Summary
Non-acquired immunodeficiency syndrome-defining malignancies (NADMs) are the crucial cause of mortality in people living with haemophilia and human immunodeficiency virus (PLWHH). We aimed to analyse the types and characters of NADMs in PLWHH after approval of direct-acting antivirals (DAA), considering that most PLWHH are infected with hepatitis C virus (HCV). We conducted a nationwide questionnaire mail survey across 395 HIV core facilities in Japan between May 2022 and February 2023. Eight-year data from 64 respondent hospitals (n = 328 PLWHH; 2015-2022) were collected; 35 NADM cases were identified and analysed. Standardised cancer incidence ratios (SCIRs) were calculated. The median age of PLWHH with NADMs was 51 years (interquartile range: 47-62 years); the SCIR was 2.08 (95% confidence interval [CI]: 1.48-2.90) for all malignancies (including carcinoma in situ). Liver cancer accounted for most NADMs (43% [15/35]). The SCIRs of liver cancer (23.09 [95% CI: 13.92- 38.30]) and papillary thyroid cancer (9.38 [2.35-37.50]) significantly increased after adjusting for general Japanese male sex and age. Among PLWHH with liver cancers, 73% (11/15) achieved HCV-sustained virological response. Notably, for patients aged ≤ 50 years, 47% (7/15) were affected by liver cancers, and 27% (4/15) succumbed to NADMs. This study presents the largest survey of NADMs in PLWHH after DAA approval. Our findings emphasised the elevated risk of malignancies in PLWHH, underscoring the need for early cancer screening and preventive measures, particularly against liver cancers, even in younger PLWHH.
Keywords: non-AIDS-defining cancers, haemophilia, HIV, HCV, liver cancer, SCIR
Introduction
Improvements in the prognosis of people living with human immunodeficiency virus (PLWHIV) have increased their risk of developing non-acquired immunodeficiency syndrome (AIDS)-defining malignancies (NADMs) (1-4). Notably, NADMs have become a major contributor to mortality (23-28%) in PLWHIV (2,3). In Japan, approximately 30% of patients with haemophilia were reportedly infected with human immunodeficiency virus (HIV) through contaminated blood products manufactured in the United States before 1986 (5). Subsequently, the number of people living with haemophilia and HIV (PLWHH) was 1,439; currently, this number is approximately 700 in Japan. PLWHH also face an increasing risk of NADMs (6,7); however, because of their relatively small global population, a few cases of NADMs have been documented, particularly after the introduction of antiretroviral therapy (ART) (6,8-11). No studies have investigated the standardised cancer incidence ratios (SCIRs) for NADMs in PLWHH in only the post-ART era.
In Japan, the introduction of ART in late 1996 greatly enhanced the prognosis of PLWHH. However, almost all Japanese patients with haemophilia during that period were also infected with the hepatitis C virus (HCV), leading to prolonged co-infection since childhood. HCV progression is accelerated in HIV co-infection compared to mono-infection (12). Despite the approval of interferon-free direct-acting antivirals (DAAs) for HCV treatment in 2014, Miuma et al. showed that liver cirrhosis remained prevalent at 45.5% (Child- Pugh grade A: 31.9%, B: 4.3%, and C: 6.4%) (13). Although PLWHH are also recommended to receive HCV treatment, multiple HCV genotypes and/or mental problems of PLWHH have hindered the achievement of sustained virological response (SVR) (13,14). HIV co-infection exacerbates liver fibrosis and hepatocellular carcinomas (HCCs) (15). Nevertheless, no data exist on NADMs in PLWHH post-DAA initiation.
Recently, breakthroughs in haemophilia treatment have markedly improved patient prognosis and increased the average life expectancy (16). The well-being of PLWHH, as well as PLWHIV transmitted through sexual means, is crucial. Therefore, identifying and preventing the risk of NADM development in PLWHH is extremely important. Furthermore, individuals coinfected with HIV and HCV experience an extremely long period of untreated infection (HIV: > 15 years, HCV: > 20 years); therefore, their immunological status is distinctive for cancer development and progression.
In this study, we aimed to elucidate the types of NADMs and the characteristics of PLWHH affected by NADMs; furthermore, we aimed to analyse SCIRs in PLWHH during the post-DAA era. By understanding SCIRs, we conducted a nationwide survey to promote comprehensive cancer care, including diagnosis, treatment, health prevention programs, and education of PLWHH.
