Abstract
BACKGROUND
The clinical effects and detailed roles of long non-coding RNA (LncRNA) steroid receptor RNA activator 1 (SRA1) in esophageal squamous cell carcinoma (ESCC) remain ambiguous. In the present study, the complementary sites between lncRNA SRA1, miRNA-363-5p, and phospholysine phosphohistidine inorganic pyrophosphate phosphatase (LHPP) predicted via bioinformatics analysis stimulated us to hypothesize that miRNA-363-5p/LHPP axis might be required for SRA1-mediated ESCC progression.
AIM
To investigate the molecular events of SRA1 in the malignant behavior in ESCC.
METHODS
Thirty-eight ESCC tissues and paired adjacent normal tissues were acquired. SRA1 expression was detected in ESCC tissues and cell lines using quantitative reverse transcription-polymerase chain reaction. Cell counting Kit-8 assay, transwell invasion assay, glycolysis assay, and xenograft tumor model were performed to address the malignant biological behaviors of ESCC cells after the introduction of SRA1. The t-test and the χ2 test were used for comparison between groups. Survival curve analysis was performed using the Kaplan-Meier method.
RESULTS
SRA1 downregulation was identified in ESCC. ESCC patients exhibiting a low SRA1 expression faced shorter overall survival than those with a high SRA1 expression. The introduction of SRA1 inhibited cell proliferation, glucose uptake, and lactate production in ESCC. In vivo, the growth of ESCC was hindered by SRA1 overexpression. Then, SRA1 overexpresses the LHPP by inhibiting miRNA-363-5p. Lastly, the introduction of small interfering RNA si-LHPP or miRNA-363-5p mimic could abrogate the inhibition roles triggered by SRA1.
CONCLUSION
SRA1 inhibits the oncogenicity of ESCC via miRNA-363-5p/LHPP axis. The SRA1/miRNA-363-5p/LHPP pathway may be a therapeutic target for ESCC.
Keywords: Steroid receptor RNA activator 1, Esophageal squamous cell carcinoma, Phospholysine phosphohistidine inorganic pyrophosphate phosphatase, Cancer therapy, MicroRNA, Long non-coding RNA
Core Tip: In this study, we identified abnormal expression of steroid receptor RNA activator 1 (SRA1) in esophageal squamous cell carcinoma (ESCC). SRA1 was significantly downregulated in ESCC tissues and cell lines, and its low expression was strongly associated with advanced tumor stage, metastasis, larger tumor size, and poor survival. Functional and rescue assays demonstrated that SRA1 could impede ESCC cell proliferation and glycolysis via miRNA-363-5p and phospholysine phosphohistidine inorganic pyrophosphate phosphatase (LHPP). Mechanistically, SRA1 elevated LHPP expression by sponging miRNA-363-5p, thereby inhibiting ESCC cell proliferation and glycolysis.
INTRODUCTION
There were 604100 new cases and 544076 deaths from esophageal squamous cell carcinoma (ESCC) estimated by 2020[1,2]. Because of the lack of obvious clinical symptoms and effective screening methods in the early stage, most patients with esophageal cancer are already in the middle and advanced stages when they are diagnosed[3,4].
Long non-coding RNAs (lncRNAs) are a class of biological macromolecules with a length greater than 200 nucleotides[5,6]. Tumor cells often use glycolysis to metabolize glucose[7]. Generally, increased glycolysis can meet the energy requirements of tumor cells and provide a favorable microenvironment for tumor occurrence and development[7]. Studies found that many lncRNAs are involved in the glycolysis process of tumors[8-11]. MiRNAs, in the form of oncogenes or tumor suppressor genes, play a role in promoting or suppressing tumors in ESCC[12]. Tumor suppressor protein, phospholysine phosphohistidine inorganic pyrophosphate phosphatase (LHPP), has the opposite effect of histidine kinase[13]. Studies found that low expression of LHPP can lead to up-regulation of histidine phosphorylation[14-16]. In the present study, the complementary sites between lncRNA steroid receptor RNA activator 1 (SRA1), miRNA-363-5p, and LHPP predicted via bioinformatics analysis stimulated us to hypothesize that miRNA-363-5p/LHPP axis might be required for SRA1-mediated ESCC progression.
In this study, the expression of SRA1 was verified in ESCC tissues. Then, we characterized the clinical implication as well as the biological function of SRA1 in ESCC. We found that SRA1 functions as a tumor suppressor gene in ESCC. In terms of mechanism, SRA1 inhibits the glycolysis and malignant biological behaviors of ESCC cells via functioning as a molecular sponge for miRNA-363-5p and upmodulating LHPP expression.
MATERIALS AND METHODS
Clinical tissues
ESCC and paired adjacent tissues (the area of tumor margin > 5 cm) were acquired from 38 ESCC patients in the Huai’an Hospital of Huai’an City from January to December 2016. The age of the patients ranged from 54 to 77 years (median age was 65 years). There were 26 males and 12 females. The inclusion criteria for ESCC patients in this study were as follows: The patient was diagnosed as ESCC by two pathologists; The patient's personal information and pathological data were complete; The patient did not receive therapy before radical ESCC surgery; The patient completed the follow-up (36 months), and obtain complete follow-up data. The exclusion criteria for patients were as follows: The patient was re-diagnosed as non-squamous cell carcinoma; The patient's personal information and pathological data were missing; Patients sign an informed consent form for participation. Study was performed under the Ethics Committee of the Huai’an Hospital of Huai’an City (No. 2016.0112).
RNA extraction
RNA extraction and purification were carried out following the manufacturer's guidelines using TRIzol reagent (Invitrogen, Carlsbad, CA, United States). The concentration and purity of RNA in each sample were assessed using a NanoDrop ND-1000. Additionally, the integrity of the RNA was evaluated using an Agilent 2100 system (Agilent, Santa Clara, CA, United States). After eliminating ribosomal RNAs, the remaining RNA molecules were subjected to high-temperature fragmentation, resulting in the generation of smaller fragments. Subsequently, the fragmented RNA pieces underwent reverse transcription to produce cDNA.
Identification of lncRNAs
Candidate lncRNAs were selected using two criteria: (1) Transcripts with a length less than 200 nucleotides and reads coverage below 3 were excluded to eliminate interference from other noncoding RNAs (such as ribosomal RNA, transfer RNA, small nucleolar RNA, and small nuclear RNA); and (2) The coding ability of the transcripts was assessed using the coding-noncoding index and coding potential calculator, and transcripts with coding potential were excluded. The remaining transcripts were then categorized as lncRNAs.
