Abstract
The relevant mechanism of tumor-associated macrophages (TAMs) in the treatment of colorectal cancer patients with immune checkpoint inhibitors (ICIs) is discussed, and the application prospects of TAMs in reversing the treatment tolerance of ICIs are discussed to provide a reference for related studies. As a class of drugs widely used in clinical tumor immunotherapy, ICIs can act on regulatory molecules on cells that play an inhibitory role-immune checkpoints-and kill tumors in the form of an immune response by activating a variety of immune cells in the immune system. The sensitivity of patients with different types of colorectal cancer to ICI treatment varies greatly. The phenotype and function of TAMs in the colorectal cancer microenvironment are closely related to the efficacy of ICIs. ICIs can regulate the phenotypic function of TAMs, and TAMs can also affect the tolerance of colorectal cancer to ICI therapy. TAMs play an important role in ICI resistance, and making full use of this target as a therapeutic strategy is expected to improve the immunotherapy efficacy and prognosis of patients with colorectal cancer.
Keywords: Colorectal cancer, Immune checkpoint inhibitor resistance, Tumor microenvironment, Tumor-associated macrophages, Review
Core Tip: This study reviews the role of tumor-associated macrophages (TAMs) in the treatment tolerance of immune checkpoint inhibitors in colorectal cancer. The effects of TAMs on immunotherapy through promoting immune escape, inhibiting T cell function, secreting pro-inflammatory factors and remodeling tumor microenvironment were discussed. In addition, the current therapeutic strategies against TAMs and their potential in improving the efficacy of immune checkpoint inhibitors are also introduced in this paper, aiming to provide new research directions and clinical application references for future colorectal cancer immunotherapy.
INTRODUCTION
The incidence of colorectal cancer ranks second among malignant tumors in China, second only to that of lung cancer[1-3]. Current first-line treatments for patients with colorectal cancer include colectomy and radical rectotomy combined with regional lymph node removal, as appropriate[4-6]. In addition, preoperative detection can be performed, and neoadjuvant therapy based on fluorouracil is recommended for patients with complete mismatch repair (MMR) function/microsatellite stability, while combining multiple treatment strategies can be considered for patients with MMR deficit (dMMR)/highly microsatellite instability (MSI-H), which can greatly improve the overall survival rate and organ retention rate[7]. In patients with hepatic metastasis, drugs targeting vascular endothelial growth factor (VEGF) and mitogen-activated extracellular signal-regulated kinase are often added to improve tumor vascular infiltration and promote the recruitment of CD8+ T cells and antigen-presenting cells[8-10]. In colorectal cancer patients with H-cell lymphoma, combination treatment with multiple immune checkpoint inhibitors (ICIs) can enhance the antitumor effect by regulating the interaction between various immune cells and tumor cells in the microenvironment[11-14]. The development of colorectal cancer is influenced by both the type of tumor cells and the specific tumor microenvironment (TME), which together constitute a dense tissue environment with high osmotic pressure[15]. The TME is composed of an extracellular matrix and a variety of stromal cells, in which the extracellular matrix is composed mainly of collagen, fibronectin, elastin and other glycoproteins, while the stromal cells are mainly mesenchymal cells and various immune cells[16-18]. As tumor-associated macrophages (TAMs) have the highest proportion of immune cells in the TME, their phenotype and functional changes are closely related to the efficacy of ICIs. Native M0 macrophages can be induced by lipopolysaccharide, tumor necrosis factor-α, and interferon-γ (IFN-γ) to differentiate into M1 macrophages with proinflammatory and antitumor effects and are influenced by the inhibitory cytokines interleukin-4 (IL-4), IL-10, and transforming growth factor-β (TGF-β)[19-23]. These cells differentiated into M2-type macrophages with a tumor-promoting effect. Relevant studies have shown that M2 macrophages can promote the hypoxia-inducible factor-alpha/Tribble 3 axis by secreting TGF-β. Thus, the β-catenin/Wnt signaling pathway is activated, and the expression of programmed cell death protein 1 (PD-1) is enhanced, thereby promoting the invasion and metastasis of rectal cancer, which indirectly proves that TAMs play an important role in immune checkpoint therapy, but the underlying mechanism is still unclear[24-26].
This study briefly reviewed the research progress on TAMs in colorectal cancer patients with ICI resistance and clarified the effects of ICI treatment on TAM phenotype and function and the mechanism by which TAM feedback regulates ICI efficacy to provide a reference for improving immune checkpoint efficacy from the perspective of TAMs.
CLINICAL APPLICATION OF ICIS IN THE TREATMENT OF COLORECTAL CANCER
Common immune checkpoints include programmed cell death ligand 1 (PD-L1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4)[27-29]. PD-L1 is expressed on the surface of antigen-presenting cells and tumor cells and binds to PD-1 to inhibit CD4+ T-cell activity, ultimately promoting immune tolerance and immune escape[30-35]. CT-LA-4 is commonly expressed in T cells, B cells and thymocyte subsets and is closely related to the mechanism that inhibits its antitumor immune effect[36]. ICIs can target a variety of lymphocytes in the regulatory immune microenvironment, block immune checkpoint ligands on the surface of immune cells, and reverse the inhibitory immune microenvironment[37-40].
There are currently seven commonly used ICI drugs approved by the United States Food and Drug Administration, of which pabolizul, navulio, cimipril and dostamumab target PD-1, and attilizul, duvarumab and avitumab target PD-L1. Currently, the main ICI drugs used to treat colorectal cancer are pabolizumab and nebuliumab, and only a very small number of dMMR/MSI-H patients can benefit from them[41-44]. In addition, ipilimumab, a monoclonal antibody targeting CTLA-4, can also be combined with the above two ICIs to help prolong overall survival and progression-free survival in patients with colorectal cancer[45]. However, most patients with colorectal cancer are not sensitive to this therapy because of its low immunogenicity, low tumor mutation load, and low infiltration of tumor-infiltrating lymphocytes in the microenvironment[46-48]. In addition, even patients with dMMR/MSI-H colorectal cancer often face the dilemma of drug resistance.
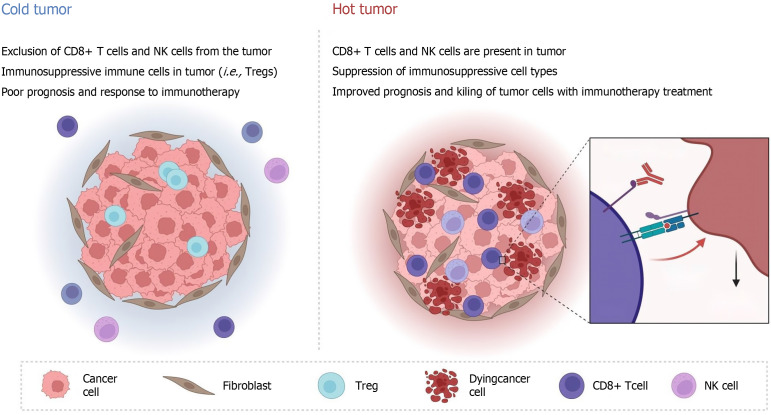
Drug resistance to ICIs can be divided into primary and secondary resistance[49]. Primary resistance occurs when the patient does not respond to ICIs and the tumor progresses rapidly, and secondary resistance occurs when the patient initially responds to ICI treatment but eventually progresses to disease[50]. Primary drug resistance is mainly related to the activation and inhibition of intracellular pathways, including loss of phosphatase and tensin homolog expression and enhancement of the IL-6/signal transducer and activator of transcription 3 (STAT3) pathway, sustained activation of the mitogen-activated protein kinases pathway, loss and mutation of IFN-γ pathway-related proteins, and upregulation of PD-L1 expression on the cell surface[51-54]. Secondary drug resistance often involves antigen presentation defects, T-cell depletion, major histocompatibility complex-I class molecular mutations, and the release of various inhibitory immune factors (such as TGF-β)[55]. These mechanisms are influenced by the immunogenicity and immune microenvironment of colorectal cancer patients’ own tumors[56]. According to the number of tumor-infiltrating lymphocytes, colorectal cancer can be roughly divided into hot tumors and cold tumors[57-59]. Only a small number of patients with strong immunogenicity and a sensitive response can respond to ICI treatment, while in the vast majority of patients with cold tumors, ICI treatment is ineffective and patients have a poor prognosis (Figure 1).
Figure 1.
Immunogenicity and effects of immune microenvironment on cold and hot. NK: Natural killer; Treg: Regulatory T cell.
THE ROLE OF TAMS IN THE CLINICAL TREATMENT TOLERANCE OF COLORECTAL CANCER
At present, the polarization characteristics of TAMs play important roles in chemotherapy resistance, radiotherapy resistance and immune escape in colorectal cancer[60-62]. Relevant studies have shown that long noncoding RNA-MIR155HG induces the polarization of M2 macrophages in colorectal cancer cells by regulating annexin A2 and promotes oxaliplatin resistance[63-65]. In addition, another study revealed that activating tyrosine kinase receptors such as Tyro3 and Mertk on the surface of TAMs can regulate the Akt signaling pathway to promote PD-L1 surface reach and chemical resistance[66-68]. By expressing receptors such as VEGF receptor 2, M2 macrophages can promote the formation of new blood vessels, enhance permeability, and accelerate the excretion of various drugs[69].
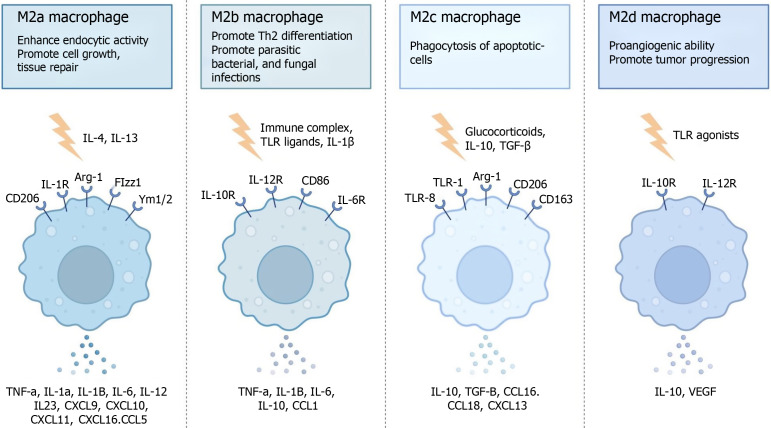
In the microenvironment, TAMs can interact with fibroblasts to promote their epithelial-mesenchymal transition through the hypoxia-inducible factor-1 alpha factor, which further leads to chemotherapy resistance[70-74]. One study showed that M2 macrophages are positively correlated with colorectal cancer radiotherapy resistance[75], and another study showed that M2 macrophages secrete arginase-1 to consume arginine, thereby preventing its breakdown to produce nitric oxide to induce radiotherapy sensitization[76-78]. However, the detailed mechanism is not yet clear. In clinical treatment, most TAMs exhibit the M2 phenotype, and most M1-type TAMs have a better prognosis, while M2-type TAMs have a worse prognosis. Most of these toxins accelerate the occurrence of the above protumor response by affecting antigen presentation, releasing inhibitory cytokines and constructing an inhibitory immune microenvironment (Figure 2).
Figure 2.
M2 macrophage subtypes. IL: Interleukin; TLR: Toll-like receptor; TGF: Transforming growth factor; CXCL: C-X-C motif chemokine ligand; CCL: C-C motif chemokine ligand; TNF: Tumor necrosis factor; VEGF: Vascular endothelial growth factor; Arg-1: Arginase-1.
MECHANISMS RELATED TO TAMS IN ICI TREATMENT TOLERANCE
At present, the application of ICIs in the clinical treatment of colorectal cancer patients is relatively limited[79]. ICIs are only used in preoperative neoadjuvant immunotherapy and palliative therapy for dMMR/MSI-H patients, and most patients have difficulty benefiting from ICIs[80]. Clinically, most patients are immunobarren patients whose immune cells are restricted by the matrix surrounding the cancer nest and for whom it is difficult to produce an inflammatory response or for whom a large number of myeloid-derived suppressor cells (MDSCs), regulatory T cells (Tregs) and M2 macrophages are present in the TME. Due to the lack of CD8+ T cells in immune rejection patients, both can be classified into the cold tumor category[81-83].