Patients and Methods
Study participants
In Japan, most PLWHIV are referred to HIV core hospitals. Physicians and other professionals engaged in HIV care in 395 HIV core facilities and clinics across Japan were contacted for this nationwide mail survey, which was conducted using a questionnaire-based tool between May and June 2022 to gather information on NADMs diagnosed from January 2015 to December 2021 (Supplemental Table S1, https://www.globalhealthmedicine.com/site/supplementaldata.html?ID=89). A second questionnaire was mailed between January and February 2023 to the facilities that responded to the initial questionnaire (questionnaire 1) for additional details on NADMs diagnosed from January to December 2022 and the numbers of PLWHH attending each facility (Supplemental Table S2, https://www.globalhealthmedicine.com/site/supplementaldata.html?ID=89). Overall, NADM data were collected over 8 years. PLWHH were defined as patients with HIV and haemophilia, or other conditions treated with unheated blood coagulation products (Supplemental Table S3, https://www.globalhealthmedicine.com/site/supplementaldata.html?ID=89). In 2014, the first interferon-free therapy for HCV, daclatasvir/ asunaprevir, was launched in Japan. In 2015, sofosbuvir, effective against all HCV genotypes, was approved. Therefore, we decided to collect data on NADMs after 2015. https://www.globalhealthmedicine.com/site/supplementaldata.html?ID=89) presents the exclusion criteria for NADMs.
Statistical analysis
Statistical analyses were performed using PRISM 8 v8.1.2 (GraphPad Software, San Diego, California, USA). Descriptive statistics comprised medians (interquartile ranges) and proportions. CD4 counts and CD4/CD8 ratio of PLWHH were analysed using the Mann-Whitney test. P values less than 0.05 were considered statistically significant. We calculated SCIRs and 95% confidence intervals (CIs) for PLWHH and compared the observed cancer incidence in PLWHH with the expected incidence, standardised based on age-stratified cancer incidence in the general Japanese male population. The sex- and age-specific cancer incidences in the general population were obtained from the National Cancer Centre website (17). If the age distribution data for the source population of PLWHH in this study could not be collected, we used the age distribution reported in other national surveillance (18). As only one female patient was present despite a significantly larger number of male patients, she was excluded from the SCIR calculation. The CIs for the SCIR were calculated using the formula exp (ln (SCIR) ± 1.96 * (1/cancer incidence1/2)) (19). If the lower limit of the CI for SCIR exceeded 1, it was considered statistically significant.
Ethics approval and consent to participate
The protocol for this research project was approved by the Ethics Board of the Institute of Medical Science, University of Tokyo (approval no.: 2021-71-1216) and adhered to the principles of the Declaration of Helsinki. The requirement for consent to participate was waived by the Institutional Review Board of the Institute of Medical Science, University of Tokyo, in accordance with national regulations.
Results
Baseline characteristics of people living with human immunodeficiency virus with haemophilia with non-acquired immunodeficiency syndrome-defining malignancies
Sixty-four HIV core hospitals responded to the questionnaire (response rate: 16.2% [64/395]). These hospitals were attended by 328 PLWHH (47.1% of the 697 PLWHH in Japan). Overall, 35 NADM cases were diagnosed for the 328 PLWHH during the 8-year study period; two PLWHH had duplicate cancers, specifically colon and tongue cancers and colon and liver cancers. The characteristics of these 35 NADM cases are presented in Tables 1 and 2. All cases except one were male, with a median age of 51 years (interquartile range: 47-62 years); the youngest PLWHH with NADM was 39 years old and had colon cancer. Furthermore, 74% of these 35 NADMs had haemophilia A, and three PLWHH had > 50 copies/mL of HIV-RNA at diagnosis. Moreover, 97% of the PLWHH with NADMs (33/34, had one missing data) exhibited HCV-antibody positivity; four PLWHH exhibited HCV-RNA positivity. At diagnosis, 6% (2/34) of the PLWHH with NADMs were heavy alcohol drinkers, 18% (6/34) were current smokers, and 29% (10/34) lived alone.
Table 1. Baseline characteristics of PLWHH with NADMs.
