Abstract
Background
An estimated 185,000 patients per year undergo an extremity amputation in the United States (over 500 amputations/day). Prolonged postoperative opioid use, defined as the presence of a filled opioid prescription between 90 and 180 days following the operative amputation procedure, affects nearly 50% of amputees. Moreover, the use of preoperative benzodiazepines, muscle relaxants, anticonvulsants, and antidepressants is strongly linked to prolonged opioid use suggesting new therapeutic strategies are needed. The goal of this study was to better understand how well post-amputation pain is currently treated by selected pharmacologic agents and the success rates of these existing agents.
Methods
The available literature on PubMed was screened for articles that used randomized-controlled trial (RCT) study designs and gabapentinoids (eg, gabapentin or pregabalin) or opioids (eg, morphine). Two morphine-related RCTs using at least 50% pain reduction responder criteria were combined and qualitatively compared with two gabapentin trials that were previously combined to understand the potential benefits of these drugs in post-amputation pain management compared with placebo.
Results
All 4 trials included measured post-amputation pain over a 4- to 6-week acute period. The combined opioid analysis demonstrated a treatment effect that favored morphine (P=0.02) over placebo and indicated the number needed to treat (NNT) of 3.9 (95% CI: 2.5, 9.3) patients. Similarly, the previously combined analysis of gabapentin trials favored gabapentin over placebo and indicated an estimated NNT of 8.9 (95% CI: 5.3, 27.8).
Conclusion
Patients undergoing limb amputation have a clear unmet need for more adequate chronic pain control. Given that post-amputation pain is often diagnosed as a chronic condition, persisting for at least 90 days, our data highlight the need for larger sample sizes and longer-term controlled trials to better understand the advantages and disadvantages of chronic use of gabapentinoids and opioids/opioid combination drugs in this patient population.
Keywords: post-amputation pain, morphine, gabapentinoids, chronic opioid use
Introduction
In the United States, an estimated 185,000 patients undergo an upper or lower limb extremity amputation each year, which translates to over 500 amputations/day.1 Nearly 80% of patients describe phantom pain post-amputation, with a higher prevalence among the lower extremity amputation population.1 Perioperative therapeutic pain relief is often administered in the form of narcotic pain medications, such as opioids. However, narcotic usage is not limited to the immediate post-operative period. Prolonged opioid use, defined as the presence of a filled opioid prescription between 90 and 180 days following the operative amputation procedure, is a problem that affects nearly 50% of patients with post-amputation pain. Moreover, use of benzodiazepines, muscle relaxants, anticonvulsants, and antidepressants post-amputation are all associated with an increased risk of prolonged postoperative narcotic use.1
Attempts to preserve or restore the quality of life for patients experiencing post-amputation pain have mainly involved pharmacologic interventions, such as opioids and anti-convulsion drugs, though an inadequate response to drug treatment has been well-documented.2 Furthermore, the dual nature of opioids as both a benefit for pain control and a risk to health, function, and well-being has also been consistently reported.3 More specifically, opioid use reduces the negative consequences of acute postsurgical pain and allows patients an easier transition back to normal function.4 However, the extended use of opioids for post-amputation pain can lead to drug dependency and unmanaged chronic pain. This chronic opioid usage is also associated with poor general health, disability, depression, social withdrawal, and the development of other comorbidities.1,5 The reality that the underlying causes of amputation such as diabetes mellitus, peripheral vascular disease, neuropathy, and trauma are unlikely to be eradicated highlights the need for new treatment modalities for patients undergoing amputations. The goal of this study was to better understand current pharmaceutical treatment approaches to post-amputation pain and the combined analyses of pain reduction from randomized controlled trials (RCTs) evaluating opioids and gabapentinoids.
Materials and Methods
The post-amputation pain literature was screened on PubMed using a four-step process. The first search used the terms “post-amputation pain” or “phantom pain” or “residual limb pain” and yielded a total of 3261 articles published from 1936 to the present. A second search using the terms “trial” or “efficacy” or “effect” narrowed the number of relevant articles to 240. The search was further narrowed to 54 articles using the term “drug” to exclude studies that evaluated nonpharmacological interventions. The remaining articles were manually screened by the authors to identify RCTs investigating gabapentinoids or opioids where ≥50% pain reduction was reported. A ≥50% pain reduction threshold was chosen based on prior analyses of 10 studies evaluating pregabalin in chronic pain (study duration range, 5–12 weeks) where >50% improvement in pain intensity numerical rating scale (NRS) score was associated with a “very much improved” outcome on the Patient Global Impression of Change instrument.6,7 Two RCTs evaluating the use of an opioid (eg, morphine) were identified.8,9 The search results from the first step in the literature search process (N = 3261 articles) were then manually rescreened for studies that evaluated gabapentinoids. Two gabapentinoid (eg, gabapentin or pregabalin) post-amputation pain RCTs10,11 were identified from a systematic review of chronic postsurgical pain that appeared in the search results.5 Although these trials did not use >50% pain reduction criteria, the authors reported average pain reduction in post-amputation pain subjects over 4–6 weeks and these studies were included for comparison.
