Abstract
Background
Patients undergoing laparoscopic renal surgery often experience significant postoperative pain. Quadratus lumborum block (QLB) provides effective postoperative pain control after laparoscopic procedures, while lidocaine administered intravenously also exerts analgesic effects for surgical patients. We design this trial to compare the effects of i.v. lidocaine infusion with QLB on postoperative analgesia in patients undergoing laparoscopic renal surgery.
Methods
In this randomized noninferiority trial, a total of 120 adult patients undergoing laparoscopic renal surgery will be randomized to receive either i.v. lidocaine or unilateral QLB for postoperative pain management. Lidocaine will be i.v. administered at 1.5 mg/kg (ideal body weight) over 10 min during anesthesia induction, followed by an infusion of 1.5 mg/kg/h intraoperatively and in a post-anesthesia care unit. Ultrasound-guided anterior QLB with 0.375% ropivacaine 30 mL will be conducted before the start of surgery. Patient-controlled i.v. sufentanil will be used for pain relief during the first 48 h after surgery. The primary outcome is the cumulative sufentanil consumption during 0–24 h postoperatively, with a noninferiority margin of 5 μg. Secondary outcomes include pain intensity at rest and on coughing at 1, 6, 24, and 48 h postoperatively; sufentanil consumption within 24–48 h after surgery; rescue analgesic use within 0–48 h after surgery; nausea and vomiting within 0–48 h postoperatively; and quality of recovery at 24 and 48 h after surgery.
Discussion
The results of this trial will add to the clinical evidence for improving postoperative pain management in patients who undergo laparoscopic renal surgery.
Trial Registration
Chinese Clinical Trial Registry (ChiCTR2400082974).
Keywords: intravenous lidocaine, quadratus lumborum block, postoperative analgesia, quality of recovery, laparoscopic renal surgery
Introduction
Laparoscopic radical or partial nephrectomy is associated with moderate-to-severe postoperative pain.1–4 Pain after these surgical procedures is multifactorial, including incisional pain, visceral pain, and sometimes pneumoperitoneum-induced shoulder pain. Significant pain decreases patient comfort, increases postoperative complications, and prolongs recovery after surgery.5,6 Optimizing pain management is a priority in perioperative care for patients undergoing laparoscopic renal surgery.
Neuraxial or regional analgesia has been widely applied as a component of multimodal analgesic strategies for patients undergoing surgery.6 Ultrasound-guided quadratus lumborum block (QLB) is an abdominal trunk block first described in 2007,7 and it has been demonstrated as an effective technique to reduce postoperative pain and opioid requirement and facilitate early recovery after laparoscopic renal surgery.2,3,8,9 The administration of intravenous lidocaine produces anti-inflammation, anti-nociceptive, and opioid-sparing effects, which has been proven to improve postoperative pain management for surgical patients in many recent studies.6,10–12 Nonetheless, the effects of perioperative i.v. lidocaine infusion and QLB on pain outcomes after laparoscopic renal surgery have not been compared head-to-head.
We perform this randomized trial with a noninferiority design to assess i.v. lidocaine vs QLB on postoperative analgesia following laparoscopic renal surgery. We hypothesize that i.v. lidocaine is noninferior to QLB regarding the primary outcome of cumulative sufentanil consumption during the first 24 h after surgery. Additionally, we will evaluate the severity of postoperative pain, need for rescue analgesia, nausea and vomiting, and recovery quality in these surgical patients.
