Abstract
In this editorial, we comment on the article by Mei et al. Nonalcoholic steatohepatitis (NASH) is a severe inflammatory subtype of nonalcoholic fatty liver disease (NAFLD) with pathological features including steatosis, hepatocellular damage, and varying degrees of fibrosis. With the epidemic of metabolic diseases and obesity, the prevalence of NAFLD in China has increased, and it is now similar to that in developed countries; thus, NAFLD has become a major chronic liver disease in China. Human epidemiological data suggest that estrogen has a protective effect on NASH in premenopausal women and that sex hormones influence the development of liver disease. This review focuses on the pathogenesis, treatment, and relationship between NASH and other diseases as well as on the relationship between NASH and sex hormone metabolism, with the aim of providing new strategies for the treatment of NASH.
Keywords: NASH, Hepatic steatosis, Sex hormone metabolism, The treatment for NASH
Core Tip: In this study, the modified Xiaoyao San (MXS) formula was found can alleviated inflammation and hepatic steatosis in nonalcoholic steatohepatitis (NASH) by suppressing male hormone metabolism and modulating inflammation/Lipid metabolism-related signaling and factors. It suggested that the regulation of sex hormone metabolism and associated signaling could be new avenues for mechanistic research on NASH and for early diagnosis and treatment. This study offers substantial evidence for the therapeutic potential of MXS in NASH and makes a valuable contribution to the development of new drugs for this condition.
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is one of the earliest manifestations of metabolic syndrome worldwide and is characterized by hepatic lipid accumulation[2,3]. In China, the incidence of NAFLD has reached 29.2% and is increasing annually[4]. Nonalcoholic steatohepatitis (NASH) is a severe nonalcoholic fatty liver disease characterized by inflammation and fat accumulation in the liver[5]. The mortality rate of NASH is predicted to double by 2030[6]. NASH is a progressive form of NAFLD, and approximately 20% of patients with NAFLD progress to NASH; however, the molecular mechanisms underlying the transition from NAFLD to NASH are complex and not fully understood[2,7]. NAFLD/NASH has become the leading cause of liver disease worldwide and can lead to liver fibrosis, cirrhosis and even hepatocellular carcinoma.
Sex dimorphism is associated not only with physical and behavioral differences between males and females but also with physiological differences that are reflected in organ metabolism[8]. Sex hormones are steroid hormones that mainly include estrogen, progesterone, and testosterone. Differences in sex hormone levels and the expression of sex hormone-specific genes are thought to contribute to certain liver diseases[9]. Studies have shown that androgens can protect against NAFLD, but other studies have shown that androgens can promote the occurrence and development of NAFLD[10-12]. Androgens have an important effect on lipid metabolism in the female liver. There are also many indications that hyperandrogenetic PCOS may indirectly increase the risk of NAFLD through obesity and insulin resistance (IR) and directly increase the risk of NAFLD through hepatotoxic effects[13].
At present, the clinical methods for detecting NAFLD mainly include biopsy, imaging, and biomarker testing, among which biopsy is still the gold standard for NAFLD detection; however, as an invasive detection method, it is not suitable for frequent detection[14,15]. Despite the high incidence and increasing impact on world health, only one drug, Rezdiffra, is currently licensed with FDA approval, and developing additional drugs has been a major challenge[16]. Therefore, exploring the pathogenesis of NAFLD at the molecular biology level and identifying effective targets that play key roles in NAFLD are important for addressing the major clinical problem of NASH.
PATHOGENIC FACTORS AND PROGRESSION OF NAFLD/NASH
Historically, the double whammy theory has been used to describe the progression from normal liver to hepatic steatosis and then to the gradual development of NASH[17]. The evolution of NASH is complex, and the initial pathological manifestation is hepatocyte steatosis (first hit); however, this is not sufficient to induce inflammation and fibrosis[18]. As the disease progresses, subsequent events, including oxidative stress, genetic variation, abnormal lipid metabolism, oxidative stress, altered immune responses and imbalances in the intestinal microflora, are necessary to exacerbate liver injury. This theory can be explained by impaired lipid metabolism, which promotes the accumulation of fatty acids in the liver, leading to hepatic steatosis[19]. Oxidative stress is the "second hit" that occurs due to increased oxidation of fatty acids, which leads to reactive oxygen species production, lipid peroxidation, DNA damage, mitochondrial dysfunction, and the release of proinflammatory cytokines that amplify inflammation, resulting in a second injury to the liver, leading to hepatocellular damage, inflammation, and fibrosis[20-22]. However, the "second hit" theory can no longer fully explain the complex pathogenesis of NASH, and currently, the pathological progression is considered to involve a "triple hit" process of steatosis, lipotoxicity, and inflammation. Over time, liver fibrosis can progress to more serious diseases, such as cirrhosis or even cancer, for which liver transplantation is the only treatment option[23].
NASH is the result of a combination of factors, including genetic variants, abnormal lipid metabolism, oxidative stress, altered immune and inflammatory responses, and imbalances in the gut microbiome[24]. While NAFLD usually occurs in the absence of excessive alcohol consumption, NAFLD is associated with an unhealthy diet and a lack of physical activity[23]. Currently, a high-fat diet is believed to lead to lipid deposition and then to the abnormal activation of the innate immune system of the liver, which is the main reason for the progression of NAFLD. At the cellular level, after lipotoxic stimulation, cells transmit signals to downstream adaptor proteins and kinases through pattern recognition receptors. After receiving signals, adaptor proteins and kinases further transmit signals to downstream effectors, eventually leading to a series of pathological changes, such as inflammation and fibrosis. During this process, important adaptor proteins and kinases in the signaling center are finely regulated at multiple cellular levels, and if abnormally activated, they trigger a downstream signaling cascade that ultimately leads to cell damage or death[25]. In NAFLD, apoptosis signal-regulating kinase 1 (ASK1) can be phosphorylated by upstream kinases to form a dimer-activated form, activating downstream apoptotic and inflammatory pathways[26]. TAK1 can be modified by K63 ubiquitination to undergo conformational changes and activate the self-phosphorylation activation process[27].
