Abstract
Nonhuman primate models are increasingly used in the screening of candidate AIDS vaccine and immunization strategies for advancement to large-scale human trials. The predictive value of such macaque studies is largely dependent upon the fidelity of the model system in mimicking human immunodeficiency virus (HIV) type 1 infection in terms of viral transmission, replication, and pathogenesis. Herein, we describe the efficient mucosal transmission of a CCR5-specific chimeric simian/human immunodeficiency virus, SHIVSF162P3. Female rhesus macaques were infected with SHIVSF162P3 after a single atraumatic application to the cervicovaginal mucosa. The disease course of SHIVSF162P3-infected monkeys is similar and as varied as natural HIV infection in terms of viral replication, gradual loss of CD4+ peripheral blood mononuclear cells, and the development of simian AIDS-defining opportunistic infections. The SHIVSF162P3/macaque model should facilitate direct preclinical assessment of HIV vaccine strategies in addition to antiviral compounds directed towards envelope target cell interactions. Furthermore, this controlled model provides the setting to investigate immunologic responses and putative host-specific susceptibility factors that alter viral transmission and subsequent disease progression.
Containment of the AIDS epidemic will require vaccine strategies that effectively decrease the worldwide spread of human immunodeficiency virus (HIV). Such approaches need to account for both the mucosal mode of viral transmission and the preferential use of the CCR5 coreceptor for viral entry. Animal models that faithfully recapitulate these characteristics of HIV transmission as well as the ensuing disease course in humans are therefore highly desirable to aid in the identification and development of effective vaccine designs for advancement to human trials. Although simian immunodeficiency virus (SIV) infection of macaques is a well-established model system for HIV infection and pathogenesis, differences between the HIV and SIV genomes complicate the direct translation of findings in macaques to humans. Of particular concern are the structural, antigenic, and immunogenic differences between the envelope proteins of HIV and SIV. These differences will restrict the utility of the SIV model when evaluating the extent of immune protection conferred by envelope-based vaccine strategies. Consequently, chimeric envelope simian/HIVs (SHIVs) are increasingly used as challenge strains in nonhuman primate vaccine studies (9, 22). Though the use of envelope SHIVs addresses envelope-related limitations of the SIV model, the SHIV strains that are currently used induce a disease course that differs significantly from natural HIV and SIV infections (4, 6, 14, 17, 18). Notably, unlike HIV infection of humans, macaques inoculated with these SHIVs suffer from a severe and sustained depletion of CD4+ PBMC within weeks after infection. This dramatic loss in the CD4+ T-cell population is likely to be related to the coreceptor specificity of the challenge strains, since the disease induced is more characteristic of late stage HIV infection or of the rare infection of Δ32 homozygous individuals (1, 2, 12, 20). Indeed, the commonly used challenge viruses utilize CXCR4 or both CCR5 and CXCR4, in contrast to the CCR5-utilizing viruses that are prevalent in natural HIV type 1 (HIV-1) transmission and disease (21, 23, 24). For these reasons, development and characterization of a SHIV that mimics natural HIV-1 infection in terms of CCR5-utilization, mucosal transmission, sustained viral replication, gradual CD4+ peripheral blood mononuclear cell (PBMC) loss and development of simian AIDS (SAIDS) would prove invaluable in preclinical evaluation of vaccine candidates in the macaque model. Herein, we describe the first cell-free transmission of SHIVSF162P3, a viral isolate the displays these desirable features.
In vivo adaptation to a pathogenic variant of SHIVSF162.
