Summary
Human immunodeficiency virus (HIV) impacts millions of individuals worldwide, and well over 2/3 of those living with HIV are accessing antiviral therapies that are successfully repressing viral replication. Most often, HIV treatments and prevention are administered in the form of daily pills as combinations of multiple drugs. An emergent and effective strategy for suppressing viral replication is the application of long-acting antiretroviral therapy (LAART), or antivirals that require less-frequent, non-daily doses. Thus far, the repertoire of LAARTs includes the widely used antiviral classes of non-nucleoside reverse transcriptase inhibitors (NNRTIs) and integrase strand transfer inhibitors (INSTIs) and has recently expanded to include a capsid-targeting antiviral. Possible future additions are nucleoside reverse transcriptase inhibitors (NRTIs) and nucleoside reverse transcriptase translocation inhibitors (NRTTIs). Here, we discuss the different strategies of using long-acting compounds to treat or prevent HIV-1 infection by targeting reverse transcriptase, integrase, and capsid.
Keywords: human immunodeficiency virus (HIV), antiretroviral therapy (ART), pre-exposure prophylaxis (PrEP), long-acting formulations, acquired immunodeficiency syndrome (AIDS)
Introduction
In 2023, around 20% of people living with human immunodeficiency virus (HIV) were not treating their infection (1). If left untreated, HIV infection progresses to acquired immunodeficiency syndrome (AIDS), which causes over 650,000 deaths annually (1-4). While there has been great success in HIV-related healthcare, with 75% of HIV-positive individuals virally suppressed, multiple global regions are underserved in terms of healthcare access and availability (1,5,6). Certain regions have a large burden of infections, especially South and West Africa, which comprise 70% of global cases. Other regions have a large proportion of untreated individuals, including the Middle East and Eastern Europe with approximately 50% untreated (1,4,6-8) (Figure 1).
Figure 1.
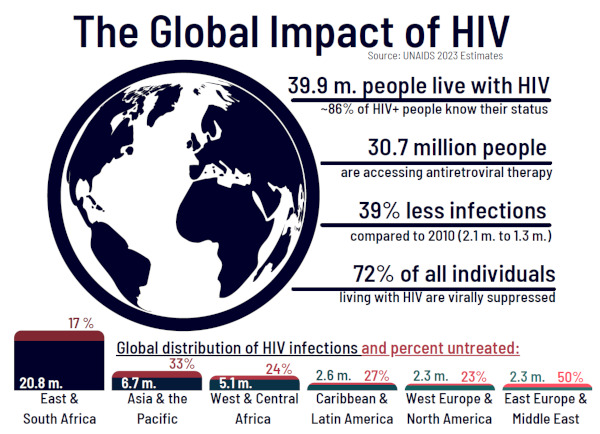
Global statistics of HIV-1 infections, treatment, and access in 2023. Regional-specific data below, showing the total estimated infections (in millions, blue) and percent of untreated individuals (red). Data from the UNAIDS 2023 report, "The urgency of now: AIDS at a crossroads" (1).
An emerging and exciting method of treating HIV infection is the use of a long-acting antiretroviral therapy (LAART) with infrequent doses compared to daily pills (4,9-13). LAART can decrease the burden of acquiring and taking a daily medication, and it has received great patient reception for those switching to and initiating LAART to treat or prevent HIV type 1 (HIV-1) infection (6,9,13-16). The main utility of LAART is in adding another tool to patient treatment. While it is not expected that all long-acting formulations are favored equally within high-incidence populations, there is a reported bias in LAART uptake toward highly informed individuals and those who practice sex with the use of preventative tools (6,14,16,17).
At present, all HIV-1 treatments are administered in combinations of at least two drugs in order to prevent the emergence of antiviral resistance. The coadministration of multiple drugs decreases the chance of antiviral resistance due to the volume of simultaneous mutations needed to escape the drugs' activities (18-21). It is possible that LAART helps decrease the occurrence of antiviral resistance by having unwavering continuity of treatment, but this also requires that patients have the opportunity to replenish the LAART at the recommended interval (10,12,15,22,23).
Opportunities introduced by LAART include less frequent dosing, avoidance of "pill fatigue", oral dosing being bypassed (with bioavailability near 100%), less adverse events, fewer drug-drug interactions, as well as protection of health privacy, avoidance of HIV-related stigma, and improved consistency of care. Challenges include large limitations due to injection volume restrictions, management of missed doses, pharmacokinetic considerations, possible development of drug resistance, management of drug-drug interactions, management of serious adverse events, and unknown dosing for children and pregnant women. Delivery routes of long-acting antivirals are oral, parenteral, and an implant/device with respective dosing frequencies of more than one week, more than one month, and more than six months (6,22,24).
Currently, the approved delivery methods for LAART are intramuscular (Cabenuva) or subcutaneous (Sunlenca) injections (Figure 2). This method of administration has been highly effective; however, there are complications and limitations to receiving injections due to patient anxiety, injection site reactions, the need for a professional healthcare worker for administration/ lack of clinical support, high cost, accessibility, and difficulty in discontinuation once the treatment is injected (10,14-17,22,25). Because of these reasons and more, other long-acting treatment options are being developed. These include subdermal implants, intravaginal rings (IVRs), microneedle array patches, long-acting hydrogels, and oral regimens that are dosed less often. Other delivery strategies have been deployed in specific areas, like the ring approved for use in Africa but not by the US FDA (25). Of note, adverse reactions can be challenging for LAART, especially for irreversible administration through subcutaneous injections; however, entry periods into the therapy with low and oral lead-in doses, when available, can be useful to test patient tolerance to the new medication before a long-term treatment is in place (13,23,25,26).
Figure 2.
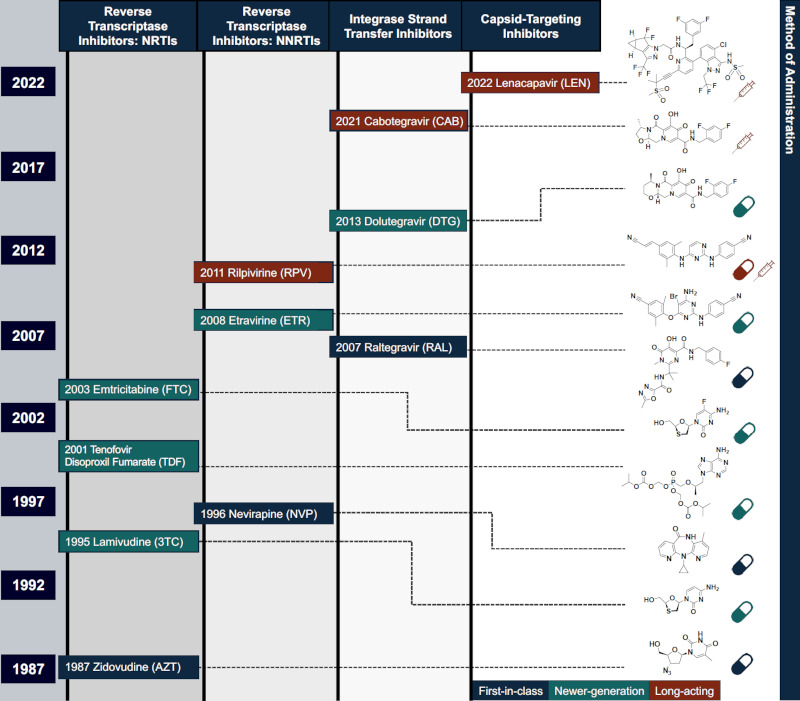
Timeline of some important discoveries, approvals, and advancements leading to long-acting HIV-1 therapeutics. Compounds are categorized as first-in-class (blue), newer-generation (green), and long-acting therapeutics (red) for treating and preventing infection. Chemical structure and delivery method(s) included for each antiviral on the right. Represented inhibitor classes are nucleoside reverse transcriptase inhibitor (NRTIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs), integrase strand transfer inhibitors (INSTIs), and capsid-targeting antivirals. Pill and syringe icons designed by Freepik.