Inflammatory and immune signaling pathways are involved in the pathogenesis of NASH, including via specific processes involving the activation of inflammatory factors; the release of hepatogenic signals; alterations in innate immune signals; changes in macrophages, T cells, platelets and neutrophils; and changes in the biology of cytokines, adipokines and chemokines. During the pathogenesis of NASH, a large number of free fatty acids bind to TLR4, activate important inflammatory pathways, including the NF-kB pathway, and trigger endoplasmic reticulum stress and inflammatory responses, ultimately leading to the production of cytokines such as tumour necrosis factor alpha (TNF-α) and interleukin (IL)-1β[28]. Cytokines are cell signaling molecules produced by a variety of cells in the body, and they are important mediators of inflammation-related diseases. Both lipid accumulation and inflammation occur in NAFLD. Cytokines may play a key role in the pathogenesis of NAFLD by stimulating liver inflammation, steatosis, apoptosis, and necrosis, as well as by inducing fibrosis[29]. Moreover, inflammasomes are activated, and different inflammasomes affect NASH via different mechanisms. Studies have shown that the pathogenesis of NASH is also associated with T-cell regeneration. In the livers of NASH patients, the numbers of T helper cell 17 (Thl7) cells and natural killer T cells are significantly increased, and Thl7 cells can promote inflammation in NASH patients through the secretion of IL-17[30].
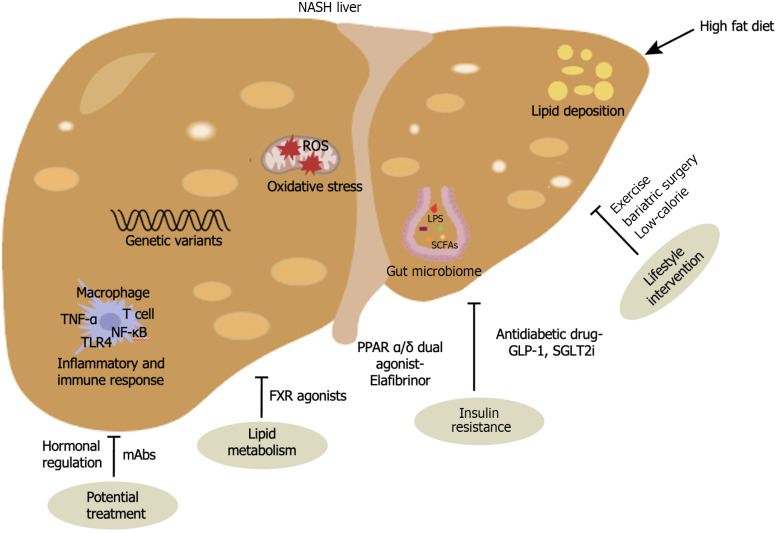
NAFLD patients have different intestinal flora compositions in different periods, and the accumulation or depletion of some specific bacteria is closely related to the progression of the disease. Lipopolysaccharide (LPS) derived from the intestinal flora can circulate through the portal vein to the liver, thereby activating toll-like receptor 4 (TLR4) on Kupffer cells and hepatic stellate cells (HSCs) and initiating a series of inflammatory signaling pathways that cause liver damage and fibrosis[31]. Short-chain fatty acids (SCFAs), including acetate, propionate and butyrate, are produced by the intestinal flora through the fermentation of dietary fiber. SCFAs are volatile fatty acids that can regulate the pH value of the intestine, inhibit the breeding of harmful bacteria, protect the homeostasis of the intestinal environment, and have an important impact on the metabolism of different organs in the body. At present, SCFAs are considered indirect indicators for determining whether the structure of the intestinal microbiota is normal. Ethyl hydrochloric acid uses cholesterol as a substrate and participates in fat synthesis. Propionate increases gluconeogenesis and then decreases hunger and cholesterol synthesis[32,33]. Studies have shown that the ratio of acetate and propionate to butyrate in normal rats is 58:26:16, and in rats with NASH induced by a high-fat diet, the ratio of the three is 74:21:5, which shows that the bacterial metabolites of the NASH model also change[34]. Genetic factors are critical in NAFLD and NASH, and five potential susceptibility sites have been identified: glucokinase regulator, tribbles pseudokinase 1, transmembrane 6 superfamily member 2, apolipoprotein E, and patatin-like phospholipase domain-containing protein 3[35]. During the progressive phase of NAFLD, the increase in oxidative stress and the lack of antioxidant defense mechanisms have been extensively studied and are thought to contribute to liver damage and the development of NASH[3]. Given the complexity of NASH pathophysiology, it is feasible to engage multiple targets and pathways to improve the outcome of drug interventions (Figure 1).
Figure 1.
Pathogenic factors and treatment of non-alcoholic steatohepatitis. NASH: Non-alcoholic steatohepatitis; SCFSs: Short-chain fatty acids; LPS: Lipopolysaccharide; TNF-α: Tumor Necrosis Factor-α; TLR4: Toll-like receptor 4; NF-κB: Nuclear factor kappa-B; GLP-1: Glucagon-like peptide-1; SGLT2i: Sodium-glucose cotransporter 2 inhibitors; PPAR α/β: Peroxisome proliferator-activated receptors α/β; FXR: Farnesoid X receptor; mAbs: Monoclonal antibodies.
TREATMENT FOR NASH
The treatment of NASH is challenging because the progression from steatosis to NASH and fibrosis can involve multiple molecular pathways in different patient subpopulations and at different stages of liver disease[36]. At present, lifestyle intervention is the most important and effective strategy for the prevention and control of NAFLD[2].
NONDRUG TREATMENT
Dietary habits may affect the development of NAFLD and NASH[37]. For example, resistant starch can reduce the levels of triglycerides (TGs), which can induce weight loss[38,39]. Excessive accumulation of TG is the main pathophysiological feature of NAFLD, suggesting that metabolic disorders involving TG or other lipids are related to the occurrence of NAFLD[37]. A low-fructose diet is preferable because dietary fructose intake is a risk factor for NASH, and a Mediterranean and low-calorie diet can reduce serum transaminase levels in patients with NAFLD[40]. Therefore, this type of diet, especially when low in saturated fat, should benefit cardiac metabolic diseases other than those of the liver[41]. Excessive caloric intake leads to obesity, and if there is purposeful weight loss, it plays a strong role in improving NAFLD. If it is difficult to achieve weight loss through diet or exercise, bariatric surgery can be an option. While lifestyle interventions have been shown to improve fatty liver disease in patients with NAFLD, liver fibrosis that progresses to advanced stages is unlikely to be cured by lifestyle changes alone, so medications specifically targeted to improve liver inflammation, fibrosis, and steatohepatitis are needed[42].