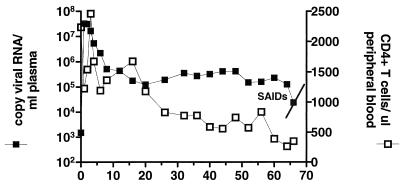
In a previously described study, two rhesus macaques were inoculated intravenously with the SHIVSF162 molecular clone, followed by three sequential blood-bone marrow transfusions into naïve rhesus macaques (3, 19). Acute plasma viremia increased with each serial passage of the virus, but more importantly, two of the late-passage macaques displayed sustained levels of viral replication. One of the passage 3 animals, T353, maintained a viral set point of 105 to 106 copies of viral RNA/ml of plasma that was accompanied by a gradual decline in CD4+ PBMCs (Fig. 1). This infection course is markedly different from formerly described pathogenic enveloped SHIVs but is reminiscent of both HIV infection in humans and experimental SIV infection in macaques. Animal T353 suffered from chronic diarrhea and severe weight loss and was euthanatized at 66 weeks postinfection. Necropsy examination disclosed severe disseminated histiocytic infiltrates due to Mycobacterium avium-Mycobacterium intracellulare (MAI) in the lamina propria of the small intestine and colon, mesenteric lymph nodes, spleen, and lung. MAI was identified by acid-fast staining and characteristic appearance. In addition severe lymphadenitis and lymphoid depletion in the thymus were noted (Table 1). The presences of an opportunistic infection in addition to sustained viral replication and T-cell depletion in macaque T353 are sufficient for clinical diagnosis of SAIDS (11).
FIG. 1.
Infection of macaque T353 with serially passaged SHIVSF162. Shown are values for plasma viremia (left y axis) and absolute number of CD3+ CD4+ PBMCs per μl of blood (right y axis) at various weeks postinfection (x axis). Mononuclear lymphocytes (LNMC) from this passage 3 macaque (T353) were purified by mechanical disruption followed by gradient centrifugation (Lymphocyte Separation Media; Bio-Whittaker, Walkersville, Md.), washed in Hanks balanced buffer solution, and cultured with an equal number of phytohemagglutinin- or recombinant interleukin 2-stimulated human PBMCs. Cultures were maintained for 10 to 14 days and monitored for the production of viral p27gag antigen using a commercially available antigen enzyme-linked immunosorbent assay as described below. Viral antigen-positive cultures were collected, subjected to light centrifugation to remove cellular debris, and passed through a 0.45-μm-pore-size filter. Cell viral supernatants designated SHIVSF162P3 were stored in 1-ml aliquots at −80°C. Stock virus was tested for TCID on stimulated human PBMC using the method of Reed and Munch (16).
TABLE 1.
Disease progression and SAIDS in macaques infected with CCR5 SHIVs
| Animal | Inoculation route | Viral inoculum | Peak viremia (set-point)a | Wk to SAIDS | SAIDS pathology |
|---|---|---|---|---|---|
| T353 | Blood-bone marrow transfusion | SHIVSF162P | 107 (105) | 66 | MAI, severe diarrhea, lymphadenitis |
| R061 | IVAG (cell-free virus) | SHIVSF162P3 | 108 (107) | 24 | PCP, cachexia, thymic atrophy |
| T290 | IVAG (cell-free virus) | SHIVSF162P3 | 106 (105) | 44 | Thymic atrophy, severe diarrhea, colitis |
| R513 | IVAG (cell-free virus) | SHIVSF162P3 | 106 (<103) | NAb | NA |
| T637 | IVAG (cell-free virus) | SHIVSF162P3 | 106 (103) | NA | NA |
Units are copies of viral RNA per milliliter of plasma. Values of <103 copies of RNA/ml of plasma are below detection limits (see text).
NA, not applicable.
Isolation and in vitro characterization of cell-free SHIVSF162P3.
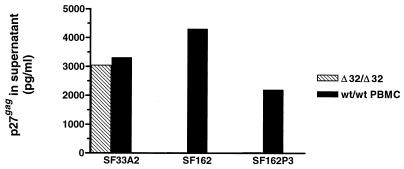
In an attempt to acquire a cell-free pathogenic CCR5-using SHIV, we recovered virus from T353 lymph node mononuclear cells (LNMC) cultures at 20 weeks postinoculation, a time postinfection that would presumably allow the outgrowth of viral species with increased pathogenicity (7). External lymph node biopsy tissue was obtained from macaque T353 infected 20 weeks earlier in a serial passage adaptation experiment of SHIVSF162, a virus that contains the tat, rev, vpu, and eny genes derived from the primary clade B isolate HIV-1SF162 (CCR5 virus) on the genomic backbone of pathogenic SIVmac239 (3, 10). Virus isolated from T353 week-20 LNMC, hereafter referred to as SHIVSF162P3, was propagated in human PBMC, its titer was determined in human PBMC, and it was characterized for coreceptor preference. Similar to the parental virus, SHIVSF162P3 grew efficiently in wild-type PBMC expressing the CCR5 protein but was unable to infect CCR5-negative PBMCs purified from Δ32/Δ32 donors. As a control, the CXCR4-specific virus SHIVSF33A.2 readily infected both wild-type and Δ32/Δ32 PBMC (Fig. 2). Furthermore, SHIVSF162P3 infected GHOST cells engineered to express the CCR5 protein but not those expressing CCR1, CXCR4, or BOB (data not shown). Collectively, these data strongly suggest that despite vigorous in vivo replication and adaptation, SHIVSF162P3 maintained CCR5 specificity, with no evidence of expanded coreceptor utilization.