Thus far, LAART has been successfully employed in clinical trials and as approved medications for HIV- 1 treatment and as a preventative measure for pre-exposure prophylaxis (PrEP) by targeting viral proteins required for an HIV-1 infection: reverse transcriptase, integrase, and capsid.
Reverse transcriptase
HIV-1 reverse transcriptase (RT) inhibitors have been used to treat infection since 1987, with the first FDA-approved HIV-1 antiviral zidovudine (AZT). Since the late 1980s, several other RT-targeting antivirals have been developed and approved (27) (Figure 2). Currently, there are five non-nucleoside reverse transcriptase inhibitors (NNRTIs) and nine nucleoside reverse transcriptase inhibitors (NRTIs) in the US market, although only five of these NRTIs are recommended (28). Another class of RT inhibitors being developed, but not yet FDA-approved, is called nucleoside reverse transcriptase translocation inhibitors (NRTTIs). These inhibitors include islatravir (ISL, EFdA, or MK-8591) and MK-8527.
All of these classes - NNRTIs, NRTIs, and NRTTIs - target HIV-1 RT. This viral enzyme is essential for the HIV-1 replication cycle as it is responsible for converting the positive-sense, single-stranded RNA genome into double-stranded DNA, which is the product that is integrated into the host genome (29). NNRTIs, NRTIs, and NRTTIs act through distinct mechanisms of action, though. NNRTIs are allosteric inhibitors that do not target the polymerase active site and instead bind to a region called the NNRTI binding pocket (NNIBP) located at the base of the thumb of RT. When these inhibitors bind, they cause conformational changes to the thumb and surrounding areas of RT, reducing the ability of RT to polymerize (30,31). NRTIs are nucleoside analogs and bind at the polymerase active site of RT (29). These inhibitors lack a 3'-OH, so once incorporated into the elongating DNA strand, another nucleotide cannot be added. Thus, NRTIs are termed immediate or obligate chain terminators (32,33). Differentially, NRTTIs retain the 3'-OH group, allowing another nucleoside to be added (34). Thus, NRTTIs can act through multiple mechanisms of action, effectively inhibiting the translocation step of reverse transcription through immediate chain termination, delayed chain termination, or increased misincorporation (35).
Non-nucleoside reverse transcriptase inhibitors
Most antiretroviral therapy (ART) treatments, including those based on RT inhibitors, are administered as once-daily oral medications. Rilpivirine (RPV), an NNRTI, was the first RT inhibitor to be approved in long-acting therapies such as Cabenuva. Initially approved in 2011, RPV is considered a next-generation NNRTI due to its capacity to overcome both resistance and safety concerns associated with earlier NNRTIs (36). With its extended half-life, RPV allows for monthly dosing using non-oral methods (37). Another promising NNRTI, doravirine (DOR), marketed as Pifeltro by Merck & Co., Inc., received FDA approval in 2018 (38). While currently available as a once-daily oral medication, efforts are underway to develop its formulation as a long-acting injectable (39). VM-1500A, another potent NNRTI, is currently in development by Viriom Inc. Elsulfavirine (ESV), the prodrug of VM-1500A, was approved in Russia in 2017 under the brand name Elpida (ESV 20 mg) as a once-daily oral medication. ESV has a long half-life (40) and is being formulated as a once-weekly oral medication and as depulfavirine in a once-monthly nanosphere drug formulation (41).
Nucleoside reverse transcriptase inhibitors
Tenofovir, an NRTI, has been used in first-line therapy worldwide for the last twenty years. It has been formulated as tenofovir alafenamide fumarate (TAF), which increases its potency and safety profile. This antiviral is currently being used as a once-daily oral medication but is in preclinical formulation as a long-acting implant for treatment up to six months (12). There are currently several implant variations being tested, which include a TAF-filled subcutaneous silicone implant with a polyvinyl alcohol (PVA) coating and orthogonal delivery channels (42), a reservoir-style biodegradable implant filled with a TAF/oil formulation (43), an implant loaded with TAF pellets sealed in a polyether urethane tube (44), and poly(ε- caprolactone) (PCL) reservoir-style implant with a TAF core formulation (45-47). Long-acting microspheres are also in development (48).
Nucleoside reverse transcriptase translocation inhibitors
ISL is a highly potent NRTTI licensed by Merck & Co. This antiviral has the potential to be long-acting due to its long half-life and favorable selectivity index. It is currently being tested in several clinical trials as a once-daily, weekly, and monthly oral medication partnered with DOR and lenacapavir (LEN) (49-70). Preclinical studies of ISL delivered as an injection, implant, and micro patch for long-acting therapies are also underway; ISL has paved the way for other NRTTI development, including MK-8527, a 7-deazadeoxyadenosine analog that is currently in phase I/II clinical trials with the potential to be used as a once-monthly oral treatment for HIV-1 (71-74).
Integrase
HIV-1 integrase protein (IN) is a target of ART formulations that contain integrase strand transfer inhibitors (INSTIs). Currently, there are five FDA-approved INSTIs used in HIV-1 treatments - first generation: raltegravir (RAL), elvitegravir (EVG); second generation: dolutegravir (DTG), bictegravir (BIC), and cabotegravir (CAB) (75-77). HIV-1 requires integration of virus-encoding nucleic acid into the host's genome as part of its replication cycle, and IN is responsible for integrating the double-stranded viral DNA (vDNA) resulting from reverse transcription of the viral positive-sense, single-stranded RNA genome (78,79). Hence, the integration process has emerged as another ART target, with multiple classes of integration-targeting compounds arising as potential treatments (reviewed in (80)). IN was first discovered within an avian retrovirus as a nucleic acid-associating protein that later was found to possess both 3'-processing and strand transfer enzymatic activities (81-86). During integration, IN oligomerizes and complexes with vDNA to form the intasome, which contains a conserved integration core (CIC) (87). Within the CIC, the IN multimer exposes 3'- OH groups on the vDNA ends, which can then catalyze a strand transfer reaction into the target host DNA (88,89). To prevent this from happening, INSTIs contain key structural moieties that block host DNA capture immediately preceding this step. These antiretrovirals have a β-diketo acid-containing a dicyclic or tricyclic pharmacophore to chelate Mg2+ ions required to catalyze reactions in the IN active site (87). Moreover, INSTIs contain a halogenated benzyl group that performs π-π stacking with the terminal vDNA base, thus preventing its interaction with host DNA (87).
RAL became the first FDA-approved INSTI in 2007, followed by EVG in 2012. In building upon these first-generation INSTIs, second generation INSTIs have seen widespread adoption due to their increased tolerability, high barrier to resistance, and low cross-reactivity (75). INSTI-containing ARTs typically administer it with two NRTIs, but these combinations can alternatively apply one NRTI and one NNRTI instead. Of these INSTIs, CAB is the only approved long-acting (LA) agent, either in combination with RPV LA as an ART (90-92) or on its own for PrEP (93,94). CAB is a structural analog of DTG and similarly has a high genetic barrier to resistance, yet it possesses a much longer half-life than DTG does (95,96). It also has potent activity at low concentrations, minimal adverse side effects, and little cross-reactivity (97-99).