Cell culture and transfection
Normal esophageal epithelial cells Het-1A and ESCC cells (TE1, Eca109, KYSE30, EC9706, KYSE180) were used. Cells were kept in DMEM under the condition of 5% CO2 and 37 °C. SRA1 was cloned into pcDNA3.1 vector to construct SRA1 overexpressing plasmid (pcDNA3.1-SRA1). The pcDNA3.1 vector was used as a negative control (NC).
Quantitative reverse transcription-polymerase chain reaction
Quantitative reverse transcription-polymerase chain reaction (QRT-PCR) was performed using the SYBR Green qPCR Kit (Thermo Fisher Scientific, Waltham, MA, United States) with a StepOne™ Real-Time PCR System (Applied Biosystems, Carlsbad, CA, United States). The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and U6 were used as control references for lncRNA/mRNA and miRNA. Their primers were purchased from Sangon Biotech (Shanghai, China), and the sequences as described previously are listed in Table 1. In this study, the amplification efficiencies of SRA1, miRNA-363-5p, LHPP, GAPDH and U6 were 97.8%, 96.9%, 99.8%, 98.4% and 99.2%, respectively.
Table 1.
Quantitative reverse transcription-polymerase chain reaction primer sequences
|
Name
|
Primer sequence
|
| lncRNA SRA1 | F: 5′-GCTGGGCACTGGGAATGTAA-3′ |
| R: 5′-CACGACCCTACAACCCTCTG-3′ | |
| miRNA-363-5p | F: 5′-GGCGAGTTTTAATTTCTATT-3′ |
| R: 5′-ATCAACTGCTCTCGTGGA-3′ | |
| LHPP | F: 5′-GACGCAGCACTCACCCATCT-3′ |
| R: 5′-CCAGGCATTCGGTGATGTG-3′ | |
| GAPDH | F: 5′-AGAAGGCTGGGGCTCATTTG-3′ |
| R: 5′-GCAGGAGGCATTGCTGATGAT-3′ | |
| U6 | F: 5′-GCTTCGGCAGCACATATACTAAAAT-3′ |
| R: 5′-CGCTTCACGAATTTGCGTGTCAT-3′ |
LncRNA: Long non-coding RNA; SRA1: Steroid receptor RNA activator 1; LHPP: Phospholysine phosphohistidine inorganic pyrophosphate phosphatase.
CCK-8 assay
After transfection, ESCC cells (2 × 103 cells/well) were plated onto 96-wells plates and cultivated for 0, 24, 48, and 72 hours, followed by addition into 10 μL CCK-8 solution (Abcam, Cambridge, MA, United States).
Analysis of glycolysis
Glycolysis assay was performed as described previously[17-19]. After transfection, 2 × 105 ESCC cells were plated onto 6-wells plates, and the levels of glycolysis were measured (BioVision, Milpitas, CA, United States).
Western blot
The membranes were incubated with LHPP (ab116175; 1:1000 dilution; Abcam, United States), glucose transport protein type 1 (GLUT1; 1:1000 dilution; ab115730, Abcam, United States), lactate dehydrogenase (LDHA; 1:1000 dilution; ab101562, Abcam, United States), GAPDH (1:3000 dilution; ab128915; Abcam, United States) antibodies.
Immunohistochemistry
Immunohistochemistry was performed in paraffin-embedded sections using an Immunohistochemistry kit (SV0002, BOSTER, China). Tissue samples were incubated with primary antibodies against Ki-67 (ab254123, Abcam, United States), followed by incubation with the secondary antibody.
Xenograft tumor model analysis
Twelve Balb/c nude mice (3-4 weeks, male) were randomly divided into pcDNA3.1-NC group and pcDNA3.1-SRA1 group. And 1 × 106 EC9706 or TE1 cells were subcutaneously injected into nude mice. After 28 days, the mice were sacrificed. All animal experiments were conducted by the guideline of the Ethical Committees of Huai’an Hospital of Huai’an City (No. 2016.A023).
Statistical analysis
The t-test and the χ2 test were used for comparison between groups. Survival curve analysis was performed using the Kaplan–Meier method. P < 0.05 indicated statistical significance.
RESULTS
SRA1 Level was decreased in ESCC
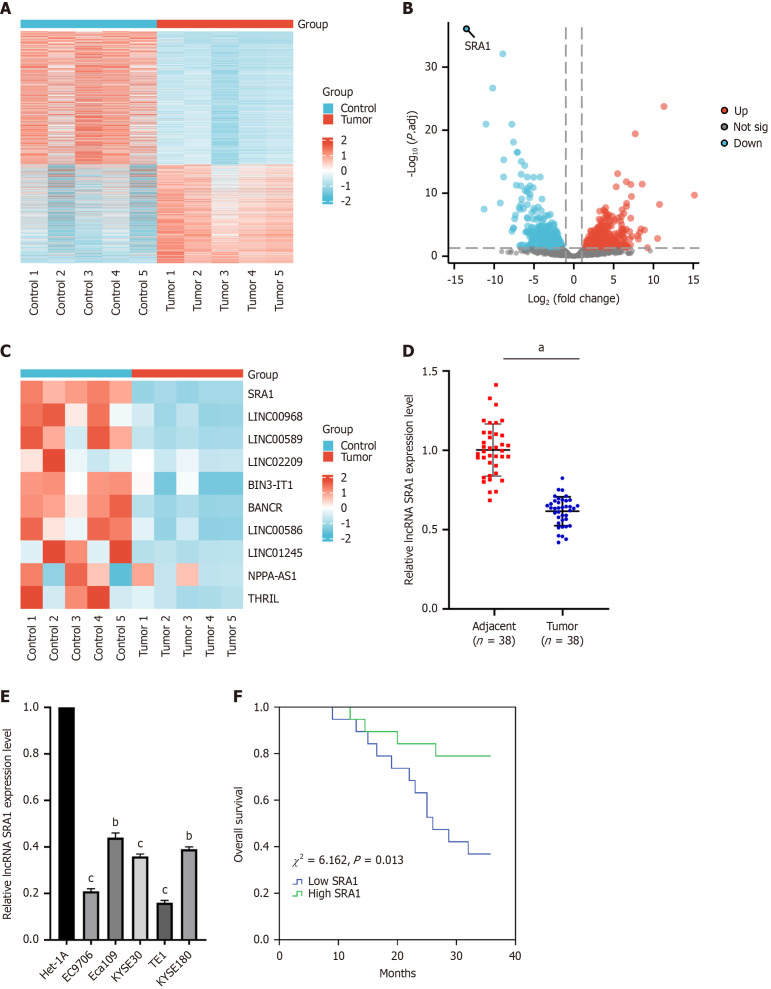
The lncRNA expression patterns were performed through hierarchical clustering between ESCC and adjacent normal tissues (Figure 1A). Changes in the lncRNA level were displayed in the volcano (Figure 1B) plot. As was observed, SRA1 had the highest fold of differential expression (Figure 1C). In clinical samples, we indicated that SRA1 expression was downregulated in ESCC tissues (Figure 1D) and cell lines (Figure 1E). Furthermore, ESCC patients with low SRA1 had shorter overall survival than patients with high SRA1 (χ2 = 6.162, P = 0.013; Figure 1F). In addition, the low SRA1 expression was correlated with lymph node metastasis, depth of tumor invasion, and TNM stage, but not with age, sex, or body mass index (Table 2).
