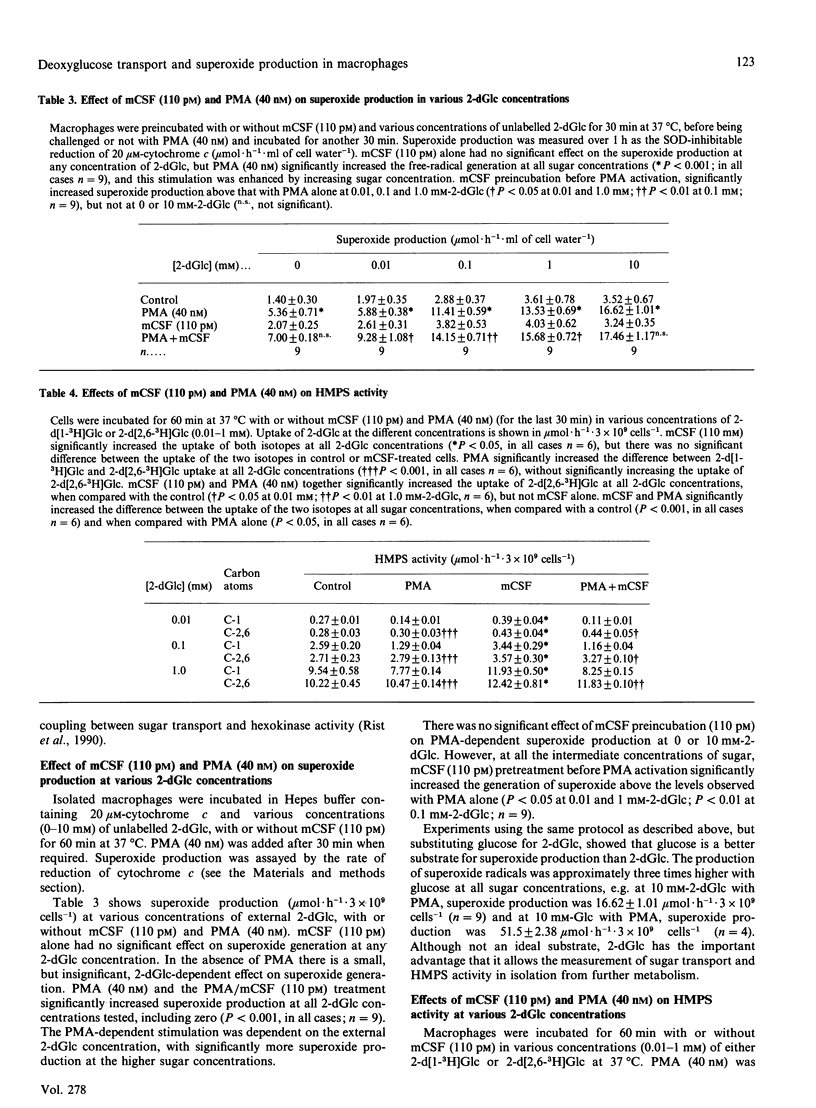
- Sampson-Johannes A., Carlino J. A. Enhancement of human monocyte tumoricidal activity by recombinant M-CSF. J Immunol. 1988 Nov 15;141(10):3680–3686. [PubMed] [Google Scholar]
- Sherr C. J. Colony-stimulating factor-1 receptor. Blood. 1990 Jan 1;75(1):1–12. [PubMed] [Google Scholar]
- Weening R. S., Wever R., Roos D. Quantitative aspects of the production of superoxide radicals by phagocytizing human granulocytes. J Lab Clin Med. 1975 Feb;85(2):245–252. [PubMed] [Google Scholar]
- Wing E. J., Ampel N. M., Waheed A., Shadduck R. K. Macrophage colony-stimulating factor (M-CSF) enhances the capacity of murine macrophages to secrete oxygen reduction products. J Immunol. 1985 Sep;135(3):2052–2056. [PubMed] [Google Scholar]
- Wing E. J., Waheed A., Shadduck R. K., Nagle L. S., Stephenson K. Effect of colony stimulating factor on murine macrophages. Induction of antitumor activity. J Clin Invest. 1982 Feb;69(2):270–276. doi: 10.1172/JCI110449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K., Tillotson L. G., Isselbacher K. J. Regulation of hexose carriers in chicken embryo fibroblasts. Effect of glucose starvation and role of protein synthesis. J Biol Chem. 1983 Aug 25;258(16):9786–9792. [PubMed] [Google Scholar]
- van GELDER B., SLATER E. C. The extinction coefficient of cytochrome c. Biochim Biophys Acta. 1962 Apr 23;58:593–595. doi: 10.1016/0006-3002(62)90073-2. [DOI] [PubMed] [Google Scholar]