CAB LA, owned by ViiV Healthcare, was approved in early 2021 in combination with Janssen's RPV LA for HIV-1 treatment under the trade name Cabenuva (100), becoming the first injectable LAART. This came after the success of the 2020 phase III Antiretroviral Therapy as Long-Acting Suppression (ATLAS) and First Long- Acting Injectable Regimen (FLAIR) studies; these trials confirmed non-inferiority of CAB LA/RPV LA against standard ART in treating and suppressing HIV- 1 infection (90,91). Following this, the 2020 phase IIIb ATLAS dosed every two months (ATLAS-2M) trial established the non-inferiority of bimonthly CAB LA/ RPV LA administration when compared to monthly treatment, leading to the approval of a bimonthly regimen as well (92,101). In late 2021, CAB LA itself was FDA-approved as the first LA injectable PrEP and was released under the trade name Apretude (102). This announcement resulted from the phase IIb/III HPTN 083 and phase III HPTN 084 studies showing non-inferiority of CAB LA against conventional PrEP treatment (93,103).
INSTIs can be given either as oral drugs or as intramuscular injections in the case of CAB LA. INSTI-containing ARTs are orally administered daily, while CAB LA/RPV LA involves a ventrogluteal injection once either monthly or bimonthly (104). However, before this treatment starts, individuals may be advised to undergo an oral lead-in period (OLI) to assess their tolerance to CAB and RPV. During OLI, individual tablets of CAB (brand name Vocabria) and RPV (brand name Edurant) are taken daily for at least four weeks. For patients enacting this optional OLI, an immediate switch to CAB and RPV initiation injections (trade names Vocabria and Rekambys, respectively) takes place on the final OLI day; otherwise, the treatment initiation period can directly begin at this step.
Initiation injection schedules for HIV-1 treatment differ between patients undergoing a monthly versus a bimonthly CAB LA + RPV LA dosing timeline. For monthly treatment, initiation injections of CAB LA and RPV LA are each given once in the month prior to the start of their monthly injection schedule at doses higher than during treatment (104). For those on a bimonthly schedule, the same monthly initiation injections are administered, though instead for two months before their bimonthly schedule begins (104). Upon successful completion of this period, the patient will then begin their prescribed injection schedule (105).
Capsid
The HIV-1 virion contains the HIV-1 capsid, the most recent molecular target of an antiviral compound, LEN (previously called GS-6207), approved in 2022 for highly treatment-experienced (HTE) patients (Figure 2). LEN targets the HIV-1 capsid protein (CA) and inhibits viral replication by perturbing the capsid core stability, assembly, and maturation (19,106-112); it was developed by Gilead Sciences to disrupt the intricately-tuned kinetics of capsid assembly by interacting with CA at the phenylalanine-glycine (FG)-binding site (3,106,113-119). The mature capsid is essential for the replication of HIV-1 in numerous ways, for example acting as a reaction vessel for RT activity and as a shuttle to carry the viral genome through the cytoplasm and to or through the nuclear pore complex (114,116,120-127). The disassembly or "uncoating" of the capsid core needs to be perfectly timed, as early or late uncoating decreases viral fitness (106,123,124,128-134). Interfering with post-entry and pre-integration events prevents the establishment of a viral infection, making the mature capsid a great target for preventing or treating HIV-1 (107,135). In fact, an interim update of the PURPOSE1 clinical trial (NCT04994509) was recently released, stating that twice-yearly LEN was shown to prevent HIV-1 infection with 100% efficacy as a PrEP regimen in cisgender women in South Africa and Uganda, with 0 reported infections among 2,134 participants who received LEN (136,137). The press release also stated that PURPOSE1 is the first phase III HIV-1 prevention trial to report zero infections (136).
Interestingly, the first compound reported to target the same site as LEN, the FG-binding site, was PF-3450074 (PF74). Similar to LEN, PF74 contains an amide group and FG scaffold to mimic the FG-containing host factors that bind to the same pocket in CA (i.e. Nup153 and CPSF6) (3,114-119,131,132,134). Due to these chemical bonds, PF74 has an exceptionally short metabolic half-life and is quickly degraded by cellular enzymes, preventing its further drug development (3,132,138,139), although more potent and more stable analogs of PF74 have been published (139,140). Intriguingly, this amide bond and general FG structure is still within the LEN molecule, and LEN maintains its exceptionally long-lasting half-life (3,106,113,141).
LEN is only approved for use in HTE patients as of June 2024, and not yet as a coformulation (109). Specifically, the patients approved for LEN treatment are already taking an ART regimen that is not successfully repressing viral replication. In fact, the CAPELLA trial (NCT04150068) with LEN found that most HTE participants that had established infections with drug-resistant viruses were able to initially suppress viremia (21 of 24), although multiple LEN-associated resistance mutations have been reported shortly after use in CA, including M66I and N74D (110,142-144). Multiple clinical trials are ongoing to investigate LEN as a form of treatment or prevention against HIV-1 with either oral formulations or subcutaneous injections in combination with BIC, ISL, emtricitabine/tenofovir disoproxil fumarate (F/TDF), F/TAF, and broadly neutralizing antibodies (bNAbs) (19,108,111,145). LEN is typically administered in 927 mg subcutaneous injections in 26- week increments and/or as 300-600 mg tablets as OLI (111,112,145,146). Overall, LEN as a first-in-class inhibitor is an exceptionally potent and long-lasting antiretroviral, though its apparent tendency to rapidly select resistance mutations in CA may be a challenge for its future applications.
Conclusions
From many oral doses administered daily and limited drug options to non-daily injections and over 30 FDA-approved antivirals with various delivery strategies, the treatment of HIV-1 infection has evolved and is becoming increasingly accessible to more patients. Many challenges remain to overcome barriers in HIV- 1 treatment and prevention. With more and varied treatment options, and numerous clinical and pre-clinical trials underway to use LAARTs, clinicians will be more able to take patient preferences into account and build applicable strategies together to effectively combat HIV- 1. Some of the developed LAART compounds expand the existing treatments in antiviral classes like NNRTIs and INSTIs; other novel classes of inhibitors have been reported with long-acting applications, like the NRTTIs and capsid-targeting compounds. As with all antiviral compounds, LAARTs create a selective pressure on the virus and thus, there are known resistance mutations associated with the independent use of a single antiretroviral. Therefore, more and alternative strategies are required to expand the LAART field and optimize combinations of antiviral drugs with applications that meet patient needs.
Funding
This research was supported in part by the National Institutes of Health (U54 AI170855, R37 AI076119, R01 AI120860, and P30 AI050409 to SGS; F31 AI174951 to W.M.M.; F31 AI172618 to A.A.S.; S.M.R., W.M.M., and A.A.S. were supported in part by T32 GM135060). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. S.G.S. acknowledges funding from the Nahmias-Schinazi Distinguished Chair in Research.
Conflict of Interest
The authors have no conflicts of interest to disclose.
References
- 1. UNAIDS. Global HIV & AIDS statistics - Fact Sheet Geneva, Switzerland 2024. https://www.unaids.org/en/resources/fact-sheet (accessed June 28, 2024).