| Baseline characteristics | All patients n = 35 |
|---|---|
| Age (years), median (IQR) | 51 (47–62) |
| Haemophilia type | |
| A | 26 (74%) |
| B | 7 (20%) |
| History of AIDS | 5/34 (15%) |
| Treatment with ART | 33 (94%) |
| Duration of ART (years), median (IQR) | 22 (17–25) |
| HIV viral load < 50 copies/mL | 32 (94%) |
| CD4 cell count (/μL), median (IQR) | 405 (281–558) |
| CD8 cell count (/μL), median (IQR) | 470 (304–665) |
| CD4/CD8 ratio, median (IQR) | 0.87 (0.58–1.11) |
| HBsAg positivity | 2/34 (6%) |
| HCV-Ab positivity | 33/34 (97%) |
| HCV-RNA positivity | 4/32 (13%) |
| Current smoking | 6/34 (18%) |
| Heavy alcohol consumption | 2/34 (6%) |
| Solitary living | 10/34 (29%) |
Abbreviations: AIDS, acquired immunodeficiency syndrome; HIV, human immunodeficiency virus; IQR, interquartile range; ART, antiretroviral therapy; HBsAg, hepatitis B surface antigen; HCV-Ab, hepatitis C virus antibody; HCV-RNA, hepatitis C virus RNA; NADM, non-acquired immunodeficiency syndrome-defining malignancy; PLWHH, people living with haemophilia and the human immunodeficiency virus.
Table 2. Characteristics and management of NADMs in PLWHH.
| Characteristics of NADMs | All patients n = 35 |
|
|---|---|---|
| Cancer type | ||
| Liver cancer | 15 (43%) | |
| Colon cancer | 5 (14%) | |
| Malignant lymphoma | 3 (9%) | |
| Papillary thyroid cancer | 2 (6%) | |
| Tongue cancer | 2 (6%) | |
| Neuroblastoma | 1 | |
| Malignant myeloma | 1 | |
| Prostate cancer | 1 | |
| Stomach cancer | 1 | |
| Renal cell carcinoma | 1 | |
| Gall bladder cancer | 1 | |
| Buccal mucosa cancer | 1 | |
| Pancreatic cancer | 1 | |
| Trigger of diagnosis | ||
| Health screening | 22 (63%) | |
| Presentation of symptoms | 8 (23%) | malignant lymphomas (n = 2), colon cancer (n = 2), tongue cancer (n = 1), buccal mucosal cancer (n = 1), neuroblastoma (n = 1), and multiple myeloma (n = 1) |
| Examination during regular visits | sit (14%) | |
| Metastasis at diagnosis | 10/34 (29%) | liver cancer (n = 5), malignant lymphomas (n = 2), buccal mucosal cancer (n = 1), neuroblastoma (n = 1), and multiple myeloma (n = 1) |
| Treatment | ||
| Only surgery | 15 (43%) | |
| Chemotherapy + radiation | 7 (20%) | |
| Only radiation | 4 (11%) | |
| Only chemotherapy | 3 (9%) | |
| Surgery + chemotherapy | 3 (9%) | |
| Surgery + chemotherapy + radiation | 2 (6%) | |
| Surgery + radiation | 1 | |
| Length of treatment | ||
| < 1 week | 11 (31%) | |
| 1 week to 6 months | 6 (17%) | |
| 6 months to 1 year | 8 (23%) | |
| 1-5 years | 8 (23%) | |
| > 5 years | 2 (6%) | |
| Outcome | ||
| Complete remission | 23/34 (68%) | |
| Partial remission | 3/34 (9%) | |
| Death due to malignancy | 6/34 (18%) | liver cancer (n=5), neuroblastoma (n = 1) |
| Death due to other causes | 1 | |
| Under treatment | 1 |
Abbreviations: AIDS, acquired immunodeficiency syndrome; HIV, human immunodeficiency virus; NADM, non-acquired immunodeficiency syndrome-defining malignancy; PLWHH, people living with haemophilia and the human immunodeficiency virus.
Characteristics of non-acquired immunodeficiency syndrome-defining malignancies in people living with human immunodeficiency virus with haemophilia
The observed 8-year cancer incidence was 35, and the corresponding annual incidence was 4.38. The most common NADMs were liver cancer (43% [15/35], one case of liver adenocarcinoma, and remaining of HCCs (Table 2); all PLWHH with liver cancers exhibited HCV-antibody positivity, and 73% (11/15) had achieved SVR. Furthermore, for patients aged ≤ 50 years, 47% (7/15) had liver cancers, and 27% (4/15) died of NADMs.
The next most common NADMs were colon cancer, malignant lymphoma, tongue cancer, and papillary thyroid cancer (in that order). One case of epithelial cancer was observed. Overall, 77% (27/35) were diagnosed during health screenings and examinations performed at regular visits. The remaining 23% (8/35) were diagnosed after symptom appearance. Metastasis was present at diagnosis in 29% (10/34) of the cases. Surgery was required for 60% (21/35); treatment duration exceeded 6 months in 51% (18/35); and complete remission occurred in 68% (23/34) of the cases. However, death from NADM occurred in 18% (6/34) of the cases.
