Abstract
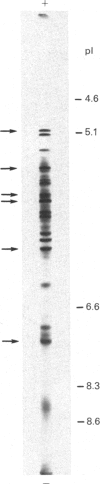
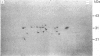
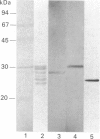
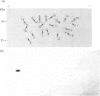
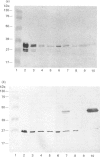
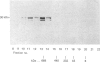
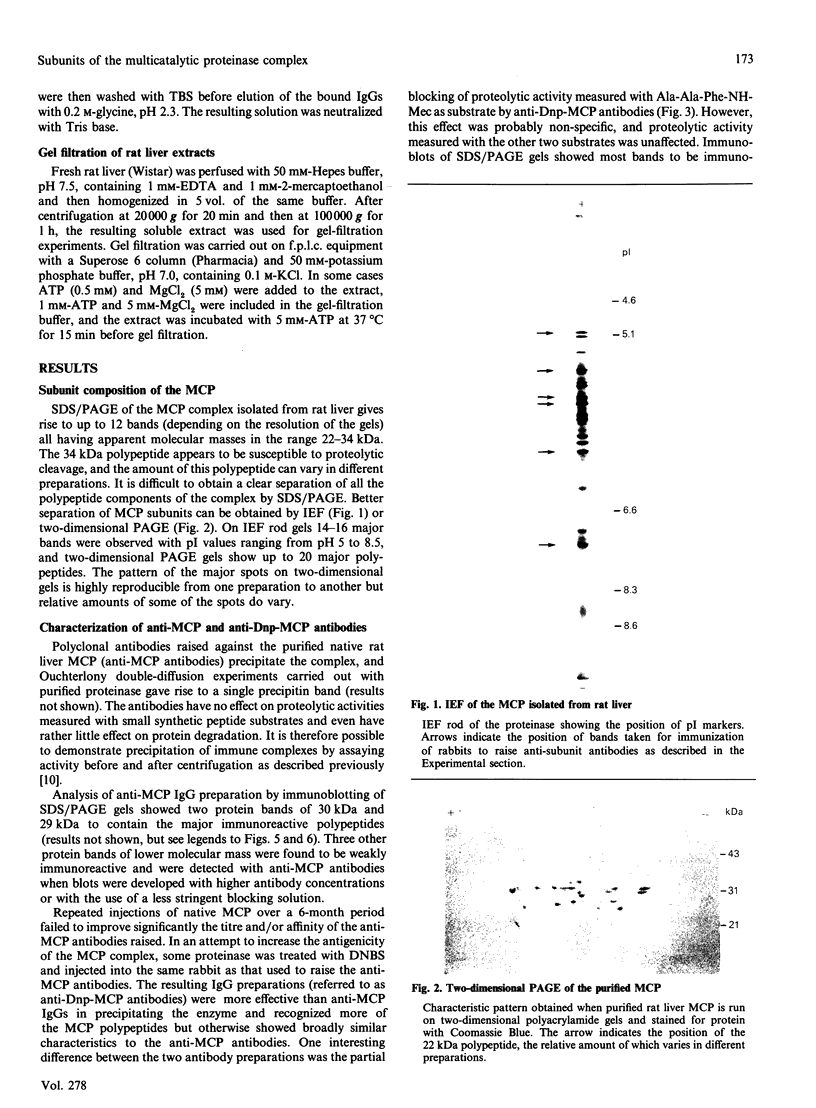
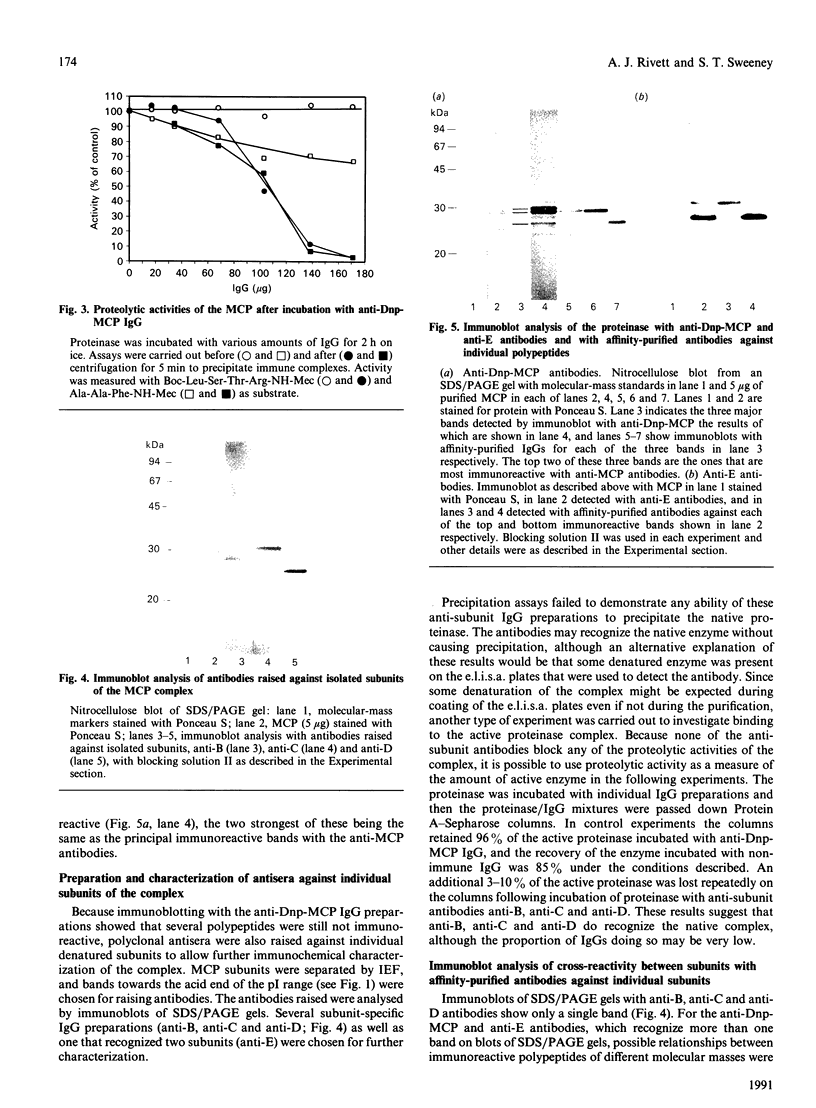
The multicatalytic proteinase (MCP) is a high-molecular-mass non-lysosomal proteinase that gives rise to a characteristic pattern of bands of molecular mass 22-34 kDa on SDS/PAGE gels. Isoelectric-focusing gels of the enzyme purified from rat liver show 16 bands with isoelectric points in the range of pH 5-8.5. Two-dimensional PAGE gels reveal that there are more than the previously reported 13 polypeptides associated with the MCP from rat liver and show a pattern of 15-20 major spots and several minor ones, similar to that of MCP isolated from some other sources. Possible relationships between the different polypeptides were investigated by immunoblot analysis of electrophoretically purified proteinase subunits with affinity-purified subunit-specific antibodies as well as antibodies raised against individual denatured subunits of the complex. The results demonstrate that many of the major polypeptide components of the MCP complex are antigenically distinct. Moreover comparison of immunoreactive material in crude cell extracts with that in purified MCP preparations has shown that the polypeptides are not derived from a smaller number of higher-molecular-mass subunits. Also, individual subunits have the same apparent molecular mass in a variety of rat tissues, suggesting close similarity between MCPs of different tissues. The highest concentrations of MCP subunits occur in liver and kidney. Gel-filtration analysis of crude extracts has demonstrated that MCP polypeptides are also associated with a higher-molecular-mass complex, which may be the 26 S proteinase that has been implicated in the degradation of ubiquitin-protein conjugates.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrigo A. P., Tanaka K., Goldberg A. L., Welch W. J. Identity of the 19S 'prosome' particle with the large multifunctional protease complex of mammalian cells (the proteasome). Nature. 1988 Jan 14;331(6152):192–194. doi: 10.1038/331192a0. [DOI] [PubMed] [Google Scholar]
- Baumeister W., Dahlmann B., Hegerl R., Kopp F., Kuehn L., Pfeifer G. Electron microscopy and image analysis of the multicatalytic proteinase. FEBS Lett. 1988 Dec 5;241(1-2):239–245. doi: 10.1016/0014-5793(88)81069-x. [DOI] [PubMed] [Google Scholar]