- 2. Adachi A, Gendelman HE, Koenig S, Folks T, Willey R, Rabson A, Martin MA. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986; 59:284-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McFadden WM, Snyder AA, Kirby KA, Tedbury PR, Raj M, Wang Z, Sarafianos SG. Rotten to the core: Antivirals targeting the HIV-1 capsid core. Retrovirology. 2021; 18:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nachega JB, Musoke P, Kilmarx PH, Gandhi M, Grinsztejn B, Pozniak A, Rawat A, Wilson L, Mills EJ, Altice FL, Mellors JW, Quinn TC. Global HIV control: Is the glass half empty or half full? The Lancet HIV. 2023; 10:e617-e622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Luis H, Fridayantara WD, Mahariski P, Wignall FS, Irwanto I, Gedela K. Evolving ART crisis for people living with HIV in Indonesia. Lancet HIV. 2020; 7:e384-e385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kadia BM, Dimala CA, Fongwen NT, Smith AD. Barriers to and enablers of uptake of antiretroviral therapy in integrated HIV and tuberculosis treatment programmes in sub-Saharan Africa: A systematic review and meta-analysis. AIDS Res Ther. 2021; 18:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gökengin D, Doroudi F, Tohme J, Collins B, Madani N. HIV/AIDS: trends in the Middle East and North Africa region. Int J Infect Dis. 2016; 44:66-73. [DOI] [PubMed] [Google Scholar]
- 8. Kteily-Hawa R, Hawa AC, Gogolishvili D, Al Akel M, Andruszkiewicz N, Vijayanathan H, Loutfy M. Understanding the epidemiological HIV risk factors and underlying risk context for youth residing in or originating from the Middle East and North Africa (MENA) region: A scoping review of the literature. PLos One. 2022; 17:e0260935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chandiwana NC, Serenata CM, Owen A, Rannard S, Pérez Casas C, Scott C, Hill A, Clayden P, Flexner C. Impact of long-acting therapies on the global HIV epidemic. AIDS. 2021; 35(Supplement 2):S137-S143. [DOI] [PubMed] [Google Scholar]
- 10. Rakhmanina N. Are we ready for long-acting HIV treatment for adolescents? Lancet HIV. 2024; 11:e200-e201. [DOI] [PubMed] [Google Scholar]
- 11. Mantsios A, Murray M, Karver TS, et al. "I feel empowered": Women's perspectives on and experiences with long-acting injectable antiretroviral therapy in the USA and Spain. Cult Health Sex. 2021; 23:1066-1078. [DOI] [PubMed] [Google Scholar]
- 12. Thoueille P, Choong E, Cavassini M, Buclin T, Decosterd LA. Long-acting antiretrovirals: A new era for the management and prevention of HIV infection. J Antimicrob Chemother. 2022; 77:290-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rivera CG, Zeuli JD, Smith BL, Johnson TM, Bhatia R, Otto AO, Temesgen Z. HIV pre-exposure prophylaxis: New and upcoming drugs to address the HIV epidemic. Drugs. 2023; 83:1677-1698. [DOI] [PubMed] [Google Scholar]
- 14. Ogunbajo A, Tsai AC, Kanki PJ, Mayer KH. Acceptability of and preferences for long-acting injectable HIV PrEP and other PrEP modalities among sexual minority men in Nigeria, Africa. AIDS Behav. 2022; 26:2363-2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sharfstein JM, Killelea A, Dangerfield D. Long-acting cabotegravir for HIV prevention: issues of access, cost, and equity. JAMA. 2022; 327:921-922. [DOI] [PubMed] [Google Scholar]
- 16. Rosen JG, Park JN, Schneider KE, White RH, Beckham SW, Glick JL, Footer KHA, Sherman SG. Mapping interests in event-driven and long-acting pre-exposure prophylaxis formulations onto the HIV risk environment of street-based female sex workers: A latent class analysis. AIDS Behav. 2022; 26:1992-2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Farooq HZ, Apea V, Kasadha B, Ullah S, Hilton-Smith G, Haley A, Scherzer J, Hand J, Paparini S, Phillips R, Orkin CM. Study protocol: The ILANA study- exploring optimal implementation strategies for long-acting antiretroviral therapy to ensure equity in clinical care and policy for women, racially minoritised people and older people living with HIV in the UK-a qualitative multiphase longitudinal study design. BMJ Open. 2023; 13:e070666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hudelson C, Cluver L. Factors associated with adherence to antiretroviral therapy among adolescents living with HIV/AIDS in low- and middle-income countries: A systematic review. AIDS Care. 2015; 27:805-816. [DOI] [PubMed] [Google Scholar]
- 19. Sever B, Otsuka M, Fujita M, Ciftci H. A review of FDA-approved anti-HIV-1 drugs, anti-gag compounds, and potential strategies for HIV-1 eradication. Int J Mol Sci. 2024; 25:3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nachega JB, Scarsi KK, Gandhi M, Scott RK, Mofenson LM, Archary M, Nachman S, Decloedt E, Geng EH, Wilson L, Rawat A, Mellors JW. Long-acting antiretrovirals and HIV treatment adherence. Lancet HIV. 2023; 10:e332-e342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pham HT, Yoo S, Mesplède T. Combination therapies currently under investigation in phase I and phase II clinical trials for HIV-1. Expert Opin Investig Drugs. 2020; 29:273-283. [DOI] [PubMed] [Google Scholar]
- 22. Xiridou M, Hoornenborg E. Long-acting PrEP: new opportunities with some drawbacks. Lancet HIV. 2023; 10:e213-e215. [DOI] [PubMed] [Google Scholar]
- 23. Tarfa A, Sayles H, Bares SH, Havens JP, Fadul N. Acceptability, feasibility, and appropriateness of implementation of long-acting injectable antiretrovirals: A national survey of Ryan White clinics in the United States. Open Forum Infect Dis. 2023; 10:ofad341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Owen A, Rannard S. Strengths, weaknesses, opportunities and challenges for long acting injectable therapies: Insights for applications in HIV therapy. Adv Drug Deliv Rev. 2016; 103:144-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van der Straten A, Agot K, Ahmed K, Weinrib R, Browne EN, Manenzhe K, Owino F, Schwartz J, Minnis A; TRIO Study Team. The Tablets, Ring, Injections as Options (TRIO) study: what young African women chose and used for future HIV and pregnancy prevention. J Int AIDS Soc. 2018; 21:e25094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Margolis DA, Gonzalez-Garcia J, Stellbrink HJ, et al. Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, non-inferiority trial. Lancet. 2017; 390:1499-1510. [DOI] [PubMed] [Google Scholar]
- 27. Tseng A, Seet J, Phillips EJ. The evolution of three decades of antiretroviral therapy: challenges, triumphs and the promise of the future. Br J Clin Pharmacol. 2015; 79:182-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Clinical Info HIV.gov. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-arv/what-start-non-nucleoside-reverse#:~:text=Summary,other%20antiretroviral%20(ARV)%20drugs. (accessed June 28, 2024).