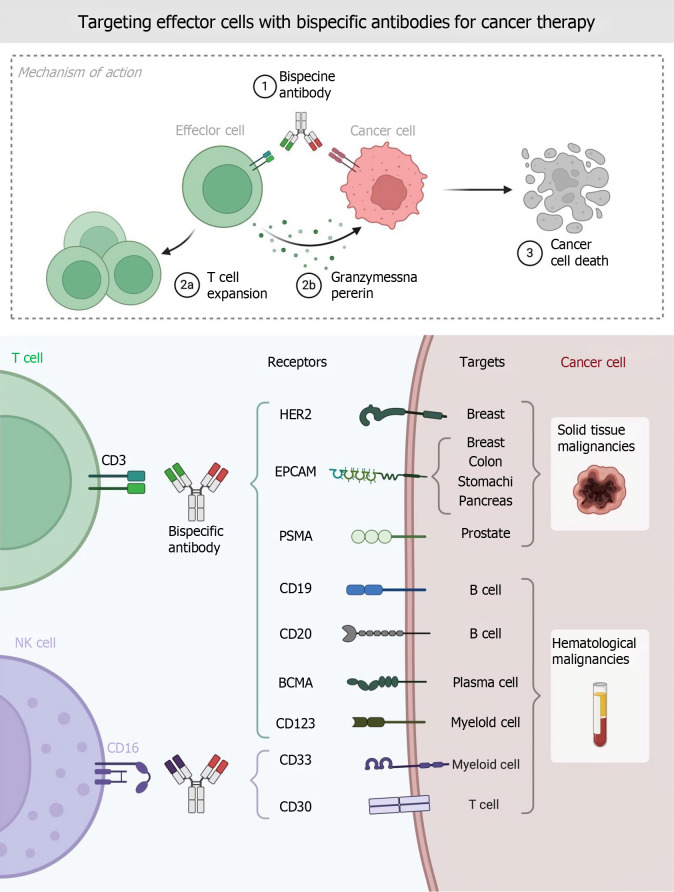
TAMs interact with a variety of cells in the TME, and TAMs can mediate M2-type macrophage differentiation through the depletion of CD8+ T cells through specific antigenic synaptic parameters; this effect is intensified under hypoxic conditions. The peroxisome proliferator-activated receptor γ-dependent increase in fatty acid oxidation[84-86]. In view of the above findings, how to target TAMs to improve the sensitivity of rectal cancer patients to ICI therapy, reverse cold tumors, and enhance the efficacy of ICI therapy in dMMR/MSI-H patients has become a new hotspot in colorectal cancer immunotherapy research (Figure 3).
Figure 3.
Targeting effector cells with bispecific antibodies for cancer therapy. NK: Natural killer; EPCAM: Epithelial cell adhesion molecule; PSMA: Prostate-specific membrane antigen; BCMA: Bulbospinal muscular atrophy.
ICIs induced phenotypic changes in TAMs
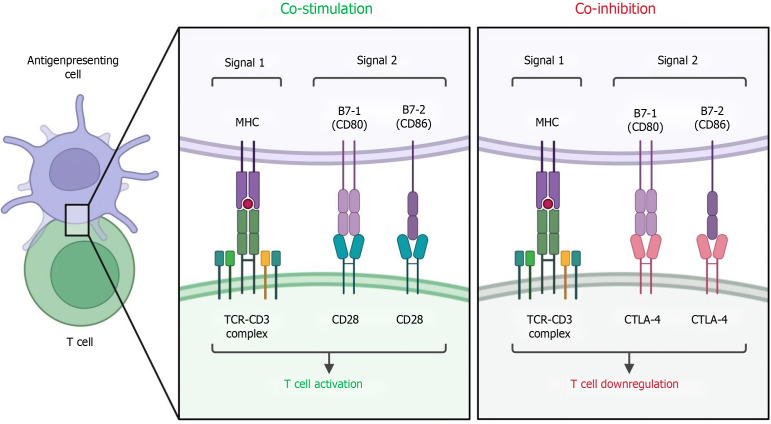
ICIs commonly used in patients with colorectal cancer mainly include pabolizumab, nabuliu, ipilimumab, attilizumab, and carbbertinimab, and the occurrence of drug resistance is mostly related to M2 differentiation of TAMs[87-90]. The greater the mutation load of tumors and the greater the infiltration degree of TAMs, the greater the possibility of ICI resistance[91]. TAMs were indirectly affected in related clinical trials by the combination of nabuliu and ipilimumab, which enhanced T-cell infiltration and facilitated immune cell interactions in the TME (Figure 4). ICIs can induce the secretion of cytokines and microRNAs (miRNAs) by tumor cells, and the soluble small molecules produced by ICIs can act on the Janus kinase/STAT pathway and phosphatidylinositol 3-kinase (PI3K)/Akt pathway, inhibit the Notch signaling pathway, affect the polarization of TAMs, and lead to the development of drug resistance[92-94].
Figure 4.
T cell co-stimulation and co-inhibition. TCR: T cell receptor; MHC: Major histocompatibility complex; CTLA: Cytotoxic T-lymphocyte-associated protein.
Colorectal cancer can be divided into exosomes containing miR-934, miR-25-3p, miR-130b-3p, miR-425-5p, and other miRNAs[95]. By inhibiting the common target gene phosphatase and tensin homolog, activation of the PI3K/Akt pathway induces an M2-polarized macrophage phenotype, and studies have shown that this pathway can increase the occurrence of liver metastasis in patients[96-98]. After ICI treatment, tumor cells can suppress the secretion of miR-148a-3p and the activation of the Notch/Jagged1 signaling pathway, further reducing the transformation of M1-type macrophages and blocking the formation of an inflammatory microenvironment[99].
TAMs regulate drug tolerance to ICIs
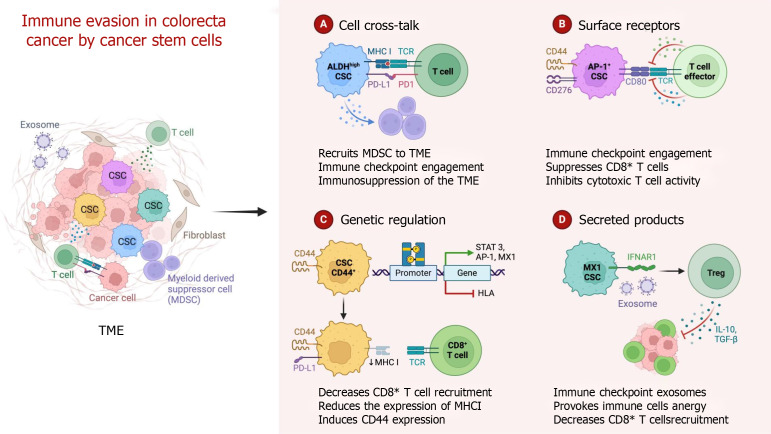
A large number of M2 macrophages accumulate in the tumor stroma, which can affect the efficacy of ICIs and lead to ICI resistance in colorectal cancer patients[100]. TAMs can increase the formation of an immunosuppressive microenvironment in the TME by secreting IL-10 and TGF-β[101-103]. M2 macrophages supply tumor cells with a large amount of ATP through fatty acid oxidation and reshape the TME by secreting VEGF and matrix metalloproteinases, promoting angiogenesis and tumor metastasis and ultimately leading to ICI resistance. In addition, TAMs also interact with tumor cells, Tregs, cytotoxic T cells and MDSCs in the TME, resulting in secondary drug resistance. First, M2-type macrophages can regulate the expression of CTLA-4 and other immune checkpoint molecules, secrete inhibitory cytokines such as TGF-β and IL-10, and secrete chemokines such as C-C motif chemokine ligand 17 (CCL17) and CCL22, which directly promote the formation of an inhibitory immune microenvironment[104-106]. Second, M2-derived prostaglandin E2, IL-10, and indoleamine 2,3-dioxygenase can induce T lymphocytes to convert to Tregs; secrete C-X-C motif chemokine ligand 12 (CXCL12), CCL5, CCL22, CCL10 and IL-8; and recruit MDSCs and Tregs into the TME[107-110]. At the same time, TAMs secrete TGF-β through the phosphate nuclear Smad2/3 protein and inhibit the mitochondrial respiratory chain, reducing the expression levels of IFN-γ and granase B in T cells, thereby inhibiting T cells from killing tumors and causing drug resistance (Figure 5).
Figure 5.
Mechanisms of immune evasion in colorectal cancer by cancer stem cells. CSC: Cancer stem cell; TME: Tumor microenvironment; TCR: T cell receptor; MHC: Major histocompatibility complex; MDSC: Myeloid-derived suppressor cell; PD-L1: Programmed cell death ligand 1; PD-1: Programmed cell death protein 1; STAT 3: Signal transducer and activator of transcription 3; AP-1: Activating protein-1; HLA: Human leukocyte antigen; IFNAR1: Interferon alpha/beta receptor 1; Treg: Regulatory T cell; IL: Interleukin; TGF: Transforming growth factor.
Targeted regulation of TAMs can improve the efficacy of ICIs in colorectal cancer patients
Targeting TAMs has become a hot research direction for reversing ICI resistance, mainly by restricting macrophage recruitment, inhibiting M2-type polarization, targeting macrophage surface immune checkpoints and chimeric antigen receptor macrophage (CAR-M) therapy[111-113].
Limiting the number of TAMs in the TME
The aggregation of the colony-stimulating factor-1 (CSF-1)/CSF 1 receptor (CSF1R) axis and CCL2/CC chemokine receptor 2-axis are important macrophage chemokines. This has been shown to increase tumor sensitivity to PD-L1 ICIs, and the use of CSF1R small molecule inhibitors can induce repolarization of TAMs from the M2 to M1 phenotype and further reduce the risk of tumor invasion and invasion[114]. Studies have shown that targeting CCL5 and CC chemokine receptor 5 has similar effects[115-118].
M2-type polarization of TAMs was inhibited
Itaconate, fritinib, fuquinitinib, and regafenib have been shown to inhibit M2-type macrophage differentiation, and cetuximab can also restore the antitumor TME by regulating and reprogramming the polarization of TAMs from the M2-like phenotype to the M1-like phenotype, including inhibiting IL-6 expression in TAMs[119-122]. The epidermal growth factor receptor axis is involved in the M2 polarization of TAMs through the PI3K/Akt/mechanistic target of rapamycin pathway, whereas inhibition of PI3K or epidermal growth factor receptor with monocrone inhibitors can reverse this process and polarize TAMs toward the antitumor M1 phenotype[123]. Murine double minute 2, a key negative regulator of p53, is highly expressed in tumors, and its antagonist APG115 can repolarize TAMs, causing them to exhibit an antitumor M1 phenotype and activate CD4+ T cells[124-126]. Moreover, inhibition of murine double minute 2 can upregulate the expression of PD-L1 on the surface of tumor cells and may lead to high immunogenicity (Figure 6).
Figure 6.
Effects of M2-type polarization from the tumor microenvironment. VEGF: Vascular endothelial growth factor; Th1: T helper 1; Treg: Regulatory T cell.
Targeting macrophage surface immune checkpoints
The presence of macrophages on the surface of the suppressive immune detection site, signal regulatory protein alpha axis/CD47, can induce tumor escape and reverse the transformation of TAMs to the M1 phenotype, and treatment targeting these macrophages has been shown to be effective in preclinical models of solid tumors[127-130].
CAR-M-cell therapy
CAR-Ms, which encode a specific gene for macrophages that enhances their tumor recognition ability, have entered clinical trials, and modified TAMs can be injected into patients to stimulate the activity of T cells in the TME and eventually reverse the immunosuppressive state[131-133]. It was found that macrophages were able to drive phagocytosis and targeted killing of tumor cells in a splenic tyrosine kinase-dependent manner without the addition of any soluble opsonics[134-136]. Adoptive cell therapy after large-scale expansion of CAR-Ms with a specific design has very good therapeutic potential, but in-depth clinical research on this topic is lacking[137-140].
In addition to the abovementioned therapeutic approaches, targeting macrophage-secreted cytokines is also a new therapeutic area, and VEGF and VEGF receptor inhibitors are widely used in the standard treatment of colorectal cancer, especially in cancer patients with RAS/RAF mutations[141-147]. An increase in CD4+Foxp3+ Tregs, M2-like macrophages and MDSCs and a decrease in CD8+ T cells are well-known immune features in tumors with high VEGFA expression[148-152]. As mentioned earlier, with respect to this cytokine-dependent immunosuppressive pathway, anti-VEGF therapy can enhance immunotherapy efficacy by reversing VEGF-mediated immunosuppression and increasing T-cell infiltration in tumors[153-155]. Anti-VEGF-r therapy may help control the immunosuppressive function of M2 TAMs in colorectal cancer[156-160].