Figure 1.
Steroid receptor RNA activator 1 expression level was decreased in human esophageal squamous cell carcinoma tissues and cell lines. A: The five pairs of esophageal squamous cell carcinoma (ESCC) and adjacent normal tissues were carried out long non-coding RNA (LncRNA) microarray analysis, and the lncRNA expression patterns were performed through hierarchical clustering; B: Changes in the lncRNA level were displayed in the volcano plot; C: LncRNA SRA1 had the highest fold of differential expression; D: Steroid receptor RNA activator 1 (SRA1) expression was apparently downregulated in ESCC tissues; E: SRA1 expression was apparently downregulated in ESCC cell line; F: ESCC patients with low SRA1 expression had shorter overall survival than patients with high SRA1 expression. aP < 0.01; bP < 0.05 vs Het-1A; cP < 0.01 vs Het-1A; LncRNA: Long non-coding RNA; SRA1: Steroid receptor RNA activator 1.
Table 2.
Correlations between steroid receptor RNA activator 1 expression and clinical characteristics in esophageal squamous cell carcinoma patients
| Characteristics | Case (n = 38) |
SRA1 expression
|
χ 2 | P value | |
|
High
|
Low
|
||||
| Sex | |||||
| Male | 26 | 12 | 14 | 0.487 | 0.485 |
| Female | 12 | 7 | 5 | ||
| Age (years) | 1.310 | 0.252 | |||
| ≤ 60 | 9 | 3 | 6 | ||
| > 60 | 29 | 16 | 13 | ||
| Pathological differentiation grade | 0.186 | 0.911 | |||
| Well | 7 | 4 | 3 | ||
| Moderately | 23 | 11 | 12 | ||
| Poorly | 8 | 4 | 4 | ||
| T Stage | 5.265 | 0.022a | |||
| T1-T2 | 20 | 13 | 7 | ||
| T3-T4 | 18 | 5 | 13 | ||
| TNM Stage | 8.622 | 0.003b | |||
| I-II | 17 | 13 | 4 | ||
| III-IV | 21 | 6 | 15 | ||
| Lymph node metastasis | 8.526 | 0.004b | |||
| Negative | 19 | 14 | 5 | ||
| Positive | 19 | 5 | 14 | ||
P < 0.05.
P < 0.01.
The χ2 test was used for comparison between groups. SRA1: Steroid receptor RNA activator 1.
SRA1 upregulation suppresses ESCC cell proliferation and glycolysis in vitro
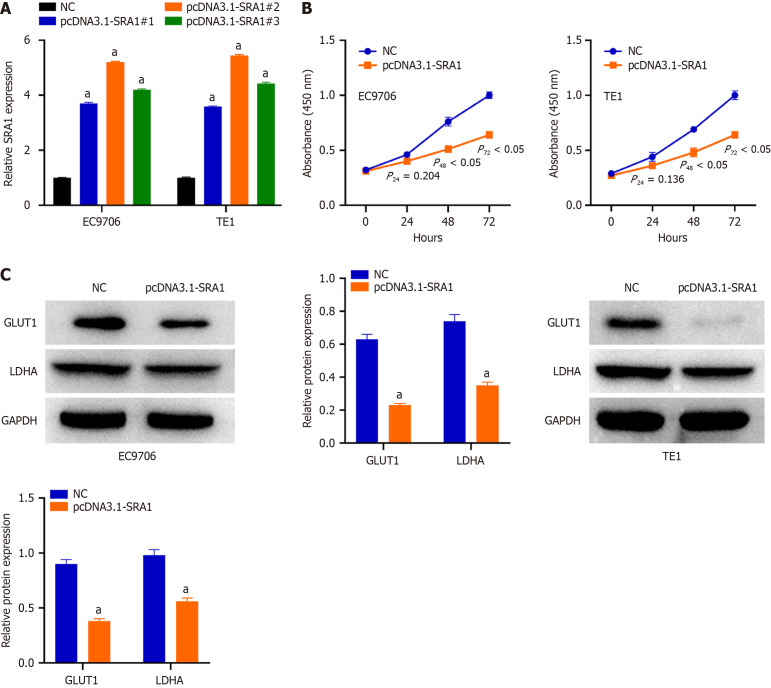
The relatively lower SRA1 was detected in the EC9706 and TE1 cells. To ascertain whether SRA1 is involved in the tumor resistance of ESCC, pcDNA3.1-SRA1 or NC was transfected into EC9706 and TE1 cells to overexpress endogenous SRA1 expression. The pcDNA3.1-SRA1#2 presented higher overexpressing efficiency; thus, the pcDNA3.1 was used (Figure 2A). Transfection with pcDNA3.1-SRA1 caused an obvious decrease in proliferation rate (Figure 2B) in EC9706 and TE1 cells. For glycolysis assay, when compared with NC group, a significant decrease in glucose consumption and lactate secretion was displayed in EC9706 and TE1 cells after pcDNA3.1-SRA1 transfection (Supplementary Figure 1). Additionally, upregulation of SRA1 triggered a decrease of GLUT1 and LDHA in EC9706 and TE1 cells than NC group (Figure 2C).
Figure 2.
Overexpression of steroid receptor RNA activator 1 suppresses esophageal squamous cell carcinoma cell proliferation and glycolysis in vitro. A: Steroid receptor RNA activator 1 (SRA1) overexpression was verified by quantitative reverse transcription-polymerase chain reaction; B: Overexpression of SRA1 caused an obvious decrease in proliferation rate in EC9706 and TE1 cells; C: Upregulation of SRA1 triggered a decrease of GLUT1 and LDHA in EC9706 and TE1 cells. aP < 0.01 vs negative control; NC: Negative control; SRA1: Steroid receptor RNA activator 1.