FIG. 2.
Coreceptor usage of SHIVSF162P3 was compared to those of molecular clones SHIVSF162 (CCR5 specific) and SHIVSF33A.2 (CXCR4 specific) for replication in PBMCs isolated from a CCR5 wild-type (wt) donor (CCR5+/+ [wt/wt PBMC]) and a donor homozygous for Δ32 (CCR5−/− [Δ32/Δ32]). Culture supernatants were collected 10 days after infection and analyzed for p27gag content by antigen enzyme-linked immunosorbent assay (Cellular Products, Buffalo N.Y.). These values represent peak p27gag production. Activated T-cell cultures were exposed to cell-free SHIVSF162P3 (multiplicity of infection of 0.1) for 4 h, washed twice in phosphate-buffered saline to remove residual inoculum, and maintained in RPMI complete medium supplemented with recombinant interleukin 2. Culture supernatants were collected at regular intervals and analyzed for p27gag production by antigen enzyme-linked immunosorbent assay.
IVAG inoculation with SHIVSF162P3.
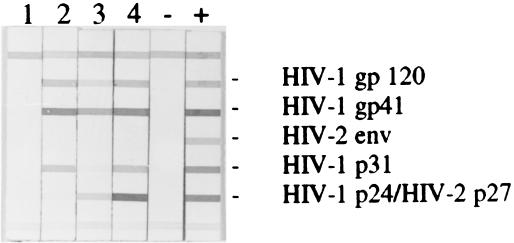
To establish mucosal transmission of SHIVSF162P3, we inoculated naïve rhesus macaques atraumatically via the cervicovaginal mucosa as previously described (4, 13). Four colony-bred Indian rhesus macaques, Macaca mulatta, were exposed to a single atraumatic intravaginal (IVAG) inoculation of 103.7 50% tissue culture infective dose (TCID50) (1-ml volume) of cell-free SHIVSF162P3. Prior to inoculation animals were determined to be serologically negative for simian type D retrovirus, SIV, and simian T-cell lymphotrophic virus; additionally, PCR analysis was performed to confirm simian type D retrovirus and SIV status. Animals were individually housed at the Tulane Regional Primate Research Center in accordance with the Guide for the Care and Use of Laboratory Animals. All procedures were approved by the institutional animal care and use committee. Animals underwent monthly physical examination that included hematology and blood chemistry measurements. Whole blood was collected at timed intervals and analyzed for viremia and antigenimia in plasma, T-cell subsets, and SHIV-specific antibodies as previously described (3, 4). All four macaques that were exposed to a single inoculum of 103.7 TCID50 of SHIVSF162P3 had significant but varying levels of viremia and antigenimia in plasma by 3 weeks after infection (Fig. 3; and data not shown). Peak viral loads ranged from 106 to 108 copies of RNA/ml of plasma with two animals (R061 and T290 [Fig. 3A and B]), reaching viral set points of >106 RNA copies/ml of plasma. Macaque T637 maintained persistent albeit low levels of viral replication 10 months after inoculation, while viral replication was below the limit of detection in macaque R513 by 12 weeks postinoculation. During acute infection, all four animals suffered a loss in the absolute number of peripheral CD4+ T cells that was followed by an inversion of the CD4/CD8 T-cell ratio in the chronic phase (Fig. 3). However, peripheral CD4+ T cells rebounded, and macaques infected with SHIVSF162P3 maintained significant numbers of circulating CD4+ PBMCs in the presence of sustained virus replication. One of the four inoculated animals, R061, demonstrated a rapid-progressor phenotype that was first described in the SIV system (5). This macaque had a peak viremia in plasma and a viral set point of 108 and 107 copies of viral RNA per ml of plasma, respectively. Unlike the other three macaques, R061 failed to develop antiviral humoral responses as measured by the radio immunoprecipitation assay-immunoblot assay (Chiron Corp.) (Fig. 4) and confirmed by the HIV-1/2 Synthetic Peptide EIA (Sanofi) (data not shown). Nevertheless, this animal maintained significant numbers of CD4+ T cells (>1,000 cells/μl of blood) up to 19 weeks postinfection, after which there was a precipitous drop in CD4+ PBMCs accompanied by dehydration and cachexia. The animal was sacrificed at 24 weeks postinfection, and a complete necropsy was performed. Tissues, including thymus, internal and external lymph nodes, brain, and spleen, were collected and either fixed for immunological or histopathologic analysis or maintained at 4°C for analysis of T-cell subsets within the lymphoid tissues (CD4/CD8 ratios: PBMC = 0.01, thymus = 0.0004, LNMC = 0.001).