Methods
Study Design
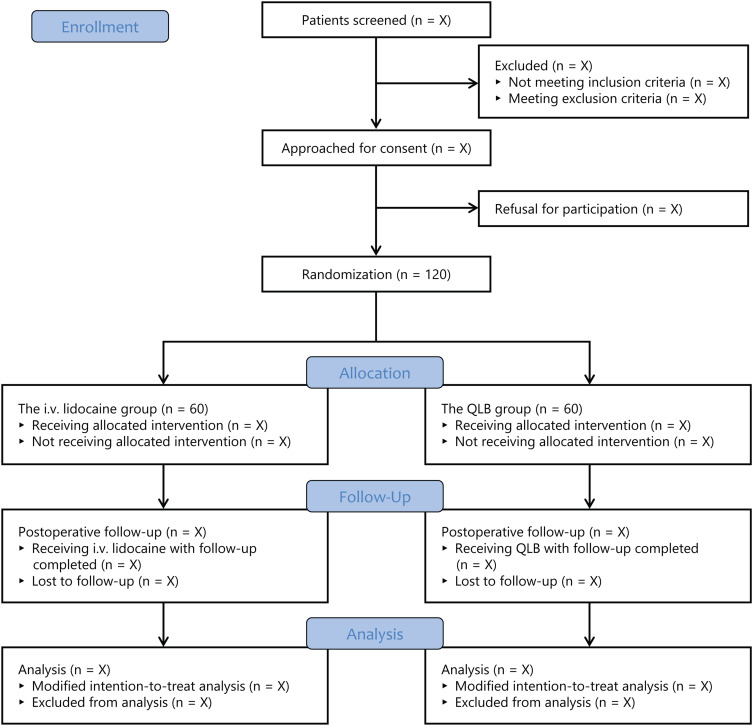
This is a prospective, single-center, randomized, controlled noninferiority trial with two parallel groups. The study protocol was approved by the Institutional Review Board of the First Affiliated Hospital of Soochow University on March 22, 2024 (approval No. 2024–069) and then registered on the Chinese Clinical Trial Registry on April 12, 2024 (identifier: ChiCTR2400082974; principal investigator: Ke Peng; available at: https://www.chictr.org.cn/showproj.html?proj=226088). This trial will be implemented in accordance with the Declaration of Helsinki. Written informed consent will be obtained from all patients. This protocol is presented according to the guideline of Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) (Supplemental File 1).13 The trial flow diagram is illustrated in Figure 1.
Figure 1.
Study flow diagram. QLB, quadratus lumborum block.
Inclusion and Exclusion Criteria
To be eligible for inclusion, patients should meet the following criteria: (1) age ≥18 years, (2) ASA physical status I to III, and (3) undergoing laparoscopic renal surgery (including nephrectomy and partial nephrectomy) under general anesthesia.
The exclusion criteria are: (1) unplanned or emergent surgery, (2) BMI ≥ 35 kg/m2, (3) allergy to anesthetic drugs or contraindications to i.v. lidocaine (electrolyte disorders, seizure disorders, neurological disorders, renal or hepatic impairment); (4) contraindications to QLB (coagulopathy, infection, or sepsis), (5) admission to intensive care unit after surgery, (6) severe cardiopulmonary or cerebrovascular diseases, (7) alcohol abuse, long-term use of opioids or other analgesics, (8) unwillingness to use patient-controlled analgesia, or (9) inability to cooperate or refusal for enrollment.
Randomization and Blinding
A research assistant without any clinical involvement performs the computer-generated block randomization, with an allocation ratio of 1:1, permuted blocks of 2, 4, and 6, and stratification by type of surgery (nephrectomy or partial nephrectomy). The randomization list is concealed in sequentially numbered sealed opaque envelopes. Upon patient arrival at a preoperative room, they receive i.v. cannulation with or without regional blocks. An investigator not involved in patient enrollment, perioperative care, or outcome assessment opens the envelopes to randomly assign patients to the i.v. lidocaine group or the QLB group (Figure 1). Anesthesiologists and surgeons are not blinded to group assignment, while all patients and trained investigators responsible for postoperative follow-up will remain blinded.
Anesthesia
Patients will receive routine monitoring with 5-lead electrocardiography, non-invasive blood pressure, and pulse oximetry. A radial arterial cannulation will also be used for continuous arterial blood pressure monitoring and blood sampling if indicated. Patients will receive standardized anesthesia care. General anesthesia induction will be performed with propofol 2 mg/kg and sufentanil 0.3 μg/kg. After endotracheal intubation with rocuronium 0.6 mg/kg, mechanical ventilation will be started. Anesthesia will be maintained with inhalation of 1–3% sevoflurane in an oxygen/air mixture, titrated to a bispectral index of 40–60. Additional sufentanil and rocuronium will be used for intraoperative analgesia and neuromuscular block. Fluid administration and hemodynamic events such as hypotension (mean blood pressure <65 mmHg) and bradycardia (heart rate <50 beats/min) will be managed at the discretion of the anesthesia team. Prophylaxis of postoperative nausea and vomiting (PONV) will be performed with dexamethasone and palonosetron. After surgery, patients will be discharged to a post-anesthesia care unit (PACU).
The multimodal pain management includes one of the study interventions (i.v. lidocaine infusion or QLB), oral acetaminophen, and patient-controlled sufentanil. Patients will receive oral acetaminophen (500 mg 1 h preoperatively and every 6 h during the first two postoperative days). The patient-controlled analgesia system will be programmed to deliver sufentanil 1 μg bolus on patient demand (a lockout interval of 5 min, no background infusion) from PACU admission to 48 h after surgery. Postoperative pain intensity will be measured using the numeric rating scale (NRS), ranging from 0 to 10 with 0 denoting no pain and 10 denoting the worst pain. If patients experience significant pain (defined as a NRS score exceeding 3), rescue analgesia with additional sufentanil 5 μg will be administered.