The two morphine-related RCTs8,9 were combined to estimate the treatment and placebo responder rates of subjects who demonstrated at least 50% pain reduction from baseline. Heterogeneity was expected and controlled for via the Fleiss method.12,13 This method uses a unique inverse variance weighting for results from the studies and also includes an adjustment for heterogeneity of the responses. The number needed to treat (NNT) for the combined analysis treatment effect of the 2 studies was calculated as the reciprocal of the decimal Total Treatment Effect rounded to the nearest whole number, where Treatment Effect = (Treatment Responder Rate [%] – Placebo Responder Rate [%])/100. NNT is an aggregate statistical measure to ascertain the number of patients needed to be treated with a specific therapeutic intervention to provide the next additional patient with the treatment effect observed in a RCT.14
The two gabapentin-related RCTs10,11 were previously combined by Wylde et al5 to estimate the mean difference between gabapentin versus placebo using pain intensity NRS scores from 0–10. The above mean difference analyses did not include a responder rate criterion or analysis, preventing the calculation of a combined analysis of NNT for gabapentin in these post-amputation pain studies. To overcome this limitation, we converted the total mean difference in NRS pain scores to an absolute-value, decimal Total Treatment Effect (measured on a scale of –1 to 1) and took the reciprocal to generate an estimated NNT for gabapentin.
Results
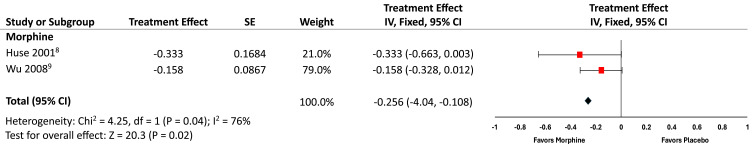
The two morphine RCTs8,9 that met our criteria for combined analysis are listed in Figure 1 and favored morphine compared with placebo (P=0.02) over the 4–6-week study period, though morphine use was associated with a higher rate of side effects in one study.9 The forest plot in Figure 1 illustrates the responder rate data in terms of Treatment Effect, with the associated 95% confidence intervals. The combined morphine Treatment Effect (absolute value = 0.256) converts to a NNT = 3.9 (95% CI: 2.5, 9.3) for post-amputation pain patients in our analysis.
Figure 1.
Combined analysis treatment effect of morphine versus placebo for treatment of chronic phantom limb pain.
Abbreviation: IV, instrumental variable.
In a previous analysis including two gabapentin-related trials for post-amputation pain,10,11 the average difference in NRS pain scores relative to placebo was −1.12 (95% CI: −1.89, −0.36), suggesting that the use of gabapentin had a favorable overall effect versus placebo (P=0.004).5 Gabapentin was well tolerated, with few10 to no11 adverse events recorded in either study. We converted the reported 1.12-point improvement in NRS pain scores to a Total Treatment Effect of −0.112 and generated an estimated NNT of 8.9 (95% CI: 5.3, 27.8) for gabapentin.
Discussion
Despite the high prevalence of post-amputation pain, our search of the existing literature reveals a paucity of RCTs evaluating the efficacy of therapeutic agents at the threshold associated with “very much improved” outcomes,6 highlighting the lack of evidence available in a patient population where current treatment approaches are highly variable.15 Similar to a previous systematic review of the management of chronic pain after surgery,5 our analysis is limited by the number of studies, the sample sizes of available studies, and the different study designs. As such, given that there are only two studies in each analysis, the combined results for opioids and for gabapentinoids are biased by the largest sample size in each study pair. Overwhelmingly, the studies above were designed for acute pain management (4–6 weeks), offering little to no insight into the benefits and risks of the chronic usage of these medications for patients with post-amputation pain. Additionally, heterogeneity in study design and outcome metrics also made the comparison challenging.