MiRNA-363-5p is sponged by SRA1 in ESCC
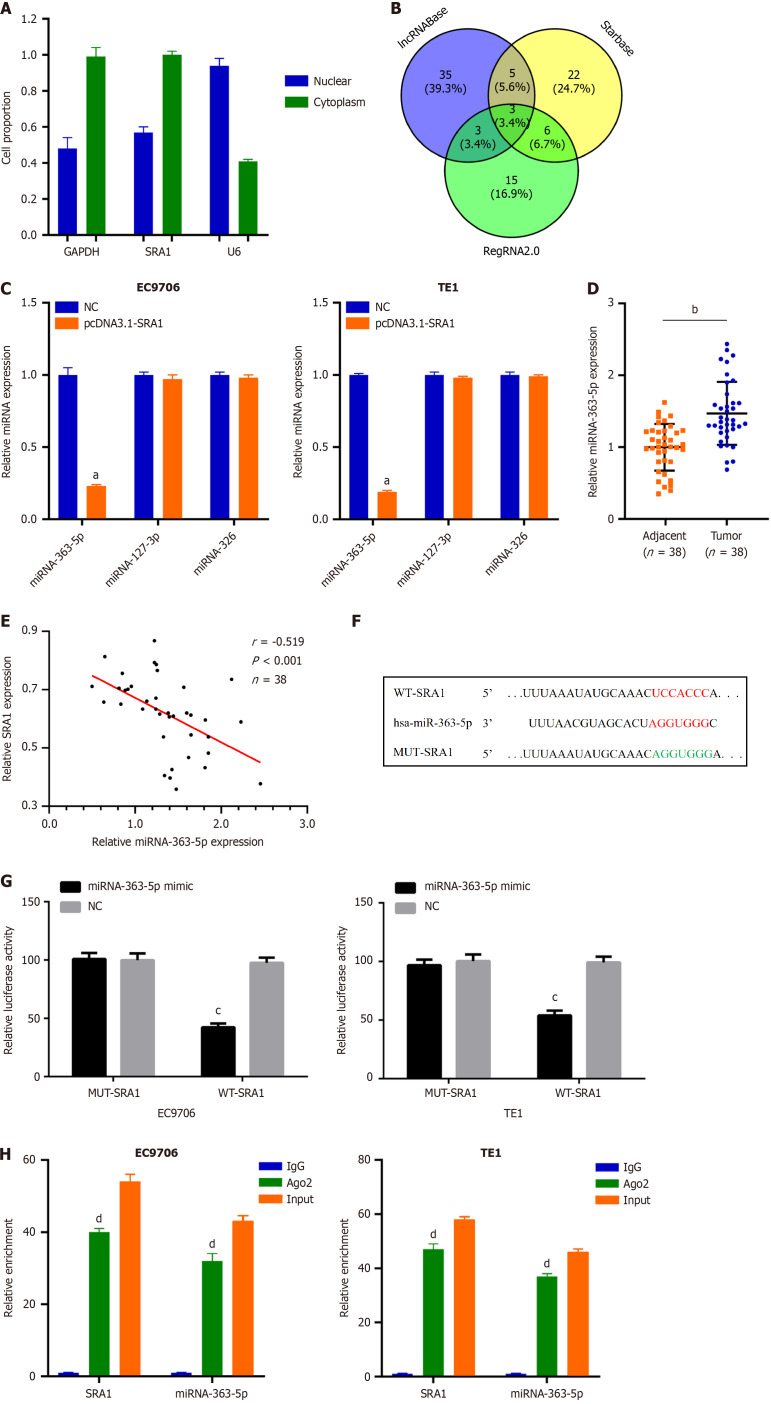
Next, SRA1 was observed in the cytoplasm (Figure 3A), which offered a theoretical basis for the ceRNA. Using lncRNABase, starBase, and RegRNA2.0, three miRNAs (miRNA-363-5p, miRNA-127-3p, and miRNA-326) were predicted to contain putative binding sites within SRA1 (Figure 3B). MiRNA-363-5p expression was inhibited in cells transfected with pcDNA3.1-SRA1 (Figure 3C). Furthermore, upregulated miRNA-363-5p was found in ESCC tissues (Figure 3D), which exhibited an inverse expression relation with SRA1 (r = -0.519, P < 0.001; Figure 3E). The Luciferase assay (Figure 3F and G) and RIP assay (Figure 3H) confirmed these findings.
Figure 3.
MiRNA-363-5p is sponged by steroid receptor RNA activator 1 in esophageal squamous cell carcinoma. A: Steroid receptor RNA activator 1 (SRA1) was observed to be enriched in the cytoplasm of esophageal squamous cell carcinoma (ESCC) cells; B: Using lncRNABase, starBase, and RegRNA2.0, a total of 3 miRNAs were predicted to contain putative binding sites within SRA1; C: MiRNA-363-5p expression was inhibited in EC9706 and TE1 cells transfected with pcDNA3.1-SRA1; D: Upregulated miRNA-363-5p was found in ESCC tissues; E: MiRNA-363-5p is negatively correlated with SRA1; F and G: Luciferase activity of WT-SRA1 instead of MUT-SRA1 was evidently decreased by miRNA-363-5p upregulation in EC9706 and TE1 cells; H: RIP assay defined that both SRA1 and miRNA-363-5p could be remarkably enriched by anti-Ago2 in EC9706 and TE1 cells. aP < 0.01 vs negative control (NC); bP < 0.01 vs Adjacent; cP < 0.05 vs NC; dP < 0.01 vs IgG; NC: Negative control; LncRNA: Long non-coding RNA; SRA1: Steroid receptor RNA activator 1.