CD4 counts and CD4/CD8 ratios at NADM diagnosis are presented in additional files (Supplemental Figure S1, https://www.globalhealthmedicine.com/site/supplementaldata.html?ID=89). PLWHH who died exhibited lower CD4 counts (298 [184-429]/μL vs. 407 [321-564]/μL) and lower CD4/CD8 ratios (0.49 [0.43- 0.89] vs. 0.89 [0.66-1.18]) than did those who survived; however, these differences were not significant (p = 0.156 [CD4 count] and 0.107 [CD4/CD8 ratio]; Supplemental Figure S1, b and c, https://www.globalhealthmedicine.com/site/supplementaldata.html?ID=89).
Age- and sex-adjusted standardised cancer incidence ratios in people living with human immunodeficiency virus with haemophilia with non-acquired immunodeficiency syndrome-defining malignancies
Figure 1 illustrates SCIRs for all malignancies, including carcinoma in situ (2.08 [1.48-2.90]). The SCIR for liver cancer was significantly higher (23.09 [95% CI: 13.92- 38.30]) than that for papillary thyroid cancer (9.38 [2.35- 37.50]). However, the association was not significant for malignant lymphoma, tongue cancer, or colon cancer. The standardised cancer death ratio was 1.38 (95% CI: 0.62-3.07), which was also not significant.
Figure 1.
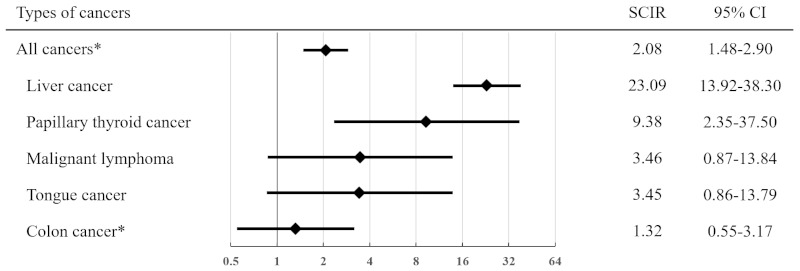
Standardised cancer incidence ratio of various cancers. Comparison of types and standardised cancer incidence ratios of non-AIDS-defining malignancies between PLWHH and the general male population in Japan. *including carcinoma in situ AIDS, acquired immunodeficiency syndrome; PLWHH, people living with haemophilia and human immunodeficiency virus; SCIR, standardised cancer incidence ratio; CI, confidence interval.
Discussion
In this study, with a SCIR of 2.08 (95% CI: 1.48-2.90) for all malignancies, including carcinoma in situ, the risk of cancer was higher in PLWHH than in the general male population in Japan, even after DAA approval. The median age at NADM diagnosis was 51 years, which is relatively young. The most common NADM was liver cancer (43%), with a notably high SCIR of 23.09 (95% CI: 13.92-38.30). In younger patients with NADM aged ≤ 50 years, 47% (7/15) had liver cancers, and 27% (4/15) died of NADMs. The SCIR for papillary thyroid cancer was also relatively high.
Papers were found following a comprehensive search in PubMed using the terms "cancer" or "malignancy" and "haemophilia" and "HIV" including the period from 2000 onwards in the study period, which was the post-ART era. Among the many studies found on cancer deaths in patients with haemophilia without HIV infection, five described cancer incidence in patients with haemophilia and HIV, all of which were small cohorts (6,8-11). Three of these reports described cancer types in PLWHH. Biron et al. reported seven cases of cancers (leukemia or hematopoietic cancer: 2, HCC: 2, urogenital: 1, respiratory system: 1, and gastrointestinal tract: 1) from France and Belgium during the observation period from 2002 to 2012 (8). Similarly, Oka et al. reported six cases (thyroid cancer: 3, HCC: 1, pancreas cancer: 1, and neuroendocrine tumor: 1) from Japan during the 2016- 2019 observation period (6). No SCIRs were reported in either case. Chen et al. reported eight cases (oral cavity: 4, non-Hodgkin lymphoma: 3, and leukemia: 1) from Taiwan with respective SCIR (95% CI) of 16.88 (4.54-43.22), 12.66 (2.54-37.00), and 4.22 (0.06-23.48), respectively (10). However, no post-DAA era reports of SCIRs for NADMs in PLWHH have been published. This nationwide survey is the largest on NADMs in PLWHH (n = 35) and the first to have determined the SCIRs after DAA approval.
