Abstract
Hepatitis B virus (HBV) infection plays an important role in the occurrence and development of hepatocellular carcinoma (HCC), and the rate of HBV infection in liver cancer patients in China is as high as 92.05%. Due to long-term exposure to chronic antigens from the gut, the liver needs to maintain a certain level of immune tolerance, both to avoid severe inflammation caused by non-pathogenic antigens and to maintain the possibility of rapid and violent responses to infection and tumors. Therefore, HBV infection interacts with the tumor microenvironment (TME) through a highly complex and intertwined signaling pathway, which results in a special TME in HCC. Due to changes in the TME, tumor cells can evade immune surveillance by inhibiting tumor-specific T cell function through cytotoxic T-lymphocy-associated protein-4 (CTLA-4) and programmed cell death 1 (PD-1)/programmed cell death ligand 1 (PD-L1). Interferons, as a class of immune factors with strong biological activity, can improve the TME of HBV-HCC through various pathways. In recent years, the systematic treatment of HCC has gradually come out of the dilemma. In addition to the continuous emergence of new multi-target anti-vascular tyrosine kinase inhibitor drugs, immune checkpoint inhibitors have opened up a new avenue for the systematic treatment of HCC. At present, immunotherapy based on PD-1/L1 inhibitors has gradually become a new direction of systematic treatment for HCC, and the disease characteristics of patients included in global clinical studies are different from those of Chinese patients. Therefore, whether a group of HCC patients with HBV background and poor prognosis in China can also benefit from immunotherapy is an issue of wide concern. This review aims to elucidate the advances of immunotherapy for HBV related HCC patients with regard to: (1) Immunotherapy based on interferons; (2) Immunotherapy based on PD-1/L1 inhibitors; (3) Immunotherapy based on CTLA4 inhibitors; (4) Adoptive cell transfer; (5) Combination immunotherapy strategy; and (6) Shortcomings of immunotherapy.
Keywords: Hepatitis B virus, Hepatocellular carcinoma, Immune checkpoint inhibitors, Peginterferon alpha, Systematic treatment
Core Tip: Hepatitis B virus (HBV) infection interacts with the tumor microenvironment (TME) through a highly complex and intertwined signaling pathway, which results in a special TME in hepatocellular carcinoma (HCC). Due to changes in the TME, tumor cells can evade immune surveillance by inhibiting tumor-specific T cell function through CTLA-4 and programmed cell death 1 (PD-1)/PD ligand 1. Interferons, as a class of immune factors with strong biological activity, can improve the TME of HBV-HCC through various pathways. Currently, the systematic treatment of HCC has gradually come out of the dilemma. In addition to the continuous emergence of new multi-target anti-vascular tyrosine kinase inhibitor drugs, immune checkpoint inhibitors have opened up a new avenue for the systematic treatment of HCC. Since the disease characteristics of patients included in global clinical studies are different from those of Chinese patients, whether a group of HCC patients with HBV background and poor prognosis in China can also benefit from immunotherapy is an issue of wide concern.
INTRODUCTION
Hepatitis B virus (HBV) infection plays an important role in the occurrence and development of hepatocellular carcinoma (HCC)[1-5]. The 2020 Global Cancer Report showed that 45.3% of global cases of liver cancer occurred in China. Unlike the alcoholic liver disease background in Europe and America and the hepatitis C background in Japan, HBV infection is the major etiology of liver cancer in Chinese patients, with an infection rate as high as 92.05%[6]. Due to long-term exposure to chronic antigens from the intestine, the liver needs to maintain a certain level of immune tolerance, avoiding severe inflammation caused by non pathogenic antigens while maintaining the possibility of rapid and intense reactions to infections and tumors. Therefore, HBV infection interacts with the tumor microenvironment (TME) through a highly complex and intertwined signaling pathway, which results in a special TME in HCC[7-9]. The occurrence and development of HBV related HCC are related to chronic inflammation, which leads to immunosuppression and forms an HCC immunosuppressive microenvironment in which multiple immune molecules and immune cells participate[10,11]. Due to changes in the TME, tumor cells can evade immune surveillance by inhibiting tumor specific T cell function through cytotoxic T-lymphocy-associated protein-4 (CTLA-4) and programmed cell death 1 (PD-1)/programmed cell death ligand-1 (PD-L1)[12,13].
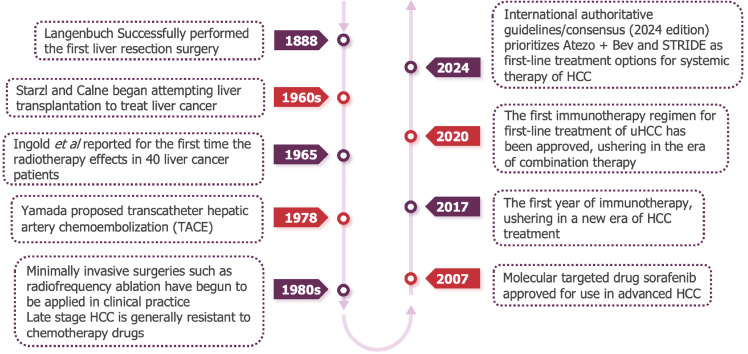
In recent years, the systematic treatment of liver cancer has gradually emerged from difficulties (Figure 1). In addition to the emergence of new multi-target anti vascular tyrosine kinase inhibitor (TKI) drugs, immune checkpoint inhibitors (ICIs) have opened up new prospects for the systematic treatment of liver cancer. At present, drug therapy based on PD-1/L1 inhibitors has gradually become a new direction for systemic treatment of liver cancer. However, the disease characteristics are different between the patient population included in global clinical studies and Chinese patients. It is of wide concern whether a group of liver cancer patients with HBV background and poor prognosis in China can also benefit from immunotherapy.
Figure 1.
The treatment methods for liver cancer are constantly advancing, evolving from surgery to systematic treatment[85-88]. HCC: Hepatocellular carcinoma; TACE: Transartical chemoembolization.
This paper reviews the progress of immunotherapy for HBV-related HCC patients, hoping to provide new ideas for clinical diagnosis and treatment of these patients.
IMMUNOTHERAPY BASED ON INTERFERONS
During chronic HBV infection, circulating HBV or HBV derived antigens promote T cell depletion[14], and most HCCs evolve in this chronic immunosuppressive necroinflammatory environment[15]. The unique TME of HCC encourages the use of immune anti-tumor drugs, among which nivolumab and pembrolizumab are the first to show potential[16]. However, the objective response rate (ORR) of patients with advanced HCC (aHCC) to ICI monotherapy is 15% to 20%, and most of them do not have significant overall survival (OS) benefits. In addition, approximately 30% of HCC cases exhibit primary resistance to ICIs. In the absence of predictive biomarkers to identify patients who may benefit from immunotherapy, exploring combination therapy strategies is well-reasoned[17]. There are mainly two such strategies: Inhibiting additional checkpoint molecules that provide inhibitory signals or stimulating checkpoint molecules that provide stimulus signals for further stimulating T cell activation, and using drugs can alter the TME to make it less hostile to cytotoxic T cells or more conducive to antigen cross presentation to T cells[17].
