Abstract
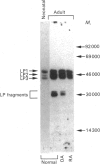
The link protein components of proteoglycan aggregates in adult human articular cartilage show heterogeneity due to proteolysis. Cleavages near the N-terminus of the intact link proteins, before residues 17, 19 and 24, generate three proteins of slightly diminished size (LP3). Cleavages within the N-terminal disulphide-bonded loop, before residues 66 and 73 of the intact link proteins, generate proteins that yield smaller degradation products upon reduction (LP fragments). In vitro, modified link protein components of a similar size to LP3 can be generated by a variety of proteinases, but of the physiologically relevant enzymes only stromelysin, cathepsin B and cathepsin G have the ability to yield modified link proteins with N-termini identical with those observed in situ. None of the proteolytic agents tested was able to produce LP fragments with N-termini identical with those observed in situ, and the majority of proteinases were not able to cleave within the disulphide-bonded loops. Cathepsin L and hydroxyl radicals can cleave within the N-terminal disulphide-bonded loop, and have the potential of initially opening the loop to allow further proteolytic processing by other agents to generate the native cleavage sites.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azzo W., Woessner J. F., Jr Purification and characterization of an acid metalloproteinase from human articular cartilage. J Biol Chem. 1986 Apr 25;261(12):5434–5441. [PubMed] [Google Scholar]
- Bayliss M. T., Ali S. Y. Studies on cathepsin B in human articular cartilage. Biochem J. 1978 Apr 1;171(1):149–154. doi: 10.1042/bj1710149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckwalter J. A., Rosenberg L. C. Electron microscopic studies of cartilage proteoglycans. Direct evidence for the variable length of the chondroitin sulfate-rich region of proteoglycan subunit core protein. J Biol Chem. 1982 Aug 25;257(16):9830–9839. [PubMed] [Google Scholar]
- Campbell I. K., Golds E. E., Mort J. S., Roughley P. J. Human articular cartilage secretes characteristic metal dependent proteinases upon stimulation by mononuclear cell factor. J Rheumatol. 1986 Feb;13(1):20–27. [PubMed] [Google Scholar]
- Caterson B., Baker J. R., Christner J. E., Lee Y., Lentz M. Monoclonal antibodies as probes for determining the microheterogeneity of the link proteins of cartilage proteoglycan. J Biol Chem. 1985 Sep 15;260(20):11348–11356. [PubMed] [Google Scholar]
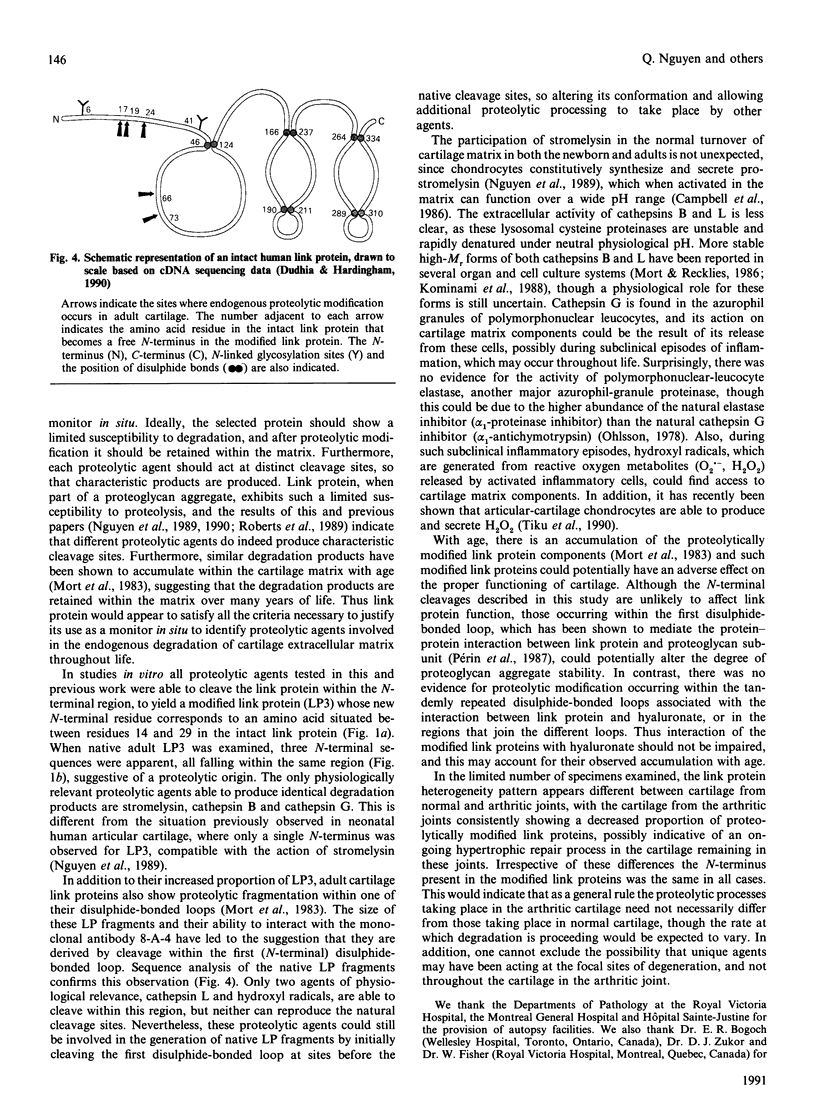