The SCIR results for all malignancies in our study are greatly influenced by the SCIR result for liver cancer. In Japan, the general population affected by liver cancers comprises older adults with complications, such as HCV and hepatitis B virus infections secondary to blood transfusion and mother-to-child transmission. Among the PLWHH with liver cancers in our study, 47% (7/15) were aged ≤ 50 years, and 33% (5/15) died (three died within 1 year of diagnosis). Accordingly, the observed young age of the affected PLWHH in this survey may be a critical finding. Co-infection with HIV and HCV, which increases the risk of HCC, may have contributed to this observation. Factors such as the HCV genotype and immune dysfunction of the patients may have also played roles. PLWHH may be infected with HCVs of multiple genotypes; for instance, HCV genotype 3 is associated with a high incidence of fatty liver, rapid fibrosis development, and HCC (20-22). Our findings revealed that 73% of the liver cancer cases in PLWHH had already achieved SVR. Other contributing factors may include rapid disease progression because of immune dysfunction.
In this study, the percentage of heavy alcohol drinkers was low, at 6%. Alcohol consumption is known to promote adipogenesis and fibrosis of the liver, particularly in those who already have liver problems due to HIV and/or HCV infection. HIV infectious disease specialists educate patients about the health risks exacerbated by alcohol consumption. Therefore, to reduce this risk, all PLWHH should avoid alcohol consumption and take steps to prevent fatty liver development for hepatoprotective purposes after achieving SVR. Four cases of HCV-RNA-positive PLWHH were also observed. Following DAA approval, SVR rates have increased; thus, collaboration with hepatologists should be considered to actively promote HCV treatment. The HCC treatment that was administered included various approaches, such as chemotherapy, radiation, and surgery; this reaffirmed the need for continued multidisciplinary treatment of HCCs involving oncologists and liver surgeons (23).
Recently, a French study revealed that the incidence of NADMs except for HCCs was higher in PLWHIV with SVR than in PLWHIV without HCV infections, even after excluding cirrhotic cases; this suggested a role of HCV infections in the development of cancers other than HCCs (24).
In our study, colon cancer was the second most common NADM; however, the SCIR for colon cancer did not differ significantly. The five most common malignancies in Japanese men are HCCs, prostate cancer, colon cancer, stomach cancer, and lung cancer (17). In the future, these malignancies may increase in PLWHH. Therefore, ensuring that PLWHH undergo screening at the same rate as the general population may be necessary.
The next most common malignancy in our study was malignant lymphomas, at 9% (Hodgkin's lymphoma, two cases; unknown, one case). Haung et al. reported that in addition to HCCs, leukaemia and lymphomas were common in people living with haemophilia before the introduction of DAA (9). The reasons for the higher incidence rates of leukaemia and lymphomas remain unclear; however, they may be linked to frequent blood transfusions in this population. Papillary thyroid cancer was the next most prevalent cancer. Notably, the affected patients were in complete remission after surgical resection. Papillary thyroid cancer generally has a relatively low malignancy, and the reasons for its high incidence in our population remain unclear. Thus, further studies are warranted to elucidate these reasons.
Studies have revealed correlations between NADM incidence and CD4 count and CD4/CD8 ratio in PLWHIV (25-27). The mean CD4 count for all PLWHH has been reported to be 546.9/μL (standard deviation: 246.4/μL) based on publicly available data for Japanese PLWHH (18); the median CD4 count for the 35 PLWHH with NADMs in our study appeared lower, at 405/μL. However, the significance of this difference was not determined. Immunological mechanisms may play a role in NADM-associated morbidity in PLWHH. The CD4 count and CD4/CD8 ratio in the six patients who died of NADMs tended to be lower than those in the 28 patients who recovered, although this difference was not significant. Disease progression in PLWHH may also be faster, which should be validated with further research.
The Joint United Nations Programme on HIV/ AIDS announced that PLWHIV should have access to integrated health services (28) and that PLWHH should be provided with health screening services and cancer treatments. Japanese men have a lifetime cancer risk of 65.5% (17). Early detection and treatment are important for PLWHH as they age. In our study, 29% of the PLWHH lived alone during NADM diagnosis, and 51% received cancer treatment for ≥ 6 months. Physical and psychological support are necessary for PLWHH at diagnosis and during treatment. Many PLWHH reportedly experience a limited range of movement secondary to haemophilic arthropathy (29); they further tend to avoid malignancy screening tests. Additionally, the strong stigma surrounding these individuals further reduces their likelihood of undergoing malignancy screening. Because PLWHH regularly attend HIV core hospitals, there is a need to establish an efficient system that can detect cancer in its early stages and promote patient education.