- Bos E. S., van der Doelen A. A., van Rooy N., Schuurs A. H. 3,3',5,5' - Tetramethylbenzidine as an Ames test negative chromogen for horse-radish peroxidase in enzyme-immunoassay. J Immunoassay. 1981;2(3-4):187–204. doi: 10.1080/15321818108056977. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Driscoll J., Goldberg A. L. The proteasome (multicatalytic protease) is a component of the 1500-kDa proteolytic complex which degrades ubiquitin-conjugated proteins. J Biol Chem. 1990 Mar 25;265(9):4789–4792. [PubMed] [Google Scholar]
- Duncan R., Hershey J. W. Evaluation of isoelectric focusing running conditions during two-dimensional isoelectric focusing/sodium dodecyl sulfate-polyacrylamide gel electrophoresis: variation of gel patterns with changing conditions and optimized isoelectric focusing conditions. Anal Biochem. 1984 Apr;138(1):144–155. doi: 10.1016/0003-2697(84)90783-8. [DOI] [PubMed] [Google Scholar]
- Eytan E., Ganoth D., Armon T., Hershko A. ATP-dependent incorporation of 20S protease into the 26S complex that degrades proteins conjugated to ubiquitin. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7751–7755. doi: 10.1073/pnas.86.20.7751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenburg P. E., Haass C., Kloetzel P. M., Niedel B., Kopp F., Kuehn L., Dahlmann B. Drosophila small cytoplasmic 19S ribonucleoprotein is homologous to the rat multicatalytic proteinase. Nature. 1988 Jan 14;331(6152):190–192. doi: 10.1038/331190a0. [DOI] [PubMed] [Google Scholar]
- Falkenburg P. E., Kloetzel P. M. Identification and characterization of three different subpopulations of the Drosophila multicatalytic proteinase (proteasome). J Biol Chem. 1989 Apr 25;264(12):6660–6666. [PubMed] [Google Scholar]
- Grossi de Sa M. F., Martins de Sa C., Harper F., Coux O., Akhayat O., Pal J. K., Florentin Y., Scherrer K. Cytolocalization of prosomes as a function of differentiation. J Cell Sci. 1988 Feb;89(Pt 2):151–165. doi: 10.1242/jcs.89.2.151. [DOI] [PubMed] [Google Scholar]
- Haass C., Kloetzel P. M. The Drosophila proteasome undergoes changes in its subunit pattern during development. Exp Cell Res. 1989 Jan;180(1):243–252. doi: 10.1016/0014-4827(89)90228-0. [DOI] [PubMed] [Google Scholar]
- Haass C., Pesold-Hurt B., Multhaup G., Beyreuther K., Kloetzel P. M. The Drosophila PROS-28.1 gene is a member of the proteasome gene family. Gene. 1990 Jun 15;90(2):235–241. doi: 10.1016/0378-1119(90)90185-t. [DOI] [PubMed] [Google Scholar]
- Hough R., Pratt G., Rechsteiner M. Purification of two high molecular weight proteases from rabbit reticulocyte lysate. J Biol Chem. 1987 Jun 15;262(17):8303–8313. [PubMed] [Google Scholar]