FIG. 3.
IVAG infection with cell-free SHIVSF162P3. Whole blood was collected at defined intervals from animals R061 (A), T290 (B), R513 (C), and T637 (D), and samples were monitored for plasma viremia (■) (copies of viral RNA per milliliter of plasma), CD4+ cell number per microliter of whole blood (□), and CD8+ cell number per microliter of whole blood (○). To detect plasma viremia, we used the branched-DNA signal amplification assay (Bayer Diagnostics, Emeryville, Calif.) with a sensitivity limit of 1,500 copies of viral RNA/ml of plasma. The following human antibodies were used: Leu-3a-phycoerythrin and Leu-2a-peridinin chlorophyll protein (Becton Dickinson, Mountainview, Calif.), which recognize the CD4 and CD8 proteins, respectively. The monoclonal antibody FN-18–fluorescein isothiocyanate (Biosource International, Camarillo, Calif.) that recognizes the monkey CD3 molecule was also used. T-cell subsets were enumerated using TruCount absolute count tubes (Becton Dickinson) according to the manufacturer. Flow cytometry analysis was performed using the FACS Caliber device. PI, postinfection.
FIG. 4.
Humoral immune responses were detected in the plasma of macaques infected with SHIVSF162P3 using a strip immunoblot assay, RIBA HIV-1/2 (Chiron Corp., Emeryville, Calif.), as per the manufacturer's instructions Lanes: 1, animal R061; 2, animal T290; 3, animal R513; 4, animal T637 −, negative control; +, positive control. Plasma was obtained 23 weeks postinoculation.
Clinical assessment and histopathology.
To determine the clinical status of infected macaques complete necropsy examinations were performed by a veterinary pathologist. Samples of all major organs were fixed in 10% neutral buffered formalin, routinely processed in paraffin, and sectioned at 5 μm. Tissue sections were stained with hematoxylin and eosin, and selected tissues were also stained with Gomorri methenamine silver, Ziehl-Neelsen acid-fast stain, tissue Gram stain, or periodic acid-Schiff stains. To assess histopathological changes, microscopic sections were examined and evaluated by an experienced veterinary pathologist. Criteria used to diagnose simian AIDS were the presence of an opportunistic infection not seen in non-SIV-infected monkeys and that is not explained by other factors. Alternatively, the presence of severe giant cell disease due to viral infection is also used as a diagnostic of SAIDS (11). Histopathological analysis of R061 lung sections revealed severe diffuse Pneumocystis carinii pneumonia (PCP) (Fig. 5). In addition, animal T061 exhibited glomerulosclerosis in the kidney, severe thymic atrophy, and bone marrow hyperplasia, changes that are typically observed in SAIDS (Table 1). Significantly, virus amplified from necropsy samples maintained CCR5-coreceptor usage and did not infect Δ32 PBMC or GHOST cells lacking CCR5 but expressing CXCR4 (data not shown). Macaque T290, which maintained a viral set point of 105 copies of viral RNA/ml, also displayed a gradual loss in peripheral CD4+ T cells. This animal exhibited clinical signs, including diarrhea and dehydration, and was sacrificed at 44 weeks postinfection. Macaque R513 was sacrificed at week 44 from complications not associated with SIV or SHIV infection.