CONCLUSION
In recent years, ICIs have become a hot topic in cancer treatment, but many cancer patients cannot benefit from ICI treatment[161-164]. The therapeutic effect of ICIs is closely related to the tumor immune microenvironment. As the main immune cells in the TME, TAMs play a central role in regulating the activation or inhibition of the immune microenvironment[165]. Therefore, summarizing the research progress on TAM and ICI treatment tolerance is helpful for providing a theoretical basis for expanding their application scope[166-170]. Promoting the transformation of TAMs into antitumor M1 macrophages, activating immunogenicity and reversing the inhibitory immune microenvironment are currently the focus of relevant research[171-173]. In addition, how to further reduce the recruitment of TAMs to the primary tumor site, discover more TAM surface molecules that play an important role in immune escape, and more effectively modify and mass-produce CAR-Ms are still problems to be overcome[174-180]. Perhaps other immune checkpoints, such as T cell immunoglobulin and mucin-domain containing 3 and lymphocyte activation gene-3, also play important roles in regulating the polarization and phagocytosis of macrophages, and combined treatment with multiple ICIs can help solve the current dilemma[181-186]. Exploring additional pathways that promote M1-type polarization, such as the toll-like receptor 4/nuclear transcription factor-kappa B pathway and IFN-γ pathway, and studying the regulation and association between these pathways to discover key proteins may also be beneficial for further improving the immunotherapy of macrophage-related colorectal cancer. It is necessary to further explore the mechanism by which TAMs promote ICI tolerance.
Footnotes
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade C
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade C
P-Reviewer: Cabezuelo AS S-Editor: Wang JJ L-Editor: A P-Editor: Zhang L
Contributor Information
Qi Fan, Intestinal Center, Chongqing University Three Gorges Hospital, Chongqing 404000, China.
Zheng-Wei Fu, Intestinal Center, Chongqing University Three Gorges Hospital, Chongqing 404000, China.
Ming Xu, Intestinal Center, Chongqing University Three Gorges Hospital, Chongqing 404000, China.
Feng Lv, Intestinal Center, Chongqing University Three Gorges Hospital, Chongqing 404000, China.
Jia-Song Shi, Intestinal Center, Chongqing University Three Gorges Hospital, Chongqing 404000, China.
Qi-Qi Zeng, Department of Gastroenterology, Nanjing Drum Tower Hospital, The Affiliated Hospital of Nanjing University Medical School, Nanjing 210008, Jiangsu Province, China.
De-Hai Xiong, Intestinal Center, Chongqing University Three Gorges Hospital, Chongqing 404000, China. 1208505185@qq.com.
References
- 1.Wang H, Tian T, Zhang J. Tumor-Associated Macrophages (TAMs) in Colorectal Cancer (CRC): From Mechanism to Therapy and Prognosis. Int J Mol Sci. 2021;22 doi: 10.3390/ijms22168470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wei C, Yang C, Wang S, Shi D, Zhang C, Lin X, Liu Q, Dou R, Xiong B. Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulating tumor cell-mediated colorectal cancer metastasis. Mol Cancer. 2019;18:64. doi: 10.1186/s12943-019-0976-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao S, Mi Y, Guan B, Zheng B, Wei P, Gu Y, Zhang Z, Cai S, Xu Y, Li X, He X, Zhong X, Li G, Chen Z, Li D. Tumor-derived exosomal miR-934 induces macrophage M2 polarization to promote liver metastasis of colorectal cancer. J Hematol Oncol. 2020;13:156. doi: 10.1186/s13045-020-00991-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu X, Liang R, Lan T, Ding D, Huang S, Shao J, Zheng Z, Chen T, Huang Y, Liu J, Pathak JL, Wei H, Wei B. Tumor-associated macrophage-specific CD155 contributes to M2-phenotype transition, immunosuppression, and tumor progression in colorectal cancer. J Immunother Cancer. 2022;10 doi: 10.1136/jitc-2021-004219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu X, Qin J, Nie J, Gao R, Hu S, Sun H, Wang S, Pan Y. ANGPTL2+cancer-associated fibroblasts and SPP1+macrophages are metastasis accelerators of colorectal cancer. Front Immunol. 2023;14:1185208. doi: 10.3389/fimmu.2023.1185208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao Y, Zhang W, Huo M, Wang P, Liu X, Wang Y, Li Y, Zhou Z, Xu N, Zhu H. XBP1 regulates the protumoral function of tumor-associated macrophages in human colorectal cancer. Signal Transduct Target Ther. 2021;6:357. doi: 10.1038/s41392-021-00761-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, Chen Z, Han J, Ma X, Zheng X, Chen J. Functional and Therapeutic Significance of Tumor-Associated Macrophages in Colorectal Cancer. Front Oncol. 2022;12:781233. doi: 10.3389/fonc.2022.781233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xun J, Du L, Gao R, Shen L, Wang D, Kang L, Chen C, Zhang Z, Zhang Y, Yue S, Feng S, Xiang R, Mi X, Tan X. Cancer-derived exosomal miR-138-5p modulates polarization of tumor-associated macrophages through inhibition of KDM6B. Theranostics. 2021;11:6847–6859. doi: 10.7150/thno.51864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang H, Liu L, Liu J, Dang P, Hu S, Yuan W, Sun Z, Liu Y, Wang C. Roles of tumor-associated macrophages in anti-PD-1/PD-L1 immunotherapy for solid cancers. Mol Cancer. 2023;22:58. doi: 10.1186/s12943-023-01725-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mizuno R, Kawada K, Itatani Y, Ogawa R, Kiyasu Y, Sakai Y. The Role of Tumor-Associated Neutrophils in Colorectal Cancer. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20030529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen S, Cui W, Chi Z, Xiao Q, Hu T, Ye Q, Zhu K, Yu W, Wang Z, Yu C, Pan X, Dai S, Yang Q, Jin J, Zhang J, Li M, Yang D, Yu Q, Wang Q, Yu X, Yang W, Zhang X, Qian J, Ding K, Wang D. Tumor-associated macrophages are shaped by intratumoral high potassium via Kir2.1. Cell Metab. 2022;34:1843–1859.e11. doi: 10.1016/j.cmet.2022.08.016. [DOI] [PubMed] [Google Scholar]
- 12.Wang X, Wang J, Zhao J, Wang H, Chen J, Wu J. HMGA2 facilitates colorectal cancer progression via STAT3-mediated tumor-associated macrophage recruitment. Theranostics. 2022;12:963–975. doi: 10.7150/thno.65411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhong Q, Fang Y, Lai Q, Wang S, He C, Li A, Liu S, Yan Q. CPEB3 inhibits epithelial-mesenchymal transition by disrupting the crosstalk between colorectal cancer cells and tumor-associated macrophages via IL-6R/STAT3 signaling. J Exp Clin Cancer Res. 2020;39:132. doi: 10.1186/s13046-020-01637-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li B, Qi ZP, He DL, Chen ZH, Liu JY, Wong MW, Zhang JW, Xu EP, Shi Q, Cai SL, Sun D, Yao LQ, Zhou PH, Zhong YS. NLRP7 deubiquitination by USP10 promotes tumor progression and tumor-associated macrophage polarization in colorectal cancer. J Exp Clin Cancer Res. 2021;40:126. doi: 10.1186/s13046-021-01920-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hou S, Zhao Y, Chen J, Lin Y, Qi X. Tumor-associated macrophages in colorectal cancer metastasis: molecular insights and translational perspectives. J Transl Med. 2024;22:62. doi: 10.1186/s12967-024-04856-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yin Y, Liu B, Cao Y, Yao S, Liu Y, Jin G, Qin Y, Chen Y, Cui K, Zhou L, Bian Z, Fei B, Huang S, Huang Z. Colorectal Cancer-Derived Small Extracellular Vesicles Promote Tumor Immune Evasion by Upregulating PD-L1 Expression in Tumor-Associated Macrophages. Adv Sci (Weinh) 2022;9:2102620. doi: 10.1002/advs.202102620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Z, Li H, Li Z, Chen S, Huang X, Zheng Z, Qian X, Zhang L, Long G, Xie J, Wang Q, Pan W, Zhang D. SHH/GLI2-TGF-β1 feedback loop between cancer cells and tumor-associated macrophages maintains epithelial-mesenchymal transition and endoplasmic reticulum homeostasis in cholangiocarcinoma. Pharmacol Res. 2023;187:106564. doi: 10.1016/j.phrs.2022.106564. [DOI] [PubMed] [Google Scholar]
- 18.Dong L, Chen C, Zhang Y, Guo P, Wang Z, Li J, Liu Y, Liu J, Chang R, Li Y, Liang G, Lai W, Sun M, Dougherty U, Bissonnette MB, Wang H, Shen L, Xu MM, Han D. The loss of RNA N(6)-adenosine methyltransferase Mettl14 in tumor-associated macrophages promotes CD8(+) T cell dysfunction and tumor growth. Cancer Cell. 2021;39:945–957.e10. doi: 10.1016/j.ccell.2021.04.016. [DOI] [PubMed] [Google Scholar]
- 19.Huang C, Wang X, Wang Y, Feng Y, Wang X, Chen S, Yan P, Liao J, Zhang Q, Mao C, Li Y, Wang L, Wang X, Yi W, Cai W, Chen S, Hong N, He W, Chen J, Jin W. Sirpα on tumor-associated myeloid cells restrains antitumor immunity in colorectal cancer independent of its interaction with CD47. Nat Cancer. 2024;5:500–516. doi: 10.1038/s43018-023-00691-z. [DOI] [PubMed] [Google Scholar]
- 20.Shi Q, Shen Q, Liu Y, Shi Y, Huang W, Wang X, Li Z, Chai Y, Wang H, Hu X, Li N, Zhang Q, Cao X. Increased glucose metabolism in TAMs fuels O-GlcNAcylation of lysosomal Cathepsin B to promote cancer metastasis and chemoresistance. Cancer Cell. 2022;40:1207–1222.e10. doi: 10.1016/j.ccell.2022.08.012. [DOI] [PubMed] [Google Scholar]
- 21.Cavalleri T, Greco L, Rubbino F, Hamada T, Quaranta M, Grizzi F, Sauta E, Craviotto V, Bossi P, Vetrano S, Rimassa L, Torri V, Bellazzi R, Mantovani A, Ogino S, Malesci A, Laghi L. Tumor-associated macrophages and risk of recurrence in stage III colorectal cancer. J Pathol Clin Res. 2022;8:307–312. doi: 10.1002/cjp2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang C, Ou R, Chen X, Zhang Y, Li J, Liang Y, Zhu X, Liu L, Li M, Lin D, Qiu J, Liu G, Zhang L, Wu Y, Tang H, Liu Y, Liang L, Ding Y, Liao W. Tumor cell-derived SPON2 promotes M2-polarized tumor-associated macrophage infiltration and cancer progression by activating PYK2 in CRC. J Exp Clin Cancer Res. 2021;40:304. doi: 10.1186/s13046-021-02108-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 24.Li Y, Shen Z, Chai Z, Zhan Y, Zhang Y, Liu Z, Liu Y, Li Z, Lin M, Zhang Z, Liu W, Guan S, Zhang J, Qian J, Ding Y, Li G, Fang Y, Deng H. Targeting MS4A4A on tumour-associated macrophages restores CD8+ T-cell-mediated antitumour immunity. Gut. 2023;72:2307–2320. doi: 10.1136/gutjnl-2022-329147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song J, Xiao T, Li M, Jia Q. Tumor-associated macrophages: Potential therapeutic targets and diagnostic markers in cancer. Pathol Res Pract. 2023;249:154739. doi: 10.1016/j.prp.2023.154739. [DOI] [PubMed] [Google Scholar]