- Ishiura S., Nomura Y., Tsukahara T., Sugita H. Addition of ATP increases the apparent molecular mass of the multicatalytic proteinase, ingensin. FEBS Lett. 1989 Oct 23;257(1):123–126. doi: 10.1016/0014-5793(89)81801-0. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt J. A., Escher C., Wolf D. H. Proteinase yscE of yeast shows homology with the 20 S cylinder particles of Xenopus laevis. FEBS Lett. 1988 Oct 24;239(1):35–40. doi: 10.1016/0014-5793(88)80540-4. [DOI] [PubMed] [Google Scholar]
- Kumatori A., Tanaka K., Tamura T., Fujiwara T., Ichihara A., Tokunaga F., Onikura A., Iwanaga S. cDNA cloning and sequencing of component C9 of proteasomes from rat hepatoma cells. FEBS Lett. 1990 May 21;264(2):279–282. doi: 10.1016/0014-5793(90)80267-m. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee L. W., Moomaw C. R., Orth K., McGuire M. J., DeMartino G. N., Slaughter C. A. Relationships among the subunits of the high molecular weight proteinase, macropain (proteasome). Biochim Biophys Acta. 1990 Feb 9;1037(2):178–185. doi: 10.1016/0167-4838(90)90165-c. [DOI] [PubMed] [Google Scholar]
- Lilley K. S., Davison M. D., Rivett A. J. N-terminal sequence similarities between components of the multicatalytic proteinase complex. FEBS Lett. 1990 Mar 26;262(2):327–329. doi: 10.1016/0014-5793(90)80220-d. [DOI] [PubMed] [Google Scholar]
- Martins de Sa C., Grossi de Sa M. F., Akhayat O., Broders F., Scherrer K., Horsch A., Schmid H. P. Prosomes. Ubiquity and inter-species structural variation. J Mol Biol. 1986 Feb 20;187(4):479–493. doi: 10.1016/0022-2836(86)90328-1. [DOI] [PubMed] [Google Scholar]
- McGuire M. J., Croall D. E., DeMartino G. N. ATP-stimulated proteolysis in soluble extracts of BHK 21/C13 cells. Evidence for multiple pathways and a role for an enzyme related to the high-molecular-weight protease, macropain. Arch Biochem Biophys. 1988 Apr;262(1):273–285. doi: 10.1016/0003-9861(88)90189-0. [DOI] [PubMed] [Google Scholar]
- Mykles D. L. Purification and characterization of a multicatalytic proteinase from crustacean muscle: comparison of latent and heat-activated forms. Arch Biochem Biophys. 1989 Oct;274(1):216–228. doi: 10.1016/0003-9861(89)90433-5. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Orlowski M., Michaud C. Pituitary multicatalytic proteinase complex. Specificity of components and aspects of proteolytic activity. Biochemistry. 1989 Nov 28;28(24):9270–9278. doi: 10.1021/bi00450a006. [DOI] [PubMed] [Google Scholar]
- Orlowski M. The multicatalytic proteinase complex, a major extralysosomal proteolytic system. Biochemistry. 1990 Nov 13;29(45):10289–10297. doi: 10.1021/bi00497a001. [DOI] [PubMed] [Google Scholar]