DRUG THERAPY
Although there are very few treatments for NASH, in March, the FDA approved Rezdiffra for treating liver fibrosis caused by nonalcoholic steatohepatitis in adults. The approval of Rezdiffra, a thyroid hormone beta receptor agonist, provides new directions for the development of drugs to treat NASH, offering a more comprehensive treatment approach[43]. However, patients should also pay attention to combining diet adjustment and strengthening exercise, but Rezdiffra cannot be used to treat patients with decompensated cirrhosis. For years, researchers have focused on targeting typical pathways involved in the progression of NASH, such as lipogenesis, oxidative stress, and inflammation[2]. Despite extensive efforts, these approaches have yet to yield any approved therapies.
In recent years, NASH therapeutics have been among most active areas of drug development, but a significant number of trials have not progressed from phase II to phase III, and most phase III trials have failed to reach their primary endpoints[44]. Although the exact pathogenesis of NASH is unclear, IR plays a key role in the development of NASH, and the prevalence of NASH is high in patients with type 2 diabetes; therefore, antidiabetic drugs are commonly used in the clinical treatment of patients with type 2 diabetes and NASH[45]. Glucagon-like peptide-1 (GLP-1) stimulates insulin secretion and suppresses the level of glucagon, and the GLP-1 agonist represented by liraglutide has been shown to improve the histological characterization of NASH[46,47]. SGLT2i is a novel oral hypoglycemic agent that inhibits renal reabsorption of glucose, thereby lowering blood glucose levels. Studies have shown that the addition of SGLT2i to common antidiabetic therapies in patients with type 2 diabetes mellitus can better reduce fat content and increase liver enzyme levels[48]. The peroxisome proliferator-activated receptor (PPAR) α/δ dual agonist Elafibrinor was shown to improve insulin resistance and liver inflammation, but the clinical phase III trial failed[49,50]. In addition, many drugs that target lipid metabolism, such as farnesoid X receptor (FXR) agonists, are under development. FXR agonists such as obeticholic acid (OCA/INT-747) have been shown to have significant lipid-lowering, anti-inflammatory, and antifibrotic effects in both mice and humans. However, it has not been approved by the FDA as a treatment for NASH, as it has been clinically shown to cause side effects such as an increase in serum LDL levels in patients[51]. Statins are common lipid-lowering drugs in clinical practice that can inhibit cholesterol synthesis[52].
While most clinical trials for NASH are based on a single drug treatment, the multiple complex mechanisms of the disease make it increasingly challenging to develop a single drug that can effectively treat most patients[53]. Therefore, combination therapy is an effective way to improve treatment efficiency, slow disease progression, and even reverse NASH. Combination therapy includes drugs from different classes, affecting multiple steps in the pathogenesis of NASH. Future combination therapies should include drugs that have been shown to be beneficial in terms of metabolism and complications as well as liver-directed therapy. For example, weight-loss drugs such as GLP1 receptor agonists and SGLT2 inhibitors have potential benefits in diabetes management and cardiovascular disease prevention, but combination therapy must be carefully weighed against the possible challenges of larger sample sizes and more types of side effects[54]. However, studies involving single drugs are still much more common than studies involving combination drugs and often focus more on their clinical course than most combinations do (Figure 1).
POTENTIAL TREATMENT
Although several drugs have multiple effects, most clinical results to date suggest that a single approach is unlikely to be a breakthrough treatment for NASH. One approach is to develop predictive biomarkers of response to a specific mechanism of action (MOA) at the time of investigational drug testing. Single-cell omics allows for the screening of novel therapeutic tools to assess on-target and off-target effects, immunophenotypes of different cell populations, and potential toxicology[55]. Another option is to use patient-derived pluripotent stem cells to generate organoids to test personalized responses to specific drugs[56]. Monoclonal antibodies have slowly begun to emerge as the preferred modality of drug treatment for the dominant drugs. However, it is inconclusive whether monoclonal antibodies have the same effect on disease progression in the treatment of metabolic disorders such as NASH. To date, only a few monoclonal antibody therapies have entered the clinical research stage, but the research has failed to live up to expectations. Some companies are using soloMER drugs to produce smaller antibody-like biologics for the treatment of advanced liver disease. This strategy offers the promise of further improving efficacy by combining biological and small-molecule drugs as a single targeted therapy.
Hormonal regulation of liver metabolism is a potential therapeutic target for the treatment of human liver disease and has been investigated in clinical trials[57]. Several studies have shown that there are sex differences in the occurrence of NAFLD, especially those related to estrogen[58,59]. Low serum levels are strongly associated with hepatic steatosis in men, suggesting that androgens protect against NAFLD[10,60]. However, other reports have shown the opposite result, with androgens contributing to the development of NAFLD[11,61]. Sex hormone-binding globulin (SHBG) is a liver factor that is produced mainly in the liver and is secreted into the blood, where it binds to circulating steroid hormones[62,63]. SHBG plays an important role in the development of NAFLD by regulating the production of fat in the liver[63]. In men, 44%-60% of testosterone binds to SHBG, whereas in women, 95% of circulating estrogen similarly binds to SHBG[64]. Therefore, SHBG can be used as a biomarker for NAFLD[8,65-67]. Another "star molecule" is formyl peptide receptor 2 (FPR2), which regulates inflammation in multiple organs. However, it is unclear whether and how FPR2 is involved in the pathophysiology of NAFLD. Most estrogen functions are mediated by its two nuclear receptors, ERα and Erβ[59]. In the liver, ERα and ERβ are expressed mainly in hepatocytes and activated hematopoietic stem cells, respectively[68,69]. Studies have shown that FPR2 is a downstream target of this gene and that estradiol stimulates estrogen expression[59]. Moreover, ERα knockout was found to induce steatosis in all mice, suggesting that ERα activation in fibrosis can be a sex-independent protective adaptation to liver injury, revealing estrogen receptors as potential drug targets for NAFLD management[70-72]. While hormone replacement therapy improves liver physiology and function in patients, it also carries risks. Therefore, while liver function can be enhanced, such therapy needs to be developed in a way that balances the benefits and risks, especially with long-term use[73] (Figure 1).