- 26.Yang C, Dou R, Wei C, Liu K, Shi D, Zhang C, Liu Q, Wang S, Xiong B. Tumor-derived exosomal microRNA-106b-5p activates EMT-cancer cell and M2-subtype TAM interaction to facilitate CRC metastasis. Mol Ther. 2021;29:2088–2107. doi: 10.1016/j.ymthe.2021.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang YJ, Yang CK, Wei PL, Huynh TT, Whang-Peng J, Meng TC, Hsiao M, Tzeng YM, Wu AT, Yen Y. Ovatodiolide suppresses colon tumorigenesis and prevents polarization of M2 tumor-associated macrophages through YAP oncogenic pathways. J Hematol Oncol. 2017;10:60. doi: 10.1186/s13045-017-0421-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang Z, Zhang M, Peng R, Liu J, Wang F, Li Y, Zhao Q, Liu J. The prognostic and clinicopathological value of tumor-associated macrophages in patients with colorectal cancer: a systematic review and meta-analysis. Int J Colorectal Dis. 2020;35:1651–1661. doi: 10.1007/s00384-020-03686-9. [DOI] [PubMed] [Google Scholar]
- 29.Shang S, Yang C, Chen F, Xiang RS, Zhang H, Dai SY, Liu J, Lv XX, Zhang C, Liu XT, Zhang Q, Lu SB, Song JW, Yu JJ, Zhou JC, Zhang XW, Cui B, Li PP, Zhu ST, Zhang HZ, Hua F. ID1 expressing macrophages support cancer cell stemness and limit CD8(+) T cell infiltration in colorectal cancer. Nat Commun. 2023;14:7661. doi: 10.1038/s41467-023-43548-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Popēna I, Ābols A, Saulīte L, Pleiko K, Zandberga E, Jēkabsons K, Endzeliņš E, Llorente A, Linē A, Riekstiņa U. Effect of colorectal cancer-derived extracellular vesicles on the immunophenotype and cytokine secretion profile of monocytes and macrophages. Cell Commun Signal. 2018;16:17. doi: 10.1186/s12964-018-0229-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei YT, Wang XR, Yan C, Huang F, Zhang Y, Liu X, Wen ZF, Sun XT, Zhang Y, Chen YQ, Gao R, Pan N, Wang LX. Thymosin α-1 Reverses M2 Polarization of Tumor-Associated Macrophages during Efferocytosis. Cancer Res. 2022;82:1991–2002. doi: 10.1158/0008-5472.CAN-21-4260. [DOI] [PubMed] [Google Scholar]
- 32.Kamerkar S, Leng C, Burenkova O, Jang SC, McCoy C, Zhang K, Dooley K, Kasera S, Zi T, Sisó S, Dahlberg W, Sia CL, Patel S, Schmidt K, Economides K, Soos T, Burzyn D, Sathyanarayanan S. Exosome-mediated genetic reprogramming of tumor-associated macrophages by exoASO-STAT6 leads to potent monotherapy antitumor activity. Sci Adv. 2022;8:eabj7002. doi: 10.1126/sciadv.abj7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bao Z, Zeng W, Zhang D, Wang L, Deng X, Lai J, Li J, Gong J, Xiang G. SNAIL Induces EMT and Lung Metastasis of Tumours Secreting CXCL2 to Promote the Invasion of M2-Type Immunosuppressed Macrophages in Colorectal Cancer. Int J Biol Sci. 2022;18:2867–2881. doi: 10.7150/ijbs.66854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang L, Zhao Y, Shan M, Wang S, Chen J, Liu Z, Xu Q. Targeting crosstalk of STAT3 between tumor-associated M2 macrophages and Tregs in colorectal cancer. Cancer Biol Ther. 2023;24:2226418. doi: 10.1080/15384047.2023.2226418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schaer DJ, Schulthess-Lutz N, Baselgia L, Hansen K, Buzzi RM, Humar R, Dürst E, Vallelian F. Hemorrhage-activated NRF2 in tumor-associated macrophages drives cancer growth, invasion, and immunotherapy resistance. J Clin Invest. 2023;134 doi: 10.1172/JCI174528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang L, Lu X, Xu Y, La X, Tian J, Li A, Li H, Wu C, Xi Y, Song G, Zhou Z, Bai W, An L, Li Z. Tumor-associated macrophages confer colorectal cancer 5-fluorouracil resistance by promoting MRP1 membrane translocation via an intercellular CXCL17/CXCL22-CCR4-ATF6-GRP78 axis. Cell Death Dis. 2023;14:582. doi: 10.1038/s41419-023-06108-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dong Y, Yang Q, Niu R, Zhang Z, Huang Y, Bi Y, Liu G. Modulation of tumor-associated macrophages in colitis-associated colorectal cancer. J Cell Physiol. 2022;237:4443–4459. doi: 10.1002/jcp.30906. [DOI] [PubMed] [Google Scholar]
- 38.Wang S, Kuai Y, Lin S, Li L, Gu Q, Zhang X, Li X, He Y, Chen S, Xia X, Ruan Z, Lin C, Ding Y, Zhang Q, Qi C, Li J, He X, Pathak JL, Zhou W, Liu S, Wang L, Zheng L. NF-κB Activator 1 downregulation in macrophages activates STAT3 to promote adenoma-adenocarcinoma transition and immunosuppression in colorectal cancer. BMC Med. 2023;21:115. doi: 10.1186/s12916-023-02791-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xue T, Yan K, Cai Y, Sun J, Chen Z, Chen X, Wu W. Prognostic significance of CD163+ tumor-associated macrophages in colorectal cancer. World J Surg Oncol. 2021;19:186. doi: 10.1186/s12957-021-02299-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Galdiero MR, Bonavita E, Barajon I, Garlanda C, Mantovani A, Jaillon S. Tumor associated macrophages and neutrophils in cancer. Immunobiology. 2013;218:1402–1410. doi: 10.1016/j.imbio.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 41.Zheng J, Dou R, Zhang X, Zhong B, Fang C, Xu Q, Di Z, Huang S, Lin Z, Song J, Wang S, Xiong B. LINC00543 promotes colorectal cancer metastasis by driving EMT and inducing the M2 polarization of tumor associated macrophages. J Transl Med. 2023;21:153. doi: 10.1186/s12967-023-04009-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li JJ, Wang JH, Tian T, Liu J, Zheng YQ, Mo HY, Sheng H, Chen YX, Wu QN, Han Y, Liao K, Pan YQ, Zeng ZL, Liu ZX, Yang W, Xu RH, Ju HQ. The liver microenvironment orchestrates FGL1-mediated immune escape and progression of metastatic colorectal cancer. Nat Commun. 2023;14:6690. doi: 10.1038/s41467-023-42332-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen J, Zhu T, Jiang G, Zeng Q, Li Z, Huang X. Target delivery of a PD-1-TREM2 scFv by CAR-T cells enhances anti-tumor efficacy in colorectal cancer. Mol Cancer. 2023;22:131. doi: 10.1186/s12943-023-01830-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang Y, Chen Z, Chu X, Yan Q, He J, Guo Y, Zhao Z, Zhang Y, Hu D, Ding H, Zhao X, Pan Y, Dong H, Wang L, Pan J. Targeting LAYN inhibits colorectal cancer metastasis and tumor-associated macrophage infiltration induced by hyaluronan oligosaccharides. Matrix Biol. 2023;117:15–30. doi: 10.1016/j.matbio.2023.02.005. [DOI] [PubMed] [Google Scholar]
- 45.Li X, Chen X, Gong S, Zhao J, Yao C, Zhu H, Xiao R, Qin Y, Li R, Sun N, Li X, Dong F, Zhao T, Pan Y, Yang J. Platelets promote CRC by activating the C5a/C5aR1 axis via PSGL-1/JNK/STAT1 signaling in tumor-associated macrophages. Theranostics. 2023;13:2040–2056. doi: 10.7150/thno.80555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gazzillo A, Polidoro MA, Soldani C, Franceschini B, Lleo A, Donadon M. Relationship between Epithelial-to-Mesenchymal Transition and Tumor-Associated Macrophages in Colorectal Liver Metastases. Int J Mol Sci. 2022;23 doi: 10.3390/ijms232416197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu M, Bai L, Liu X, Peng S, Xie Y, Bai H, Yu H, Wang X, Yuan P, Ma R, Lin J, Wu L, Huang M, Li Y, Luo Y. Silence of a dependence receptor CSF1R in colorectal cancer cells activates tumor-associated macrophages. J Immunother Cancer. 2022;10 doi: 10.1136/jitc-2022-005610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shibutani M, Nakao S, Maeda K, Nagahara H, Kashiwagi S, Hirakawa K, Ohira M. The Impact of Tumor-associated Macrophages on Chemoresistance via Angiogenesis in Colorectal Cancer. Anticancer Res. 2021;41:4447–4453. doi: 10.21873/anticanres.15253. [DOI] [PubMed] [Google Scholar]
- 49.Cao D, Liang L, Xu Y, Sun J, Lei M, Wang M, Wei Y, Sun Z. Tumor associated macrophages and angiogenesis dual-recognizable nanoparticles for enhanced cancer chemotherapy. Nanomedicine. 2018;14:651–659. doi: 10.1016/j.nano.2017.12.018. [DOI] [PubMed] [Google Scholar]
- 50.Song M, Qian C, Zhang T, Tang Y, Zhou Y, Wei Z, Wang A, Zhong C, Zhao Y, Lu Y. Salvia mitiorrhiza Bunge aqueous extract attenuates infiltration of tumor-associated macrophages and potentiates anti-PD-L1 immunotherapy in colorectal cancer through modulating Cox2/PGE2 cascade. J Ethnopharmacol. 2023;316:116735. doi: 10.1016/j.jep.2023.116735. [DOI] [PubMed] [Google Scholar]
- 51.He S, Song W, Cui S, Li J, Jiang Y, Chen X, Peng L. Modulation of miR-146b by N6-methyladenosine modification remodels tumor-associated macrophages and enhances anti-PD-1 therapy in colorectal cancer. Cell Oncol (Dordr) 2023;46:1731–1746. doi: 10.1007/s13402-023-00839-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu L, Zheng Y, Liu J, Luo R, Wu D, Xu P, Wu D, Li X. Comprehensive evaluation of the efficacy and safety of LPV/r drugs in the treatment of SARS and MERS to provide potential treatment options for COVID-19. Aging (Albany NY) 2021;13:10833–10852. doi: 10.18632/aging.202860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Väyrynen JP, Haruki K, Lau MC, Väyrynen SA, Zhong R, Dias Costa A, Borowsky J, Zhao M, Fujiyoshi K, Arima K, Twombly TS, Kishikawa J, Gu S, Aminmozaffari S, Shi S, Baba Y, Akimoto N, Ugai T, Da Silva A, Guerriero JL, Song M, Wu K, Chan AT, Nishihara R, Fuchs CS, Meyerhardt JA, Giannakis M, Ogino S, Nowak JA. The Prognostic Role of Macrophage Polarization in the Colorectal Cancer Microenvironment. Cancer Immunol Res. 2021;9:8–19. doi: 10.1158/2326-6066.CIR-20-0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang R, Qi F, Zhao F, Li G, Shao S, Zhang X, Yuan L, Feng Y. Cancer-associated fibroblasts enhance tumor-associated macrophages enrichment and suppress NK cells function in colorectal cancer. Cell Death Dis. 2019;10:273. doi: 10.1038/s41419-019-1435-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Inagaki K, Kunisho S, Takigawa H, Yuge R, Oka S, Tanaka S, Shimamoto F, Chayama K, Kitadai Y. Role of tumor-associated macrophages at the invasive front in human colorectal cancer progression. Cancer Sci. 2021;112:2692–2704. doi: 10.1111/cas.14940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu H, Liang Z, Zhou C, Zeng Z, Wang F, Hu T, He X, Wu X, Wu X, Lan P. Mutant KRAS triggers functional reprogramming of tumor-associated macrophages in colorectal cancer. Signal Transduct Target Ther. 2021;6:144. doi: 10.1038/s41392-021-00534-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu Y, Chen X, Xu Y, Yang T, Wang H, Wang Z, Hu Z, Chen L, Zhang Z, Wu Y. CTHRC1 promotes colorectal cancer progression by recruiting tumor-associated macrophages via up-regulation of CCL15. J Mol Med (Berl) 2024;102:81–94. doi: 10.1007/s00109-023-02399-0. [DOI] [PubMed] [Google Scholar]