FIG. 5.
Histologic section of lung obtained at necropsy form macaque R061. Alveolar septae are thickened, and hypercellular white alveolar spaces are filled with eosinic, foamy material. These changes are typical of interstitial PCP. Hematoxylin and eosin stain was used. The presence of P. carinii was confirmed by Gomorri methenamine silver staining.
A system wherein mucosal transmission and subsequent disease progression in macaques infected with cell-free, CCR5-specific envelope SHIV is now established. Our data demonstrate that SHIVSF162P3 crosses the vaginal mucosa efficiently (four of four animals) and replicates to high levels without the precipitous peripheral CD4+ T-cell loss that is characteristic of other pathogenic SHIVs. Similar to HIV and SIV infections in humans and macaques, respectively, infection of adult rhesus macaques with SHIVSF162P3 caused a gradual loss in the total number of circulating CD4+ T cells, except in terminal stages of disease in a rapid progressor when the CD4+ T cell population declined severely. Furthermore, a range of viral set points, immune responses, and disease outcome were seen in the infected animals. As with HIV and SIV infections, viral load was predictive of disease progression (5, 8). R061, the animal that sustained the highest viral set point, showed a rapid disease progression and died of SAIDS without seroconversion typical of a rapid progressor (Table 1). Macaque T290, with a viral set point of 105 copies of viral RNA/ml, developed viral specific humoral immune responses within 3 weeks of inoculation, and the antibody responses broadened and strengthened over time. However, these responses were not protective against disease progression, as the animal suffered from gradual CD4+ T-cell loss and was sacrificed 44 weeks postinoculation. Postmortem examination revealed thymic atrophy, lymphoid hyperplasia, and colitis (Table 1). Of the remaining macaques, both had a peak viral replication of 106 copies of viral RNA/ml of plasma within 3 weeks of viral inoculation; however, these animals controlled viral replication such that viral set points were below or near the assay detection limit (Fig. 3).
In addition to providing a controlled setting for modeling HIV-1-induced disease, our findings underscore the utility of the SHIVSF162P3/macaque system in the preclinical testing of immunogens and immunization protocols. In the absence of any defined correlates of protective immunity, vaccine efficacy will be largely measured by its ability to prevent infection, decrease viral replication, and delay the onset of disease. The distinctive properties of SHIVSF162P3, including vaginal mucosal transmission, CCR5 envelope specificity, and neutralization resistance (data not shown), make this SHIV comparable to most HIV-1 strains isolated in the early and chronic phases of infection. Furthermore, the replication kinetics and disease patterns seen in animals vaginally inoculated with SHIVSH162P3 are qualitatively similar to and as variable as HIV infection in humans. These characteristics of SHIVSF162P3 make it a highly desirable and suitable challenge virus in the evaluation of envelope-based vaccine efficacy. Moreover, as SHIVSF162P3 also expresses the Tat, Rev, and Vpu proteins of HIV-1, this virus will also be useful in testing the efficacy of vaccines targeted against these nonstructural proteins (15). Lastly, since the envelope of SHIVSF162P3 functions with CCR5, it can be used to assess the therapeutic potential of anti-CCR5 drugs that are currently being developed. Of interest will be whether the use of these drugs selects for viruses that use CXCR4 and hence have an impact on viral pathogenesis.
Acknowledgments
We acknowledge the veterinary and animal husbandry staff at the Tulane Regional Primate Research Center for excellent care of animals; Jenny Booth, Casey Wingfield, and Lynette Sawyer for technical assistance with the branched-DNA assay; and Joann Yee at the California RPRC for assistance with enzyme immunoassay. We thank the Preclinical Research Branch of the NIAID for supplying animals used in this study and Lisa Chakrabarti for critical review and helpful comments.
This work was supported by NIH grants AI41945, AI46980, and AI46278.