Interferons (IFNs) are a class of immune factors with strong biological activity that can improve the TME of HBV-HCC through various pathways. First, IFNs are a type of core anti-HBV drug that can induce viral serological transformation and reduce HBsAg levels or even eliminate this antigen. High levels of HBV DNA and HBsAg are high-risk factors for HCC recurrence[18,19]. Second, IFNs inhibit the proliferation of HBV-HCC cells and induces their apoptosis by interfering with the cyclin (G1) of cell clones to block the cell cycle[20-22]. Third, IFNs can regulate various immune cells, including natural killer (NK) cells, dendritic cells (DCs), myeloid derived suppressive cells (MDSCs), B cells, CD4+ T cells, CD8+ T cells, macrophages, etc., enhancing the antigen presentation process, amplifying the specific anti-tumor effect of T cells, and enhancing their killing effect on tumor cells[23,24]. Fourth, IFNs can inhibit the expression of VEGF and MAPK, thereby inhibiting the angiogenesis pathway[25,26]. In summary, IFNs reshape immune surveillance and the TME through various mechanisms such as antiviral action, activation of positive immunity, inhibition of tumor cell proliferation, and inhibition of angiogenesis, and have unique application value in HBV-HCC.
The application of IFNs in postoperative adjuvant therapy for HBV-HCC has been widely recognized. In the Chinese Guidelines for the Diagnosis and Treatment of Primary Liver Cancer (2022 edition) and the Chinese Expert Consensus on Postoperative Adjuvant Therapy for Liver Cancer (2023 edition), IFNs are recommended as a postoperative adjuvant therapy, with a high level of evidence[27,28]. In a randomized controlled study conducted in China, which included HBV-HCC patients (n = 236) to explore the adjuvant value of IFNs after surgery[29], IFNs significantly improved patient OS compared to the untreated group (63.8 mo vs 38.8 mo, P = 0.0003). In a randomized controlled study conducted by Korean scholars on HBV-HCC patients at high risk of postoperative recurrence (n = 93)[30], the IFN treated group showed a significant improvement in 2-year OS (100% vs 87%; P < 0.05). In addition, similar conclusions were also obtained in multiple meta-analyses[31-33]. The treatment of HCC using IFNs has been widely reported, and multiple studies have shown that IFNs can be used for immunotherapy of HCC through various mechanisms[34-36]. In the era that immunotherapy dominates HCC treatment, IFNs as an adjuvant therapy for HBV-HCC after surgery may be further optimized, e.g., the selection of dominant groups or the exploration of combination therapy with ICIs or other targeted drugs.
The application of IFNs combined with ICIs in the treatment of unresectable HBV-HCC is showing signs of dawn. In order to further improve the efficacy of ICIs and overcome primary drug resistance, various immunotherapy combinations have been developed, mainly including ICIs combined with bevacizumab, ICIs combined with TKIs, and a combination of two types of ICIs[17]. However, combining ICIs with IFNs has similar mechanisms. In a study published in Cancer Discovery[37], unresectable HCC patients (87% HBV related) received a combination therapy of an IFN and PD-1 antibody, with an ORR of 40.0% and disease control rate of 80.0%. In a mouse model, researchers found that the combination therapy significantly prolonged OS and completely eliminated lung metastases. Further mechanistic exploration suggested that the IFN inhibits glucose metabolism in tumor cells and increases T cell glycolysis, thereby promoting PD-1 antibody induced immune response, enhancing T cell immune killing ability, and producing a 1 + 1 > 2 effect. Numata et al[38] have indicated that the mechanism of resistance of HCC cells to immunotherapy is affected by IFN-γ[38].
Another study published in Cellular & Molecular Immunology[39] explored the effects of IFN-α on tumor infiltrating immune cells and PD-1 expression in the HCC immune microenvironment, as well as the mechanism of IFN-α combined with PD-1 antibody in vivo: IFN-α recruits cytotoxic CD8+ T cells to infiltrate the TME by inducing liver cancer cells to secrete CCL4, and upregulates the expression of PD-1 on CD8+ T cells through the IFN α-IFNAR1-JAK1-STAT3 pathway. However, long-term use of IFNs can easily lead to the depletion of CD8+ T cells. Therefore, IFNs combined with PD-1 antibody can restore or even enhance the cell count of CD8+ T cells. That is, they have a synergistic anti-tumor effect.
IMMUNOTHERAPY BASED ON PD-1/L1 INHIBITORS
PD-1/PD-L1 inhibitors are believed to be effective in activating the existing immune response for HCC[40]. PD-1, as an immune checkpoint molecule, is a member of the CD28 family and is expressed on multiple immune cells, including activated T cells, B cells, NK cells, and DCs. PD-1 is a common immunosuppressive factor on the surface of T cells, which represses T cell receptor (TCR) stimulation signals, and plays a vital role in downregulating immune system function and promoting tolerance. PD-L1 is overexpressed on the surface of malignant tumor cells and binds to PD-1, inhibiting T cell proliferation and activation, resulting in T cell inactivation and inducing immune escape, and ultimately leading to the failure of treatment[41].
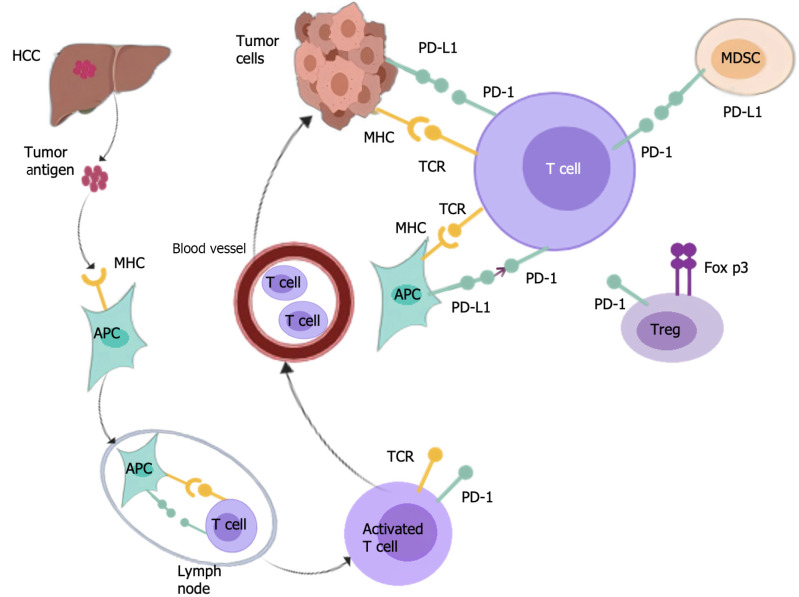
PD-1 can also inhibit T cell function by augmenting the expression of transcription factors and antagonizing the transcription effector, thereby affecting the activation, lifespan, and proliferation of T cells, leading to a decrease of tumor necrosis factor (TNF), interleukin-2 (IL-2), and other cytokines, and assisting tumor cells in evading immune responses[42]. According to reports, PD-1/PD-L1 is expressed in regulatory T cells (Tregs), and PD-L1 induces differentiation, maintenance, and function of induced Tregs by augmenting Foxp3 expression, which intensifies the inhibition and extinction of TME immune status[43]. After PD-1 is activated, SHP-2 is recruited to PD-1, causing the dephosphorylation of BCR pathway molecules, thereby inhibiting PI3K, ERK, and other pathways, and B cell growth in B cells[44]. Reportedly, PD-1 overexpression in B cells can induce T cell dysfunction via an IL-10 dependent pathway, resulting in the progression of tumors[45]. In addition, in vitro, PD-1+ B cells can inhibit the expansion of T cells and reduce their survival ability[46]. In MDSCs, the specific microenvironment induces the expression of PD-L1[47], which by binding to PD-1 on T cells, suppresses the activation of T cells. Additionally, the PI3K/AKT/NF-κB pathway in B cells can be activated by MDSCs via the PD-1/PD-L1 axis[48]. Reportedly, PD-L1 is preferentially expressed on macrophages rather than cancer cells[49]. Targeting macrophages expressing PD-L1 in HCC can serve as a strategy to improve the efficacy of immunotherapy. Consequently, the PD-1/PD-L1 axis plays a vital role in the immunosuppressive microenvironment of HCC (Figure 2).