- Dudhia J., Hardingham T. E. The primary structure of human cartilage link protein. Nucleic Acids Res. 1990 Mar 11;18(5):1292–1292. doi: 10.1093/nar/18.5.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunja-Smith Z., Nagase H., Woessner J. F., Jr Purification of the neutral proteoglycan-degrading metalloproteinase from human articular cartilage tissue and its identification as stromelysin matrix metalloproteinase-3. Biochem J. 1989 Feb 15;258(1):115–119. doi: 10.1042/bj2580115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. E., Muir H. Hyaluronic acid in cartilage and proteoglycan aggregation. Biochem J. 1974 Jun;139(3):565–581. doi: 10.1042/bj1390565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. E. The role of link-protein in the structure of cartilage proteoglycan aggregates. Biochem J. 1979 Jan 1;177(1):237–247. doi: 10.1042/bj1770237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascall V. C., Heinegård D. Aggregation of cartilage proteoglycans. I. The role of hyaluronic acid. J Biol Chem. 1974 Jul 10;249(13):4232–4241. [PubMed] [Google Scholar]
- King J., Laemmli U. K. Polypeptides of the tail fibres of bacteriophage T4. J Mol Biol. 1971 Dec 28;62(3):465–477. doi: 10.1016/0022-2836(71)90148-3. [DOI] [PubMed] [Google Scholar]
- Kominami E., Tsukahara T., Hara K., Katunuma N. Biosyntheses and processing of lysosomal cysteine proteinases in rat macrophages. FEBS Lett. 1988 Apr 11;231(1):225–228. doi: 10.1016/0014-5793(88)80736-1. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Mochan E., Keler T. Plasmin degradation of cartilage proteoglycan. Biochim Biophys Acta. 1984 Aug 21;800(3):312–315. doi: 10.1016/0304-4165(84)90412-4. [DOI] [PubMed] [Google Scholar]
- Mort J. S., Poole A. R., Decker R. S. Immunofluorescent localization of cathepsins B and D in human fibroblasts. J Histochem Cytochem. 1981 May;29(5):649–657. doi: 10.1177/29.5.6788835. [DOI] [PubMed] [Google Scholar]
- Mort J. S., Poole A. R., Roughley P. J. Age-related changes in the structure of proteoglycan link proteins present in normal human articular cartilage. Biochem J. 1983 Jul 15;214(1):269–272. doi: 10.1042/bj2140269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mort J. S., Recklies A. D. Interrelationship of active and latent secreted human cathepsin B precursors. Biochem J. 1986 Jan 1;233(1):57–63. doi: 10.1042/bj2330057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neame P. J., Christner J. E., Baker J. R. The primary structure of link protein from rat chondrosarcoma proteoglycan aggregate. J Biol Chem. 1986 Mar 15;261(8):3519–3535. [PubMed] [Google Scholar]
- Nguyen Q., Mort J. S., Roughley P. J. Cartilage proteoglycan aggregate is degraded more extensively by cathepsin L than by cathepsin B. Biochem J. 1990 Mar 1;266(2):569–573. [PMC free article] [PubMed] [Google Scholar]