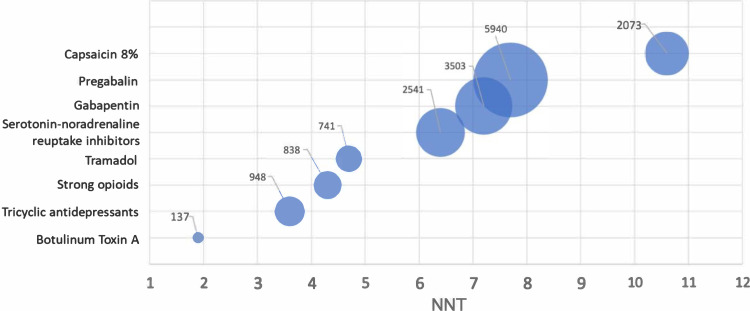
Despite these limitations, our NNT = 3.9 from a combined analysis of two post-amputation pain opioid trials and an estimated NNT of 8.9 based on the combined analysis by Wylde et al of two post-amputation pain gabapentin trials are similar to those found in a systematic review using a responder rate efficacy criterion of at least 50% pain reduction in adult neuropathic pain.2,5 NNT data from the above review (summarized in Figure 2) showed a large range for at least 50% pain relief in neuropathic pain studies for eight commonly prescribed drugs.2 These range from the strong opioid NNT of 4.3 (95% CI: 3.4, 5.8) to the gabapentinoid NNTs of 7.2 (95% CI: 5.9, 9.1) for gabapentin and 7.7 (95% CI: 6.5, 9.4) for pregabalin. Taken together, although the sample sizes were small in the combined analyses for opioid8,9 and gabapentinoids10,11 in post-amputation pain, there is general NNT agreement for opioids and gabapentinoids in our analysis with the larger neuropathic pain analysis.
Figure 2.
NNT bubble plot to achieve at least 50% pain relief for peripheral neuropathic pain drugs*.
Notes: *Bubbles represent total number of subjects analyzed, data from Finnerup et al.2
Abbreviation: NNT, number needed to treat.
Qualitatively, the two studies that used gabapentin for post-amputation pain corroborate the higher-than-opioid NNT for gabapentinoids given that treatment with gabapentin was described as better than placebo in one study,10 although it did not substantially affect pain in the other study.11 Figure 2 also shows that – despite higher NNTs – 56% (9443/16,721) of the total neuropathic pain subjects analyzed in the prior systematic review were studied in RCTs with gabapentin and pregabalin.2 This majority sample size may represent a hypothesized benefit-to-risk ratio of moderate neuropathic pain relief without opioid-like dependency. However, morphine/opioid treatment in post-amputation pain8,9 and in neuropathic pain (Figure 2) showed greater efficacy and provided a lower NNT, respectively, indicating a more favorable effect on pain management. While opioids are well accepted for the treatment of acute pain and terminal pain, opioid use for the treatment of chronic non-cancer pain remains controversial.16 Mild side effects (eg, constipation, nausea) are commonly acknowledged and reported in acute pain trials,17 but more severe side effects (eg, opioid-induced respiratory depression) are common in patients with multiple comorbidities such as amputees.18 Further, physical dependence and addiction remain critical factors in evaluating the benefits and risks of opioid therapy, particularly with regard to acute versus chronic use.3
Conclusion
While our analysis found that both opioids and gabapentinoids are more effective than placebo in managing post-amputation pain, very few studies met the inclusion criterion of reporting ≥50% pain reduction, a threshold indicating substantial improvement.6 Patients undergoing limb amputation have a clear unmet need for more adequate chronic pain control. Given that post-amputation pain is often diagnosed as a chronic condition, persisting for at least 90 days, our data highlight the need for larger sample sizes and longer-term controlled trials to better understand the advantages and disadvantages of chronic use of gabapentinoids and opioids/opioid combination drugs in this patient population.
Acknowledgments
The authors thank Julie Broderick, RAC and Daniel Sessler, MD for their helpful comments and review of this manuscript. JetPub Scientific Communications LLC assisted the authors in the preparation of this manuscript.
Funding Statement
Funding for this work was provided by Neuros Medical, Inc.
Disclosure
Dr. Arthur is a consultant for Arsenal, Balt, Johnson and Johnson, Medtronic, Microvention, Neuros, Penumbra, Perfuze, Scientia, Siemens, Stryker; reports research support from Balt, Medtronic, Microvention, Penumbra and Siemens; and is a shareholder in Azimuth, Bendit, Cerebrotech, Endostream, Magneto, Mentice, Neurogami, Neuros, Perfuze, Revbio, Scientia, Serenity, Synchron, Tulavi, Vastrax, and VizAI. Dr. Chiacchierini is a consultant to Neuros Medical but does not have a financial interest in any companies with opioid or non-opioid pain relief products. Dr. Hargus is the VP, Clinical and Medical Affairs at Neuros Medical, Inc. Dr. Patterson was the Chief Technology Officer at Neuros Medical, Inc. at the time of this study. Dr. Kapural reports financial interest in Neuros, Nevro, Biotronik, Nalu, Medtronic, Xalud, Saul Therapeutics, Saluda Medical, NeuraLace, Gimer Medical, and Avanos; is a member of the scientific advisory board for Presidio, Saluda Medical, and Biotronik; Paid Speaker for Nevro and Saluda Medical. The authors report no other conflicts of interest in this work.