REFERENCES
- 1.Biti R, French R, Young J, Bennetts B, Stewart G, Liang T. HIV-1 infection in an individual homozygous for the CCR5 deletion allele. Nat Med. 1997;3:252–253. doi: 10.1038/nm0397-252. [DOI] [PubMed] [Google Scholar]
- 2.Connor R I, Sheridan K E, Ceradini D, Choe S, Landau N R. Change in coreceptor use correlates with disease progression in HIV-1-infected individuals. J Exp Med. 1997;185:621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harouse J M, Gettie A, Tan R C, Blanchard J, Cheng-Mayer C. Distinct pathogenic sequela in rhesus macaques infected with CCR5 or CXCR4 utilizing SHIVs. Science. 1999;284:816–819. doi: 10.1126/science.284.5415.816. [DOI] [PubMed] [Google Scholar]
- 4.Harouse J M, Tan R C, Gettie A, Dailey P, Marx P A, Luciw P A, Cheng-Mayer C. Mucosal transmission of pathogenic CXCR4-utilizing SHIVSF33A variants in rhesus macaques. Virology. 1998;248:95–107. doi: 10.1006/viro.1998.9236. [DOI] [PubMed] [Google Scholar]
- 5.Hirsch V M, Fuerst T R, Sutter G, Carroll M W, Yang L C, Goldstein S, Piatak M, Jr, Elkins W R, Alvord W G, Montefiori D C, Moss B, Lifson J D. Patterns of viral replication correlate with outcome in simian immunodeficiency virus (SIV)-infected macaques: effect of prior immunization with a trivalent SIV vaccine in modified vaccinia virus Ankara. J Virol. 1996;70:3741–3752. doi: 10.1128/jvi.70.6.3741-3752.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joag S V, Li Z, Foresman L, Stephens E B, Zhao L J, Adany I, Pinson D M, McClure H M, Narayan O. Chimeric simian/human immunodeficiency virus that causes progressive loss of CD4+ T cells and AIDS in pig-tailed macaques. J Virol. 1996;70:3189–3197. doi: 10.1128/jvi.70.5.3189-3197.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kimata J T, Kuller L, Anderson D B, Dailey P, Overbaugh J. Emerging cytopathic and antigenic simian immunodeficiency virus variants influence AIDS progression. Nat Med. 1999;5:535–541. doi: 10.1038/8414. [DOI] [PubMed] [Google Scholar]
- 8.Lifson J D, Nowak M A, Goldstein S, Rossio J L, Kinter A, Vasquez G, Wiltrout T A, Brown C, Schneider D, Wahl L, Lloyd A L, Williams J, Elkins W R, Fauci A S, Hirsch V M. The extent of early viral replication is a critical determinant of the natural history of simian immunodeficiency virus infection. J Virol. 1997;71:9508–9514. doi: 10.1128/jvi.71.12.9508-9514.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu Y, Salvato M S, Pauza C D, Li J, Sodroski J, Manson K, Wyand M, Letvin N, Jenkins S, Touzjian N, Chutkowski C, Kushner N, LeFaile M, Payne L G, Roberts B. Utility of SHIV for testing HIV-1 vaccine candidates in macaques. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;12:99–106. doi: 10.1097/00042560-199606010-00001. [DOI] [PubMed] [Google Scholar]
- 10.Luciw P A, Pratt-Lowe E, Shaw K E S, Levy J A, Cheng-Mayer C. Persistent infection of rhesus macaques with T-cell-line-tropic and macrophage-tropic clones of simian/human immunodeficiency viruses (SHIV) Proc Natl Acad Sci USA. 1995;92:7490–7494. doi: 10.1073/pnas.92.16.7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mansfield K, King N. Viral diseases. In: Bennett B T, Abee C R, Henrickson R, editors. Nonhuman primates in biomedical research. I. Diseases. New York, N.Y: Academic Press; 1998. pp. 1–48. [Google Scholar]