- Nguyen Q., Murphy G., Roughley P. J., Mort J. S. Degradation of proteoglycan aggregate by a cartilage metalloproteinase. Evidence for the involvement of stromelysin in the generation of link protein heterogeneity in situ. Biochem J. 1989 Apr 1;259(1):61–67. doi: 10.1042/bj2590061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole A. R., Mort J. S. Biochemical and immunological studies of lysosomal and related proteinases in health and disease. J Histochem Cytochem. 1981 Mar;29(3A):494–502. doi: 10.1177/29.3.494. [DOI] [PubMed] [Google Scholar]
- Périn J. P., Bonnet F., Thurieau C., Jollès P. Link protein interactions with hyaluronate and proteoglycans. Characterization of two distinct domains in bovine cartilage link proteins. J Biol Chem. 1987 Sep 25;262(27):13269–13272. [PubMed] [Google Scholar]
- Roberts C. R., Mort J. S., Roughley P. J. Treatment of cartilage proteoglycan aggregate with hydrogen peroxide. Relationship between observed degradation products and those that occur naturally during aging. Biochem J. 1987 Oct 15;247(2):349–357. doi: 10.1042/bj2470349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts C. R., Roughley P. J., Mort J. S. Degradation of human proteoglycan aggregate induced by hydrogen peroxide. Protein fragmentation, amino acid modification and hyaluronic acid cleavage. Biochem J. 1989 May 1;259(3):805–811. doi: 10.1042/bj2590805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughley P. J., Mort J. S. Ageing and the aggregating proteoglycans of human articular cartilage. Clin Sci (Lond) 1986 Oct;71(4):337–344. doi: 10.1042/cs0710337. [DOI] [PubMed] [Google Scholar]
- Roughley P. J., Poole A. R., Mort J. S. The heterogeneity of link proteins isolated from human articular cartilage proteoglycan aggregates. J Biol Chem. 1982 Oct 25;257(20):11908–11914. [PubMed] [Google Scholar]
- Roughley P. J., White R. J., Poole A. R. Identification of a hyaluronic acid-binding protein that interferes with the preparation of high-buoyant-density proteoglycan aggregates from adult human articular cartilage. Biochem J. 1985 Oct 1;231(1):129–138. doi: 10.1042/bj2310129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandy J. D., Sriratana A., Brown H. L., Lowther D. A. Evidence for polymorphonuclear-leucocyte-derived proteinases in arthritic cartilage. Biochem J. 1981 Jan 1;193(1):193–202. doi: 10.1042/bj1930193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J. W., Hascall V. C. N-terminal carbamylation of the hyaluronic acid-binding region and the link protein from the chondrosarcoma proteoglycan aggregate. J Biol Chem. 1986 Nov 25;261(33):15442–15449. [PubMed] [Google Scholar]
- Tiku M. L., Liesch J. B., Robertson F. M. Production of hydrogen peroxide by rabbit articular chondrocytes. Enhancement by cytokines. J Immunol. 1990 Jul 15;145(2):690–696. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woessner J. F., Jr, Selzer M. G. Two latent metalloproteases of human articular cartilage that digest proteoglycan. J Biol Chem. 1984 Mar 25;259(6):3633–3638. [PubMed] [Google Scholar]