Study Interventions
In the i.v. lidocaine group, patients will receive a loading dose of lidocaine (1.5 mg/kg) intravenously administered over 10 min at anesthesia induction, followed by a continuous infusion (1.5 mg/kg/h) during surgery and until 1 h after arrival at the PACU. We select this dosage based on our pilot observation and recent literature.10,11,14 The dose of lidocaine will be calculated using the patient’s ideal body weight (male: height [cm] – 100; female: height [cm] – 105).15
In the QLB group, a single-shot anterior QLB with the target lumbar level of L2 will be performed before anesthesia induction.16 Patients will be placed in the lateral decubitus position with the surgical side up. An ultrasound transducer (SonoSite, Bothell, WA, USA) will be placed transversely between the twelfth rib and the iliac crest to identify the quadratus lumborum muscle. A 22-gauge 100-mm needle will be advanced in-plane into the fascial interspace posterior to the quadratus lumborum muscle. After negative aspiration, 0.9% normal saline (1–2 mL) will be injected to confirm the correct needle tip positioning, and then 0.375% ropivacaine (30 mL) will be incrementally injected. All QLB procedures will be performed by an experienced attending anesthesiologist who has performed this technique for more than 5 years.
Study Outcomes
The primary outcome is the cumulative sufentanil consumption during 0–24 h postoperatively. The secondary outcomes include: (1) pain intensity at rest and on coughing at 1, 6, 24, and 48 h postoperatively, assessed using the NRS; (2) sufentanil consumption within 24–48 h after surgery; (3) rescue analgesic use within 0–48 h after surgery; (4) PONV within 0–48 hours after surgery; and (5) quality of recovery at 24 and 48 h after surgery, assessed using the 15-item quality of recovery scale (ranging 0–150 with a higher score denoting a better quality of recovery).17–19 In addition, safety outcomes include local anesthetic toxicity (such as dizziness, tinnitus, metallic taste, and arrhythmias) and muscle weakness.
Data Management
Table 1 presents the scheduled process of patient enrollment, interventions, and assessments according to the SPIRIT guideline. During the preoperative visit, a trial investigator not involved in anesthesia or postoperative follow-up will screen for eligible patients, collect baseline data (including sex, age, weight, height, ASA classification, preoperative medications, comorbidities, and preoperative renal function), and obtain written informed consent. Intraoperative and anesthesia data (including type of surgery, anesthetic and analgesic doses, blood pressure and heart rate values, hemodynamic events, length of anesthesia, length of surgery, length of PACU stay, and length of postoperative hospital stay) will be extracted from the electronic anesthesia information system (DoCare V5.0, Suzhou MedicalSystem Company, Suzhou, China). Trained investigators blinded to group allocation will assess study outcomes (including sufentanil consumption, pain intensity, rescue analgesia, PONV, and quality of recovery) and document adverse events (symptoms of local anesthetic toxicity and severe postoperative complications) during the postoperative follow-up.
Table 1.
Schedule of Patient Enrollment, Interventions, and Assessments According to SPIRIT Statement
| Study Period | |||||||
|---|---|---|---|---|---|---|---|
| Timepoint | Enrollment | Allocation | Post-Allocation | Closeout | |||
| Preoperative Visit | Before Surgery | During Surgery | Postoperative Care Unit | Day 1 | Day 2 | Hospital Discharge | |
| Enrollment | |||||||
| Inclusion criteria | × | ||||||
| Exclusion criteria | × | ||||||
| Written informed consent | × | ||||||
| Baseline data | × | ||||||
| Randomization | × | ||||||
| Allocation | × | ||||||
| Interventions | |||||||
| Intravenous lidocaine | × | × | |||||
| Quadratus lumborum block | × | ||||||
| Assessments | |||||||
| Sufentanil consumption | × | × | |||||
| Pain at rest | × | × | × | ||||
| Pain on coughing | × | × | × | ||||
| Rescue analgesia | × | × | × | ||||
| Postoperative nausea and vomiting | × | × | × | ||||
| Quality of recovery | × | × | |||||
| Adverse events | × | × | |||||
| Postoperative hospital stay | × | ||||||
Notes: According to SPIRIT statement of defining standard protocol items for clinical trials.