- 29. Sarafianos SG, Marchand B, Das K, Himmel DM, Parniak MA, Hughes SH, Arnold E. Structure and function of HIV-1 reverse transcriptase: Molecular mechanisms of polymerization and inhibition. J Mol Biol. 2009; 385:693-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hsiou Y, Ding J, Das K, Clark AD, Jr., Hughes SH, Arnold E. Structure of unliganded HIV-1 reverse transcriptase at 2.7 A resolution: Implications of conformational changes for polymerization and inhibition mechanisms. Structure. 1996; 4:853-860. [DOI] [PubMed] [Google Scholar]
- 31. Singh AK, De Wijngaert B, Bijnens M, Uyttersprot K, Nguyen H, Martinez SE, Schols D, Herdewijn P, Pannecouque C, Arnold E, Das K. Cryo-EM structures of wild-type and E138K/M184I mutant HIV-1 RT/DNA complexed with inhibitors doravirine and rilpivirine. Proc Natl Acad Sci U S A. 2022; 119:e2203660119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cilento ME, Kirby KA, Sarafianos SG. Avoiding drug resistance in HIV reverse transcriptase. Chemical Rev. 2021; 121:3271-3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Singh K, Marchand B, Kirby KA, Michailidis E, Sarafianos SG. Structural aspects of drug resistance and inhibition of HIV-1 reverse transcriptase. Viruses. 2010; 2:606-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Deval J. Antimicrobial strategies: Inhibition of viral polymerases by 3'-hydroxyl nucleosides. Drugs. 2009; 69:151-166. [DOI] [PubMed] [Google Scholar]
- 35. Michailidis E, Huber AD, Ryan EM, Ong YT, Leslie MD, Matzek KB, Singh K, Marchand B, Hagedorn AN, Kirby KA, Rohan LC, Kodama EN, Mitsuya H, Parniak MA, Sarafianos SG. 4'-Ethynyl-2-fluoro-2'-deoxyadenosine (EFdA) inhibits HIV-1 reverse transcriptase with multiple mechanisms. J Biol Chem. 2014; 289:24533-24548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sharma M, Saravolatz LD. Rilpivirine: A new non-nucleoside reverse transcriptase inhibitor. J Antimicrob Chemother. 2012; 68:250-256. [DOI] [PubMed] [Google Scholar]
- 37. Ford N, Lee J, Andrieux-Meyer I, Calmy A. Safety, efficacy, and pharmacokinetics of rilpivirine: Systematic review with an emphasis on resource-limited settings. HIV AIDS (Auckl). 2011; 3:35-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Deeks ED. Doravirine: first global approval. Drugs. 2018; 78:1643-1650. [DOI] [PubMed] [Google Scholar]
- 39. Yee KL, Mittal S, Fan L, Triantafyllou I, Dockendorf MF, Fackler PH, Stoch SA, Khalilieh SG, Iwamoto M. Pharmacokinetics, safety and tolerability of long-acting parenteral intramuscular injection formulations of doravirine. J Clin Pharm Ther. 2020; 45:1098-1105. [DOI] [PubMed] [Google Scholar]
- 40. Levin J. Pharmacokinetics of VM-1500 20 mg and 40 mg in healthy and HIV-infected patients. 20th International AIDS Conference. https://www.natap.org/2014/IAC/ IAC_108 htm (accessed June 28, 2024) .
- 41. Viriom. Pipeline. https://www.viriom.com/pipeline (accessed June 28, 2024).
- 42. Gunawardana M, Remedios-Chan M, Miller CS, Fanter R, Yang F, Marzinke MA, Hendrix CW, Beliveau M, Moss JA, Smith TJ, Baum MM. Pharmacokinetics of long-acting tenofovir alafenamide (GS-7340) subdermal implant for HIV prophylaxis. Antimicrob Agents Chemother. 2015; 59:3913-3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Johnson LM, Krovi SA, Li L, Girouard N, Demkovich ZR, Myers D, Creelman B, van der Straten A. Characterization of a reservoir-style implant for sustained release of tenofovir alafenamide (TAF) for HIV pre-exposure prophylaxis (PrEP). Pharmaceutics. 2019; 11:315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Simpson SM, Widanapathirana L, Su JT, Sung S, Watrous D, Qiu J, Pearson E, Evanoff A, Karunakaran D, Chacon JE, Kiser PF. Design of a drug-eluting subcutaneous implant of the antiretroviral tenofovir alafenamide fumarate. Pharm Res. 2020; 37:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li L, Johnson LM, Krovi SA, Demkovich ZR, van der Straten A. Performance and stability of tenofovir alafenamide formulations within subcutaneous biodegradable implants for HIV pre-exposure prophylaxis (PrEP). Pharmaceutics. 2020; 12:1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li L, Gatto GJ, Brand RM, Krovi SA, Cottrell ML, Norton C, van der Straten A, Johnson LM. Long-acting biodegradable implant for sustained delivery of antiretrovirals (ARVs) and hormones. J Control Release. 2021; 340:188-199. [DOI] [PubMed] [Google Scholar]
- 47. Gatto G, Krovi A, Li L, et al. Comparative pharmacokinetics and local tolerance of tenofovir alafenamide (TAF) from subcutaneous implant in rabbits, dogs, and macaques. Front Pharmacol. 2022; 13:923954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pawar MA, Abadi LF, Rojekar SV, Yawalkar AN, Kulkarni SS, Vavia PR. Tenofovir alafenamide fumarate loaded long-acting microsphere for HIV pre-exposure prophylaxis. J Drug Deliv Sci Technol. 2023; 87:104762. [Google Scholar]
- 49. ClinicalTrials.gov. DOR/ISL in HIV-1 antiretroviral treatment-naïve participants (MK-8591A-053). https://classic.clinicaltrials.gov/show/NCT05705349 (accessed June 28, 2024).
- 50. ClinicalTrials.gov. A switch to doravirine/islatravir (DOR/ ISL) in participants with human immunodeficiency virus type 1 (HIV-1) who are virologically suppressed on bictegravir/emtricitabine/tenofovir alafenamide (BIC/ FTC/TAF) (MK-8591A-052). https://classic.clinicaltrials.gov/show/NCT05630755 (accessed June 28, 2024).
- 51. ClinicalTrials.gov. Oral ISL QM as PrEP in cisgender women at high risk for HIV-1 infection (MK-8591-022). https://classic.clinicaltrials.gov/show/NCT04644029 (accessed June 28, 2024).
- 52. ClinicalTrials.gov. Radiopaque matrix MK-8591 implant in participants at low-risk for human immunodeficiency virus type 1 (HIV-1) infection (MK-8591-043). https://classic.clinicaltrials.gov/show/NCT05115838 (accessed June 28, 2024).
- 53. ClinicalTrials.gov. Study evaluating the safety and efficacy of islatravir in combination with lenacapavir in virologically suppressed people with HIV. https://classic.clinicaltrials.gov/show/NCT05052996 (accessed June 28, 2024).
- 54. ClinicalTrials.gov. Islatravir (MK-8591) with doravirine and lamivudine in participants infected with human immunodeficiency virus type 1 (MK-8591-011). https://classic.clinicaltrials.gov/show/NCT03272347 (accessed June 28, 2024).
- 55. ClinicalTrials.gov. Oral islatravir (MK-8591) once-monthly as preexposure prophylaxis (PrEP) in men and transgender women who are at high risk for HIV-1 infection (MK-8591-024). https://classic.clinicaltrials.gov/show/NCT04652700 (accessed June 28, 2024).
- 56. ClinicalTrials.gov. A study of islatravir (MK-8591) in anti-retroviral therapy-naive, human immunodeficiency virus-1 infected participants (MK-8591-003). https://classic.clinicaltrials.gov/show/NCT02217904 (accessed June 28, 2024).
- 57. ClinicalTrials.gov. Dose ranging, switch study of islatravir (ISL) and ulonivirine (MK-8507) once-weekly in virologically-suppressed adults with human immunodeficiency virus type 1 (HIV-1) MK-8591-013. https://classic.clinicaltrials.gov/show/NCT04564547 (accessed June 28, 2024).
- 58. ClinicalTrials.gov. Study of doravirine/islatravir (DOR/ ISL 100 mg/0. 75 mg) to evaluate the antiretroviral activity, safety, and tolerability in treatment-naïve participants with human immunodeficiency virus type 1 (HIV-1) infection (MK-8591A-020). https://classic.clinicaltrials.gov/show/NCT04233879 (accessed June 28, 2024).