CONCLUSION
NAFLD has become a new challenge and major public health problem in the fields of liver disease and metabolism worldwide. Lifestyle intervention is an important basic treatment, and the regulation of glycolipid metabolism remains the focus in the treatment of NAFLD. I think that lifestyle modification and surgery are the best treatment strategies in the long run, but there are some drawbacks that cannot be ignored compared with some of the potential methods that are currently being explored. An unhealthy or irregular approach to weight loss that causes rapid weight loss can sometimes backfire. This can cause the body to break down fat too quickly, which can induce or exacerbate inflammatory infiltration or fibrosis of the liver. Bariatric surgery can be considered for patients with NASH who are not sensitive to behavior modification, effectively improving the quality of life and survival time of NASH patients. However, due to the safety issues faced by surgery, further research and verification are still needed.
Various novel metabolic drugs, such as PPAR agonists, FXR agonists and THRβ agonists, are also under development and are in clinical trials. However, clinical trials of most therapeutic agents have not been satisfactory, and liver histological endpoints have not been achieved. The reasons for NASH bottlenecks in the drug development process can be summarized as follows: First, target selection is a problem. On the one hand, there are multiple pathways involved from the sensing of lipotoxic stimuli to the final effect, and there are cross-interactions between signaling pathways. Thus, unless a critical component involved in the regulation of the signaling network is targeted, when the upstream drivers of the disease are still present, the other pathways remain active or even produce compensatory effects even though one pathway is inhibited[74]. On the other hand, many targets not only play key roles in the pathogenesis of NAFLD but also play important roles under normal physiological conditions. Therefore, approaches that interfere with the pathological process of NAFLD will also affect physiological function to varying degrees. Drug side effects often become one of the main obstacles in the translation of targets that have been verified in some animal experiments into clinical practice[75]. Second, there are unreasonable indicators for the observation of subjects. For example, the ASKI inhibitor selonsertib, the PPARα/δ agonist elafibranor, and the CCR2-CCR5 antagonist cenicriviroc, which were highly evaluated in early trials, ultimately failed in phase III trials[76]. Third, there is a lack of in-depth exploration of the complexity of disease pathogenesis. Failure of the pancaspase inhibitor emricasan is an example. The mechanism of action of emricasan involves the inhibition of hepatocyte apoptosis, which can improve liver function indicators in the short term but exacerbate liver fibrosis and hepatocyte balloon formation in the long term. Therefore, NAFLD/NASH therapy should be anchored to its underlying cause—lipotoxic stimulus—or have pleiotropic effects at different locations in the disease cascade[77]. To date, the use of pioglitazone, bariatric surgery, and GLP1 have successfully supported this idea[78]. An in-depth understanding of the pathogenesis of NAFLD and NASH will lead to more disease-related targets and is expected to yield safer and more effective approaches for controlling these diseases.
Footnotes
Conflict-of-interest statement: All authors declare no competing financial interest.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade C, Grade C
Novelty: Grade B, Grade C
Creativity or Innovation: Grade C, Grade C
Scientific Significance: Grade B, Grade C
P-Reviewer: Cheong KL; Tai DI S-Editor: Liu JH L-Editor: A P-Editor: Zhang XD
Contributor Information
Guan-Yue Shan, Institute of Translational Medicine, The First Hospital of Jilin University, Changchun 130061, Jilin Province, China.
Hui Wan, Institute of Translational Medicine, The First Hospital of Jilin University, Changchun 130061, Jilin Province, China.
Yu-Xin Zhang, Institute of Translational Medicine, The First Hospital of Jilin University, Changchun 130061, Jilin Province, China.
Jun-Ya Cheng, Department of Bioengineering, Pharmacy School of Jilin University, Changchun 130061, Jilin Province, China.
Duan-Rui Qiao, Institute of Translational Medicine, The First Hospital of Jilin University, Changchun 130061, Jilin Province, China.
Yi-Ying Liu, Institute of Translational Medicine, The First Hospital of Jilin University, Changchun 130061, Jilin Province, China.
Wen-Na Shi, Institute of Translational Medicine, The First Hospital of Jilin University, Changchun 130061, Jilin Province, China.
Hai-Jun Li, Institute of Liver Diseases, Institute of Translational Medicine, The First Hospital of Jilin University, Changchun 130061, Jilin Province, China. hjli2012@jlu.edu.cn.
References
- 1.Mei XL, Wu SY, Wu SL, Luo XL, Huang SX, Liu R, Qiang Z. Hepatoprotective effects of Xiaoyao San formula on hepatic steatosis and inflammation via regulating the sex hormones metabolism. World J Hepatol. 2024;16:1051–1066. doi: 10.4254/wjh.v16.i7.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu X, Poulsen KL, Wu L, Liu S, Miyata T, Song Q, Wei Q, Zhao C, Lin C, Yang J. Targeted therapeutics and novel signaling pathways in non-alcohol-associated fatty liver/steatohepatitis (NAFL/NASH) Signal Transduct Target Ther. 2022;7:287. doi: 10.1038/s41392-022-01119-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raza S, Rajak S, Upadhyay A, Tewari A, Anthony Sinha R. Current treatment paradigms and emerging therapies for NAFLD/NASH. Front Biosci (Landmark Ed) 2021;26:206–237. doi: 10.2741/4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Michelotti GA, Machado MV, Diehl AM. NAFLD, NASH and liver cancer. Nat Rev Gastroenterol Hepatol. 2013;10:656–665. doi: 10.1038/nrgastro.2013.183. [DOI] [PubMed] [Google Scholar]