Figure 2.
Mechanisms of programmed cell death 1/programmed cell death ligand 1 axis in hepatocellular carcinoma. HCC: Hepatocellular carcinoma; TCR: T cell receptor; PD-1: Programmed cell death 1; PD-L1: Programmed cell death ligand 1; MDSC: Myeloid derived suppressive cell.
Since 2017, PD-1 inhibitors, as second-line therapies, have been recommended for the treatment of aHCC. CheckMate 040 has shown that PD-1 inhibitors can achieve a 20% ORR in aHCC[50]. The phase III randomized controlled trial CheckMate 459 for first-line therapy have suggested that PD-1 blockades prolong OS, with a higher ORR and safety[51]. The latest KEYNOTE-224 study has found that as a second-line treatment for aHCC, PD-1 inhibitors can achieve an 18.3% ORR, with median progression free survival (PFS) and OS at 4.9 and 13.2 mo, respectively[52]. Among KEYNOTE-394 patients included in Asia, PD-1 inhibitors significantly improved OS, PFS, and ORR[53]. The breakthrough study of IMbrave150 indicated that during preliminary analysis, the PD-1/PD-L1 inhibitor combined with anti-VEGF drug treatment regimen significantly improved OS and PFS in the treatment of early aHCC[54]. This first successful phase III randomized controlled trial indicated that immunotargeted therapy has strong efficacy and controllable safety, making a new breakthrough in the treatment of aHCC. The median OS in the Chinese population subgroup reached an unprecedented 24 mo[54], suggesting that this regimen has truly extended the survival time of patients with aHCC. Recently, Chinese effort on PD-1 and PD-L1 blockers has proved useful[55]. Their efficacy and safety in HCC patients are under research.
IMMUNOTHERAPY BASED ON CTLA4 INHIBITORS
Another inhibitory immune checkpoint, CTLA-4, as a CD28 homolog, is located in resting immature T cells and expressed on activated T cells. The activation of immature T cells is regulated by co-inhibition and co-stimulation[56,57]. The combination of CD28 and B7-1/2 can activate T cells, while the combination of CTLA-4 and CD80/86 inhibits T cell activation and proliferation. Moreover, anti-CTLA-4 antibodies can enhance the anti-tumor activity of T cells by preventing the combination of CTLA-4 on T cells and CD80/86 on antigen-presenting cells (APCs). CTLA-4 also interferes with the combination of B7 and CD28 by directly transmitting inhibitory signals to T cells, which inhibits T cell responses. Additionally, Tregs can control the effector T cell function and play a crucial role in maintaining peripheral tolerance. CTLA-4 is also expressed on Tregs and plays a vital role in regulating their function. CTLA-4 on Tregs acts as a co-inhibitory receptor, blocking co-stimulatory signaling by binding to CD80/86 on APCs, leading to impaired anti-tumor immune response and promoted immune evasion of tumor cells[58]. Moreover, by blocking CTLA-4, Treg function is weakened and their anti-tumor effect is enhanced.
A study has suggested that anti-CTLA-4 drugs can deplete Tregs through Fc mediated antibody dependent cytotoxicity, thereby enhancing anti-tumor immune effects[59]. In 31 HCC patients, it was found that the addition of anti-CTLA-4 antibodies increased the frequency of tumor associated antigen specific cytotoxic T cells in 60% of patients, enhancing the anti-tumor activity of tumor specific T cells[60]. The dysfunction of Treg specific CTLA-4 can affect Treg inhibitory function in vivo and promote anti-tumor immune effects. Research has shown that upregulation of CTLA-4 may mediate the inhibitory function of Tregs through small-scale graft injury, which is beneficial for the recurrence of HCC after liver transplantation[61]. After only 4 years of pilot clinical trials, it was first demonstrated that CTLA4 blockers can induce persistent objective remission in HCC and HCV infected patients. This is the first clinical trial of HCC immunotherapy, demonstrating the activity of CTLA4 blockers[62]. Therefore, anti-CTLA-4 agents are very promising drugs that can promote immune cell proliferation and enhance anti-tumor immune response by blocking ligand binding sites or inhibiting the expression of CTLA-4 in HCC patients during treatment. Based on the above research, ICIs are effective in treating HCC patients.
ADOPTIVE CELL TRANSFER
Adoptive cell transfer (ACT) is a treatment method that involves sensitizing effector cells (most commonly lymphocytes), propagating them in vitro, and then injecting them into the patient's body[63]. The most commonly used lymphocytes in ACT are gene encoded T cells, which can specifically recognize and target tumor cells. Moreover, the key core of adoptive cell immunotherapy is the number and activity of target lymphocytes cultured in vitro. The main strategy of ACT includes transgenic tumor antigen-specific TCRs or chimeric antigen receptors. Cell based immunotherapy, such as tumor infiltrating lymphocytes or cytokine-induced killer (CIK) cell adoptive immunotherapy, has strong anti-tumor effects and low toxicity to normal cells. Yang et al[64] suggested that CIK cells from HCC patients exhibited cytotoxicity and inhibited human HCC both in vitro and in vivo[64]. In a study of 230 HCC patients who underwent surgical resection or radiofrequency ablation (RFA) therapy, the efficacy and safety of activated CIK cells in adjuvant therapy were evaluated. The results showed that neither the CIK treatment group nor the control group achieved OS, but the CIK treatment group had a longer OS[65]. Research has indicated that in HCC patients receiving potential curative treatment, adjuvant CIK cell immunotherapy is more cost-effective with regard to prolonged survival and reduced HCC recurrence[66]. Prior to the development of bispecific antibody coupled CIK immunotherapy, anti-PD-1 antibody therapy may be effective and safe for HCC patients[67]. The combination therapy of DC-CIK therapy and microwave ablation (MWA) can remarkably reduce recurrence and prolong disease-free survival[68]. Chimeric antigen receptor (CAR)-T cells have shown optimistic results in the treatment of hematological malignancies, but their use for solid tumor treatment is still under development[69]. The tumor specific antigen glypian-3 (GPC3) is the most significant target for CAR-T cell therapy in HCC[70]. Currently, clinical trials are being conducted to ensure the safety and efficacy of GPC3 mediated CAR-T cells[71]. Generally speaking, patients receiving ACT treatment receive a pre-treatment regimen of cyclophosphamide and fludarabine to induce depletion of lymphocytes, thereby supporting in vivo expansion of adoptive cells. Adoptive cell immunotherapy, especially T cell-based adoptive immunotherapy, is a very promising anti-cancer immunotherapy and has shown good therapeutic effects in some cancer patients[63].
Due to the lack of efficacy and relative complexity of the technology, the initial attempts to develop ACT in HCC did not meet clinical expectations. A large number of single arm or control experiments with different defects have shown that CIK cells have anti-HCC activity. Despite these positive results, ACT has not been used as an adjuvant therapy in most centers, possibly limited by some facilities for in vivo cell therapy.
COMBINATION IMMUNOTHERAPY STRATEGY
In the treatment of HCC, immunotherapy is based on the support of the liver’s immune tolerance microenvironment and the main immune suppression of HCC[72]. Although single-agent immunotherapy has achieved effective results in the treatment of HCC, its response rate is still not satisfactory. In cancer research, combining the immune initiation stage suppressed by CTLA-4 or the immune response stage suppressed by PD-1/PD-L1 with targeted therapy has become a more influential treatment method.