Our study had some limitations. First, the small number of PLWHH with NADMs weakened the statistical power of our study. Although data on NADM morbidity were collected, the cancer screening may have been inadequate, potentially leading to undiagnosed NADMs in several cases. It is possible that prostate, colon, stomach, and lung cancers, which are more frequent in our country, might have been reported more if the cancer screening had been conducted adequately. Second, because we did not collect detailed data on liver conditions, we could not directly determine the differences in liver fibrosis between PLWHH with HCCs and those with non-HCC NADMs. Finally, we could not obtain patient blood samples; thus, no immune cell- or serum-based analyses were performed. Further immunological in vitro studies are required to elucidate the mechanism of carcinogenesis and progression in PLWHH.
In conclusion, our study investigated the characteristics of PLWHH with NADMs using national surveillance data and focused on morbidities over 8 years (2015-2022); it involved the largest amount of data on NADMs in PLWHH in the post-DAA era. PLWHH were found to be at an increased risk for malignancies. The median age at NADM diagnosis was relatively low, at 51 years, indicating an earlier onset. Among various NADMs, liver cancers were the most prevalent, followed by papillary thyroid cancer. These findings emphasise the importance of cancer screening and preventive measures even in younger PLWHH because early detection is crucial for addressing the elevated risk of NADMs in PLWHH.
Acknowledgements
The authors are grateful to all individuals who participated in the questionnaire survey and to all medical staff for the time and effort that they spent during the surveillance. We would like to thank the following institutes as well: Hokkaido University Hospital, Obihiro Kosei Hospital, Aomori Prefectural Central Hospital, Iwate Prefectural Central Hospital, NHO Sendai Medical Centre, Tohoku University Hospital, National Hospital Organization Sendai Nishitaga Hospital, Ohta General Hospital, Fukushima Rosai Hospital, National Hospital Organization Tochigi Medical Centre, Gunma University Hospital, National Defence Medical College Hospital, Chiba University Hospital, the Jikei University Hospital, IMSUT Hospital, Tokyo Metropolitan Cancer and Infectious Diseases Centre Komagome Hospital, Juntendo University Hospital, the University of Tokyo Hospital, Tokyo Metropolitan Bokutoh Hospital, Nippon Medical School Tama Nagayama Hospital, Yokohama City University Hospital, NHO Tokyo Medical Centre, Tokyo Medical University Hospital, Teikyo University Hospital, Tokyo Metropolitan Toshima Hospital, Yokohama Municipal Citizen's Hospital, NHO Kofu National Hospital, Tsuru Municipal General Hospital, Niigata University Medical & Dental Hospital, NHO Nishiniigata Chuo Hospital, Niigata Prefectural Shibata Hospital, Toyama University Hospital, Ishikawa Prefectural Central Hospital, Fujieda Municipal General Hospital, Shimada General Medical Centre, NHO Nagoya Medical Centre, Social Medical Corporation Kojunkai Daido Hospital, Kyoto University Hospital, NHO Maizuru Medical Centre, NHO Osaka National Hospital, Osaka University Hospital, Rinku General Medical Centre, Hyogo Medical University Hospital, NHO Himeji Medical Centre, Nara Medical University Hospital, Tottori University Hospital, Shimane Prefectural Central Hospital, Hiroshima Prefectural Hospital, Hiroshima University Hospital, Yamaguchi University Hospital, Tokushima Prefectural Miyoshi Hospital, Ehime University Hospital, NHO Ehime Medical Centre, Ehime Prefectural Central Hospital, NHO Kyushu Medical Centre, Kyushu University Hospital, Nagasaki University Hospital, Kumamoto University Hospital, NHO Beppu Medical Centre, University of the Ryukyus Hospital, Shinjuku Higashiguchi Clinic, Taniguchi International Clinic, Kasai Clinic, and Oda Internal Medicine Clinic.
Funding
This study was supported in part by research grants from the Japanese Ministry of Health, Labour and Welfare (21HB2005) and JSPS KAKENHI (21K07314).
Conflict of Interest
K.T. has received financial support for lectures from Shionogi Pharma Co, Ltd, ViiV Healthcare. R.M. has received financial support for lectures from ViiV Healthcare, Gilead Sciences, Inc. T.F. has received consulting fees from Gilead Sciences, Inc. and ViiV Healthcare and speakers bureaus from ViiV Healthcare and Gilead Sciences, Inc. D.W. has received grants for clinical trials from Gilead Sciences, ViiV Healthcare, GlaxoSmithKline, MSD, and Cmic out of this work, and honoraria from Gilead Science, ViiV Healthcare, MSD, and Janssen Pharmaceutical out of this work. All other authors declare no conflicts of interest.