- 58.Lv J, Jiang Z, Yuan J, Zhuang M, Guan X, Liu H, Yin Y, Ma Y, Liu Z, Wang H, Wang X. Pan-cancer analysis identifies PD-L2 as a tumor promotor in the tumor microenvironment. Front Immunol. 2023;14:1093716. doi: 10.3389/fimmu.2023.1093716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li J, Li L, Li Y, Long Y, Zhao Q, Ouyang Y, Bao W, Gong K. Tumor-associated macrophage infiltration and prognosis in colorectal cancer: systematic review and meta-analysis. Int J Colorectal Dis. 2020;35:1203–1210. doi: 10.1007/s00384-020-03593-z. [DOI] [PubMed] [Google Scholar]
- 60.Wu L, Zhong Y, Wu D, Xu P, Ruan X, Yan J, Liu J, Li X. Immunomodulatory Factor TIM3 of Cytolytic Active Genes Affected the Survival and Prognosis of Lung Adenocarcinoma Patients by Multi-Omics Analysis. Biomedicines. 2022;10 doi: 10.3390/biomedicines10092248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li H, Pan L, Guo J, Lao J, Wei M, Huang F. Integration of single-cell and bulk RNA sequencing to establish a prognostic signature based on tumor-associated macrophages in colorectal cancer. BMC Gastroenterol. 2023;23:385. doi: 10.1186/s12876-023-03035-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jiang M, Cui H, Liu Z, Zhou X, Zhang L, Cao L, Wang M. The Role of Amino Acid Metabolism of Tumor Associated Macrophages in the Development of Colorectal Cancer. Cells. 2022;11 doi: 10.3390/cells11244106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dai E, Han L, Liu J, Xie Y, Kroemer G, Klionsky DJ, Zeh HJ, Kang R, Wang J, Tang D. Autophagy-dependent ferroptosis drives tumor-associated macrophage polarization via release and uptake of oncogenic KRAS protein. Autophagy. 2020;16:2069–2083. doi: 10.1080/15548627.2020.1714209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Peng J, Zhou J, Sun R, Chen Y, Pan D, Wang Q, Chen Y, Gong Z, Du Q. Dual-targeting of artesunate and chloroquine to tumor cells and tumor-associated macrophages by a biomimetic PLGA nanoparticle for colorectal cancer treatment. Int J Biol Macromol. 2023;244:125163. doi: 10.1016/j.ijbiomac.2023.125163. [DOI] [PubMed] [Google Scholar]
- 65.Wu L, Liu Q, Ruan X, Luan X, Zhong Y, Liu J, Yan J, Li X. Multiple Omics Analysis of the Role of RBM10 Gene Instability in Immune Regulation and Drug Sensitivity in Patients with Lung Adenocarcinoma (LUAD) Biomedicines. 2023;11 doi: 10.3390/biomedicines11071861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lan H, Liu Y, Liu J, Wang X, Guan Z, Du J, Jin K. Tumor-Associated Macrophages Promote Oxaliplatin Resistance via METTL3-Mediated m(6)A of TRAF5 and Necroptosis in Colorectal Cancer. Mol Pharm. 2021;18:1026–1037. doi: 10.1021/acs.molpharmaceut.0c00961. [DOI] [PubMed] [Google Scholar]
- 67.Lan J, Sun L, Xu F, Liu L, Hu F, Song D, Hou Z, Wu W, Luo X, Wang J, Yuan X, Hu J, Wang G. M2 Macrophage-Derived Exosomes Promote Cell Migration and Invasion in Colon Cancer. Cancer Res. 2019;79:146–158. doi: 10.1158/0008-5472.CAN-18-0014. [DOI] [PubMed] [Google Scholar]
- 68.Pinto ML, Rios E, Durães C, Ribeiro R, Machado JC, Mantovani A, Barbosa MA, Carneiro F, Oliveira MJ. The Two Faces of Tumor-Associated Macrophages and Their Clinical Significance in Colorectal Cancer. Front Immunol. 2019;10:1875. doi: 10.3389/fimmu.2019.01875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhong X, Chen B, Yang Z. The Role of Tumor-Associated Macrophages in Colorectal Carcinoma Progression. Cell Physiol Biochem. 2018;45:356–365. doi: 10.1159/000486816. [DOI] [PubMed] [Google Scholar]
- 70.Ge S, Sun X, Sang L, Zhang M, Yan X, Ju Q, Ma X, Xu M. Curcumin inhibits malignant behavior of colorectal cancer cells by regulating M2 polarization of tumor-associated macrophages and metastasis associated in colon cancer 1 (MACC1) expression. Chem Biol Drug Des. 2023;102:1202–1212. doi: 10.1111/cbdd.14330. [DOI] [PubMed] [Google Scholar]
- 71.Wu L, Zheng Y, Ruan X, Wu D, Xu P, Liu J, Wu D, Li X. Long-chain noncoding ribonucleic acids affect the survival and prognosis of patients with esophageal adenocarcinoma through the autophagy pathway: construction of a prognostic model. Anticancer Drugs. 2022;33:e590–e603. doi: 10.1097/CAD.0000000000001189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li D, Zhang Q, Li L, Chen K, Yang J, Dixit D, Gimple RC, Ci S, Lu C, Hu L, Gao J, Shan D, Li Y, Zhang J, Shi Z, Gu D, Yuan W, Wu Q, Yang K, Zhao L, Qiu Z, Lv D, Gao W, Yang H, Lin F, Wang Q, Man J, Li C, Tao W, Agnihotri S, Qian X, Shi Y, You Y, Zhang N, Rich JN, Wang X. β2-Microglobulin Maintains Glioblastoma Stem Cells and Induces M2-like Polarization of Tumor-Associated Macrophages. Cancer Res. 2022;82:3321–3334. doi: 10.1158/0008-5472.CAN-22-0507. [DOI] [PubMed] [Google Scholar]
- 73.Wang H, Shao Q, Wang J, Zhao L, Wang L, Cheng Z, Yue C, Chen W, Wang H, Zhang Y. Decreased CXCR2 expression on circulating monocytes of colorectal cancer impairs recruitment and induces Re-education of tumor-associated macrophages. Cancer Lett. 2022;529:112–125. doi: 10.1016/j.canlet.2022.01.004. [DOI] [PubMed] [Google Scholar]
- 74.Wen B, Li S, Ruan L, Yang Y, Chen Z, Zhang B, Yang X, Jie H, Li S, Zeng Z, Liu S. Engulfment and cell motility protein 1 fosters reprogramming of tumor-associated macrophages in colorectal cancer. Cancer Sci. 2023;114:410–422. doi: 10.1111/cas.15628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Colby JK, Jaoude J, Liu F, Shureiqi I. Oxygenated lipid signaling in tumor-associated macrophages-focus on colon cancer. Cancer Metastasis Rev. 2018;37:289–315. doi: 10.1007/s10555-018-9743-z. [DOI] [PubMed] [Google Scholar]
- 76.Kou Y, Li Z, Sun Q, Yang S, Wang Y, Hu C, Gu H, Wang H, Xu H, Li Y, Han B. Prognostic value and predictive biomarkers of phenotypes of tumour-associated macrophages in colorectal cancer. Scand J Immunol. 2022;95:e13137. doi: 10.1111/sji.13137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shinohara H, Kuranaga Y, Kumazaki M, Sugito N, Yoshikawa Y, Takai T, Taniguchi K, Ito Y, Akao Y. Regulated Polarization of Tumor-Associated Macrophages by miR-145 via Colorectal Cancer-Derived Extracellular Vesicles. J Immunol. 2017;199:1505–1515. doi: 10.4049/jimmunol.1700167. [DOI] [PubMed] [Google Scholar]
- 78.Gao X, Zhou S, Qin Z, Li D, Zhu Y, Ma D. Upregulation of HMGB1 in tumor-associated macrophages induced by tumor cell-derived lactate further promotes colorectal cancer progression. J Transl Med. 2023;21:53. doi: 10.1186/s12967-023-03918-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cheruku S, Rao V, Pandey R, Rao Chamallamudi M, Velayutham R, Kumar N. Tumor-associated macrophages employ immunoediting mechanisms in colorectal tumor progression: Current research in Macrophage repolarization immunotherapy. Int Immunopharmacol. 2023;116:109569. doi: 10.1016/j.intimp.2022.109569. [DOI] [PubMed] [Google Scholar]
- 80.Ito M, Mimura K, Nakajima S, Okayama H, Saito K, Nakajima T, Kikuchi T, Onozawa H, Fujita S, Sakamoto W, Saito M, Momma T, Saze Z, Kono K. M2 tumor-associated macrophages resist to oxidative stress through heme oxygenase-1 in the colorectal cancer tumor microenvironment. Cancer Immunol Immunother. 2023;72:2233–2244. doi: 10.1007/s00262-023-03406-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Waniczek D, Lorenc Z, Śnietura M, Wesecki M, Kopec A, Muc-Wierzgoń M. Tumor-Associated Macrophages and Regulatory T Cells Infiltration and the Clinical Outcome in Colorectal Cancer. Arch Immunol Ther Exp (Warsz) 2017;65:445–454. doi: 10.1007/s00005-017-0463-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Scheurlen KM, Billeter AT, O'Brien SJ, Galandiuk S. Metabolic dysfunction and early-onset colorectal cancer - how macrophages build the bridge. Cancer Med. 2020;9:6679–6693. doi: 10.1002/cam4.3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cheng D, Wang H, Cheng H, Zhang H, Dong G, Yan F, Ning Z, Wang C, Wei L, Zhang X, Zhang J, Xiong H. IL-28B reprograms tumor-associated macrophages to promote anti-tumor effects in colon cancer. Int Immunopharmacol. 2022;109:108799. doi: 10.1016/j.intimp.2022.108799. [DOI] [PubMed] [Google Scholar]
- 84.Mola S, Pandolfo C, Sica A, Porta C. The Macrophages-Microbiota Interplay in Colorectal Cancer (CRC)-Related Inflammation: Prognostic and Therapeutic Significance. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21186866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hinshaw DC, Hanna A, Lama-Sherpa T, Metge B, Kammerud SC, Benavides GA, Kumar A, Alsheikh HA, Mota M, Chen D, Ballinger SW, Rathmell JC, Ponnazhagan S, Darley-Usmar V, Samant RS, Shevde LA. Hedgehog Signaling Regulates Metabolism and Polarization of Mammary Tumor-Associated Macrophages. Cancer Res. 2021;81:5425–5437. doi: 10.1158/0008-5472.CAN-20-1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Freire Valls A, Knipper K, Giannakouri E, Sarachaga V, Hinterkopf S, Wuehrl M, Shen Y, Radhakrishnan P, Klose J, Ulrich A, Schneider M, Augustin HG, Ruiz de Almodovar C, Schmidt T. VEGFR1(+) Metastasis-Associated Macrophages Contribute to Metastatic Angiogenesis and Influence Colorectal Cancer Patient Outcome. Clin Cancer Res. 2019;25:5674–5685. doi: 10.1158/1078-0432.CCR-18-2123. [DOI] [PubMed] [Google Scholar]
- 87.Wu L, Zhong Y, Yu X, Wu D, Xu P, Lv L, Ruan X, Liu Q, Feng Y, Liu J, Li X. Selective poly adenylation predicts the efficacy of immunotherapy in patients with lung adenocarcinoma by multiple omics research. Anticancer Drugs. 2022;33:943–959. doi: 10.1097/CAD.0000000000001319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chang J, Feng Q, Mao Y, Zhang Z, Xu Y, Chen Y, Zheng P, Lin S, Shen F, Zhang Z, Zhang Z, He G, Xu J, Wei Y. Siglec9 + tumor-associated macrophages predict prognosis and therapeutic vulnerability in patients with colon cancer. Int Immunopharmacol. 2024;130:111771. doi: 10.1016/j.intimp.2024.111771. [DOI] [PubMed] [Google Scholar]
- 89.Liu Q, Song J, Pan Y, Shi D, Yang C, Wang S, Xiong B. Wnt5a/CaMKII/ERK/CCL2 axis is required for tumor-associated macrophages to promote colorectal cancer progression. Int J Biol Sci. 2020;16:1023–1034. doi: 10.7150/ijbs.40535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wu L, Li X, Yan J. Commentary: Machine learning developed an intratumor heterogeneity signature for predicting prognosis and immunotherapy benefits in cholangiocarcinoma. Transl Oncol. 2024;45:101995. doi: 10.1016/j.tranon.2024.101995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lv Q, Zhang Y, Gao W, Wang J, Hu Y, Yang H, Xie Y, Lv Y, Zhang H, Wu D, Hu L, Wang J. CSF1R inhibition reprograms tumor-associated macrophages to potentiate anti-PD-1 therapy efficacy against colorectal cancer. Pharmacol Res. 2024;202:107126. doi: 10.1016/j.phrs.2024.107126. [DOI] [PubMed] [Google Scholar]