- 59. ClinicalTrials.gov. Switch to doravirine/islatravir (DOR/ISL) in human immunodeficiency virus 1 (HIV- 1) participants treated with bictegravir/emtricitabine/ tenofovir alafenamide (BIC/FTC/TAF) (MK-8591A-018). https://classic.clinicaltrials.gov/show/NCT04223791 (accessed June 28, 2024).
- 60. ClinicalTrials.gov. A switch to doravirine/islatravir (DOR/ ISL) in participants with human immunodeficiency virus type 1 (HIV-1) who are virologically suppressed on antiretroviral therapy (ART) (MK-8591A-051). https://classic.clinicaltrials.gov/show/NCT05631093 (accessed June 28, 2024).
- 61. ClinicalTrials.gov. A study of doravirine/islatravir (DOR/ISL, MK-8591A) for the treatment of human immunodeficiency virus 1 (HIV-1) infection in participants who previously received DOR/ISL (MK-8591A-054). https://classic.clinicaltrials.gov/show/NCT05766501 (accessed June 28, 2024).
- 62. ClinicalTrials.gov. Safety and efficacy of a switch to doravirine/islatravir in participants with HIV-1 (MK- 8591A-017). https://classic.clinicaltrials.gov/show/NCT04223778 (accessed June 28, 2024).
- 63. ClinicalTrials.gov. Doravirine/islatravir (DOR/ISL) in pediatric participants with human immunodeficiency virus type 1 (HIV-1) who are < 18 years of age and weigh ≥ 35 kg (MK-8591A-028). https://classic.clinicaltrials.gov/show/NCT04295772 (accessed June 28, 2024).
- 64. ClinicalTrials.gov. Doravirine/islatravir (DOR/ISL) in heavily treatment-experienced (HTE) participants for human immunodeficiency virus type 1 (HIV-1) infection (MK-8591A-019). https://classic.clinicaltrials.gov/show/NCT04233216 (accessed June 28, 2024).
- 65. ClinicalTrials.gov. Islatravir and methadone pharmacokinetics (MK-8591-029). https://classic.clinicaltrials.gov/show/NCT04568603 (accessed June 28, 2024).
- 66. ClinicalTrials.gov. Open-label, follow-up of doravirine/ islatravir for participants with human immunodeficiency virus-1 (HIV-1) infection (MK-8591A-033). https://classic.clinicaltrials.gov/show/NCT04776252 (accessed June 28, 2024).
- 67. ClinicalTrials.gov. Safety and pharmacokinetics of oral islatravir (MK-8591) once monthly in participants at low risk of human immunodeficiency virus 1 (HIV-1) infection (MK-8591-016). https://classic.clinicaltrials.gov/show/NCT04003103 (accessed June 28, 2024).
- 68. ClinicalTrials.gov. Single-dose islatravir in moderate hepatic impairment (MK-8591-030). https://classic.clinicaltrials.gov/show/NCT04515641 (accessed June 28, 2024).
- 69. ClinicalTrials.gov. Pharmacokinetics of islatravir in participants with severe renal impairment (MK-8591- 026). https://classic.clinicaltrials.gov/show/NCT04303156 (accessed June 28, 2024).
- 70. ClinicalTrials.gov. A study of islatravir (MK-8591) in trans and gender diverse participants (MK-8591-035). https://classic.clinicaltrials.gov/show/NCT05130086 (accessed June 28, 2024).
- 71. MERCK. Merck to initiate new phase 3 clinical program with lower dose of daily oral islatravir in combination with doravirine for treatment of people with HIV-1 infection. https://www.merck.com/news/merck-to-initiate-new-phase-3-clinical-program-with-lower-dose-of-daily-oral-islatravir-in-combination-with-doravirine-for-treatment-of-people-with-hiv-1-infection/ (accessed June 28, 2024).
- 72. ClinicalTrials.gov. A study of MK-8527 in human immunodeficiency type 1 virus (HIV-1) infected participants (MK-8527-002). https://classic.clinicaltrials.gov/show/NCT03615183 (accessed June 28, 2024).
- 73. ClinicalTrials.gov. MK-8527 single-dose trial in HIV- 1 infected participants (MK-8527-004). https://classic.clinicaltrials.gov/show/NCT05494736 (accessed June 28, 2024).
- 74. ClinicalTrials.gov. Safety and pharmacokinetic study of oral MK-8527 QM in participants at low-risk for HIV- 1 infection (MK-8527-007). https://classic.clinicaltrials.gov/show/NCT06045507 (accessed June 28, 2024).
- 75. Zhao AV, Crutchley RD, Guduru RC, Ton K, Lam T, Min AC. A clinical review of HIV integrase strand transfer inhibitors (INSTIs) for the prevention and treatment of HIV-1 infection. Retrovirology. 2022; 19:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Prather C, Jeon C. Cabotegravir: The first long-acting injectable for HIV pre-exposure prophylaxis. Am J Health Syst Pharm. 2022; 79:1898-1905. [DOI] [PubMed] [Google Scholar]
- 77. Taki E, Soleimani F, Asadi A, Ghahramanpour H, Namvar A, Heidary M. Cabotegravir/Rilpivirine: The last FDA-approved drug to treat HIV. Expert Rev Anti Infect Ther. 2022; 20:1135-1147. [DOI] [PubMed] [Google Scholar]
- 78. Donehower LA, Varmus HE. A mutant murine leukemia virus with a single missense codon in pol is defective in a function affecting integration. Proc Natl Acad Sci U S A. 1984; 81:6461-6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Goodarzi G, Im GJ, Brackmann K, Grandgenett D. Concerted integration of retrovirus-like DNA by human immunodeficiency virus type 1 integrase. J Virol. 1995; 69:6090-6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Engelman AN, Singh PK. Cellular and molecular mechanisms of HIV-1 integration targeting. Cell Mol Life Sci. 2018; 75:2491-2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Grandgenett DP, Vora AC, Schiff RD. A 32,000-Dalton nucleic acid-binding protein from avian retravirus cores possesses DNA endonuclease activity. Virology. 1978; 89:119-132. [DOI] [PubMed] [Google Scholar]
- 82. Grandgenett DP, Vora AC, Swanstrom R, Olsen JC. Nuclease mechanism of the avian retrovirus pp32 endonuclease. J Virol. 1986; 58:970-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Brown PO, Bowerman B, Varmus HE, Bishop JM. Retroviral integration: Structure of the initial covalent product and its precursor, and a role for the viral IN protein. Proc Natl Acad Sci U S A. 1989; 86:2525-2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Sherman PA, Fyfe JA. Human immunodeficiency virus integration protein expressed in Escherichia coli possesses selective DNA cleaving activity. Proc Natl Acad Sci U S A. 1990; 87:5119-5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Katz RA, Merkel G, Kulkosky J, Leis J, Skalka AM. The avian retroviral IN protein is both necessary and sufficient for integrative recombination in vitro. Cell. 1990; 63:87-95. [DOI] [PubMed] [Google Scholar]