- 5.Wei W, Wong CC, Jia Z, Liu W, Liu C, Ji F, Pan Y, Wang F, Wang G, Zhao L, Chu ESH, Zhang X, Sung JJY, Yu J. Parabacteroides distasonis uses dietary inulin to suppress NASH via its metabolite pentadecanoic acid. Nat Microbiol. 2023;8:1534–1548. doi: 10.1038/s41564-023-01418-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Estes C, Anstee QM, Arias-Loste MT, Bantel H, Bellentani S, Caballeria J, Colombo M, Craxi A, Crespo J, Day CP, Eguchi Y, Geier A, Kondili LA, Kroy DC, Lazarus JV, Loomba R, Manns MP, Marchesini G, Nakajima A, Negro F, Petta S, Ratziu V, Romero-Gomez M, Sanyal A, Schattenberg JM, Tacke F, Tanaka J, Trautwein C, Wei L, Zeuzem S, Razavi H. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J Hepatol. 2018;69:896–904. doi: 10.1016/j.jhep.2018.05.036. [DOI] [PubMed] [Google Scholar]
- 7.Hardy T, Oakley F, Anstee QM, Day CP. Nonalcoholic Fatty Liver Disease: Pathogenesis and Disease Spectrum. Annu Rev Pathol. 2016;11:451–496. doi: 10.1146/annurev-pathol-012615-044224. [DOI] [PubMed] [Google Scholar]
- 8.Song MJ, Choi JY. Androgen dysfunction in non-alcoholic fatty liver disease: Role of sex hormone binding globulin. Front Endocrinol (Lausanne) 2022;13:1053709. doi: 10.3389/fendo.2022.1053709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kur P, Kolasa-Wołosiuk A, Misiakiewicz-Has K, Wiszniewska B. Sex Hormone-Dependent Physiology and Diseases of Liver. Int J Environ Res Public Health. 2020;17 doi: 10.3390/ijerph17082620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang H, Liu Y, Wang L, Li Z, Zhang H, Wu J, Rahman N, Guo Y, Li D, Li N, Huhtaniemi I, Tsang SY, Gao GF, Li X. Differential effects of estrogen/androgen on the prevention of nonalcoholic fatty liver disease in the male rat. J Lipid Res. 2013;54:345–357. doi: 10.1194/jlr.M028969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones H, Sprung VS, Pugh CJ, Daousi C, Irwin A, Aziz N, Adams VL, Thomas EL, Bell JD, Kemp GJ, Cuthbertson DJ. Polycystic ovary syndrome with hyperandrogenism is characterized by an increased risk of hepatic steatosis compared to nonhyperandrogenic PCOS phenotypes and healthy controls, independent of obesity and insulin resistance. J Clin Endocrinol Metab. 2012;97:3709–3716. doi: 10.1210/jc.2012-1382. [DOI] [PubMed] [Google Scholar]
- 12.Schwingel PA, Zoppi CC, Cotrim HP. Increased liver steatosis in anabolic-androgenic steroid users: more evidence towards toxicant-associated fatty liver disease development. Liver Int. 2011;31:1240–1241. doi: 10.1111/j.1478-3231.2011.02552.x. [DOI] [PubMed] [Google Scholar]
- 13.Bohdanowicz-Pawlak A, Lenarcik-Kabza A, Brona A, Kuliczkowska-Płaksej J, Łaczmański Ł, Zaleska-Dorobisz U, Milewicz A. Non-alcoholic fatty liver disease in women with polycystic ovary syndrome - clinical and metabolic aspects and lipoprotein lipase gene polymorphism. Endokrynol Pol. 2014;65:416–421. doi: 10.5603/EP.2014.0058. [DOI] [PubMed] [Google Scholar]
- 14.Ajmera V, Cepin S, Tesfai K, Hofflich H, Cadman K, Lopez S, Madamba E, Bettencourt R, Richards L, Behling C, Sirlin CB, Loomba R. A prospective study on the prevalence of NAFLD, advanced fibrosis, cirrhosis and hepatocellular carcinoma in people with type 2 diabetes. J Hepatol. 2023;78:471–478. doi: 10.1016/j.jhep.2022.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bazick J, Donithan M, Neuschwander-Tetri BA, Kleiner D, Brunt EM, Wilson L, Doo E, Lavine J, Tonascia J, Loomba R. Clinical Model for NASH and Advanced Fibrosis in Adult Patients With Diabetes and NAFLD: Guidelines for Referral in NAFLD. Diabetes Care. 2015;38:1347–1355. doi: 10.2337/dc14-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keam SJ. Resmetirom: First Approval. Drugs. 2024;84:729–735. doi: 10.1007/s40265-024-02045-0. [DOI] [PubMed] [Google Scholar]
- 17.Gentile CL, Pagliassotti MJ. The role of fatty acids in the development and progression of nonalcoholic fatty liver disease. J Nutr Biochem. 2008;19:567–576. doi: 10.1016/j.jnutbio.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vancells Lujan P, Viñas Esmel E, Sacanella Meseguer E. Overview of Non-Alcoholic Fatty Liver Disease (NAFLD) and the Role of Sugary Food Consumption and Other Dietary Components in Its Development. Nutrients. 2021;13 doi: 10.3390/nu13051442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hwang JH, Stein DT, Barzilai N, Cui MH, Tonelli J, Kishore P, Hawkins M. Increased intrahepatic triglyceride is associated with peripheral insulin resistance: in vivo MR imaging and spectroscopy studies. Am J Physiol Endocrinol Metab. 2007;293:E1663–E1669. doi: 10.1152/ajpendo.00590.2006. [DOI] [PubMed] [Google Scholar]
- 20.Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest. 2004;114:147–152. doi: 10.1172/JCI22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seki S, Kitada T, Sakaguchi H. Clinicopathological significance of oxidative cellular damage in non-alcoholic fatty liver diseases. Hepatol Res. 2005;33:132–134. doi: 10.1016/j.hepres.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 22.Korenblat KM, Fabbrini E, Mohammed BS, Klein S. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology. 2008;134:1369–1375. doi: 10.1053/j.gastro.2008.01.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fraile JM, Palliyil S, Barelle C, Porter AJ, Kovaleva M. Non-Alcoholic Steatohepatitis (NASH) - A Review of a Crowded Clinical Landscape, Driven by a Complex Disease. Drug Des Devel Ther. 2021;15:3997–4009. doi: 10.2147/DDDT.S315724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loomba R, Friedman SL, Shulman GI. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell. 2021;184:2537–2564. doi: 10.1016/j.cell.2021.04.015. [DOI] [PubMed] [Google Scholar]