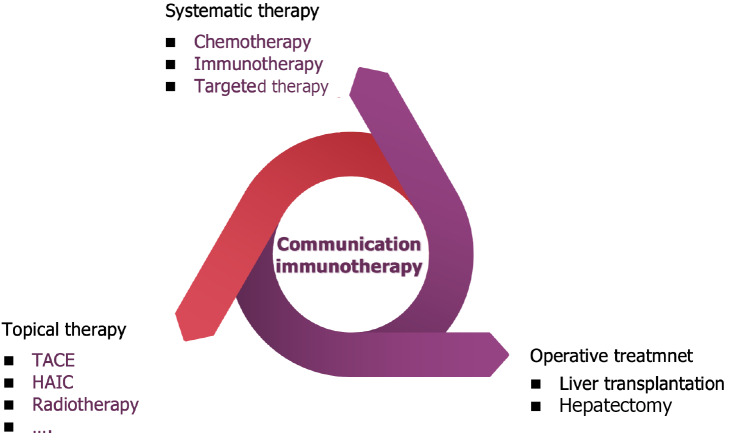
A characteristic of HCC is strong vascularization of arterial blood vessels, secondary to overexpression of angiogenic endothelial growth factor A and platelet derived growth factor[73,74]. Basic research has shown that combining PD-1 inhibitors with TKIs or anti-VEGF antibodies can enhance anti-tumor effects by increasing lymphocyte infiltration, weakening immunosuppressive status, and promoting vascular normalization[75,76]. Compared with classical anti-tumor therapy, the combination of PD-L1 inhibitors and anti-VEGF inhibitors significantly prolongs OS and PFS. Therefore, this combination therapy represents a new systemic treatment approach for HCC[77]. Other immune based combination therapies include the combination therapy of ICI and TKIs[78]. TKIs may not only have anti-angiogenic effects, but also have immunomodulatory effects. In addition, two different combinations of ICIs have been shown to be effective against other solid tumors[79]. Studies have shown that combining PD-1/PD-L1 inhibitors with CTLA-4 inhibitors can produce non-redundant effects[80]. Check-mate040[81] is a multicenter, open label, multi-cohort phase I/II trial, and the results of this study are consistent with the different but complementary roles of PD-1/L1 and CTLA-4 pathways in negatively regulating immune activity. Based on this study, the Food and Drug Administration approved the combination of nivolumab and ipilimumab for second-line treatment of aHCC in March 2020. Multiple studies have confirmed that the combination of systemic therapy and transartical chemoembolization (TACE) is more effective in treating aHCC than TACE or TKIs alone[82,83]. In addition, available local combination therapies include radiotherapy, TACE, selective internal radiotherapy, RFA, and MWA[84] (Figure 3).
Figure 3.
Immunotherapy-based combination therapy for liver cancer. TACE: Transartical chemoembolization.
In order to improve the efficacy of CAR-T in cancer, combination therapy may be used in the future to enhance efficacy, such as improving local metabolism, inhibiting inflammatory factors or immunosuppressive cells, blocking immune checkpoints, and combining CAR-T cells with anti-tumor drugs or immune modulators.
SHORTCOMINGS OF IMMUNOTHERAPY
In recent years, ICIs represented by anti-PD-1/PD-L1 agents have made breakthroughs in the field of liver cancer treatment, significantly improving patient survival rates and gradually becoming the preferred treatment option recommended by guidelines. However, the effective rate of single drug ICI treatment does not exceed 20%, and the efficacy can be effectively improved by combining anti-angiogenic drugs or TKIs, though it still does not exceed 40%. Although some new immunotherapy methods have shown good potential in laboratory research, they still face many challenges in clinical application, such as safety, tolerability, and cost. In the future, with a deeper understanding of the mechanisms of HBV infection and liver cancer, as well as the continuous development of immunotherapy technology, the immunotherapy of HBV related liver cancer is expected to have a further breakthrough, providing patients with more treatment options and hope. In summary, immunotherapy for HBV related liver cancer is a challenging and opportunity filled field. With the deepening of research and technological progress, we have reason to believe that immunotherapy will bring revolutionary changes to the treatment of HBV related liver cancer.
CONCLUSION
HBV related HCC patients have a heavy disease burden, including viral infections and malignant tumors. ICIs also play a promoting role in antiviral therapy when exerting anti-tumor mechanisms. It is worth noting that in the synergy of disease treatment and life saving, anti-tumor and antiviral treatments complement each other, and comprehensive treatment plans need to be personalized for patients to improve overall efficacy. HBV related liver cancer has entered the era of comprehensive treatment. With the continuous development of immunotherapy, the comprehensive treatment mode of liver cancer has also changed, with the aim of prolonging the OS of patients. Immunotherapy, combined with traditional local therapies such as TACE and radiofrequency, has a synergistic effect and may help reduce tumor burden and improve patient prognosis. The multidisciplinary research of liver cancer and the full process management will bring OS benefits to patients with HBV related liver cancer.
Footnotes
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade C
Novelty: Grade C
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Han T S-Editor: Li L L-Editor: Wang TQ P-Editor: Zhang XD
Contributor Information
Wei-Hua Cao, Department of Hepatology Division 2, Beijing Ditan Hospital, Capital Medical University, Beijing 100015, China.
Ya-Qin Zhang, Department of Hepatology Division 2, Beijing Ditan Hospital, Capital Medical University, Beijing 100015, China.
Xin-Xin Li, Department of Hepatology Division 2, Beijing Ditan Hospital, Capital Medical University, Beijing 100015, China.
Zi-Yu Zhang, Department of Hepatology Division 2, Beijing Ditan Hospital, Capital Medical University, Beijing 100015, China.
Ming-Hui Li, Department of Hepatology Division 2, Beijing Ditan Hospital, Capital Medical University, Beijing 100015, China; Department of Hepatology Division 2, Beijing Ditan Hospital, Capital Medical University, Peking University Ditan Teaching Hospital, Beijing 100015, China. wuhm2000@sina.com.