Data will be recorded in the care report forms and then entered in an online database (https://www.toolset8.com/e) with password protection. After the completion of this trial, the dataset without identifiable patient personal information will be sent to a statistician for formal analysis. Our institutional data monitoring committee will supervise the implementation of this study.
Sample Size
The noninferiority design of this trial is based on the primary outcome (sufentanil consumption during 0–24 h after surgery). In our pilot observation, the mean ± standard deviation (SD) 24-h sufentanil consumption was 36 ± 7 μg in patients receiving i.v. lidocaine infusion and 35 ± 6 μg in those receiving QLB (n = 15 in each group). According to the recent data from a meta-analysis comparing QLB with control (sham block or no block) for patients undergoing nephrectomy, the mean reduction in morphine equivalents was 11.6 mg (equal to sufentanil 11.6 μg).20 We assume that a noninferiority margin of 5 μg would be acceptable from the clinical perspective. Considering a possible 5% loss to follow-up rate, a total of 120 patients (n = 60 in each group) are needed to test the noninferiority of lidocaine with a power of 90% at a significance level of 0.025. We calculated the sample size using the PASS (version 15.0, NCSs, LLC).
Statistical Analysis
Statistical analyses will be performed with SPSS 26.0 (IBM SPSS, Chicago, IL, USA). Data will be presented as mean ± SD, median and interquartile range, and number (%), depending on the type of data. The between-group comparisons will be tested with student t test, Mann–Whitney U-test, χ2 test, or Fisher’s exact test. The mean/median difference or relative risk with 95% confidence intervals (CI) will be used to analyze the treatment effects of i.v. lidocaine vs QLB.
For the primary outcome of 24-h sufentanil consumption, the noninferiority of i.v. lidocaine will be concluded when the 95% CI upper boundary of the between-group difference is less than 5 μg (the noninferiority margin). In addition, we will conduct a superiority test for this comparison. The prespecified subgroup analyses will be performed based on age (< 60 years vs ≥ 60 years), sex (male vs female), type of surgery (nephrectomy vs partial nephrectomy), and use of dexmedetomidine (yes vs no). For secondary outcomes, corrections for multiple comparisons are not planned; thus, these outcome data will necessarily be interpreted as exploratory.
All study results will be analyzed in the full analysis set (including patients randomly allocated to receive i.v. lidocaine or QLB) and in the per-protocol set (including patients completing the trial without significant protocol violation). Missing data will not be imputed. A P value < 0.05 is deemed statistically significant.
Discussion
In this randomized controlled noninferiority trial, we will include a total of 120 adult patients undergoing laparoscopic renal surgery to compare i.v. lidocaine with anterior QLB. The primary focus is postoperative pain control, assessed using the cumulative sufentanil consumption during the first 24 h postoperatively. We will also observe the treatment effect on pain intensity during 0–48 h postoperatively, need for rescue analgesia, PONV, and the quality of recovery at 24 and 48 h after surgery.
We design this noninferiority trial investigating i.v. lidocaine vs QLB for these reasons: (1) the analgesic effect of QLB for patients undergoing renal surgery has been well recognized. Data from recent meta-analyses confirmed that QLB compared with sham block or no block reduced postoperative opioid consumption and improved analgesia in the first 24 h after renal surgery;20,21 and (2) QLB has been frequently performed in our clinical practice, as a component of multimodal analgesia for patients undergoing various procedures including renal surgery. Therefore, we do not set a placebo group, and QLB will be performed as the standard intervention to ensure adequate postoperative pain control.
Lidocaine i.v. administration may improve postoperative pain and recovery, but the existing evidence is inconsistent.15,22–25 According to the international consensus statement, the ideal body weight should be used for dose calculation of i.v. lidocaine.15 Thus, we will follow this statement to calculate the dose of lidocaine in our patients. We design this trial with the primary hypothesis that i.v. lidocaine is noninferior to QLB in terms of postoperative sufentanil consumption. However, it is possible that i.v. lidocaine may be superior to QLB for postoperative analgesia in these patients. In addition to the noninferiority test, we will also conduct a superiority test to further analyze the analgesic effects of i.v. lidocaine vs QLB.