- 92.Wan G, Xie M, Yu H, Chen H. Intestinal dysbacteriosis activates tumor-associated macrophages to promote epithelial-mesenchymal transition of colorectal cancer. Innate Immun. 2018;24:480–489. doi: 10.1177/1753425918801496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Krijgsman D, De Vries NL, Andersen MN, Skovbo A, Tollenaar RAEM, Møller HJ, Hokland M, Kuppen PJK. CD163 as a Biomarker in Colorectal Cancer: The Expression on Circulating Monocytes and Tumor-Associated Macrophages, and the Soluble Form in the Blood. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21165925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hong SM, Lee AY, Kim BJ, Lee JE, Seon SY, Ha YJ, Ng JT, Yoon G, Lim SB, Morgan MJ, Cha JH, Lee D, Kim YS. NAMPT-Driven M2 Polarization of Tumor-Associated Macrophages Leads to an Immunosuppressive Microenvironment in Colorectal Cancer. Adv Sci (Weinh) 2024;11:e2303177. doi: 10.1002/advs.202303177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dost Gunay FS, Kırmızı BA, Ensari A, İcli F, Akbulut H. Tumor-associated Macrophages and Neuroendocrine Differentiation Decrease the Efficacy of Bevacizumab Plus Chemotherapy in Patients With Advanced Colorectal Cancer. Clin Colorectal Cancer. 2019;18:e244–e250. doi: 10.1016/j.clcc.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 96.Yuan Y, Wu D, Hou Y, Zhang Y, Tan C, Nie X, Zhao Z, Hou J. Wnt signaling: Modulating tumor-associated macrophages and related immunotherapeutic insights. Biochem Pharmacol. 2024;223:116154. doi: 10.1016/j.bcp.2024.116154. [DOI] [PubMed] [Google Scholar]
- 97.Liu C, Zhang W, Wang J, Si T, Xing W. Tumor-associated macrophage-derived transforming growth factor-β promotes colorectal cancer progression through HIF1-TRIB3 signaling. Cancer Sci. 2021;112:4198–4207. doi: 10.1111/cas.15101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zheng X, Chen J, Nan T, Zheng L, Lan J, Jin X, Cai Y, Liu H, Chen W. FAM198B promotes colorectal cancer progression by regulating the polarization of tumor-associated macrophages via the SMAD2 signaling pathway. Bioengineered. 2022;13:12435–12445. doi: 10.1080/21655979.2022.2075300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pei W, Wei K, Wu Y, Qiu Q, Zhu H, Mao L, Shi X, Zhang S, Shi Y, Tao S, Mao H, Pang S, Wang J, Liu M, Wang W, Yang Q, Chen C. Colorectal cancer tumor cell-derived exosomal miR-203a-3p promotes CRC metastasis by targeting PTEN-induced macrophage polarization. Gene. 2023;885:147692. doi: 10.1016/j.gene.2023.147692. [DOI] [PubMed] [Google Scholar]
- 100.Su Y, Choi HS, Choi JH, Kim HS, Jang YS, Seo JW. 7S,15R-Dihydroxy-16S,17S-epoxy-docosapentaenoic Acid Overcomes Chemoresistance of 5-Fluorouracil by Suppressing the Infiltration of Tumor-Associated Macrophages and Inhibiting the Activation of Cancer Stem Cells in a Colorectal Cancer Xenograft Model. Mar Drugs. 2023;21 doi: 10.3390/md21020080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Min AKT, Mimura K, Nakajima S, Okayama H, Saito K, Sakamoto W, Fujita S, Endo H, Saito M, Saze Z, Momma T, Ohki S, Kono K. Therapeutic potential of anti-VEGF receptor 2 therapy targeting for M2-tumor-associated macrophages in colorectal cancer. Cancer Immunol Immunother. 2021;70:289–298. doi: 10.1007/s00262-020-02676-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wu L, Li H, Liu Y, Fan Z, Xu J, Li N, Qian X, Lin Z, Li X, Yan J. Research progress of 3D-bioprinted functional pancreas and in vitro tumor models. Int J Bioprinting. 2024;10:1256. [Google Scholar]
- 103.Zhang A, Xu Y, Xu H, Ren J, Meng T, Ni Y, Zhu Q, Zhang WB, Pan YB, Jin J, Bi Y, Wu ZB, Lin S, Lou M. Lactate-induced M2 polarization of tumor-associated macrophages promotes the invasion of pituitary adenoma by secreting CCL17. Theranostics. 2021;11:3839–3852. doi: 10.7150/thno.53749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang N, Wang S, Wang X, Zheng Y, Yang B, Zhang J, Pan B, Gao J, Wang Z. Research trends in pharmacological modulation of tumor-associated macrophages. Clin Transl Med. 2021;11:e288. doi: 10.1002/ctm2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xu H, Zhang Y, Peña MM, Pirisi L, Creek KE. Six1 promotes colorectal cancer growth and metastasis by stimulating angiogenesis and recruiting tumor-associated macrophages. Carcinogenesis. 2017;38:281–292. doi: 10.1093/carcin/bgw121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sougiannis AT, VanderVeen B, Chatzistamou I, Kubinak JL, Nagarkatti M, Fan D, Murphy EA. Emodin reduces tumor burden by diminishing M2-like macrophages in colorectal cancer. Am J Physiol Gastrointest Liver Physiol. 2022;322:G383–G395. doi: 10.1152/ajpgi.00303.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang X, Luo X, Chen C, Tang Y, Li L, Mo B, Liang H, Yu S. The Ap-2α/Elk-1 axis regulates Sirpα-dependent tumor phagocytosis by tumor-associated macrophages in colorectal cancer. Signal Transduct Target Ther. 2020;5:35. doi: 10.1038/s41392-020-0124-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Taniyama D, Taniyama K, Kuraoka K, Yamamoto H, Zaitsu J, Saito A, Sakamoto N, Sentani K, Oue N, Yasui W. CD204-Positive Tumor-associated Macrophages Relate to Malignant Transformation of Colorectal Adenoma. Anticancer Res. 2019;39:2767–2775. doi: 10.21873/anticanres.13403. [DOI] [PubMed] [Google Scholar]
- 109.Jiang X, Cao G, Gao G, Wang W, Zhao J, Gao C. Triptolide decreases tumor-associated macrophages infiltration and M2 polarization to remodel colon cancer immune microenvironment via inhibiting tumor-derived CXCL12. J Cell Physiol. 2021;236:193–204. doi: 10.1002/jcp.29833. [DOI] [PubMed] [Google Scholar]
- 110.Cai J, Xia L, Li J, Ni S, Song H, Wu X. Tumor-Associated Macrophages Derived TGF-β‒Induced Epithelial to Mesenchymal Transition in Colorectal Cancer Cells through Smad2,3-4/Snail Signaling Pathway. Cancer Res Treat. 2019;51:252–266. doi: 10.4143/crt.2017.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chiou YS, Lan YM, Lee PS, Lin Q, Nagabhushanam K, Ho CT, Pan MH. Piceatannol Prevents Colon Cancer Progression via Dual-Targeting to M2-Polarized Tumor-Associated Macrophages and the TGF-β1 Positive Feedback Signaling Pathway. Mol Nutr Food Res. 2022;66:e2200248. doi: 10.1002/mnfr.202200248. [DOI] [PubMed] [Google Scholar]
- 112.Wu L, Li X, Qian X, Wang S, Liu J, Yan J. Lipid Nanoparticle (LNP) Delivery Carrier-Assisted Targeted Controlled Release mRNA Vaccines in Tumor Immunity. Vaccines (Basel) 2024;12 doi: 10.3390/vaccines12020186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zeng J, Sun Y, Sun S, Jiang M, Zhang D, Li W, Liu Z, Shang H, Guan X, Zhang W. Leveraging Nanodrug Delivery System for Simultaneously Targeting Tumor Cells and M2 Tumor-Associated Macrophages for Efficient Colon Cancer Therapy. ACS Appl Mater Interfaces. 2022;14:50475–50484. doi: 10.1021/acsami.2c11534. [DOI] [PubMed] [Google Scholar]
- 114.Zhang T, Liu L, Lai W, Zeng Y, Xu H, Lan Q, Su P, Chu Z. Interaction with tumorassociated macrophages promotes PRL3induced invasion of colorectal cancer cells via MAPK pathwayinduced EMT and NFκB signalinginduced angiogenesis. Oncol Rep. 2019;41:2790–2802. doi: 10.3892/or.2019.7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li Y, Zhao Y, Gao Y, Li Y, Liu M, Xu N, Zhu H. Age-related macrophage alterations are associated with carcinogenesis of colorectal cancer. Carcinogenesis. 2022;43:1039–1049. doi: 10.1093/carcin/bgac088. [DOI] [PubMed] [Google Scholar]
- 116.Pacheco-Fernández T, Juárez-Avelar I, Illescas O, Terrazas LI, Hernández-Pando R, Pérez-Plasencia C, Gutiérrez-Cirlos EB, Ávila-Moreno F, Chirino YI, Reyes JL, Maldonado V, Rodriguez-Sosa M. Macrophage Migration Inhibitory Factor Promotes the Interaction between the Tumor, Macrophages, and T Cells to Regulate the Progression of Chemically Induced Colitis-Associated Colorectal Cancer. Mediators Inflamm. 2019;2019:2056085. doi: 10.1155/2019/2056085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang B, Guo X, Huang L, Zhang Y, Li Z, Su D, Lin L, Zhou P, Ye H, Lu Y, Zhou Q. Tumour-associated macrophages and Schwann cells promote perineural invasion via paracrine loop in pancreatic ductal adenocarcinoma. Br J Cancer. 2024;130:542–554. doi: 10.1038/s41416-023-02539-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wei Z, Yang M, Feng M, Wu Z, Rosin-Arbesfeld R, Dong J, Zhu D. Inhibition of BCL9 Modulates the Cellular Landscape of Tumor-Associated Macrophages in the Tumor Immune Microenvironment of Colorectal Cancer. Front Pharmacol. 2021;12:713331. doi: 10.3389/fphar.2021.713331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wu L, Chen X, Zeng Q, Lai Z, Fan Z, Ruan X, Li X, Yan J. NR5A2 gene affects the overall survival of LUAD patients by regulating the activity of CSCs through SNP pathway by OCLR algorithm and immune score. Heliyon. 2024;10:e28282. doi: 10.1016/j.heliyon.2024.e28282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Feng Q, Chang W, Mao Y, He G, Zheng P, Tang W, Wei Y, Ren L, Zhu D, Ji M, Tu Y, Qin X, Xu J. Tumor-associated Macrophages as Prognostic and Predictive Biomarkers for Postoperative Adjuvant Chemotherapy in Patients with Stage II Colon Cancer. Clin Cancer Res. 2019;25:3896–3907. doi: 10.1158/1078-0432.CCR-18-2076. [DOI] [PubMed] [Google Scholar]
- 121.Tu W, Gong J, Zhou Z, Tian D, Wang Z. TCF4 enhances hepatic metastasis of colorectal cancer by regulating tumor-associated macrophage via CCL2/CCR2 signaling. Cell Death Dis. 2021;12:882. doi: 10.1038/s41419-021-04166-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cheng H, Sun L, Shen D, Ren A, Ma F, Tai G, Fan L, Zhou Y. Beta-1,6 glucan converts tumor-associated macrophages into an M1-like phenotype. Carbohydr Polym. 2020;247:116715. doi: 10.1016/j.carbpol.2020.116715. [DOI] [PubMed] [Google Scholar]
- 123.Li N, Liang X, Li J, Zhang D, Li T, Guo Z. C-C motif chemokine ligand 14 inhibited colon cancer cell proliferation and invasion through suppressing M2 polarization of tumor-associated macrophages. Histol Histopathol. 2021;36:743–752. doi: 10.14670/HH-18-348. [DOI] [PubMed] [Google Scholar]
- 124.Shao LN, Zhu BS, Xing CG, Yang XD, Young W, Cao JP. Effects of autophagy regulation of tumor-associated macrophages on radiosensitivity of colorectal cancer cells. Mol Med Rep. 2016;13:2661–2670. doi: 10.3892/mmr.2016.4820. [DOI] [PubMed] [Google Scholar]