References
- 1.Capasso M, Cossiga V, Guarino M, Ranieri L, Morisco F. The Role of Hepatitis Viruses as Drivers of Hepatocancerogenesis. Cancers (Basel) 2024;16 doi: 10.3390/cancers16081505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ataman E, Harputluoglu M, Carr BI, Gozukara H, Ince V, Yilmaz S. HBV viral load and tumor and non-tumor factors in patients with HBV-associated HCC. Hepatol Forum. 2024;5:73–76. doi: 10.14744/hf.2023.2023.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Battista D, Yakymi R, Scheibe E, Sato S, Gerstein H, Markowitz TE, Lack J, Mereu R, Manieli C, Zamboni F, Farci P. Identification of Two Distinct Immune Subtypes in Hepatitis B Virus (HBV)-Associated Hepatocellular Carcinoma (HCC) Cancers (Basel) 2024;16 doi: 10.3390/cancers16071370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sartorius K, Sartorius B, Winkler C, Chuturgoon A, Shen TW, Zhao Y, An P. Serum microRNA Profiles and Pathways in Hepatitis B-Associated Hepatocellular Carcinoma: A South African Study. Int J Mol Sci. 2024;25 doi: 10.3390/ijms25020975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agustiningsih A, Rasyak MR, Turyadi, Jayanti S, Sukowati C. The oncogenic role of hepatitis B virus X gene in hepatocarcinogenesis: recent updates. Explor Target Antitumor Ther. 2024;5:120–134. doi: 10.37349/etat.2024.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xia C, Dong X, Li H, Cao M, Sun D, He S, Yang F, Yan X, Zhang S, Li N, Chen W. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl) 2022;135:584–590. doi: 10.1097/CM9.0000000000002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pessino G, Scotti C, Maggi M Immuno-Hub Consortium. Hepatocellular Carcinoma: Old and Emerging Therapeutic Targets. Cancers (Basel) 2024;16 doi: 10.3390/cancers16050901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ren Y, Qian Y, Zhang Q, Li X, Li M, Li W, Yang P, Ren H, Li H, Weng Y, Li D, Xu K, Yu W. High LGALS3 expression induced by HCP5/hsa-miR-27b-3p correlates with poor prognosis and tumor immune infiltration in hepatocellular carcinoma. Cancer Cell Int. 2024;24:142. doi: 10.1186/s12935-024-03309-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mroweh M, Decaens T, Marche PN, Macek Jilkova Z, Clément F. Modulating the Crosstalk between the Tumor and Its Microenvironment Using RNA Interference: A Treatment Strategy for Hepatocellular Carcinoma. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21155250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hou J, Zhang H, Sun B, Karin M. The immunobiology of hepatocellular carcinoma in humans and mice: Basic concepts and therapeutic implications. J Hepatol. 2020;72:167–182. doi: 10.1016/j.jhep.2019.08.014. [DOI] [PubMed] [Google Scholar]
- 11.Tagliamonte M, Petrizzo A, Tornesello ML, Ciliberto G, Buonaguro FM, Buonaguro L. Combinatorial immunotherapy strategies for hepatocellular carcinoma. Curr Opin Immunol. 2016;39:103–113. doi: 10.1016/j.coi.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Min HY, Lee HY. Molecular targeted therapy for anticancer treatment. Exp Mol Med. 2022;54:1670–1694. doi: 10.1038/s12276-022-00864-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tchen J, Simon Q, Chapart L, Thaminy MK, Vibhushan S, Saveanu L, Lamri Y, Saidoune F, Pacreau E, Pellefigues C, Bex-Coudrat J, Karasuyama H, Miyake K, Hidalgo J, Fallon PG, Papo T, Blank U, Benhamou M, Hanouna G, Sacre K, Daugas E, Charles N. PD-L1- and IL-4-expressing basophils promote pathogenic accumulation of T follicular helper cells in lupus. Nat Commun. 2024;15:3389. doi: 10.1038/s41467-024-47691-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ye B, Liu X, Li X, Kong H, Tian L, Chen Y. T-cell exhaustion in chronic hepatitis B infection: current knowledge and clinical significance. Cell Death Dis. 2015;6:e1694. doi: 10.1038/cddis.2015.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donne R, Lujambio A. The liver cancer immune microenvironment: Therapeutic implications for hepatocellular carcinoma. Hepatology. 2023;77:1773–1796. doi: 10.1002/hep.32740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu SJ. Immunotherapy for hepatocellular carcinoma: Recent advances and future targets. Pharmacol Ther. 2023;244:108387. doi: 10.1016/j.pharmthera.2023.108387. [DOI] [PubMed] [Google Scholar]
- 17.Rimassa L, Finn RS, Sangro B. Combination immunotherapy for hepatocellular carcinoma. J Hepatol. 2023;79:506–515. doi: 10.1016/j.jhep.2023.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Sohn W, Paik YH, Kim JM, Kwon CH, Joh JW, Cho JY, Gwak GY, Choi MS, Lee JH, Koh KC, Paik SW, Yoo BC. HBV DNA and HBsAg levels as risk predictors of early and late recurrence after curative resection of HBV-related hepatocellular carcinoma. Ann Surg Oncol. 2014;21:2429–2435. doi: 10.1245/s10434-014-3621-x. [DOI] [PubMed] [Google Scholar]
- 19.Yoo S, Kim JY, Lim YS, Han S, Choi J. Impact of HBsAg seroclearance on late recurrence of hepatitis B virus-related hepatocellular carcinoma after surgical resection. J Hepatol. 2022;77:939–946. doi: 10.1016/j.jhep.2022.05.014. [DOI] [PubMed] [Google Scholar]
- 20.Zhang S, Gao S, Zhao M, Liu Y, Bu Y, Jiang Q, Zhao Q, Ye L, Zhang X. Anti-HBV drugs suppress the growth of HBV-related hepatoma cells via down-regulation of hepatitis B virus X protein. Cancer Lett. 2017;392:94–104. doi: 10.1016/j.canlet.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Yano H, Ogasawara S, Momosaki S, Akiba J, Kojiro S, Fukahori S, Ishizaki H, Kuratomi K, Basaki Y, Oie S, Kuwano M, Kojiro M. Growth inhibitory effects of pegylated IFN alpha-2b on human liver cancer cells in vitro and in vivo. Liver Int. 2006;26:964–975. doi: 10.1111/j.1478-3231.2006.01321.x. [DOI] [PubMed] [Google Scholar]
- 22.Maeda S, Wada H, Naito Y, Nagano H, Simmons S, Kagawa Y, Naito A, Kikuta J, Ishii T, Tomimaru Y, Hama N, Kawamoto K, Kobayashi S, Eguchi H, Umeshita K, Ishii H, Doki Y, Mori M, Ishii M. Interferon-α acts on the S/G2/M phases to induce apoptosis in the G1 phase of an IFNAR2-expressing hepatocellular carcinoma cell line. J Biol Chem. 2014;289:23786–23795. doi: 10.1074/jbc.M114.551879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Q, Sun B, Zhuo Y, Jiang Z, Li R, Lin C, Jin Y, Gao Y, Wang D. Interferon and interferon-stimulated genes in HBV treatment. Front Immunol. 2022;13:1034968. doi: 10.3389/fimmu.2022.1034968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Afzal MZ, Pinnamaneni V, Birendra KC, Davis AT, Koehler TJ, Lakhani N. A retrospective analysis of tolerance and outcomes of cutaneous malignant melanoma in patients receiving adjuvant interferon-alpha 2B: a community oncology perspective. J Exp Ther Oncol. 2017;11:91–96. [PubMed] [Google Scholar]