- 25.Lawan A, Bennett AM. Mitogen-Activated Protein Kinase Regulation in Hepatic Metabolism. Trends Endocrinol Metab. 2017;28:868–878. doi: 10.1016/j.tem.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X, Ni W, Ge X, Zhang J, Ma H, Cao K. Proteomic identification of potential target proteins regulated by an ASK1-mediated proteolysis pathway. Cell Res. 2006;16:489–498. doi: 10.1038/sj.cr.7310060. [DOI] [PubMed] [Google Scholar]
- 27.Singh AK, Haque M, O'Sullivan K, Chourasia M, Ouseph MM, Ahmed S. Suppression of monosodium urate crystal-induced inflammation by inhibiting TGF-β-activated kinase 1-dependent signaling: role of the ubiquitin proteasome system. Cell Mol Immunol. 2021;18:162–170. doi: 10.1038/s41423-019-0284-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tazi KA, Quioc JJ, Saada V, Bezeaud A, Lebrec D, Moreau R. Upregulation of TNF-alpha production signaling pathways in monocytes from patients with advanced cirrhosis: possible role of Akt and IRAK-M. J Hepatol. 2006;45:280–289. doi: 10.1016/j.jhep.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 29.Kitaura H, Marahleh A, Ohori F, Noguchi T, Nara Y, Pramusita A, Kinjo R, Ma J, Kanou K, Mizoguchi I. Role of the Interaction of Tumor Necrosis Factor-α and Tumor Necrosis Factor Receptors 1 and 2 in Bone-Related Cells. Int J Mol Sci. 2022;23 doi: 10.3390/ijms23031481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharpton SR, Ajmera V, Loomba R. Emerging Role of the Gut Microbiome in Nonalcoholic Fatty Liver Disease: From Composition to Function. Clin Gastroenterol Hepatol. 2019;17:296–306. doi: 10.1016/j.cgh.2018.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ye J, Lv L, Wu W, Li Y, Shi D, Fang D, Guo F, Jiang H, Yan R, Ye W, Li L. Butyrate Protects Mice Against Methionine-Choline-Deficient Diet-Induced Non-alcoholic Steatohepatitis by Improving Gut Barrier Function, Attenuating Inflammation and Reducing Endotoxin Levels. Front Microbiol. 2018;9:1967. doi: 10.3389/fmicb.2018.01967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Leeuwen PT, Brul S, Zhang J, Wortel MT. Synthetic microbial communities (SynComs) of the human gut: design, assembly, and applications. FEMS Microbiol Rev. 2023;47 doi: 10.1093/femsre/fuad012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chambers ES, Viardot A, Psichas A, Morrison DJ, Murphy KG, Zac-Varghese SE, MacDougall K, Preston T, Tedford C, Finlayson GS, Blundell JE, Bell JD, Thomas EL, Mt-Isa S, Ashby D, Gibson GR, Kolida S, Dhillo WS, Bloom SR, Morley W, Clegg S, Frost G. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut. 2015;64:1744–1754. doi: 10.1136/gutjnl-2014-307913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahmed LA, Salem MB, Seif El-Din SH, El-Lakkany NM, Ahmed HO, Nasr SM, Hammam OA, Botros SS, Saleh S. Gut microbiota modulation as a promising therapy with metformin in rats with non-alcoholic steatohepatitis: Role of LPS/TLR4 and autophagy pathways. Eur J Pharmacol. 2020;887:173461. doi: 10.1016/j.ejphar.2020.173461. [DOI] [PubMed] [Google Scholar]
- 35.Ghodsian N, Abner E, Emdin CA, Gobeil É, Taba N, Haas ME, Perrot N, Manikpurage HD, Gagnon É, Bourgault J, St-Amand A, Couture C, Mitchell PL, Bossé Y, Mathieu P, Vohl MC, Tchernof A, Thériault S, Khera AV, Esko T, Arsenault BJ. Electronic health record-based genome-wide meta-analysis provides insights on the genetic architecture of non-alcoholic fatty liver disease. Cell Rep Med. 2021;2:100437. doi: 10.1016/j.xcrm.2021.100437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Musso G, Saba F, Cassader M, Gambino R. Lipidomics in pathogenesis, progression and treatment of nonalcoholic steatohepatitis (NASH): Recent advances. Prog Lipid Res. 2023;91:101238. doi: 10.1016/j.plipres.2023.101238. [DOI] [PubMed] [Google Scholar]
- 37.Wei S, Wang L, Evans PC, Xu S. NAFLD and NASH: etiology, targets and emerging therapies. Drug Discov Today. 2024;29:103910. doi: 10.1016/j.drudis.2024.103910. [DOI] [PubMed] [Google Scholar]
- 38.Ni Y, Qian L, Siliceo SL, Long X, Nychas E, Liu Y, Ismaiah MJ, Leung H, Zhang L, Gao Q, Wu Q, Zhang Y, Jia X, Liu S, Yuan R, Zhou L, Wang X, Li Q, Zhao Y, El-Nezami H, Xu A, Xu G, Li H, Panagiotou G, Jia W. Resistant starch decreases intrahepatic triglycerides in patients with NAFLD via gut microbiome alterations. Cell Metab. 2023;35:1530–1547.e8. doi: 10.1016/j.cmet.2023.08.002. [DOI] [PubMed] [Google Scholar]
- 39.Liu D, Huang Y, Huang C, Yang S, Wei X, Zhang P, Guo D, Lin J, Xu B, Li C, He H, He J, Liu S, Shi L, Xue Y, Zhang H. Calorie Restriction with or without Time-Restricted Eating in Weight Loss. N Engl J Med. 2022;386:1495–1504. doi: 10.1056/NEJMoa2114833. [DOI] [PubMed] [Google Scholar]
- 40.Mosca A, Nobili V, De Vito R, Crudele A, Scorletti E, Villani A, Alisi A, Byrne CD. Serum uric acid concentrations and fructose consumption are independently associated with NASH in children and adolescents. J Hepatol. 2017;66:1031–1036. doi: 10.1016/j.jhep.2016.12.025. [DOI] [PubMed] [Google Scholar]
- 41.Yki-Järvinen H, Luukkonen PK, Hodson L, Moore JB. Dietary carbohydrates and fats in nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2021;18:770–786. doi: 10.1038/s41575-021-00472-y. [DOI] [PubMed] [Google Scholar]