References
- 1. Chiao EY, Coghill A, Kizub D, Fink V, Ndlovu N, Mazul A, Sigel K. The effect of non-AIDS-defining cancers on people living with HIV. Lancet Oncol. 2021; 22:e240-e253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Smith CJ, Ryom L, Weber R, et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): A multicohort collaboration. Lancet. 2014; 384:241-248. [DOI] [PubMed] [Google Scholar]
- 3. Oka S, Ikeda K, Takano M, Ogane M, Tanuma J, Tsukada K, Gatanaga H. Pathogenesis, clinical course, and recent issues in HIV-1-infected Japanese hemophiliacs: A three-decade follow-up. Glob Health Med. 2020; 2:9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tsuda H, Koga M, Nojima M, Senkoji T, Kubota M, Kikuchi T, Adachi E, Ikeuchi K, Tsutsumi T, Koibuchi T, Yotsuyanagi H. Changes in survival and causes of death among people living with HIV: Three decades of surveys from Tokyo, one of the Asian metropolitan cities. J Infect Chemother. 2021; 27:949-956. [DOI] [PubMed] [Google Scholar]
- 5. Kurimura T, Kawatani T, Hattori N, Tsuchie H. Prevalence and transmission of human immunodeficiency virus in Japan. AIDS Res. 1986; 2 Suppl 1:S163-S166. [PubMed] [Google Scholar]
- 6. Oka S, Ogata M, Takano M, Minamimoto R, Hotta M, Tajima T, Nagata N, Tsukada K, Teruya K, Kikuchi Y, Gatanaga H, Cancer Screening in Hemophiliac/HIV Patient Study Group. Non-AIDS-defining malignancies in Japanese hemophiliacs with HIV-1 infection. Glob Health Med. 2019; 1:49-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hassan S, Monahan RC, Mauser-Bunschoten EP, et al. Mortality, life expectancy, and causes of death of persons with hemophilia in the Netherlands 2001-2018. J Thromb Haemost. 2021; 19:645-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Biron-Andreani C, de Moerloose P, D'oiron R, Chambost H, Schved JF, Hermans C. Cancer detection and management in patients with haemophilia: A retrospective European multicentre study. Haemophilia. 2014; 20:78-82. [DOI] [PubMed] [Google Scholar]
- 9. Huang YC, Tsan YT, Chan WC, Wang JD, Chu WM, Fu YC, Tong KM, Lin CH, Chang ST, Hwang WL. Incidence and survival of cancers among 1,054 hemophilia patients: A nationwide and 14-year cohort study. Am J Hematol. 2015; 90:E55-E59. [DOI] [PubMed] [Google Scholar]
- 10. Chen M, Jen I, Sharp GB, Chen YM. The incidence rates and standardized incidence ratios of cancer in hemophilic HIV/AIDS patients in Taiwan. J Acquir Immune Defic Syndr. 2016; 71:e114-e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tagliaferri A, Di Perna C, Santoro C, Schinco P, Santoro R, Rossetti G, Coppola A, Morfini M, Franchini M; Italian Association of Hemophilia Centers. Cancers in patients with hemophilia: A retrospective study from the Italian Association of Hemophilia Centers. J Thromb Haemost. 2012; 10:90-95. [DOI] [PubMed] [Google Scholar]
- 12. de Lédinghen V, Barreiro P, Foucher J, Labarga P, Castéra L, Vispo ME, Bernard PH, Martin-Carbonero L, Neau D, García-Gascó P, Merrouche W, Soriano V. Liver fibrosis on account of chronic hepatitis C is more severe in HIV-positive than HIV-negative patients despite antiretroviral therapy. J Viral Hepat. 2008; 15:427-433. [DOI] [PubMed] [Google Scholar]
- 13. Miuma S, Hidaka M, Takatsuki M, Natsuda K, Soyama A, Miyaaki H, Kanda Y, Tamada Y, Shibata H, Ozawa E, Taura N, Eguchi S, Nakao K. Current characteristics of hemophilia patients co-infected with HIV/HCV in Japan. Exp Ther Med. 2018; 15:2148-2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yotsuyanagi H, Kikuchi Y, Tsukada K, Nishida K, Kato M, Sakai H, Takamatsu J, Hige S, Chayama K, Moriya K, Koike K. Chronic hepatitis C in patients co-infected with human immunodeficiency virus in Japan: A retrospective multicenter analysis. Hepatol Res. 2009; 39:657-663. [DOI] [PubMed] [Google Scholar]
- 15. Pinato DJ, Allara E, Chen TY, et al. Influence of HIV infection on the natural history of hepatocellular carcinoma: Results from a global multicohort study. J Clin Oncol. 2019; 37:296-304. [DOI] [PubMed] [Google Scholar]
- 16. Srivastava A, Santagostino E, Dougall A, et al. WFH Guidelines for the Management of Hemophilia, 3rd edition. Haemophilia. 2020; 26 Suppl 6:1-158. [DOI] [PubMed] [Google Scholar]
- 17. Cancer Information Service. Latest cancer statistic. https://ganjoho.jp/reg_stat/statistics/stat/summary.html (accessed January 7, 2024). (in Japanese) .