There are some limitations of this study. First, the dosage of i.v. lidocaine is selected based on the previous studies and our pilot observation. The optimal dosage of i.v. lidocaine for patients undergoing laparoscopic renal surgery needs more studies. Second, we do not measure the plasma concentration of lidocaine. This dose of i.v. lidocaine (1.5 mg/kg followed by 1 mg/kg/h intraoperatively) is relatively low with a good safety profile. Third, anesthesiologists and surgeons are not blinded to group assignment; however, all patients and investigators responsible for postoperative follow-up will remain blinded. Last, there are several different QLB approaches reported in the literature, and our approach may not be the preferred choice by other practitioners or in other clinical settings.
In summary, we will conduct this randomized noninferiority trial to compare i.v. lidocaine infusion with QLB for the purpose of optimizing pain management and improving postoperative recovery in patients undergoing laparoscopic renal surgery.
Funding Statement
This work will be supported by Suzhou Medical Health Science and Technology Innovation Project (SKY2022136), National Natural Science Foundation of China (82471290), Key Medical Research Projects in Jiangsu Province (ZD2022021), and Suzhou Clinical Medical Center for Anesthesiology (Szlcyxzxj202102). The funders have no role in the study design, data collection, data analysis, interpretation, or writing of this manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Kaya C, Dost B, Turunc E, Dokmeci H. Comparison of the effects of subcostal anterior quadratus lumborum block and thoracic paravertebral block in laparoscopic nephrectomy: a randomized study. Minerva Anestesiol. 2023;89(11):986–995. doi: 10.23736/S0375-9393.23.17433-5 [DOI] [PubMed] [Google Scholar]
- 2.Li X, Xu ZZ, Li YT, Lin ZM, Liu ZY, Wang DX. Analgesic efficacy of two approaches of ultrasound-guided quadratus lumborum block for laparoscopic renal surgery: a randomised controlled trial. Eur J Anaesthesiol. 2021;38(3):265–274. doi: 10.1097/EJA.0000000000001433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Y, Wang L, Sun J, et al. Effects of subcostal anterior quadratus lumborum block with and without dexmedetomidine on postoperative rehabilitation in patients undergoing laparoscopic renal surgery: a prospective double-blinded randomized controlled study. Drug Des Devel Ther. 2023;17:3281–3293. doi: 10.2147/DDDT.S422356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peng F, Li Y, Ai Y, Yang J, Wang Y. Application of preoperative assessment of pain induced by venous cannulation in predicting postoperative pain in patients under laparoscopic nephrectomy: a prospective observational study. BMC Anesthesiol. 2020;20(1):86. doi: 10.1186/s12871-020-01003-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kehlet H. Postoperative pain, analgesia, and recovery-bedfellows that cannot be ignored. Pain. 2018;159:S11–S16. [DOI] [PubMed] [Google Scholar]
- 6.Pirie K, Traer E, Finniss D, Myles PS, Riedel B. Current approaches to acute postoperative pain management after major abdominal surgery: a narrative review and future directions. Br J Anaesth. 2022;129(3):378–393. doi: 10.1016/j.bja.2022.05.029 [DOI] [PubMed] [Google Scholar]
- 7.Dhanjal S, Tonder S. Quadratus Lumborum Block. In: StatPearls. Treasure Island (FL): StatPearls; 2024. [PubMed] [Google Scholar]
- 8.Borys M, Szajowska P, Jednakiewicz M, et al. Quadratus lumborum block reduces postoperative opioid consumption and decreases persistent postoperative pain severity in patients undergoing both open and laparoscopic nephrectomies-A randomized controlled trial. J Clin Med. 2021;10(16). doi: 10.3390/jcm10163590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prasad Mantha S, Nair A, Kodisharapu PK, Anne P, Naik VM, Rayani BK. Ultrasound-guided continuous transmuscular quadratus lumborum block for postoperative analgesia in patients undergoing radical nephrectomy: a randomized controlled trial. Cureus. 2021;13(10):e19120. doi: 10.7759/cureus.19120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dewinter G, Coppens S, Van de Velde M, et al. Quadratus lumborum block versus perioperative intravenous lidocaine for postoperative pain control in patients undergoing laparoscopic colorectal surgery: a prospective, randomized, double-blind controlled clinical trial. Ann Surg. 2018;268(5):769–775. doi: 10.1097/SLA.0000000000002888 [DOI] [PubMed] [Google Scholar]