- 42.Vinker S, Frese T, Hummers E, Ketiš ZK, Windak A, Petrazzuoli F, Bueno-Ortiz JM, Asenova R, Poppleton A, Méndez-Sánchez N. WONCA Europe position statement on the redefinition of fatty liver disease. Lancet Gastroenterol Hepatol. 2022;7:1076–1077. doi: 10.1016/S2468-1253(22)00333-8. [DOI] [PubMed] [Google Scholar]
- 43.Alshehade SA. Resmetirom's approval: Highlighting the need for comprehensive approaches in NASH therapeutics. Clin Res Hepatol Gastroenterol. 2024;48:102377. doi: 10.1016/j.clinre.2024.102377. [DOI] [PubMed] [Google Scholar]
- 44.Shi YW, Fan JG. Current status and challenges in the drug treatment for fibrotic nonalcoholic steatohepatitis. Acta Pharmacol Sin. 2022;43:1191–1199. doi: 10.1038/s41401-021-00822-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ali A, Amin MJ, Ahmed MU, Taj A, Aasim M, Tabrez E. Frequency of non-alcoholic fatty liver disease (NAFLD) and its associated risk factors among Type-2 diabetics. Pak J Med Sci. 2022;38:28–33. doi: 10.12669/pjms.38.1.4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mashayekhi M, Nian H, Mayfield D, Devin JK, Gamboa JL, Yu C, Silver HJ, Niswender K, Luther JM, Brown NJ. Weight Loss-Independent Effect of Liraglutide on Insulin Sensitivity in Individuals With Obesity and Prediabetes. Diabetes. 2024;73:38–50. doi: 10.2337/db23-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gou X, Qin L, Wu D, Xie J, Lu Y, Zhang Q, He Y. Research Progress of Takeda G Protein-Coupled Receptor 5 in Metabolic Syndrome. Molecules. 2023;28 doi: 10.3390/molecules28155870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li S, Xiong F, Zhang S, Liu J, Gao G, Xie J, Wang Y. Oligonucleotide therapies for nonalcoholic steatohepatitis. Mol Ther Nucleic Acids. 2024;35:102184. doi: 10.1016/j.omtn.2024.102184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Westerouen Van Meeteren MJ, Drenth JPH, Tjwa ETTL. Elafibranor: a potential drug for the treatment of nonalcoholic steatohepatitis (NASH) Expert Opin Investig Drugs. 2020;29:117–123. doi: 10.1080/13543784.2020.1668375. [DOI] [PubMed] [Google Scholar]
- 50.Liu R, Li Y, Zheng Q, Ding M, Zhou H, Li X. Epigenetic modification in liver fibrosis: Promising therapeutic direction with significant challenges ahead. Acta Pharm Sin B. 2024;14:1009–1029. doi: 10.1016/j.apsb.2023.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, Chalasani N, Dasarathy S, Diehl AM, Hameed B, Kowdley KV, McCullough A, Terrault N, Clark JM, Tonascia J, Brunt EM, Kleiner DE, Doo E NASH Clinical Research Network. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956–965. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang S, Ren X, Zhang B, Lan T, Liu B. A Systematic Review of Statins for the Treatment of Nonalcoholic Steatohepatitis: Safety, Efficacy, and Mechanism of Action. Molecules. 2024;29 doi: 10.3390/molecules29081859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dufour JF, Caussy C, Loomba R. Combination therapy for non-alcoholic steatohepatitis: rationale, opportunities and challenges. Gut. 2020;69:1877–1884. doi: 10.1136/gutjnl-2019-319104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alkhouri N, Herring R, Kabler H, Kayali Z, Hassanein T, Kohli A, Huss RS, Zhu Y, Billin AN, Damgaard LH, Buchholtz K, Kjær MS, Balendran C, Myers RP, Loomba R, Noureddin M. Safety and efficacy of combination therapy with semaglutide, cilofexor and firsocostat in patients with non-alcoholic steatohepatitis: A randomised, open-label phase II trial. J Hepatol. 2022;77:607–618. doi: 10.1016/j.jhep.2022.04.003. [DOI] [PubMed] [Google Scholar]
- 55.Nassar SF, Raddassi K, Wu T. Single-Cell Multiomics Analysis for Drug Discovery. Metabolites. 2021;11 doi: 10.3390/metabo11110729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kuret T, Sodin-Šemrl S, Leskošek B, Ferk P. Single Cell RNA Sequencing in Autoimmune Inflammatory Rheumatic Diseases: Current Applications, Challenges and a Step Toward Precision Medicine. Front Med (Lausanne) 2021;8:822804. doi: 10.3389/fmed.2021.822804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kasarinaite A, Sinton M, Saunders PTK, Hay DC. The Influence of Sex Hormones in Liver Function and Disease. Cells. 2023;12 doi: 10.3390/cells12121604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee J, Lee JY, Yang YJ. Sex-Specific Association between Sodium Intake Estimated by 24-Hour Urinary Sodium Excretion and Nonalcoholic Fatty Liver Disease: The Community-Based Prospective Cohort Study. Nutrients. 2024;16 doi: 10.3390/nu16040548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee C, Kim J, Han J, Oh D, Kim M, Jeong H, Kim TJ, Kim SW, Kim JN, Seo YS, Suzuki A, Kim JH, Jung Y. Formyl peptide receptor 2 determines sex-specific differences in the progression of nonalcoholic fatty liver disease and steatohepatitis. Nat Commun. 2022;13:578. doi: 10.1038/s41467-022-28138-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tebbens M, Schutte M, Troelstra MA, Bruinstroop E, de Mutsert R, Nederveen AJ, den Heijer M, Bisschop PH. Sex Steroids Regulate Liver Fat Content and Body Fat Distribution in Both Men and Women: A Study in Transgender Persons. J Clin Endocrinol Metab. 2023;109:e280–e290. doi: 10.1210/clinem/dgad409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang X, Wang Y, Liu P. Omic studies reveal the pathogenic lipid droplet proteins in non-alcoholic fatty liver disease. Protein Cell. 2017;8:4–13. doi: 10.1007/s13238-016-0327-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bourebaba N, Ngo T, Śmieszek A, Bourebaba L, Marycz K. Sex hormone binding globulin as a potential drug candidate for liver-related metabolic disorders treatment. Biomed Pharmacother. 2022;153:113261. doi: 10.1016/j.biopha.2022.113261. [DOI] [PubMed] [Google Scholar]