- 25.Kudo M. Impact of interferon therapy after curative treatment of hepatocellular carcinoma. Oncology. 2008;75 Suppl 1:30–41. doi: 10.1159/000173422. [DOI] [PubMed] [Google Scholar]
- 26.Wang W, Edington HD, Jukic DM, Rao UN, Land SR, Kirkwood JM. Impact of IFNalpha2b upon pSTAT3 and the MEK/ERK MAPK pathway in melanoma. Cancer Immunol Immunother. 2008;57:1315–1321. doi: 10.1007/s00262-008-0466-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhong JH. Adjuvant therapy for hepatocellular carcinoma: Dilemmas at the start of a new era. World J Gastroenterol. 2024;30:806–810. doi: 10.3748/wjg.v30.i8.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou J, Sun H, Wang Z, Cong W, Wang J, Zeng M, Zhou W, Bie P, Liu L, Wen T, Han G, Wang M, Liu R, Lu L, Ren Z, Chen M, Zeng Z, Liang P, Liang C, Chen M, Yan F, Wang W, Ji Y, Yun J, Cai D, Chen Y, Cheng W, Cheng S, Dai C, Guo W, Hua B, Huang X, Jia W, Li Y, Li Y, Liang J, Liu T, Lv G, Mao Y, Peng T, Ren W, Shi H, Shi G, Tao K, Wang W, Wang X, Wang Z, Xiang B, Xing B, Xu J, Yang J, Yang J, Yang Y, Yang Y, Ye S, Yin Z, Zhang B, Zhang B, Zhang L, Zhang S, Zhang T, Zhao Y, Zheng H, Zhu J, Zhu K, Liu R, Shi Y, Xiao Y, Dai Z, Teng G, Cai J, Wang W, Cai X, Li Q, Shen F, Qin S, Dong J, Fan J. Guidelines for the Diagnosis and Treatment of Hepatocellular Carcinoma (2019 Edition) Liver Cancer. 2020;9:682–720. doi: 10.1159/000509424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun HC, Tang ZY, Wang L, Qin LX, Ma ZC, Ye QH, Zhang BH, Qian YB, Wu ZQ, Fan J, Zhou XD, Zhou J, Qiu SJ, Shen YF. Postoperative interferon alpha treatment postponed recurrence and improved overall survival in patients after curative resection of HBV-related hepatocellular carcinoma: a randomized clinical trial. J Cancer Res Clin Oncol. 2006;132:458–465. doi: 10.1007/s00432-006-0091-y. [DOI] [PubMed] [Google Scholar]
- 30.Lee D, Chung YH, Kim JA, Park WH, Jin YJ, Shim JH, Ryu SH, Jang MK, Yu E, Lee YJ. Safety and efficacy of adjuvant pegylated interferon therapy for metastatic tumor antigen 1-positive hepatocellular carcinoma. Cancer. 2013;119:2239–2246. doi: 10.1002/cncr.28082. [DOI] [PubMed] [Google Scholar]
- 31.Jiang S, Liu Y, Wang L, Duan C, Liu M. A meta-analysis and systematic review: adjuvant interferon therapy for patients with viral hepatitis-related hepatocellular carcinoma. World J Surg Oncol. 2013;11:240. doi: 10.1186/1477-7819-11-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu J, Li J, Chen J, Liu ZJ. Effect of adjuvant interferon therapy on hepatitis b/c virus-related hepatocellular carcinoma after curative therapy - meta-analysis. Adv Clin Exp Med. 2015;24:331–340. doi: 10.17219/acem/29760. [DOI] [PubMed] [Google Scholar]
- 33.Luo JX, Zhang Y, Hu XY, Xiang N. Interferon therapy improves survival in patients with hepatitis B virus-related hepatocellular carcinoma after curative surgery: a meta-analysis. Hepatol Int. 2024;18:63–72. doi: 10.1007/s12072-023-10618-6. [DOI] [PubMed] [Google Scholar]
- 34.Peng C, Ye Z, Ju Y, Huang X, Zhan C, Wei K, Zhang Z. Mechanism of action and treatment of type I interferon in hepatocellular carcinoma. Clin Transl Oncol. 2024;26:326–337. doi: 10.1007/s12094-023-03266-7. [DOI] [PubMed] [Google Scholar]
- 35.Li H, Liu Y, Cheng C, Wu Y, Liang SH, Wu L, Wang H, Tu CY, Yao HH, Meng FZ, Zhang B, Wang W, Wang JB, Liu LX. UBE2O reduces the effectiveness of interferon-α via degradation of IFIT3 in hepatocellular carcinoma. Cell Death Dis. 2023;14:854. doi: 10.1038/s41419-023-06369-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu WP, He XG, Zhu HX, Wang LR, Lin ZH, Wang M, Wang L. Identification of miRNAs, mRNAs, lncRNAs, and circRNAs associated with hepatocellular carcinoma recurrence after interferon treatment. J Biol Regul Homeost Agents. 2021;35 doi: 10.23812/21-173-A. [DOI] [PubMed] [Google Scholar]
- 37.Hu B, Yu M, Ma X, Sun J, Liu C, Wang C, Wu S, Fu P, Yang Z, He Y, Zhu Y, Huang C, Yang X, Shi Y, Qiu S, Sun H, Zhu AX, Zhou J, Xu Y, Zhu D, Fan J. IFNα Potentiates Anti-PD-1 Efficacy by Remodeling Glucose Metabolism in the Hepatocellular Carcinoma Microenvironment. Cancer Discov. 2022;12:1718–1741. doi: 10.1158/2159-8290.CD-21-1022. [DOI] [PubMed] [Google Scholar]
- 38.Numata Y, Akutsu N, Ishigami K, Koide H, Wagatsuma K, Motoya M, Sasaki S, Nakase H. Synergistic effect of IFN-γ and IL-1β on PD-L1 expression in hepatocellular carcinoma. Biochem Biophys Rep. 2022;30:101270. doi: 10.1016/j.bbrep.2022.101270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu Y, Chen M, Xu D, Li TE, Zhang Z, Li JH, Wang XY, Yang X, Lu L, Jia HL, Dong QZ, Qin LX. The combination of PD-1 blockade with interferon-α has a synergistic effect on hepatocellular carcinoma. Cell Mol Immunol. 2022;19:726–737. doi: 10.1038/s41423-022-00848-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Riaz N, Havel JJ, Makarov V, Desrichard A, Urba WJ, Sims JS, Hodi FS, Martín-Algarra S, Mandal R, Sharfman WH, Bhatia S, Hwu WJ, Gajewski TF, Slingluff CL Jr, Chowell D, Kendall SM, Chang H, Shah R, Kuo F, Morris LGT, Sidhom JW, Schneck JP, Horak CE, Weinhold N, Chan TA. Tumor and Microenvironment Evolution during Immunotherapy with Nivolumab. Cell. 2017;171:934–949.e16. doi: 10.1016/j.cell.2017.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu J, Chen Z, Li Y, Zhao W, Wu J, Zhang Z. PD-1/PD-L1 Checkpoint Inhibitors in Tumor Immunotherapy. Front Pharmacol. 2021;12:731798. doi: 10.3389/fphar.2021.731798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Daassi D, Mahoney KM, Freeman GJ. The importance of exosomal PDL1 in tumour immune evasion. Nat Rev Immunol. 2020;20:209–215. doi: 10.1038/s41577-019-0264-y. [DOI] [PubMed] [Google Scholar]
- 43.Li B, Yan C, Zhu J, Chen X, Fu Q, Zhang H, Tong Z, Liu L, Zheng Y, Zhao P, Jiang W, Fang W. Anti-PD-1/PD-L1 Blockade Immunotherapy Employed in Treating Hepatitis B Virus Infection-Related Advanced Hepatocellular Carcinoma: A Literature Review. Front Immunol. 2020;11:1037. doi: 10.3389/fimmu.2020.01037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cai J, Wang D, Zhang G, Guo X. The Role Of PD-1/PD-L1 Axis In Treg Development And Function: Implications For Cancer Immunotherapy. Onco Targets Ther. 2019;12:8437–8445. doi: 10.2147/OTT.S221340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xiao X, Lao XM, Chen MM, Liu RX, Wei Y, Ouyang FZ, Chen DP, Zhao XY, Zhao Q, Li XF, Liu CL, Zheng L, Kuang DM. PD-1hi Identifies a Novel Regulatory B-cell Population in Human Hepatoma That Promotes Disease Progression. Cancer Discov. 2016;6:546–559. doi: 10.1158/2159-8290.CD-15-1408. [DOI] [PubMed] [Google Scholar]