- 12.Michael N L, Nelson J A E, KewalRamani V N, Chang G, O'Brien S J, Mascola J R, Volsky B, Louder M, White G C, II, Littman D R, Swanstrom R, O'Brien T R. Exclusive and persistent use of the entry coreceptor CXCR4 by human immunodeficiency virus type 1 from a subject homozygous for CCR Δ32. J Virol. 1998;72:6040–6047. doi: 10.1128/jvi.72.7.6040-6047.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller C J. Mucosal transmission of simian immunodeficiency virus. Curr Top Microbiol Immunol. 1994;188:107–122. doi: 10.1007/978-3-642-78536-8_6. [DOI] [PubMed] [Google Scholar]
- 14.Miller C J, Marthas M, Greenier J, Lu D, Dailey P J, Lu Y. In vivo replication rather than in vitro macrophage tropism predicts efficiency of vaginal transmission of simian immunodeficiency virus or simian/human immunodeficiency virus in rhesus macaques. J Virol. 1998;72:3248–3258. doi: 10.1128/jvi.72.4.3248-3258.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pauza C D, Trivedi P, Wallace M, Ruckwardt T J, Le Buanec H, Lu W, Bizzini B, Burny A, Zagury D, Gallo R C. Vaccination with tat toxoid attenuates disease in simian/HIV-challenged macaques. Proc Natl Acad Sci USA. 2000;97:3515–3519. doi: 10.1073/pnas.070049797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reed L J, Meunch H. A simple method for estimating 50 percent endpoints. Am J Hyg. 1938;27:493–499. [Google Scholar]
- 17.Reimann K A, Li J T, Veazey R, Halloran M, Park I-W, Karlsson G B, Sodroski J, Letvin N L. A chimeric simian/human immunodeficiency virus expressing a primary patient human immunodeficiency virus type 1 isolate env causes an AIDS-like disease after in vivo passage in rhesus monkeys. J Virol. 1996;70:6922–6928. doi: 10.1128/jvi.70.10.6922-6928.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shibata R, Maldarelli F, Siemon C, Matano T, Parta M, Miller G, Fredrickson T, Martin M A. Infection and pathogenicity of chimeric simian-human immunodeficiency viruses in macaques: determinants of high virus loads and CD4 cell killing. J Infect Dis. 1997;176:362–373. doi: 10.1086/514053. [DOI] [PubMed] [Google Scholar]
- 19.Tan R C, Harouse J M, Gettie A, Cheng-Mayer C. In vivo adaptation of SHIV(SF162): chimeric virus expressing a NSI, CCR5-specific envelope protein. J Med Primatol. 1999;28:164–168. doi: 10.1111/j.1600-0684.1999.tb00265.x. [DOI] [PubMed] [Google Scholar]
- 20.Theodoru I, Meyer L, Magierowska M, Katlama C, Rouzioux C. HIV-1 infection in an individual homozygous for CCR5 delta 32: Seroco study group. Lancet. 1997;349:1219–1220. [PubMed] [Google Scholar]
- 21.van't Wout A B, Blaak H, Ran L J, Brouwer M, Kuiken C, Schuitemaker H. Evolution of syncytium-inducing and non-syncytium-inducing biological virus clones in relation to replication kinetics during course of human immunodeficiency virus infection. J Virol. 1998;72:5099–5107. doi: 10.1128/jvi.72.6.5099-5107.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warren J T, Levinson M A. AIDS preclinical vaccine development: biennial survey of HIV, SIV, and SHIV challenge studies in vaccinated nonhuman primates. J Med Primatol. 1999;28:249–273. doi: 10.1111/j.1600-0684.1999.tb00276.x. [DOI] [PubMed] [Google Scholar]
- 23.Wolinsky S M, Wike C M, Korber B T M, Hutto C, Parks W P, Rosenblum L L, Kunstman K J, Furtado M R, Munoz J L. Selective transmission of human immunodeficiency virus type-1 variants from mothers to infants. Science. 1992;255:1134–1137. doi: 10.1126/science.1546316. [DOI] [PubMed] [Google Scholar]
- 24.Zhu T, Wang N, Carr A, Nam D S, Moor-Jankowski R, Cooper D A, Ho D D. Genetic characterization of human immunodeficiency virus type 1 in blood and genital secretions: evidence for viral compartmentalization and selection during sexual transmission. J Virol. 1996;70:3098–3107. doi: 10.1128/jvi.70.5.3098-3107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]