- 63.Hu R, Long S, Luo M, Tang B, Tan T, Dong W, Wang Q, Zhang J. Hyperglycemia Inhibits Hepatic SHBG Synthesis Through the NGBR-AMPK-HNF4 Pathway in Rats with Polycystic Ovary Syndrome Induced by Letrozole in Combination with a High-Fat Diet. Mol Nutr Food Res. 2024;68:e2300915. doi: 10.1002/mnfr.202300915. [DOI] [PubMed] [Google Scholar]
- 64.Simó R, Sáez-López C, Barbosa-Desongles A, Hernández C, Selva DM. Novel insights in SHBG regulation and clinical implications. Trends Endocrinol Metab. 2015;26:376–383. doi: 10.1016/j.tem.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 65.Grasa MDM, Gulfo J, Camps N, Alcalá R, Monserrat L, Moreno-Navarrete JM, Ortega FJ, Esteve M, Remesar X, Fernández-López JA, Fernández-Real JM, Alemany M. Modulation of SHBG binding to testosterone and estradiol by sex and morbid obesity. Eur J Endocrinol. 2017;176:393–404. doi: 10.1530/EJE-16-0834. [DOI] [PubMed] [Google Scholar]
- 66.Dong J, Liu C, Lu J, Wang L, Xie S, Ji L, Lu B. The relationship between sex hormone-binding protein and non-alcoholic fatty liver disease using Mendelian randomisation. Eur J Clin Invest. 2024;54:e14082. doi: 10.1111/eci.14082. [DOI] [PubMed] [Google Scholar]
- 67.Wang X, Xie J, Pang J, Zhang H, Chen X, Lin J, Li Q, Chen Q, Ma J, Xu X, Yang Y, Ling W, Chen Y. Serum SHBG Is Associated With the Development and Regression of Nonalcoholic Fatty Liver Disease: A Prospective Study. J Clin Endocrinol Metab. 2020;105 doi: 10.1210/clinem/dgz244. [DOI] [PubMed] [Google Scholar]
- 68.Bravo González-Blas C, Matetovici I, Hillen H, Taskiran II, Vandepoel R, Christiaens V, Sansores-García L, Verboven E, Hulselmans G, Poovathingal S, Demeulemeester J, Psatha N, Mauduit D, Halder G, Aerts S. Single-cell spatial multi-omics and deep learning dissect enhancer-driven gene regulatory networks in liver zonation. Nat Cell Biol. 2024;26:153–167. doi: 10.1038/s41556-023-01316-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang B, Zhang CG, Ji LH, Zhao G, Wu ZY. Estrogen receptor β selective agonist ameliorates liver cirrhosis in rats by inhibiting the activation and proliferation of hepatic stellate cells. J Gastroenterol Hepatol. 2018;33:747–755. doi: 10.1111/jgh.13976. [DOI] [PubMed] [Google Scholar]
- 70.Hart-Unger S, Arao Y, Hamilton KJ, Lierz SL, Malarkey DE, Hewitt SC, Freemark M, Korach KS. Hormone signaling and fatty liver in females: analysis of estrogen receptor α mutant mice. Int J Obes (Lond) 2017;41:945–954. doi: 10.1038/ijo.2017.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qiu S, Vazquez JT, Boulger E, Liu H, Xue P, Hussain MA, Wolfe A. Hepatic estrogen receptor α is critical for regulation of gluconeogenesis and lipid metabolism in males. Sci Rep. 2017;7:1661. doi: 10.1038/s41598-017-01937-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zahr T, Boda VK, Ge J, Yu L, Wu Z, Que J, Li W, Qiang L. Small molecule conjugates with selective estrogen receptor β agonism promote anti-aging benefits in metabolism and skin recovery. Acta Pharm Sin B. 2024;14:2137–2152. doi: 10.1016/j.apsb.2024.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tassinari R, Tammaro A, Lori G, Tait S, Martinelli A, Cancemi L, Frassanito P, Maranghi F. Risk Assessment of Transgender People: Development of Rodent Models Mimicking Gender-Affirming Hormone Therapies and Identification of Sex-Dimorphic Liver Genes as Novel Biomarkers of Sex Transition. Cells. 2023;12 doi: 10.3390/cells12030474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Harrison SA, Wong VW, Okanoue T, Bzowej N, Vuppalanchi R, Younes Z, Kohli A, Sarin S, Caldwell SH, Alkhouri N, Shiffman ML, Camargo M, Li G, Kersey K, Jia C, Zhu Y, Djedjos CS, Subramanian GM, Myers RP, Gunn N, Sheikh A, Anstee QM, Romero-Gomez M, Trauner M, Goodman Z, Lawitz EJ, Younossi Z STELLAR-3; STELLAR-4 Investigators. Selonsertib for patients with bridging fibrosis or compensated cirrhosis due to NASH: Results from randomized phase III STELLAR trials. J Hepatol. 2020;73:26–39. doi: 10.1016/j.jhep.2020.02.027. [DOI] [PubMed] [Google Scholar]
- 75.Harrison SA, Abdelmalek MF, Caldwell S, Shiffman ML, Diehl AM, Ghalib R, Lawitz EJ, Rockey DC, Schall RA, Jia C, McColgan BJ, McHutchison JG, Subramanian GM, Myers RP, Younossi Z, Ratziu V, Muir AJ, Afdhal NH, Goodman Z, Bosch J, Sanyal AJ GS-US-321-0105 and GS-US-321-0106 Investigators. Simtuzumab Is Ineffective for Patients With Bridging Fibrosis or Compensated Cirrhosis Caused by Nonalcoholic Steatohepatitis. Gastroenterology. 2018;155:1140–1153. doi: 10.1053/j.gastro.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 76.Loomba R, Lawitz E, Mantry PS, Jayakumar S, Caldwell SH, Arnold H, Diehl AM, Djedjos CS, Han L, Myers RP, Subramanian GM, McHutchison JG, Goodman ZD, Afdhal NH, Charlton MR GS-US-384-1497 Investigators. The ASK1 inhibitor selonsertib in patients with nonalcoholic steatohepatitis: A randomized, phase 2 trial. Hepatology. 2018;67:549–559. doi: 10.1002/hep.29514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Harrison SA, Goodman Z, Jabbar A, Vemulapalli R, Younes ZH, Freilich B, Sheikh MY, Schattenberg JM, Kayali Z, Zivony A, Sheikh A, Garcia-Samaniego J, Satapathy SK, Therapondos G, Mena E, Schuppan D, Robinson J, Chan JL, Hagerty DT, Sanyal AJ. A randomized, placebo-controlled trial of emricasan in patients with NASH and F1-F3 fibrosis. J Hepatol. 2020;72:816–827. doi: 10.1016/j.jhep.2019.11.024. [DOI] [PubMed] [Google Scholar]
- 78.Vuppalanchi R, Noureddin M, Alkhouri N, Sanyal AJ. Therapeutic pipeline in nonalcoholic steatohepatitis. Nat Rev Gastroenterol Hepatol. 2021;18:373–392. doi: 10.1038/s41575-020-00408-y. [DOI] [PubMed] [Google Scholar]