MiRNA-363-5p directly targets LHPP in ESCC cells
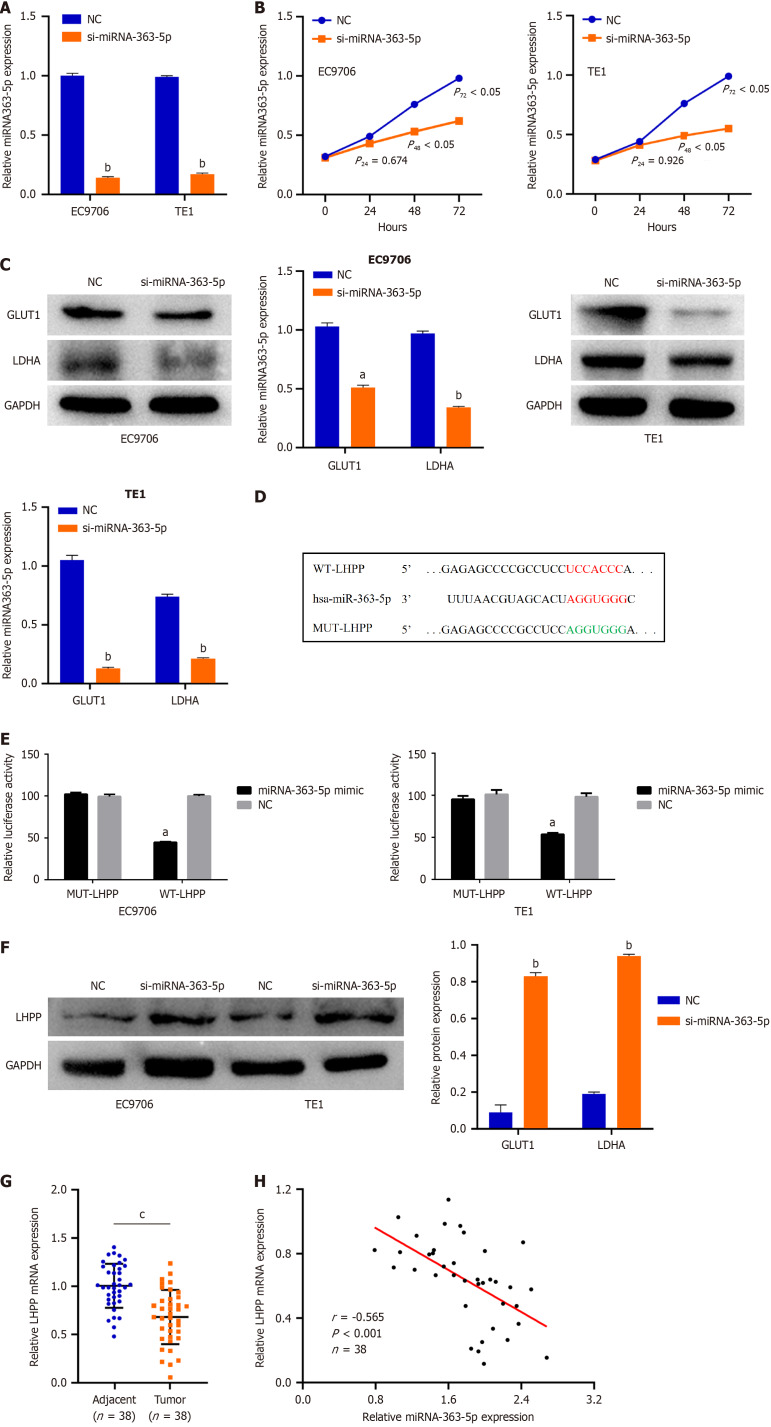
We next tried to ascertain the roles of miRNA-363-5p in ESCC cells. si-miRNA-363-5p was transfected into EC9706 and TE1 cells. MiRNA-363-5p remarkably decreased after si-miRNA-363-5p transfection (Figure 4A). Inhibition of miRNA-363-5p expression significantly attenuated proliferation (Figure 4B) and glycolysis (Figure 4C, Supplementary Figure 2) in EC9706 and TE1 cells.
Figure 4.
MiRNA-363-5p directly targets phospholysine phosphohistidine inorganic pyrophosphate phosphatase in esophageal squamous cell carcinoma cells. A: Quantitative reverse transcription-polymerase chain reaction analysis indicated that miRNA-363-5p expression remarkably decreased after si-miRNA-363-5p transfection; B: Inhibition of miRNA-363-5p expression significantly attenuated proliferation in EC9706 and TE1 cells; C: Inhibition of miRNA-363-5p expression significantly attenuated glycolysis in EC9706 and TE1 cells; D: Phospholysine phosphohistidine inorganic pyrophosphate phosphatase (LHPP) was predicted to have binding sequences for miRNA-363-5p; E: Luciferase reporter turned out that miRNA-363-5p mimic clearly weakened the luciferase activity of WT-LHPP; F: Downregulating miRNA-363-5p enhanced the LHPP protein levels in EC9706 and TE1 cells; G: LHPP expression was low in esophageal squamous cell carcinoma tissues compared to adjacent normal tissues; H: MiRNA-363-5p is negatively correlated with LHPP. aP < 0.05 vs NC; bP < 0.01 vs NC; cP < 0.01 vs Adjacent. NC: Negative control; LHPP: Phospholysine phosphohistidine inorganic pyrophosphate phosphatase.
To clarify the mechanism of miRNA-363-5p on ESCC, bioinformatics analysis was performed to explore the target of miRNA-363-5p. LHPP was predicted to have binding sequences for miRNA-363-5p (Figure 4D). Then, luciferase reporter turned out that miRNA-363-5p mimic weakened the luciferase activity of WT-LHPP (Figure 4E). Furthermore, downregulating miRNA-363-5p enhanced the LHPP protein (Figure 4F) levels in EC9706 and TE1 cells. Besides, LHPP expression was low in ESCC tissues compared to adjacent normal tissues (Figure 4G). An inverse correlation between miRNA-363-5p and LHPP was found in ESCC (r = -0.565, P < 0.001; Figure 4H). Collectively, miRNA-363-5p directly targets LHPP in ESCC cells.