- 86. Engelman A, Bushman FD, Craigie R. Identification of discrete functional domains of HIV-1 integrase and their organization within an active multimeric complex. EMBO J. 1993; 12:3269-3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Jóźwik IK, Passos DO, Lyumkis D. Structural biology of HIV integrase strand transfer inhibitors. Trends Pharmacol Sci. 2020; 41:611-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Craigie R, Fujiwara T, Bushman F. The IN protein of Moloney murine leukemia virus processes the viral DNA ends and accomplishes their integration in vitro. Cell. 1990; 62:829-837. [DOI] [PubMed] [Google Scholar]
- 89. Engelman A, Mizuuchi K, Craigie R. HIV-1 DNA integration: Mechanism of viral DNA cleavage and DNA strand transfer. Cell. 1991; 67:1211-1221. [DOI] [PubMed] [Google Scholar]
- 90. Orkin C, Arasteh K, Górgolas Hernández-Mora M, et al. Long-acting cabotegravir and rilpivirine after oral induction for HIV-1 infection. N Engl J Med. 2020; 382:1124-1135. [DOI] [PubMed] [Google Scholar]
- 91. Swindells S, Andrade-Villanueva J-F, Richmond GJ, et al. Long-acting cabotegravir and rilpivirine for maintenance of HIV-1 suppression. New Engl J Med. 2020; 382:1112-1123. [DOI] [PubMed] [Google Scholar]
- 92. Overton ET, Richmond G, Rizzardini G, et al. Long-acting cabotegravir and rilpivirine dosed every 2 months in adults with HIV-1 infection (ATLAS-2M), 48-week results: a randomised, multicentre, open-label, phase 3b, non-inferiority study. Lancet. 2021; 396:1994-2005. [DOI] [PubMed] [Google Scholar]
- 93. Landovitz RJ, Donnell D, Clement ME, et al. Cabotegravir for HIV prevention in cisgender men and transgender women. New Engl J Med. 2021; 385:595-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Landovitz RJ, Hanscom BS, Clement ME, et al. Efficacy and safety of long-acting cabotegravir compared with daily oral tenofovir disoproxil fumarate plus emtricitabine to prevent HIV infection in cisgender men and transgender women who have sex with men 1 year after study unblinding: A secondary analysis of the phase 2b and 3 HPTN 083 randomised controlled trial. Lancet HIV. 2023; 10:e767-e778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Trezza C, Ford SL, Spreen W, Pan R, Piscitelli S. Formulation and pharmacology of long-acting cabotegravir. Curr Opin HIV AIDS. 2015; 10:239-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Spreen W, Min S, Ford SL, Chen S, Lou Y, Bomar M, St Clair M, Piscitelli S, Fujiwara T. Pharmacokinetics, safety, and monotherapy antiviral activity of GSK1265744, an HIV integrase strand transfer inhibitor. HIV Clin Trials. 2013; 14:192-203. [DOI] [PubMed] [Google Scholar]
- 97. Spreen W, Ford SL, Chen S, Wilfret D, Margolis D, Gould E, Piscitelli S. GSK1265744 pharmacokinetics in plasma and tissue after single-dose long-acting injectable administration in healthy subjects. J Acquir Immune Defic Syndr. 2014; 67:481-486. [DOI] [PubMed] [Google Scholar]
- 98. Spreen WR, Margolis DA, Pottage JC. Long-acting injectable antiretrovirals for HIV treatment and prevention. Curr Opin HIV AIDS. 2013; 8:565-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ford SL, Gould E, Chen S, Margolis D, Spreen W, Crauwels H, Piscitelli S. Lack of pharmacokinetic interaction between rilpivirine and integrase inhibitors dolutegravir and GSK1265744. Antimicrob Agents Chemother. 2013; 57:5472-5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. ViiV Healthcare. ViiV Healthcare announces FDA approval of Cabenuva (Cabotegravir, Rilpivirine), the first and only complete long-acting regimen for HIV treatment. https://viivhealthcare.com/hiv-news-and-media/news/press-releases/2021/january/viiv-healthcare-announces-fda-approval-of-cabenuva/ (accessed June 28, 2024).
- 101. ViiV Healthcare. ViiV Healthcare announces US FDA approval of Cabenuva (Cabotegravir, Rilpivirine) for use every two months, expanding the label of the first and only complete long-acting HIV treatment. https://viivhealthcare.com/hiv-news-and-media/news/press-releases/2022/january/viiv-healthcare-announces-fda-approval-of-cabenuva-for-use-every-two-months/ (accessed June 28, 2024).
- 102. ViiV Healthcare. ViiV Healthcare announces FDA approval of Apretude (Cabotegravir extended-release injectable suspension), the first and only long-acting injectable option for HIV prevention. https://viivhealthcare.com/hiv-news-and-media/news/press-releases/2021/december/viiv-healthcare-announces-fda-approval-of-apretude/ (accessed June 28, 2024).
- 103. Delany-Moretlwe S, Hughes JP, Bock P, et al. Cabotegravir for the prevention of HIV-1 in women: results from HPTN 084, a phase 3, randomised clinical trial. Lancet. 2022; 399:1779-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Patel P, Teichner P, Elliot E, Boffito M, Murray M, Polli JW, Baker M, Ford SL, Han K, Russu A, Crauwels H, D'Amico RD, Spreen WR, van Wyk J. Practical dosing guidance for the management of clinician-administered injections of long-acting cabotegravir and rilpivirine. Ther Adv Infect Dis. 2023; 10:20499361231214626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. ViiV Healthcare. Dosing and administration of long-acting cabotegravir when used as pre-exposure prophylaxis. https://medinfo.gsk.com/5f95dbd7-245e-4e65-9f36-1a99e28e5bba/e28f393c-fec0-4746-bd45-df61388fe935/e28f393c-fec0-4746-bd45-df61388fe935_viewable_rendition__v.pdf?medcommid=MED--US-9176 (accessed June 28, 2024).
- 106. Link JO, Rhee MS, Tse WC, et al. Clinical targeting of HIV capsid protein with a long-acting small molecule. Nature. 2020; 584:614-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. McFadden WM, Sarafianos SG. Targeting the HIV-1 and HBV capsids, an EnCore. Viruses. 2023; 15:896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Patel PC, Beasley HK, Hinton A, Wanjalla CN. Lenacapavir (Sunlenca®) for the treatment of HIV-1. Trends Pharmacol Sci. 2023; 44:553-554. [DOI] [PubMed] [Google Scholar]
- 109. HIV.gov. FDA approves new HIV drug for adults with limited treatment options. https://www.hiv.gov/blog/fda-approves-new-hiv-drug-for-adults-with-limited-treatment-options (accessed June 28, 2024).