- 46.Wang X, Wang G, Wang Z, Liu B, Han N, Li J, Lu C, Liu X, Zhang Q, Yang Q, Wang G. PD-1-expressing B cells suppress CD4(+) and CD8(+) T cells via PD-1/PD-L1-dependent pathway. Mol Immunol. 2019;109:20–26. doi: 10.1016/j.molimm.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 47.Lu C, Redd PS, Lee JR, Savage N, Liu K. The expression profiles and regulation of PD-L1 in tumor-induced myeloid-derived suppressor cells. Oncoimmunology. 2016;5:e1247135. doi: 10.1080/2162402X.2016.1247135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu M, Wei F, Wang J, Yu W, Shen M, Liu T, Zhang D, Wang Y, Ren X, Sun Q. Myeloid-derived suppressor cells regulate the immunosuppressive functions of PD-1(-)PD-L1(+) Bregs through PD-L1/PI3K/AKT/NF-κB axis in breast cancer. Cell Death Dis. 2021;12:465. doi: 10.1038/s41419-021-03745-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou D, Luan J, Huang C, Li J. Tumor-Associated Macrophages in Hepatocellular Carcinoma: Friend or Foe? Gut Liver. 2021;15:500–516. doi: 10.5009/gnl20223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, Meyer T, Kang YK, Yeo W, Chopra A, Anderson J, Dela Cruz C, Lang L, Neely J, Tang H, Dastani HB, Melero I. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, Kudo M, Harding JJ, Merle P, Rosmorduc O, Wyrwicz L, Schott E, Choo SP, Kelley RK, Sieghart W, Assenat E, Zaucha R, Furuse J, Abou-Alfa GK, El-Khoueiry AB, Melero I, Begic D, Chen G, Neely J, Wisniewski T, Tschaika M, Sangro B. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022;23:77–90. doi: 10.1016/S1470-2045(21)00604-5. [DOI] [PubMed] [Google Scholar]
- 52.Kudo M, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer DH, Verslype C, Zagonel V, Fartoux L, Vogel A, Sarker D, Verset G, Chan SL, Knox J, Daniele B, Yau T, Gurary EB, Siegel AB, Wang A, Cheng AL, Zhu AX KEYNOTE-224 Investigators. Updated efficacy and safety of KEYNOTE-224: a phase II study of pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib. Eur J Cancer. 2022;167:1–12. doi: 10.1016/j.ejca.2022.02.009. [DOI] [PubMed] [Google Scholar]
- 53.Qin S, Fang W, Ren Z, Ou S, Lim HY, Zhang F, Lee KC, Choi HJ, Tong J, Tao M, Xu A, Cheng A, Lu CH, Chiu CF, Abdul Wahid MI, Kamble S, Norquist JM, Zhong W, Li C, Chen Z. A Phase 3 Study of Pembrolizumab versus Placebo for Previously Treated Patients from Asia with Hepatocellular Carcinoma: Health-Related Quality of Life Analysis from KEYNOTE-394. Liver Cancer. 2024;13:389–400. doi: 10.1159/000535338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Lim HY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Ma N, Nicholas A, Wang Y, Li L, Zhu AX, Finn RS. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76:862–873. doi: 10.1016/j.jhep.2021.11.030. [DOI] [PubMed] [Google Scholar]
- 55.Hu M, Yao W, Shen Q. Advances and challenges of immunocheckpoint inhibitors in the treatment of primary liver cancer. Front Genet. 2022;13:1005658. doi: 10.3389/fgene.2022.1005658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zenke S, Palm MM, Braun J, Gavrilov A, Meiser P, Böttcher JP, Beyersdorf N, Ehl S, Gerard A, Lämmermann T, Schumacher TN, Beltman JB, Rohr JC. Quorum Regulation via Nested Antagonistic Feedback Circuits Mediated by the Receptors CD28 and CTLA-4 Confers Robustness to T Cell Population Dynamics. Immunity. 2020;52:313–327.e7. doi: 10.1016/j.immuni.2020.01.018. [DOI] [PubMed] [Google Scholar]
- 57.Ganesan A, Moon TC, Barakat KH. Revealing the atomistic details behind the binding of B7-1 to CD28 and CTLA-4: A comprehensive protein-protein modelling study. Biochim Biophys Acta Gen Subj. 2018;1862:2764–2778. doi: 10.1016/j.bbagen.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 58.Nishikawa H, Koyama S. Mechanisms of regulatory T cell infiltration in tumors: implications for innovative immune precision therapies. J Immunother Cancer. 2021;9 doi: 10.1136/jitc-2021-002591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA, Bergerhoff K, Arce F, Roddie C, Henry JY, Yagita H, Wolchok JD, Peggs KS, Ravetch JV, Allison JP, Quezada SA. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med. 2013;210:1695–1710. doi: 10.1084/jem.20130579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mizukoshi E, Nakamoto Y, Arai K, Yamashita T, Sakai A, Sakai Y, Kagaya T, Yamashita T, Honda M, Kaneko S. Comparative analysis of various tumor-associated antigen-specific t-cell responses in patients with hepatocellular carcinoma. Hepatology. 2011;53:1206–1216. doi: 10.1002/hep.24149. [DOI] [PubMed] [Google Scholar]
- 61.Li CX, Ling CC, Shao Y, Xu A, Li XC, Ng KT, Liu XB, Ma YY, Qi X, Liu H, Liu J, Yeung OW, Yang XX, Liu QS, Lam YF, Zhai Y, Lo CM, Man K. CXCL10/CXCR3 signaling mobilized-regulatory T cells promote liver tumor recurrence after transplantation. J Hepatol. 2016;65:944–952. doi: 10.1016/j.jhep.2016.05.032. [DOI] [PubMed] [Google Scholar]
- 62.Sangro B, Gomez-Martin C, de la Mata M, Iñarrairaegui M, Garralda E, Barrera P, Riezu-Boj JI, Larrea E, Alfaro C, Sarobe P, Lasarte JJ, Pérez-Gracia JL, Melero I, Prieto J. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59:81–88. doi: 10.1016/j.jhep.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 63.Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348:62–68. doi: 10.1126/science.aaa4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang CK, Huang CH, Hu CH, Fang JH, Chen TC, Lin YC, Lin CY. Immunophenotype and antitumor activity of cytokine-induced killer cells from patients with hepatocellular carcinoma. PLoS One. 2023;18:e0280023. doi: 10.1371/journal.pone.0280023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee JH, Lee JH, Lim YS, Yeon JE, Song TJ, Yu SJ, Gwak GY, Kim KM, Kim YJ, Lee JW, Yoon JH. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology. 2015;148:1383–91.e6. doi: 10.1053/j.gastro.2015.02.055. [DOI] [PubMed] [Google Scholar]
- 66.Cho JY, Kwon SH, Lee EK, Lee JH, Kim HL. Cost-Effectiveness of Adjuvant Immunotherapy With Cytokine-Induced Killer Cell for Hepatocellular Carcinoma Based on a Randomized Controlled Trial and Real-World Data. Front Oncol. 2021;11:728740. doi: 10.3389/fonc.2021.728740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu T, Zhang L, Zeng Z, Yan T, Cheng J, Miao X, Lu Y. Complete Response to PD-1 Inhibitor in Primary Hepatocellular Carcinoma Patients Post-Progression on Bi-Specific Antibody Conjugated CIK Cell Treatment: A Report of Two Cases. Onco Targets Ther. 2021;14:5447–5453. doi: 10.2147/OTT.S333604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang L, Li X, Dong XJ, Yu XL, Zhang J, Cheng ZG, Han ZY, Liu FY, Yu J, Liang P. Dendritic cell-cytokine killer combined with microwave ablation reduced recurrence for hepatocellular carcinoma compared to ablation alone. Technol Health Care. 2024;32:1819–1834. doi: 10.3233/THC-230871. [DOI] [PubMed] [Google Scholar]