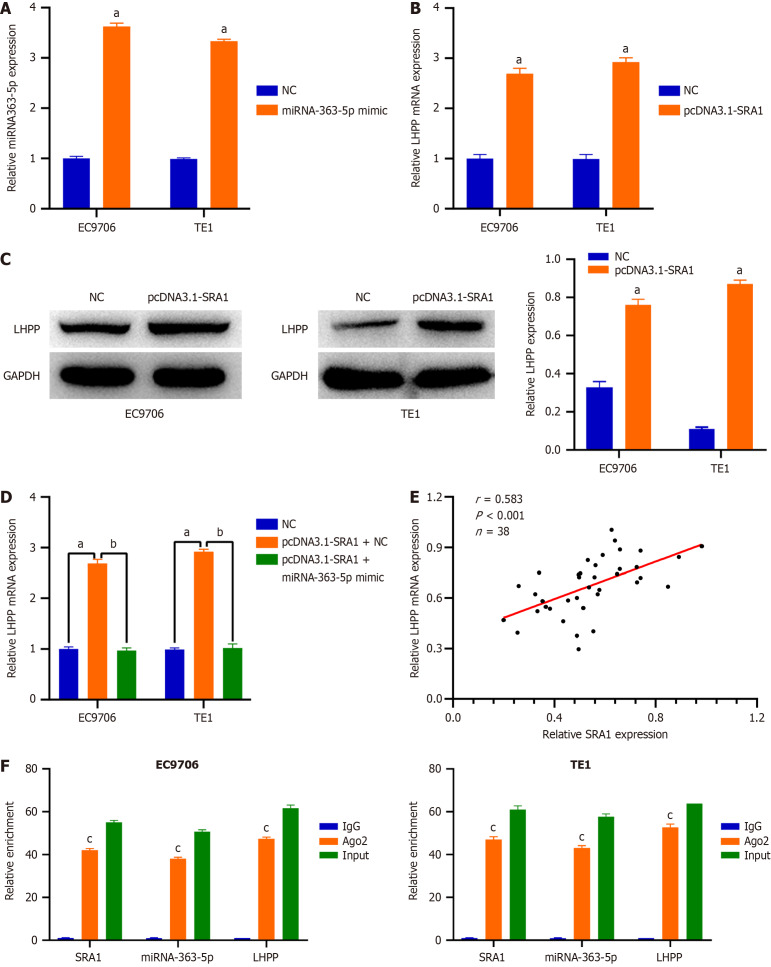
SRA1 overexpression promotes LHPP level in ESCC by sponging miRNA-363-5p
Then, EC9706 and TE1 cells were transfected with pcDNA3.1-SRA1 and miRNA-363-5p mimic either alone or in combination. The overexpression efficiency of miRNA-363-5p mimic in ESCC cells was tested by qRT-PCR. The delivery of miRNA-363-5p mimic caused a prominent miRNA-363-5p upregulation in EC9706 and TE1 cells (Figure 5A). SRA1 upregulation triggered an obvious overexpressed effect on the endogenous LHPP mRNA (Figure 5B) and protein (Figure 5C) levels in EC9706 and TE1 cells, whereas the delivery of miRNA-363-5p mimic counteracted such effect (Figure 5D). Furthermore, LHPP expression was positively correlated with SRA1 expression in ESCC tissues (r = 0.583, P < 0.001; Figure 5E). Besides, compared with the anti-IgG NC group, SRA1, miRNA-363-5p, and LHPP were preferentially concentrated in the anti-Ago2 precipitate (Figure 5F).
Figure 5.
Steroid receptor RNA activator 1 overexpression promotes phospholysine phosphohistidine inorganic pyrophosphate phosphatase expression in esophageal squamous cell carcinoma cells by sponging miRNA-363-5p. A: The delivery of miRNA-363-5p mimic caused a prominent miRNA-363-5p upregulation in EC9706 and TE1 cells; B-D: Steroid receptor RNA activator 1 (SRA1) upregulation triggered an obvious overexpressed effect on the endogenous phospholysine phosphohistidine inorganic pyrophosphate phosphatase (LHPP) mRNA and protein levels in EC9706 and TE1 cells, whereas the delivery of miRNA-363-5p mimic counteracted such effect; E: LHPP expression was positively correlated with SRA1 expression in ESCC tissues; F: SRA1, miRNA-363-5p, and LHPP were preferentially concentrated in the anti-Ago2 precipitate. aP < 0.01 vs NC; bP < 0.01 vs pcDNA3.1-SRA1 + miRNA-363-5p mimic; cP < 0.01 vs IgG; NC: Negative control; SRA1: Steroid receptor RNA activator 1; LHPP: Phospholysine phosphohistidine inorganic pyrophosphate phosphatase.
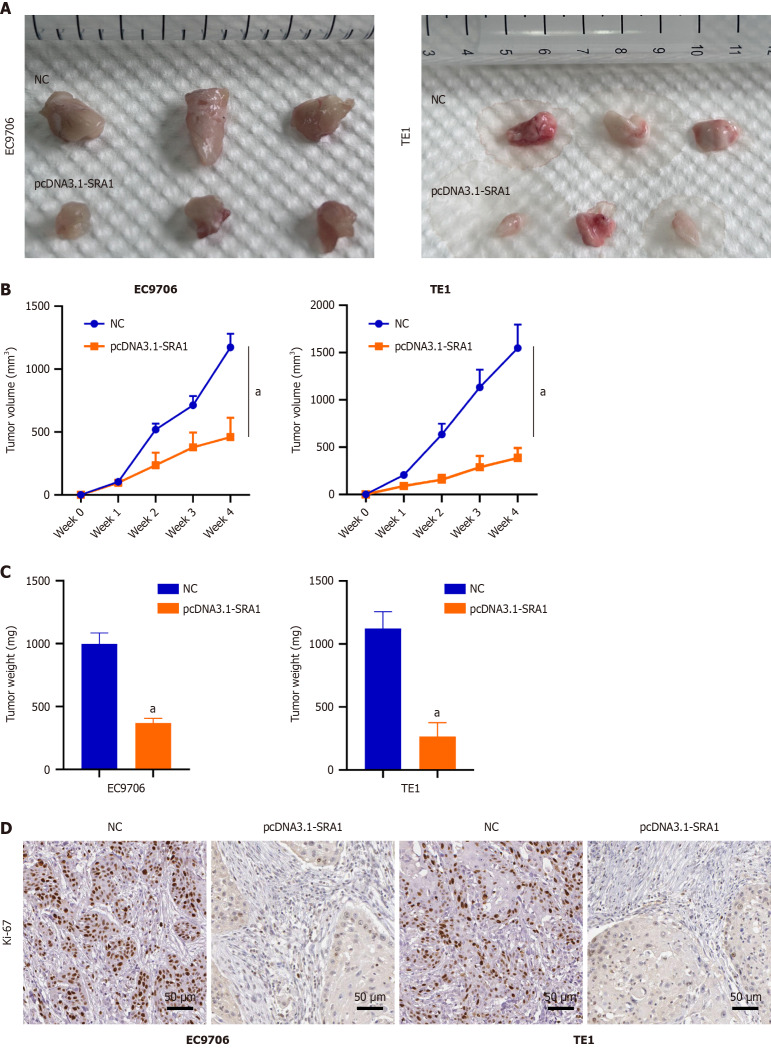
SRA1 overexpression inhibits ESCC tumor growth in vivo
We implanted EC9706 and TE1 ESCC cells infected with pcDNA3.1-SRA1 into the nude mouse. Overexpressing SRA1 exhibited weaker tumorigenic ability (Figure 6A-C). The immunohistochemical staining found a low level of Ki-67 in the tumors with overexpressing SRA1 (Figure 6D).
Figure 6.
Steroid receptor RNA activator 1 overexpression inhibits esophageal squamous cell carcinoma tumor growth in vivo. A-C: Overexpressing steroid receptor RNA activator 1 (SRA1) exhibited weaker tumorigenic ability; D: Immunohistochemical staining confirmed the relatively low level of proliferation marker Ki-67 in the tumors with overexpressing SRA1. Scale bar = 50μm. aP < 0.01 vs negative control; NC: Negative control.