- 125.Tang Y, Hu S, Li T, Qiu X. Tumor cells-derived exosomal circVCP promoted the progression of colorectal cancer by regulating macrophage M1/M2 polarization. Gene. 2023;870:147413. doi: 10.1016/j.gene.2023.147413. [DOI] [PubMed] [Google Scholar]
- 126.Huang J, Pan H, Sun J, Wu J, Xuan Q, Wang J, Ke S, Lu S, Li Z, Feng Z, Hua Y, Yu Q, Yin B, Qian B, Zhou M, Xu Y, Bai M, Zhang Y, Wu Y, Ma Y, Jiang H, Dai W. TMEM147 aggravates the progression of HCC by modulating cholesterol homeostasis, suppressing ferroptosis, and promoting the M2 polarization of tumor-associated macrophages. J Exp Clin Cancer Res. 2023;42:286. doi: 10.1186/s13046-023-02865-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shi W, Zhang F, Chen X, Wang S, Zhang H, Yang Z, Wang G, Zheng Y, Han Y, Sun Y, Gao A. Tumor-derived immunoglobulin like transcript 5 induces suppressive immunocyte infiltration in colorectal cancer. Cancer Sci. 2022;113:1939–1954. doi: 10.1111/cas.15360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang Y, Zhao Y, Li Q, Wang Y. Macrophages, as a Promising Strategy to Targeted Treatment for Colorectal Cancer Metastasis in Tumor Immune Microenvironment. Front Immunol. 2021;12:685978. doi: 10.3389/fimmu.2021.685978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zheng H, Yu S, Zhu C, Guo T, Liu F, Xu Y. HIF1α promotes tumor chemoresistance via recruiting GDF15-producing TAMs in colorectal cancer. Exp Cell Res. 2021;398:112394. doi: 10.1016/j.yexcr.2020.112394. [DOI] [PubMed] [Google Scholar]
- 130.Yi B, Zhang S, Yan S, Liu Y, Feng Z, Chu T, Liu J, Wang W, Xue J, Zhang C, Wang Y. Marsdenia tenacissima enhances immune response of tumor infiltrating T lymphocytes to colorectal cancer. Front Immunol. 2023;14:1238694. doi: 10.3389/fimmu.2023.1238694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Malier M, Gharzeddine K, Laverriere MH, Marsili S, Thomas F, Decaens T, Roth G, Millet A. Hypoxia Drives Dihydropyrimidine Dehydrogenase Expression in Macrophages and Confers Chemoresistance in Colorectal Cancer. Cancer Res. 2021;81:5963–5976. doi: 10.1158/0008-5472.CAN-21-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jiang X, Zhang H, Zhang H, Wang F, Wang X, Ding T, Zhang X, Wang T. Microcystin-LR-Induced Interaction between M2 Tumor-Associated Macrophage and Colorectal Cancer Cell Promotes Colorectal Cancer Cell Migration through Regulating the Expression of TGF-β1 and CST3. Int J Mol Sci. 2023;24 doi: 10.3390/ijms241310527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chen W, Xu Y, Zhong J, Wang H, Weng M, Cheng Q, Wu Q, Sun Z, Jiang H, Zhu M, Ren Y, Xu P, Chen J, Miao C. MFHAS1 promotes colorectal cancer progress by regulating polarization of tumor-associated macrophages via STAT6 signaling pathway. Oncotarget. 2016;7:78726–78735. doi: 10.18632/oncotarget.12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Fei R, Zhang Y, Wang S, Xiang T, Chen W. α7 nicotinic acetylcholine receptor in tumor-associated macrophages inhibits colorectal cancer metastasis through the JAK2/STAT3 signaling pathway. Oncol Rep. 2017;38:2619–2628. doi: 10.3892/or.2017.5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liu LQ, Nie SP, Shen MY, Hu JL, Yu Q, Gong D, Xie MY. Tea Polysaccharides Inhibit Colitis-Associated Colorectal Cancer via Interleukin-6/STAT3 Pathway. J Agric Food Chem. 2018;66:4384–4393. doi: 10.1021/acs.jafc.8b00710. [DOI] [PubMed] [Google Scholar]
- 136.Cavnar MJ, Turcotte S, Katz SC, Kuk D, Gönen M, Shia J, Allen PJ, Balachandran VP, D'Angelica MI, Kingham TP, Jarnagin WR, DeMatteo RP. Tumor-Associated Macrophage Infiltration in Colorectal Cancer Liver Metastases is Associated With Better Outcome. Ann Surg Oncol. 2017;24:1835–1842. doi: 10.1245/s10434-017-5812-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yang Y, Hu YE, Huang YQ, Jiang YF, Fu X, You FM. [Tongxie Yaofang regulates tumor-associated macrophage polarization in colorectal cancer under chronic stress] Zhongguo Zhong Yao Za Zhi. 2023;48:6142–6153. doi: 10.19540/j.cnki.cjcmm.20230811.703. [DOI] [PubMed] [Google Scholar]
- 138.Scheurlen KM, Snook DL, Walter MN, Cook CN, Fiechter CR, Pan J, Beal RJ, Galandiuk S. Itaconate and leptin affecting PPARγ in M2 macrophages: A potential link to early-onset colorectal cancer. Surgery. 2022;171:650–656. doi: 10.1016/j.surg.2021.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rao G, Wang H, Li B, Huang L, Xue D, Wang X, Jin H, Wang J, Zhu Y, Lu Y, Du L, Chen Q. Reciprocal interactions between tumor-associated macrophages and CD44-positive cancer cells via osteopontin/CD44 promote tumorigenicity in colorectal cancer. Clin Cancer Res. 2013;19:785–797. doi: 10.1158/1078-0432.CCR-12-2788. [DOI] [PubMed] [Google Scholar]
- 140.Kinouchi M, Miura K, Mizoi T, Ishida K, Fujibuchi W, Sasaki H, Ohnuma S, Saito K, Katayose Y, Naitoh T, Motoi F, Shiiba K, Egawa S, Shibata C, Unno M. Infiltration of CD40-positive tumor-associated macrophages indicates a favorable prognosis in colorectal cancer patients. Hepatogastroenterology. 2013;60:83–88. doi: 10.5754/hge12372. [DOI] [PubMed] [Google Scholar]
- 141.Woldemeskel M, Hawkins I, Whittington L. Ki-67 protein expression and tumor associated inflammatory cells (macrophages and mast cells) in canine colorectal carcinoma. BMC Vet Res. 2017;13:111. doi: 10.1186/s12917-017-1030-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Qiao T, Yang W, He X, Song P, Chen X, Liu R, Xiao J, Yang X, Li M, Gao Y, Chen G, Lu Y, Zhang J, Leng J, Ren H. Dynamic differentiation of F4/80+ tumor-associated macrophage and its role in tumor vascularization in a syngeneic mouse model of colorectal liver metastasis. Cell Death Dis. 2023;14:117. doi: 10.1038/s41419-023-05626-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Larionova I, Patysheva M, Iamshchikov P, Kazakova E, Kazakova A, Rakina M, Grigoryeva E, Tarasova A, Afanasiev S, Bezgodova N, Kiselev A, Dobrodeev A, Kostromitskiy D, Cherdyntseva N, Kzhyshkowska J. PFKFB3 overexpression in monocytes of patients with colon but not rectal cancer programs pro-tumor macrophages and is indicative for higher risk of tumor relapse. Front Immunol. 2022;13:1080501. doi: 10.3389/fimmu.2022.1080501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhu M, Zhu Z, Jiang P, Zheng J, Yan F, Feng J. CircMERTK modulates the suppressive capacity of tumor-associated macrophage via targeting IL-10 in colorectal cancer. Hum Cell. 2023;36:276–285. doi: 10.1007/s13577-022-00792-4. [DOI] [PubMed] [Google Scholar]
- 145.de Carvalho TG, Lara P, Jorquera-Cordero C, Aragão CFS, de Santana Oliveira A, Garcia VB, de Paiva Souza SV, Schomann T, Soares LAL, da Matta Guedes PM, de Araújo Júnior RF. Inhibition of murine colorectal cancer metastasis by targeting M2-TAM through STAT3/NF-kB/AKT signaling using macrophage 1-derived extracellular vesicles loaded with oxaliplatin, retinoic acid, and Libidibia ferrea. Biomed Pharmacother. 2023;168:115663. doi: 10.1016/j.biopha.2023.115663. [DOI] [PubMed] [Google Scholar]
- 146.Shibutani M, Maeda K, Nagahara H, Fukuoka T, Nakao S, Matsutani S, Hirakawa K, Ohira M. The peripheral monocyte count is associated with the density of tumor-associated macrophages in the tumor microenvironment of colorectal cancer: a retrospective study. BMC Cancer. 2017;17:404. doi: 10.1186/s12885-017-3395-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chen W, Zhou M, Guan B, Xie B, Liu Y, He J, Zhao J, Zhao Q, Yan D. Tumour-associated macrophage-derived DOCK7-enriched extracellular vesicles drive tumour metastasis in colorectal cancer via the RAC1/ABCA1 axis. Clin Transl Med. 2024;14:e1591. doi: 10.1002/ctm2.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kaidi D, Szeponik L, Yrlid U, Wettergren Y, Bexe Lindskog E. Impact of thymidine phosphorylase and CD163 expression on prognosis in stage II colorectal cancer. Clin Transl Oncol. 2022;24:1818–1827. doi: 10.1007/s12094-022-02839-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Liu F, Ran F, He H, Chen L. Astragaloside IV Exerts Anti-tumor Effect on Murine Colorectal Cancer by Re-educating Tumor-Associated Macrophage. Arch Immunol Ther Exp (Warsz) 2020;68:33. doi: 10.1007/s00005-020-00598-y. [DOI] [PubMed] [Google Scholar]
- 150.Sunakawa Y, Stintzing S, Cao S, Heinemann V, Cremolini C, Falcone A, Yang D, Zhang W, Ning Y, Stremitzer S, Matsusaka S, Yamauchi S, Parekh A, Okazaki S, Berger MD, Graver S, Mendez A, Scherer SJ, Loupakis F, Lenz HJ. Variations in genes regulating tumor-associated macrophages (TAMs) to predict outcomes of bevacizumab-based treatment in patients with metastatic colorectal cancer: results from TRIBE and FIRE3 trials. Ann Oncol. 2015;26:2450–2456. doi: 10.1093/annonc/mdv474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chen X, Jia Z, Wen Y, Huang Y, Yuan X, Chen Y, Liu Y, Liu J. Bidirectional anisotropic palladium nanozymes reprogram macrophages to enhance collaborative chemodynamic therapy of colorectal cancer. Acta Biomater. 2022;151:537–548. doi: 10.1016/j.actbio.2022.08.020. [DOI] [PubMed] [Google Scholar]
- 152.Cui YL, Li HK, Zhou HY, Zhang T, Li Q. Correlations of tumor-associated macrophage subtypes with liver metastases of colorectal cancer. Asian Pac J Cancer Prev. 2013;14:1003–1007. doi: 10.7314/apjcp.2013.14.2.1003. [DOI] [PubMed] [Google Scholar]
- 153.Wei TT, Lin YT, Tseng RY, Shun CT, Lin YC, Wu MS, Fang JM, Chen CC. Prevention of Colitis and Colitis-Associated Colorectal Cancer by a Novel Polypharmacological Histone Deacetylase Inhibitor. Clin Cancer Res. 2016;22:4158–4169. doi: 10.1158/1078-0432.CCR-15-2379. [DOI] [PubMed] [Google Scholar]
- 154.Katholnig K, Schütz B, Fritsch SD, Schörghofer D, Linke M, Sukhbaatar N, Matschinger JM, Unterleuthner D, Hirtl M, Lang M, Herac M, Spittler A, Bergthaler A, Schabbauer G, Bergmann M, Dolznig H, Hengstschläger M, Magnuson MA, Mikula M, Weichhart T. Inactivation of mTORC2 in macrophages is a signature of colorectal cancer that promotes tumorigenesis. JCI Insight. 2019;4 doi: 10.1172/jci.insight.124164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Frión-Herrera Y, Gabbia D, Scaffidi M, Zagni L, Cuesta-Rubio O, De Martin S, Carrara M. Cuban Brown Propolis Interferes in the Crosstalk between Colorectal Cancer Cells and M2 Macrophages. Nutrients. 2020;12 doi: 10.3390/nu12072040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ni H, Chen Y, Xia W, Wang C, Hu C, Sun L, Tang W, Cui H, Shen T, Liu Y, Li J. SATB2 Defect Promotes Colitis and Colitis-associated Colorectal Cancer by Impairing Cl-/HCO3- Exchange and Homeostasis of Gut Microbiota. J Crohns Colitis. 2021;15:2088–2102. doi: 10.1093/ecco-jcc/jjab094. [DOI] [PubMed] [Google Scholar]