- 69.Sangro B, Sarobe P, Hervás-Stubbs S, Melero I. Advances in immunotherapy for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2021;18:525–543. doi: 10.1038/s41575-021-00438-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gao H, Li K, Tu H, Pan X, Jiang H, Shi B, Kong J, Wang H, Yang S, Gu J, Li Z. Development of T cells redirected to glypican-3 for the treatment of hepatocellular carcinoma. Clin Cancer Res. 2014;20:6418–6428. doi: 10.1158/1078-0432.CCR-14-1170. [DOI] [PubMed] [Google Scholar]
- 71.Sun L, Gao F, Gao Z, Ao L, Li N, Ma S, Jia M, Li N, Lu P, Sun B, Ho M, Jia S, Ding T, Gao W. Shed antigen-induced blocking effect on CAR-T cells targeting Glypican-3 in Hepatocellular Carcinoma. J Immunother Cancer. 2021;9 doi: 10.1136/jitc-2020-001875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ringelhan M, Pfister D, O'Connor T, Pikarsky E, Heikenwalder M. The immunology of hepatocellular carcinoma. Nat Immunol. 2018;19:222–232. doi: 10.1038/s41590-018-0044-z. [DOI] [PubMed] [Google Scholar]
- 73.Chiang DY, Villanueva A, Hoshida Y, Peix J, Newell P, Minguez B, LeBlanc AC, Donovan DJ, Thung SN, Solé M, Tovar V, Alsinet C, Ramos AH, Barretina J, Roayaie S, Schwartz M, Waxman S, Bruix J, Mazzaferro V, Ligon AH, Najfeld V, Friedman SL, Sellers WR, Meyerson M, Llovet JM. Focal gains of VEGFA and molecular classification of hepatocellular carcinoma. Cancer Res. 2008;68:6779–6788. doi: 10.1158/0008-5472.CAN-08-0742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yu JH, Kim JM, Kim JK, Choi SJ, Lee KS, Lee JW, Chang HY, Lee JI. Platelet-derived growth factor receptor α in hepatocellular carcinoma is a prognostic marker independent of underlying liver cirrhosis. Oncotarget. 2017;8:39534–39546. doi: 10.18632/oncotarget.17134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.D'Alessio A, Rimassa L, Cortellini A, Pinato DJ. PD-1 Blockade for Hepatocellular Carcinoma: Current Research and Future Prospects. J Hepatocell Carcinoma. 2021;8:887–897. doi: 10.2147/JHC.S284440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Borst J, Busselaar J, Bosma DMT, Ossendorp F. Mechanism of action of PD-1 receptor/ligand targeted cancer immunotherapy. Eur J Immunol. 2021;51:1911–1920. doi: 10.1002/eji.202048994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang N, Yang X, Piao M, Xun Z, Wang Y, Ning C, Zhang X, Zhang L, Wang Y, Wang S, Chao J, Lu Z, Yang X, Wang H, Zhao H. Biomarkers and prognostic factors of PD-1/PD-L1 inhibitor-based therapy in patients with advanced hepatocellular carcinoma. Biomark Res. 2024;12:26. doi: 10.1186/s40364-023-00535-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, Okusaka T, Kobayashi M, Kumada H, Kaneko S, Pracht M, Mamontov K, Meyer T, Kubota T, Dutcus CE, Saito K, Siegel AB, Dubrovsky L, Mody K, Llovet JM. Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients With Unresectable Hepatocellular Carcinoma. J Clin Oncol. 2020;38:2960–2970. doi: 10.1200/JCO.20.00808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, Cowey CL, Schadendorf D, Wagstaff J, Dummer R, Ferrucci PF, Smylie M, Hogg D, Hill A, Márquez-Rodas I, Haanen J, Guidoboni M, Maio M, Schöffski P, Carlino MS, Lebbé C, McArthur G, Ascierto PA, Daniels GA, Long GV, Bastholt L, Rizzo JI, Balogh A, Moshyk A, Hodi FS, Wolchok JD. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med. 2019;381:1535–1546. doi: 10.1056/NEJMoa1910836. [DOI] [PubMed] [Google Scholar]
- 80.Fife BT, Bluestone JA. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev. 2008;224:166–182. doi: 10.1111/j.1600-065X.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 81.Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, Melero I, Kudo M, Hou MM, Matilla A, Tovoli F, Knox JJ, Ruth He A, El-Rayes BF, Acosta-Rivera M, Lim HY, Neely J, Shen Y, Wisniewski T, Anderson J, Hsu C. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients With Advanced Hepatocellular Carcinoma Previously Treated With Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol. 2020;6:e204564. doi: 10.1001/jamaoncol.2020.4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Peng Z, Fan W, Zhu B, Wang G, Sun J, Xiao C, Huang F, Tang R, Cheng Y, Huang Z, Liang Y, Fan H, Qiao L, Li F, Zhuang W, Peng B, Wang J, Li J, Kuang M. Lenvatinib Combined With Transarterial Chemoembolization as First-Line Treatment for Advanced Hepatocellular Carcinoma: A Phase III, Randomized Clinical Trial (LAUNCH) J Clin Oncol. 2023;41:117–127. doi: 10.1200/JCO.22.00392. [DOI] [PubMed] [Google Scholar]
- 83.Zhu HD, Li HL, Huang MS, Yang WZ, Yin GW, Zhong BY, Sun JH, Jin ZC, Chen JJ, Ge NJ, Ding WB, Li WH, Huang JH, Mu W, Gu SZ, Li JP, Zhao H, Wen SW, Lei YM, Song YS, Yuan CW, Wang WD, Huang M, Zhao W, Wu JB, Wang S, Zhu X, Han JJ, Ren WX, Lu ZM, Xing WG, Fan Y, Lin HL, Zhang ZS, Xu GH, Hu WH, Tu Q, Su HY, Zheng CS, Chen Y, Zhao XY, Fang ZT, Wang Q, Zhao JW, Xu AB, Xu J, Wu QH, Niu HZ, Wang J, Dai F, Feng DP, Li QD, Shi RS, Li JR, Yang G, Shi HB, Ji JS, Liu YE, Cai Z, Yang P, Zhao Y, Zhu XL, Lu LG, Teng GJ CHANCE001 Investigators. Transarterial chemoembolization with PD-(L)1 inhibitors plus molecular targeted therapies for hepatocellular carcinoma (CHANCE001) Signal Transduct Target Ther. 2023;8:58. doi: 10.1038/s41392-022-01235-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Duffy AG, Ulahannan SV, Makorova-Rusher O, Rahma O, Wedemeyer H, Pratt D, Davis JL, Hughes MS, Heller T, ElGindi M, Uppala A, Korangy F, Kleiner DE, Figg WD, Venzon D, Steinberg SM, Venkatesan AM, Krishnasamy V, Abi-Jaoudeh N, Levy E, Wood BJ, Greten TF. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol. 2017;66:545–551. doi: 10.1016/j.jhep.2016.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 86.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 87.Neuzillet C, de Mestier L, Rousseau B, Mir O, Hebbar M, Kocher HM, Ruszniewski P, Tournigand C. Unravelling the pharmacologic opportunities and future directions for targeted therapies in gastro-intestinal cancers part 2: Neuroendocrine tumours, hepatocellular carcinoma, and gastro-intestinal stromal tumours. Pharmacol Ther. 2018;181:49–75. doi: 10.1016/j.pharmthera.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 88.Gordan JD, Kennedy EB, Abou-Alfa GK, Beal E, Finn RS, Gade TP, Goff L, Gupta S, Guy J, Hoang HT, Iyer R, Jaiyesimi I, Jhawer M, Karippot A, Kaseb AO, Kelley RK, Kortmansky J, Leaf A, Remak WM, Sohal DPS, Taddei TH, Wilson Woods A, Yarchoan M, Rose MG. Systemic Therapy for Advanced Hepatocellular Carcinoma: ASCO Guideline Update. J Clin Oncol. 2024;42:1830–1850. doi: 10.1200/JCO.23.02745. [DOI] [PubMed] [Google Scholar]