References
- 1.Park RH, Liston JM, Samuel AR, Forster GL, Degeorge BR. Risk Factors for Prolonged Opioid Consumption in Lower Extremity Amputees. Plast Reconstr Surg Glob Open. 2022;10(2):E4026. doi: 10.1097/GOX.0000000000004026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: systematic review, meta-analysis and updated NeuPSIG recommendations. Lancet Neurol. 2015;14(2):162. doi: 10.1016/S1474-4422(14)70251-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Humphreys K, Shover CL, Andrews CM, et al. Responding to the opioid crisis in North America and beyond: recommendations of the Stanford-Lancet Commission. Lancet. 2022;399(10324):555–604. doi: 10.1016/S0140-6736(21)02252-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horn R, Hendrix JM, Kramer J Postoperative Pain Control. 2024. Available from: https://www.ncbi.nlm.nih.gov/books/NBK544298/. Accessed April 8, 2024. [PubMed]
- 5.Wylde V, Dennis J, Beswick AD, et al. Systematic review of management of chronic pain after surgery. Br J Surg. 2017;104(10):1293–1306. doi: 10.1002/BJS.10601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farrar JT, Young JP, LaMoreaux L, et al. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149–158. doi: 10.1016/S0304-3959(01)00349-9 [DOI] [PubMed] [Google Scholar]
- 7.Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105–121. doi: 10.1016/j.jpain.2007.09.005 [DOI] [PubMed] [Google Scholar]
- 8.Huse E, Larbig W, Flor H, Birbaumer N. The effect of opioids on phantom limb pain and cortical reorganization. Pain. 2001;90(1–2):47–55. doi: 10.1016/S0304-3959(00)00385-7 [DOI] [PubMed] [Google Scholar]
- 9.Wu CL, Agarwal S, Tella PK, et al. Morphine versus mexiletine for treatment of postamputation pain: a randomized, placebo-controlled, crossover trial. Anesthesiology. 2008;109(2):289–296. doi: 10.1097/ALN.0B013E31817F4523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bone M, Critchley P, Buggy DJ. Gabapentin in postamputation phantom limb pain: a randomized, double-blind, placebo-controlled, cross-over study. Reg Anesth Pain Med. 2002;27(5):481–486. doi: 10.1053/rapm.2002.3516911 [DOI] [PubMed] [Google Scholar]
- 11.Smith DG, Ehde DM, Hanley MA, et al. Efficacy of gabapentin in treating chronic phantom limb and residual limb pain. J Rehabil Res Dev. 2005;42(5):645–654. doi: 10.1682/JRRD.2005.05.0082 [DOI] [PubMed] [Google Scholar]
- 12.Fleiss JL. The statistical basis of meta-analysis. Stat Methods Med Res. 1993;2(2):121–145. doi: 10.1177/096228029300200202 [DOI] [PubMed] [Google Scholar]
- 13.Fleiss JL, Levin B, Cho Paik M. Statistical Methods for Rates and Proportions. In: Statistical Methods for Rates and Proportions. Third Edition. 2004:1–760. doi: 10.1002/0471445428 [DOI] [Google Scholar]
- 14.Nguyen C, Naunton M, Thomas J, Todd L, McEwen J, Bushell M. Availability and use of number needed to treat (NNT) based decision aids for pharmaceutical interventions. Exploratory Res Clin Soc Pharm. 2021;2:100039. doi: 10.1016/J.RCSOP.2021.100039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stone AB, Hollmann MW, Terwindt LE, et al. Chronic post amputation pain: pathophysiology and prevention options for a heterogenous phenomenon. Curr Opin Anaesthesiol. 2023;36(5):572–579. doi: 10.1097/ACO.0000000000001298 [DOI] [PubMed] [Google Scholar]
- 16.Häuser W, Bock F, Engeser P, Tölle T, Willweber-Strumpfe A, Petzke F. Long-term opioid use in non-cancer pain. Dtsch Arztebl Int. 2014;111(43):732–740. doi: 10.3238/ARZTEBL.2014.0732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Viscusi ER. Clinical Overview and Considerations for the Management of Opioid-induced Constipation in Patients With Chronic Noncancer Pain. Clin J Pain. 2019;35(2):174–188. doi: 10.1097/AJP.0000000000000662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Algera MH, Kamp J, van der Schrier R, et al. Opioid-induced respiratory depression in humans: a review of pharmacokinetic-pharmacodynamic modelling of reversal. Br J Anaesth. 2019;122(6):e168–e179. doi: 10.1016/J.BJA.2018.12.023 [DOI] [PubMed] [Google Scholar]