- 157.Li X, Lan Q, Lai W, Wu H, Xu H, Fang K, Chu Z, Zeng Y. Exosome-derived lnc-HOXB8-1:2 induces tumor-associated macrophage infiltration to promote neuroendocrine differentiated colorectal cancer progression by sponging hsa-miR-6825-5p. BMC Cancer. 2022;22:928. doi: 10.1186/s12885-022-09926-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Xu G, Jiang L, Ye C, Qin G, Luo Z, Mo Y, Chen J. The Ratio of CD86+/CD163+ Macrophages Predicts Postoperative Recurrence in Stage II-III Colorectal Cancer. Front Immunol. 2021;12:724429. doi: 10.3389/fimmu.2021.724429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.De la Fuente López M, Landskron G, Parada D, Dubois-Camacho K, Simian D, Martinez M, Romero D, Roa JC, Chahuán I, Gutiérrez R, Lopez-K F, Alvarez K, Kronberg U, López S, Sanguinetti A, Moreno N, Abedrapo M, González MJ, Quera R, Hermoso-R MA. The relationship between chemokines CCL2, CCL3, and CCL4 with the tumor microenvironment and tumor-associated macrophage markers in colorectal cancer. Tumour Biol. 2018;40:1010428318810059. doi: 10.1177/1010428318810059. [DOI] [PubMed] [Google Scholar]
- 160.Zhang L, Shi P, Jin P, Chen Z, Hu B, Cao C, Wang X, Sheng J. Ganodermanontriol regulates tumor-associated M2 macrophage polarization in gastric cancer. Aging (Albany NY) 2024;16:1390–1398. doi: 10.18632/aging.205434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Suarez-Lopez L, Kong YW, Sriram G, Patterson JC, Rosenberg S, Morandell S, Haigis KM, Yaffe MB. MAPKAP Kinase-2 Drives Expression of Angiogenic Factors by Tumor-Associated Macrophages in a Model of Inflammation-Induced Colon Cancer. Front Immunol. 2020;11:607891. doi: 10.3389/fimmu.2020.607891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kee JY, Ito A, Hojo S, Hashimoto I, Igarashi Y, Tsuneyama K, Tsukada K, Irimura T, Shibahara N, Takasaki I, Inujima A, Nakayama T, Yoshie O, Sakurai H, Saiki I, Koizumi K. CXCL16 suppresses liver metastasis of colorectal cancer by promoting TNF-α-induced apoptosis by tumor-associated macrophages. BMC Cancer. 2014;14:949. doi: 10.1186/1471-2407-14-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wang Y, Tiruthani K, Li S, Hu M, Zhong G, Tang Y, Roy S, Zhang L, Tan J, Liao C, Liu R. mRNA Delivery of a Bispecific Single-Domain Antibody to Polarize Tumor-Associated Macrophages and Synergize Immunotherapy against Liver Malignancies. Adv Mater. 2021;33:e2007603. doi: 10.1002/adma.202007603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Aasebø K, Bruun J, Bergsland CH, Nunes L, Eide GE, Pfeiffer P, Dahl O, Glimelius B, Lothe RA, Sorbye H. Prognostic role of tumour-infiltrating lymphocytes and macrophages in relation to MSI, CDX2 and BRAF status: a population-based study of metastatic colorectal cancer patients. Br J Cancer. 2022;126:48–56. doi: 10.1038/s41416-021-01586-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zinovkin DA, Kose SY, Nadyrov EA, Achinovich SL, Los' DM, Gavrilenko TE, Gavrilenko DI, Yuzugulen J, Pranjol MZI. Potential role of tumor-infiltrating T-, B-lymphocytes, tumor-associated macrophages and IgA-secreting plasma cells in long-term survival in the rectal adenocarcinoma patients. Life Sci. 2021;286:120052. doi: 10.1016/j.lfs.2021.120052. [DOI] [PubMed] [Google Scholar]
- 166.Stadler M, Pudelko K, Biermeier A, Walterskirchen N, Gaigneaux A, Weindorfer C, Harrer N, Klett H, Hengstschläger M, Schüler J, Sommergruber W, Oehler R, Bergmann M, Letellier E, Dolznig H. Stromal fibroblasts shape the myeloid phenotype in normal colon and colorectal cancer and induce CD163 and CCL2 expression in macrophages. Cancer Lett. 2021;520:184–200. doi: 10.1016/j.canlet.2021.07.006. [DOI] [PubMed] [Google Scholar]
- 167.Ammendola M, Patruno R, Sacco R, Marech I, Sammarco G, Zuccalà V, Luposella M, Zizzo N, Gadaleta C, Porcelli M, Gadaleta CD, Ribatti D, Ranieri G. Mast cells positive to tryptase and tumour-associated macrophages correlate with angiogenesis in locally advanced colorectal cancer patients undergone to surgery. Expert Opin Ther Targets. 2016;20:533–540. doi: 10.1517/14728222.2016.1158811. [DOI] [PubMed] [Google Scholar]
- 168.Zeng YJ, Lai W, Wu H, Liu L, Xu HY, Wang J, Chu ZH. Neuroendocrine-like cells -derived CXCL10 and CXCL11 induce the infiltration of tumor-associated macrophage leading to the poor prognosis of colorectal cancer. Oncotarget. 2016;7:27394–27407. doi: 10.18632/oncotarget.8423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Tacconi C, Ungaro F, Correale C, Arena V, Massimino L, Detmar M, Spinelli A, Carvello M, Mazzone M, Oliveira AI, Rubbino F, Garlatti V, Spanò S, Lugli E, Colombo FS, Malesci A, Peyrin-Biroulet L, Vetrano S, Danese S, D'Alessio S. Activation of the VEGFC/VEGFR3 Pathway Induces Tumor Immune Escape in Colorectal Cancer. Cancer Res. 2019;79:4196–4210. doi: 10.1158/0008-5472.CAN-18-3657. [DOI] [PubMed] [Google Scholar]
- 170.Lin X, Yi Z, Diao J, Shao M, Zhao L, Cai H, Fan Q, Yao X, Sun X. ShaoYao decoction ameliorates colitis-associated colorectal cancer by downregulating proinflammatory cytokines and promoting epithelial-mesenchymal transition. J Transl Med. 2014;12:105. doi: 10.1186/1479-5876-12-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Pancione M, Forte N, Sabatino L, Tomaselli E, Parente D, Febbraro A, Colantuoni V. Reduced beta-catenin and peroxisome proliferator-activated receptor-gamma expression levels are associated with colorectal cancer metastatic progression: correlation with tumor-associated macrophages, cyclooxygenase 2, and patient outcome. Hum Pathol. 2009;40:714–725. doi: 10.1016/j.humpath.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 172.Pansa MF, Lamberti MJ, Cogno IS, Correa SG, Rumie Vittar NB, Rivarola VA. Contribution of resident and recruited macrophages to the photodynamic intervention of colorectal tumor microenvironment. Tumour Biol. 2016;37:541–552. doi: 10.1007/s13277-015-3768-5. [DOI] [PubMed] [Google Scholar]
- 173.Soldani C, De Simone G, Polidoro MA, Morabito A, Franceschini B, Colombo FS, Anselmo A, Milana F, Lleo A, Torzilli G, Pastorelli R, Donadon M, Brunelli L. Riboflavin-LSD1 axis participates in the in vivo tumor-associated macrophage morphology in human colorectal liver metastases. Cancer Immunol Immunother. 2024;73:63. doi: 10.1007/s00262-024-03645-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Small DM, Burden RE, Jaworski J, Hegarty SM, Spence S, Burrows JF, McFarlane C, Kissenpfennig A, McCarthy HO, Johnston JA, Walker B, Scott CJ. Cathepsin S from both tumor and tumor-associated cells promote cancer growth and neovascularization. Int J Cancer. 2013;133:2102–2112. doi: 10.1002/ijc.28238. [DOI] [PubMed] [Google Scholar]
- 175.Illemann M, Laerum OD, Hasselby JP, Thurison T, Høyer-Hansen G, Nielsen HJ Danish Study Group on Early Detection of Colorectal Cancer, Christensen IJ. Urokinase-type plasminogen activator receptor (uPAR) on tumor-associated macrophages is a marker of poor prognosis in colorectal cancer. Cancer Med. 2014;3:855–864. doi: 10.1002/cam4.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Che N, Li M, Liu X, Cui CA, Gong J, Xuan Y. Macelignan prevents colorectal cancer metastasis by inhibiting M2 macrophage polarization. Phytomedicine. 2024;122:155144. doi: 10.1016/j.phymed.2023.155144. [DOI] [PubMed] [Google Scholar]
- 177.Wei B, Pan J, Yuan R, Shao B, Wang Y, Guo X, Zhou S. Polarization of Tumor-Associated Macrophages by Nanoparticle-Loaded Escherichia coli Combined with Immunogenic Cell Death for Cancer Immunotherapy. Nano Lett. 2021;21:4231–4240. doi: 10.1021/acs.nanolett.1c00209. [DOI] [PubMed] [Google Scholar]
- 178.Gao J, Liang Y, Wang L. Shaping Polarization Of Tumor-Associated Macrophages In Cancer Immunotherapy. Front Immunol. 2022;13:888713. doi: 10.3389/fimmu.2022.888713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lan Q, Lai W, Zeng Y, Liu L, Li S, Jin S, Zhang Y, Luo X, Xu H, Lin X, Chu Z. CCL26 Participates in the PRL-3-Induced Promotion of Colorectal Cancer Invasion by Stimulating Tumor-Associated Macrophage Infiltration. Mol Cancer Ther. 2018;17:276–289. doi: 10.1158/1535-7163.MCT-17-0507. [DOI] [PubMed] [Google Scholar]
- 180.Wang X, Yuwen TJ, Zhong Y, Li ZG, Wang XY. A new method for predicting the prognosis of colorectal cancer patients through a combination of multiple tumor-associated macrophage markers at the invasive front. Heliyon. 2023;9:e13211. doi: 10.1016/j.heliyon.2023.e13211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lv Q, Pan X, Wang D, Rong Q, Ma B, Xie X, Zhang Y, Wang J, Hu L. Discovery of (Z)-1-(3-((1H-Pyrrol-2-yl)methylene)-2-oxoindolin-6-yl)-3-(isoxazol-3-yl)urea Derivatives as Novel and Orally Highly Effective CSF-1R Inhibitors for Potential Colorectal Cancer Immunotherapy. J Med Chem. 2021;64:17184–17208. doi: 10.1021/acs.jmedchem.1c01184. [DOI] [PubMed] [Google Scholar]
- 182.Malier M, Gharzeddine K, Laverriere MH, Decaens T, Roth G, Millet A. [Tumor-associated macrophages: New targets to thwart 5-FU chemoresistance in colorectal cancers?] Med Sci (Paris) 2022;38:243–245. doi: 10.1051/medsci/2022017. [DOI] [PubMed] [Google Scholar]
- 183.Liu Y, Wen Y, Chen X, Zhu X, Yu Q, Gong Y, Yuan G, Liu J, Qin X. Inflammation-responsive functional Ru nanoparticles combining a tumor-associated macrophage repolarization strategy with phototherapy for colorectal cancer therapy. J Mater Chem B. 2019;7:6210–6223. doi: 10.1039/c9tb01613a. [DOI] [PubMed] [Google Scholar]
- 184.Hu L, Huang S, Chen G, Li B, Li T, Lin M, Huang Y, Xiao Z, Shuai X, Su Z. Nanodrugs Incorporating LDHA siRNA Inhibit M2-like Polarization of TAMs and Amplify Autophagy to Assist Oxaliplatin Chemotherapy against Colorectal Cancer. ACS Appl Mater Interfaces. 2022;14:31625–31633. doi: 10.1021/acsami.2c05841. [DOI] [PubMed] [Google Scholar]
- 185.Lackner C, Jukic Z, Tsybrovskyy O, Jatzko G, Wette V, Hoefler G, Klimpfinger M, Denk H, Zatloukal K. Prognostic relevance of tumour-associated macrophages and von Willebrand factor-positive microvessels in colorectal cancer. Virchows Arch. 2004;445:160–167. doi: 10.1007/s00428-004-1051-z. [DOI] [PubMed] [Google Scholar]
- 186.Luput L, Licarete E, Sesarman A, Patras L, Alupei MC, Banciu M. Tumor-associated macrophages favor C26 murine colon carcinoma cell proliferation in an oxidative stress-dependent manner. Oncol Rep. 2017;37:2472–2480. doi: 10.3892/or.2017.5466. [DOI] [PubMed] [Google Scholar]