- 11.Casas-Arroyave FD, Osorno-Upegui SC, Zamudio-Burbano MA. Therapeutic efficacy of intravenous lidocaine infusion compared with thoracic epidural analgesia in major abdominal surgery: a noninferiority randomised clinical trial. Br J Anaesth. 2023;131(5):947–954. doi: 10.1016/j.bja.2023.07.032 [DOI] [PubMed] [Google Scholar]
- 12.Xu Y, Ye M, Liu F, et al. Efficacy of prolonged intravenous lidocaine infusion for postoperative movement-evoked pain following hepatectomy: a double-blinded, randomised, placebo-controlled trial. Br J Anaesth. 2023;131(1):113–121. doi: 10.1016/j.bja.2023.03.026 [DOI] [PubMed] [Google Scholar]
- 13.Chan AW, Tetzlaff JM, Gøtzsche PC, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346:e7586. doi: 10.1136/bmj.e7586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eipe N, Gupta S, Penning J. Intravenous lidocaine for acute pain: an evidence-based clinical update. BJA Educ. 2016;16(9):292–298. doi: 10.1093/bjaed/mkw008 [DOI] [Google Scholar]
- 15.Foo I, Macfarlane AJR, Srivastava D, et al. The use of intravenous lidocaine for postoperative pain and recovery: international consensus statement on efficacy and safety. Anaesthesia. 2021;76(2):238–250. doi: 10.1111/anae.15270 [DOI] [PubMed] [Google Scholar]
- 16.El-Boghdadly K, Wolmarans M, Stengel AD, et al. Standardizing nomenclature in regional anesthesia: an ASRA-ESRA Delphi consensus study of abdominal wall, paraspinal, and chest wall blocks. Reg Anesth Pain Med. 2021;46(7):571–580. doi: 10.1136/rapm-2020-102451 [DOI] [PubMed] [Google Scholar]
- 17.Stark P, Myles P, Burke J. Development and psychometric evaluation of a postoperative quality of recovery score: the QoR-15. Anesthesiology. 2013;118(6):1332–1340. doi: 10.1097/ALN.0b013e318289b84b [DOI] [PubMed] [Google Scholar]
- 18.Myles P, Shulman M, Reilly J, Kasza J, Romero L. Measurement of quality of recovery after surgery using the 15-item quality of recovery scale: a systematic review and meta-analysis. Br J Anaesth. 2022;128(6):1029–1039. doi: 10.1016/j.bja.2022.03.009 [DOI] [PubMed] [Google Scholar]
- 19.Bu X, Zhang J, Zuo Y. Validation of the Chinese version of the quality of recovery-15 score and its comparison with the post-operative quality recovery scale. Patient. 2016;9(3):251–259. doi: 10.1007/s40271-015-0148-6 [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Lin C, Liu J. Ultrasound-guided quadratus lumborum block for postoperative analgesia in renal surgery: a systematic review and meta-analysis of randomized controlled trials. J Anesth. 2022;36(2):254–264. doi: 10.1007/s00540-022-03040-z [DOI] [PubMed] [Google Scholar]
- 21.Jin Z, Liu J, Li R, Gan TJ, He Y, Lin J. Single injection quadratus lumborum block for postoperative analgesia in adult surgical population: a systematic review and meta-analysis. J Clin Anesthes. 2020;62:109715. doi: 10.1016/j.jclinane.2020.109715 [DOI] [PubMed] [Google Scholar]
- 22.Hussain N, Brull R, Weber L, et al. The analgesic effectiveness of perioperative lidocaine infusions for acute and chronic persistent postsurgical pain in patients undergoing breast cancer surgery: a systematic review and meta-analysis. Br J Anaesth. 2024;132(3):575–587. doi: 10.1016/j.bja.2023.12.005 [DOI] [PubMed] [Google Scholar]
- 23.Lemoine A, Witdouck A, Beloeil H, Bonnet F, Pwgotesor A, Pain T. PROSPECT guidelines update for evidence-based pain management after prostatectomy for cancer. Anaesth Crit Care Pain Med. 2021;40(4):100922. doi: 10.1016/j.accpm.2021.100922 [DOI] [PubMed] [Google Scholar]
- 24.Yu JM, Tao QY, He Y, Liu D, Niu JY, Zhang Y. Opioid-free anesthesia for pain relief after laparoscopic cholecystectomy: a prospective randomized controlled trial. J Pain Res. 2023;16:3625–3632. doi: 10.2147/JPR.S432601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yurttas T, Djurdjevic M, Schnider TW, Filipovic M. Analgesic efficacy of systemic lidocaine using lean body mass based dosing regime versus placebo in bariatric surgery: a prospective, randomised, double-blind, placebo-controlled, single-centre study. Br J Anaesth. 2023;131(1):122–129. doi: 10.1016/j.bja.2023.03.027 [DOI] [PubMed] [Google Scholar]