DISCUSSION
In this study, we found the abnormally expressed SRA1 in ESCC. Here, SRA1 was clearly downmodulated in ESCC tissues and cell lines, and low expression of SRA1 was significantly associated with advanced tumor stage, metastasis, larger tumor size, and poor survival. Function and rescue assays demonstrated that SRA1 could impede cell proliferation and glycolysis of ESCC cells via miRNA-363-5p and LHPP. Regarding the mechanism, SRA1 elevated LHPP expression by sponging miRNA-363-5p, leading to inhibition of ESCC cell proliferation and glycolysis (Figure 7).
Figure 7.
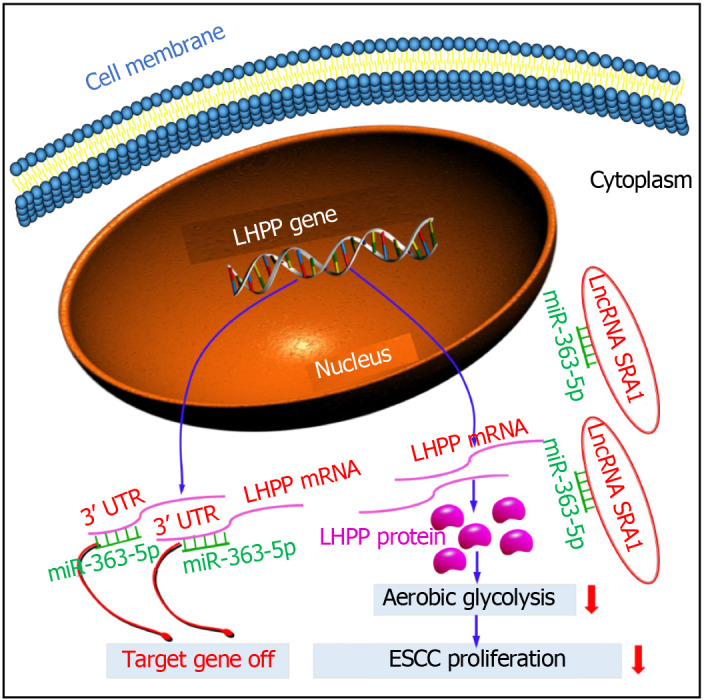
Schematic diagram showing the regulatory mechanism of steroid receptor RNA activator 1 in esophageal squamous cell carcinoma. LncRNA: Long non-coding RNA; SRA1: Steroid receptor RNA activator 1; ESCC: Esophageal squamous cell carcinoma; LHPP: Phospholysine phosphohistidine inorganic pyrophosphate phosphatase.
Increasing studies have demonstrated that non-coding RNAs, including miRNAs, lncRNAs and circRNAs, play a pivotal role in ESCC formation, and may be used as therapeutic targets or diagnostic and prognostic biomarkers for ESCC patients[20-26]. At present, there are many reports on SRA1 and tumor progression, but its role in different tumors reflects tumor heterogeneity. There have been many reports on the research of SRA1 in some gynecological solid tumors. These studies have found that SRA1 plays an oncogene role, such as cervical cancer[27], endometrial cancer[28], breast cancer[29]. However, a number of studies in several tumors have pointed out that SRA1 acts as a tumor suppressor gene, such as hepatocellular carcinoma[30] and prostate cancer[31]. Moreover, using online bioinformatics software and qRT-PCR analysis, we screened a few miRNAs sharing binding sites with SRA1. In the present study, our bioinformatics analyses showed that miR-363-5p has binding sites for SRA1, and further experiments showed SRA1 knockdown strongly accelerated miR-363-5p expression in ESCC cells. And miR-363-5p was found to be upmodulated, and was inversely correlated with SRA1 Levels in ESCC tissues. Importantly, using luciferase reporter and RIP assays, SRA1 still has a targeting relationship with miR-363-5p. Further, we conducted the loss-of-function and rescue experiments, and found that the suppressive effects of SRA1 overexpression on cell proliferation, glucose uptake, and lactate production of ESCC cells were rescued by miR-363-5p mimic. The partial reversal effect of miR-363-5p mimic on pcDNA3.1-SRA1 function proved that miR-363-5p was one of the downstream miRNAs of SRA1.
However, this study has some limitations. (1) Our research explored and found for the first time that SRA1 inhibits the carcinogenicity of ESCC cells by targeting the miRNA-363-5p/LHPP axis. However, the downstream regulatory molecules of LHPP need to be further studied; and (2) Finally, it is worthy of our attention that we only detected the expression of SRA1/miRNA-363-5p/LHPP in ESCC tissues. Then, by expanding the number of ESCC patients, whether the detection of serum SRA1/miRNA-363-5p/LHPP in ESCC patients can discover early noninvasive markers of ESCC patients remains to be explored.
CONCLUSION
In conclusion, we found that SRA1 inhibits the oncogenicity of ESCC via miRNA-363-5p/LHPP axis. The SRA1/miRNA-363-5p/LHPP pathway may be a therapeutic target for ESCC.
Footnotes
Institutional review board statement: The study was reviewed and approved by the Ethics Committee of the Huai’an Hospital of Huai’an Institutional Review Board.
Institutional animal care and use committee statement: All procedures involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of the Ethical Committees of Huai’an Hospital of Huai’an (IACUC protocol No. 2016.A023).
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
ARRIVE guidelines statement: The authors have read the ARRIVE Guidelines, and the manuscript was prepared and revised according to the ARRIVE Guidelines.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade C
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Groisman I S-Editor: Li L L-Editor: A P-Editor: Zheng XM
Contributor Information
Ming He, Department of Radiation Oncology, Huai’an Hospital of Huai’an, Huai’an 223299, Jiangsu Province, China.
Ye Qi, Department of Nursing, Huai’an Hospital of Huai’an, Huai’an 223299, Jiangsu Province, China.
Ze-Mao Zheng, Department of Radiation Oncology, Huai’an Hospital of Huai’an, Huai’an 223299, Jiangsu Province, China.
Min Sha, Institute of Clinical Medicine, Taizhou People's Hospital Affiliated of Nantong University of Medicine, Taizhou 225300, Zhejiang Province, China.
Xiang Zhao, Department of Radiation Oncology, Huai’an Hospital of Huai’an, Huai’an 223299, Jiangsu Province, China.
Yu-Rao Chen, Department of Radiation Oncology, Huai’an Hospital of Huai’an, Huai’an 223299, Jiangsu Province, China.
Zheng-Hai Chen, Department of Thoracic Surgery, Huai’an Hospital of Huai’an, Huai’an 223299, Jiangsu Province, China.
Rong-Yu Qian, Department of Radiation Oncology, Huai’an Hospital of Huai’an, Huai’an 223299, Jiangsu Province, China.