- 18. Nationwide survey on coagulation disorders 2021. Project entrusted by Ministry of Health, Labour and Welfare: Japan Foundation for AIDS Prevention. https://api-net.jfap.or.jp/image/data/blood/r03_research/r03_research.pdf (accessed January 7, 2024). (in Japanese) .
- 19. Greenland S, Rothman KJ. Introduction to categorical statistics. In: Rothman KJ, Greenland S, Lash TL, editors. Modern epidemiology. Lippincott Williams & Wilkins; 2008; pp.238-257. [Google Scholar]
- 20. Leandro G, Mangia A, Hui J, et al. Relationship between steatosis, inflammation, and fibrosis in chronic hepatitis C: A meta-analysis of individual patient data. Gastroenterology. 2006; 130:1636-1642. [DOI] [PubMed] [Google Scholar]
- 21. Probst A, Dang T, Bochud M, Egger M, Negro F, Bochud PY. Role of hepatitis C virus genotype 3 in liver fibrosis progression--a systematic review and meta-analysis: HCV genotype 3 and fibrosis progression. J Viral Hepat. 2011; 18:745-759. [DOI] [PubMed] [Google Scholar]
- 22. Nkontchou G, Ziol M, Aout M, Lhabadie M, Baazia Y, Mahmoudi A, Roulot D, Ganne-Carrie N, Grando- Lemaire V, Trinchet JC, Gordien E, Vicaut E, Baghad I, Beaugrand M. HCV genotype 3 is associated with a higher hepatocellular carcinoma incidence in patients with ongoing viral C cirrhosis: HCV genotype 3 and HCC. J Viral Hepat. 2011; 18:e516-e522. [DOI] [PubMed] [Google Scholar]
- 23. Takatsuki M, Natsuda K, Hidaka M, et al. The treatment choices and outcome of hepatocellular carcinoma in hemophilic patients with human immunodeficiency virus/hepatitis C virus (HIV/HCV) coinfection due to contaminated blood products in Japan. J Gastrointest Oncol. 2021; 12:2952-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Requena MB, Grabar S, Lanoy E, Pialoux G, Billaud E, Duvivier C, Merle P, Piroth L, Tattevin P, Salmon D, Weiss L, Costagliola D, Lacombe K. Mortality in hepatitis C virus-cured vs. hepatitis C virus-uninfected people with HIV AIDS. 2023; 37:1297-1306. [DOI] [PubMed] [Google Scholar]
- 25. Chammartin F, Mocroft A, Egle A, et al. Measures of longitudinal immune dysfunction and risk of AIDS and non-AIDS defining malignancies in antiretroviral-treated people with human immunodeficiency virus. Clin Infect Dis. 2024; 78:995-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sigel K, Wisnivesky J, Crothers K, Gordon K, Brown ST, Rimland D, Rodriguez-Barradas MC, Gibert C, Goetz MB, Bedimo R, Park LS, Dubrow R. Immunological and infectious risk factors for lung cancer in US veterans with HIV: A longitudinal cohort study. Lancet HIV. 2017; 4:e67-e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hema MN, Ferry T, Dupon M, Cuzin L, Verdon R, Thiébaut R, Protopopescu C, Leport C, Raffi F, Le Moing V; ANRS CO 8 (APROCO/COPILOTE) study group. Low CD4/CD8 ratio is associated with non AIDS-defining cancers in patients on antiretroviral therapy: ANRS CO8 (aproco/copilote) prospective cohort study. PLoS One. 2016; 11:e0161594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. UNAIDS. Global AIDS strategy 2021-2026, End Inequalities. End AIDS. https://www.unaids.org/sites/default/files/media_asset/global-AIDS-strategy-2021-2026_en.pdf (accessed January 7, 2024).
- 29. Fujii T, Fujii T, Takedani H. Long-term impact of haemarthrosis on arthropathy and activities of daily living in Japanese persons with haemophilia. Haemophilia. 2020; 26:e124-e127. [DOI] [PubMed] [Google Scholar]