- Ray K., Harris H. Comparative studies on lens neutral endopeptidase and pituitary neutral proteinase: two closely similar enzymes. FEBS Lett. 1986 Jan 1;194(1):91–95. doi: 10.1016/0014-5793(86)80057-6. [DOI] [PubMed] [Google Scholar]
- Ray K., Harris H. Lens neutral endopeptidase occurs in other bovine and human tissues. Biochem J. 1987 Dec 15;248(3):643–648. doi: 10.1042/bj2480643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivett A. J. Purification of a liver alkaline protease which degrades oxidatively modified glutamine synthetase. Characterization as a high molecular weight cysteine proteinase. J Biol Chem. 1985 Oct 15;260(23):12600–12606. [PubMed] [Google Scholar]
- Rivett A. J. The multicatalytic proteinase of mammalian cells. Arch Biochem Biophys. 1989 Jan;268(1):1–8. doi: 10.1016/0003-9861(89)90558-4. [DOI] [PubMed] [Google Scholar]
- Rivett A. J. The multicatalytic proteinase. Multiple proteolytic activities. J Biol Chem. 1989 Jul 25;264(21):12215–12219. [PubMed] [Google Scholar]
- Skilton H. E., Eperon I. C., Rivett A. J. Co-purification of a small RNA species with multicatalytic proteinase (proteasome) from rat liver. FEBS Lett. 1991 Feb 25;279(2):351–355. doi: 10.1016/0014-5793(91)80185-6. [DOI] [PubMed] [Google Scholar]
- Sweeney S. T., Rivett A. J. Immunological properties of the multicatalytic proteinase. Biochem Soc Trans. 1989 Dec;17(6):1126–1127. doi: 10.1042/bst0171126. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Ii K., Ichihara A., Waxman L., Goldberg A. L. A high molecular weight protease in the cytosol of rat liver. I. Purification, enzymological properties, and tissue distribution. J Biol Chem. 1986 Nov 15;261(32):15197–15203. [PubMed] [Google Scholar]
- Tanaka K., Yoshimura T., Ichihara A., Ikai A., Nishigai M., Morimoto Y., Sato M., Tanaka N., Katsube Y., Kameyama K. Molecular organization of a high molecular weight multi-protease complex from rat liver. J Mol Biol. 1988 Oct 20;203(4):985–996. doi: 10.1016/0022-2836(88)90123-4. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Yoshimura T., Kumatori A., Ichihara A., Ikai A., Nishigai M., Kameyama K., Takagi T. Proteasomes (multi-protease complexes) as 20 S ring-shaped particles in a variety of eukaryotic cells. J Biol Chem. 1988 Nov 5;263(31):16209–16217. [PubMed] [Google Scholar]
- Tomek W., Adam G., Schmid H. P. Prosomes, small cytoplasmic RNP particles, contain glycoproteins. FEBS Lett. 1988 Oct 24;239(1):155–158. doi: 10.1016/0014-5793(88)80564-7. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner B. J., Margolis J. W. Common epitopes of bovine lens multicatalytic-proteinase-complex subunits. Biochem J. 1989 Jan 1;257(1):265–269. doi: 10.1042/bj2570265. [DOI] [PMC free article] [PubMed] [Google Scholar]