- 110. Segal-Maurer S, DeJesus E, Stellbrink HJ, et al. Capsid inhibition with lenacapavir in multidrug-resistant HIV-1 infection. N Engl J Med. 2022; 386:1793-1803. [DOI] [PubMed] [Google Scholar]
- 111. Gupta SK, Berhe M, Crofoot G, et al. Lenacapavir administered every 26 weeks or daily in combination with oral daily antiretroviral therapy for initial treatment of HIV: A randomised, open-label, active-controlled, phase 2 trial. Lancet HIV. 2023; 10:e15-e23. [DOI] [PubMed] [Google Scholar]
- 112. Paik J. Lenacapavir: First approval. Drugs. 2022; 82:1499-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Bester SM, Wei G, Zhao H, et al. Structural and mechanistic bases for a potent HIV-1 capsid inhibitor. Science. 2020; 370:360-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Rankovic S, Ramalho R, Aiken C, Rousso I. PF74 reinforces the HIV-1 capsid to impair reverse transcription-induced uncoating. J Virol. 2018; 92:e00845-00818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Xu S, Sun L, Zalloum WA, et al. From design to biological mechanism evaluation of phenylalanine-bearing HIV-1 capsid inhibitors targeting a vital assembly interface. Chin Chem Lett. 2023; 34:107611. [Google Scholar]
- 116. Highland CM, Tan A, Ricaña CL, Briggs JAG, Dick RA. Structural insights into HIV-1 polyanion-dependent capsid lattice formation revealed by single particle cryo-EM. Proc Natl Acad Sci U S A. 2023; 120:e2220545120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Blair WS, Pickford C, Irving SL, et al. HIV capsid is a tractable target for small molecule therapeutic intervention. PLoS Pathog. 2010; 6:e1001220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Yamashita M, Engelman AN. Capsid-dependent host factors in HIV-1 infection. Trends Microbiol. 2017; 25:741-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Bruce A, Adebomi V, Czabala P, Palmer J, McFadden WM, Lorson ZC, Slack RL, Bhardwaj G, Sarafianos SG, Raj M. A tag-free platform for synthesis and screening of cyclic peptide libraries. Angew Chem Int Ed Engl. 2024; 63:e202320045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Price AJ, Fletcher AJ, Schaller T, Elliott T, Lee K, KewalRamani VN, Chin JW, Towers GJ, James LC. CPSF6 defines a conserved capsid interface that modulates HIV-1 replication. PLoS Pathog. 2012; 8:e1002896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Matreyek KA, Yücel SS, Li X, Engelman A. Nucleoporin NUP153 phenylalanine-glycine motifs engage a common binding pocket within the HIV-1 capsid protein to mediate lentiviral infectivity. PLoS Pathog. 2013; 9:e1003693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Gres AT, Kirby KA, McFadden WM, Bryer AJ, Perilla JR, Shi J, Aiken C, Fu X, Zhang P, Francis AC, Melikyan GB, Sarafianos SG. Multidisciplinary studies with mutated HIV-1 capsid proteins reveal structural mechanisms of lattice stabilization. Nat Commun. 2023; 14:5614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Gifford LB, Melikyan GB. HIV-1 capsid uncoating is a multistep process that proceeds through defect formation followed by disassembly of the capsid lattice. ACS Nano. 2024. 18:2928-2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Müller TG, Zila V, Müller B, Kräusslich H-G. Nuclear capsid uncoating and reverse transcription of HIV-1. Annu Rev Virol. 2022; 9:261-284. [DOI] [PubMed] [Google Scholar]
- 125. Jacques DA, McEwan WA, Hilditch L, Price AJ, Towers GJ, James LC. HIV-1 uses dynamic capsid pores to import nucleotides and fuel encapsidated DNA synthesis. Nature. 2016; 536:349-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Zhuang S, Torbett BE. Interactions of HIV-1 capsid with host factors and their implications for developing novel therapeutics. Viruses. 2021; 13:417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Novikova M, Zhang Y, Freed EO, Peng K. Multiple roles of HIV-1 capsid during the virus replication cycle. Virol Sin. 2019; 34:119-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Rihn SJ, Wilson SJ, Loman NJ, Alim M, Bakker SE, Bhella D, Gifford RJ, Rixon FJ, Bieniasz PD. Extreme genetic fragility of the HIV-1 capsid. PLoS Pathog. 2013; 9:e1003461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Barklis E, Alfadhli A, McQuaw C, Yalamuri S, Still A, Barklis RL, Kukull B, López CS. Characterization of the in vitro HIV-1 capsid assembly pathway. J Mol Biol. 2009; 387:376-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Shi J, Zhou J, Halambage UD, Shah VB, Burse MJ, Wu H, Blair WS, Butler SL, Aiken C. Compensatory cubstitutions in the HIV-1 capsid reduce the fitness cost associated with resistance to a capsid-targeting small-molecule inhibitor. J Virol. 2015; 89:208-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Gres AT, Kirby KA, KewalRamani VN, Tanner JJ, Pornillos O, Sarafianos SG. X-ray crystal structures of native HIV-1 capsid protein reveal conformational variability. Science. 2015; 349:99-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Bhattacharya A, Alam SL, Fricke T, Zadrozny K, Sedzicki J, Taylor AB, Demeler B, Pornillos O, Ganser-Pornillos BK, Diaz-Griffero F, Ivanov DN, Yeager M. Structural basis of HIV-1 capsid recognition by PF74 and CPSF6. Proc Natl Acad Sci U S A. 2014; 111:18625-18630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Lanman J, Sexton J, Sakalian M, Prevelige PE Jr. Kinetic analysis of the role of intersubunit interactions in human immunodeficiency virus type 1 capsid protein assembly in vitro. J Virol. 2002; 76:6900-6908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Faysal KMR, Walsh JC, Renner N, Márquez CL, Shah VB, Tuckwell AJ, Christie MP, Parker MW, Turville SG, Towers GJ, James LC, Jacques DA, Böcking T. Pharmacologic hyperstabilisation of the HIV-1 capsid lattice induces capsid failure. eLife. 2024; 13:e83605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011; 365:493-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. GILEAD. Gilead's twice-yearly lenacapavir demonstrated 100% efficacy and superiority to daily Truvada® for HIV prevention. https://www.gilead.com/news-and-press/press-room/press-releases/2024/6/gileads-twiceyearly-lenacapavir-demonstrated-100-efficacy-and-superiority-to-daily-truvada-for-hiv-prevention (accessed June 28, 2024).
- 137. Bekker L-G, Das M, Karim QA, et al. Twice-yearly lenacapavir or daily F/TAF for HIV prevention in cisgender women. New Engl J Med. 2024. [DOI] [PubMed] [Google Scholar]
- 138. Xu S, Sun L, Ding D, Zhang X, Liu X, Zhan P. Metabolite identification of HIV-1 capsid modulators PF74 and 11L in human liver microsomes. Metabolites. 2022; 12:752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Vernekar SKV, Sahani RL, Casey MC, Kankanala J, Wang L, Kirby KA, Du H, Zhang H, Tedbury PR, Xie J, Sarafianos SG, Wang Z. Toward structurally novel and metabolically stable HIV-1 capsid-targeting small molecules. Viruses. 2020; 12:452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Wang L, Casey MC, Vernekar SKV, Sahani RL, Kirby KA, Du H, Zhang H, Tedbury PR, Xie J, Sarafianos SG, Wang Z. Novel PF74-like small molecules targeting the HIV-1 capsid protein: Balance of potency and metabolic stability. Acta Pharm Sin B. 2021; 11:810-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Conference Reports for NATAP. Begley R, Rhee M, West S, Worth A, Ling J, German P. PK, food effect, and safety of oral GS-6207, a novel HIV-1 capsid inhibitor. https://www.natap.org/2020/CROI/croi_69.htm (accessed June 28, 2024).
- 142. Wirden M, Pouderoux C, Peytavin G, Abdi B, Fayçal A, Palich R, Valantin MA, Seang S, Katlama C, Calvez V, Pourcher V, Marcelin AG. Ultra-rapid selection of the N74D capsid inhibitor resistance mutation after 3 weeks on lenacapavir. J Antimicrob Chemother. 2024; 79:1706-1707. [DOI] [PubMed] [Google Scholar]
- 143. Sun Q, Levy RM, Kirby KA, Wang Z, Sarafianos SG, Deng N. Molecular dynamics free energy simulations reveal the mechanism for the antiviral resistance of the M66I HIV-1 capsid mutation. Viruses. 2021; 13:920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Troyano-Hernáez P, Reinosa R, Holguín Á. HIV capsid protein genetic diversity across HIV-1 variants and impact on new capsid-inhibitor lenacapavir. Front Microbiol. 2022; 13:854974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Tailor MW, Chahine EB, Koren D, Sherman EM. Lenacapavir: A novel long-acting capsid inhibitor for HIV. Ann Pharmacother. 2024; 58:185-195. [DOI] [PubMed] [Google Scholar]
- 146. Tuan J, Ogbuagu O. Lenacapavir: A twice-yearly treatment for adults with multidrug-resistant HIV infection and limited treatment options. Expert Rev Anti Infect Ther. 2023; 21:565-570. [DOI] [PubMed] [Google Scholar]