Juan Yao, Department of Radiation Oncology, Huai’an Hospital of Huai’an, Huai’an 223299, Jiangsu Province, China. tzsry110120@163.com.
Zheng-Dong Yang, Department of Thoracic Surgery, Huai’an Hospital of Huai’an, Huai’an 223299, Jiangsu Province, China.
Data sharing statement
Technical appendix, statistical code, and dataset available from the corresponding author at tzsry110120@163.com.
References
- 1.Chu LY, Peng YH, Weng XF, Xie JJ, Xu YW. Blood-based biomarkers for early detection of esophageal squamous cell carcinoma. World J Gastroenterol. 2020;26:1708–1725. doi: 10.3748/wjg.v26.i15.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Gao R, Wang Z, Liu Q, Yang C. MicroRNA-105 plays an independent prognostic role in esophageal cancer and acts as an oncogene. Cancer Biomark. 2020;27:173–180. doi: 10.3233/CBM-180. [DOI] [PubMed] [Google Scholar]
- 4.Deng W, Chen J, Xiao Z. ASO Author Reflections: Prognostic Stratification and the Value of Adjuvant Therapy in Thoracic Esophageal Squamous Cell Carcinoma Patients After Esophagectomy. Ann Surg Oncol. 2019;26:802–803. doi: 10.1245/s10434-019-07728-7. [DOI] [PubMed] [Google Scholar]
- 5.Niu Y, Guo Y, Li Y, Shen S, Liang J, Guo W, Dong Z. LncRNA GATA2-AS1 suppresses esophageal squamous cell carcinoma progression via the mir-940/PTPN12 axis. Exp Cell Res. 2022;416:113130. doi: 10.1016/j.yexcr.2022.113130. [DOI] [PubMed] [Google Scholar]
- 6.Zhang H, Hua Y, Jiang Z, Yue J, Shi M, Zhen X, Zhang X, Yang L, Zhou R, Wu S. Cancer-associated Fibroblast-promoted LncRNA DNM3OS Confers Radioresistance by Regulating DNA Damage Response in Esophageal Squamous Cell Carcinoma. Clin Cancer Res. 2019;25:1989–2000. doi: 10.1158/1078-0432.CCR-18-0773. [DOI] [PubMed] [Google Scholar]
- 7.Gao W, Zhang Y, Luo H, Niu M, Zheng X, Hu W, Cui J, Xue X, Bo Y, Dai F, Lu Y, Yang D, Guo Y, Guo H, Li H, Zhang Y, Yang T, Li L, Zhang L, Hou R, Wen S, An C, Ma T, Jin L, Xu W, Wu Y. Targeting SKA3 suppresses the proliferation and chemoresistance of laryngeal squamous cell carcinoma via impairing PLK1-AKT axis-mediated glycolysis. Cell Death Dis. 2020;11:919. doi: 10.1038/s41419-020-03104-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xue ST, Zheng B, Cao SQ, Ding JC, Hu GS, Liu W, Chen C. Long non-coding RNA LINC00680 functions as a ceRNA to promote esophageal squamous cell carcinoma progression through the miR-423-5p/PAK6 axis. Mol Cancer. 2022;21:69. doi: 10.1186/s12943-022-01539-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang J, Ren B, Yang G, Wang H, Chen G, You L, Zhang T, Zhao Y. The enhancement of glycolysis regulates pancreatic cancer metastasis. Cell Mol Life Sci. 2020;77:305–321. doi: 10.1007/s00018-019-03278-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu T, Liu H, Liang Z, Wang F, Zhou C, Zheng X, Zhang Y, Song Y, Hu J, He X, Xiao J, King RJ, Wu X, Lan P. Tumor-intrinsic CD47 signal regulates glycolysis and promotes colorectal cancer cell growth and metastasis. Theranostics. 2020;10:4056–4072. doi: 10.7150/thno.40860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang C, Xie L, Fu Y, Yang J, Cui Y. lncRNA MIAT promotes esophageal squamous cell carcinoma progression by regulating miR-1301-3p/INCENP axis and interacting with SOX2. J Cell Physiol. 2020;235:7933–7944. doi: 10.1002/jcp.29448. [DOI] [PubMed] [Google Scholar]
- 12.Yu J, Chen S, Niu Y, Liu M, Zhang J, Yang Z, Gao P, Wang W, Han X, Sun G. Functional Significance and Therapeutic Potential of miRNA-20b-5p in Esophageal Squamous Cell Carcinoma. Mol Ther Nucleic Acids. 2020;21:315–331. doi: 10.1016/j.omtn.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hindupur SK, Colombi M, Fuhs SR, Matter MS, Guri Y, Adam K, Cornu M, Piscuoglio S, Ng CKY, Betz C, Liko D, Quagliata L, Moes S, Jenoe P, Terracciano LM, Heim MH, Hunter T, Hall MN. The protein histidine phosphatase LHPP is a tumour suppressor. Nature. 2018;555:678–682. doi: 10.1038/nature26140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Z, Zhou X, Zhu H, Song X, Gao H, Niu Z, Lu J. Purpurin binding interacts with LHPP protein that inhibits PI3K/AKT phosphorylation and induces apoptosis in colon cancer cells HCT-116. J Biochem Mol Toxicol. 2021;35:e22665. doi: 10.1002/jbt.22665. [DOI] [PubMed] [Google Scholar]
- 15.Sun W, Qian K, Guo K, Chen L, Xiang J, Li D, Wu Y, Ji Q, Sun T, Wang Z. LHPP inhibits cell growth and migration and triggers autophagy in papillary thyroid cancer by regulating the AKT/AMPK/mTOR signaling pathway. Acta Biochim Biophys Sin (Shanghai) 2020;52:382–389. doi: 10.1093/abbs/gmaa015. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X, Kang H, Xiao J, Shi B, Li X, Chen G. LHPP Inhibits the Proliferation and Metastasis of Renal Cell Carcinoma. Biomed Res Int. 2020;2020:7020924. doi: 10.1155/2020/7020924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu T, Ma K, Zhan C, Yang X, Shi Y, Jiang W, Wang H, Wang S, Wang Q, Tan L. Downregulation of long non-coding RNA LINP1 inhibits the malignant progression of esophageal squamous cell carcinoma. Ann Transl Med. 2020;8:675. doi: 10.21037/atm-20-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Q, Guan F, Fan T, Li S, Ma S, Zhang Y, Guo W, Liu H. LncRNA WDFY3-AS2 suppresses proliferation and invasion in oesophageal squamous cell carcinoma by regulating miR-2355-5p/SOCS2 axis. J Cell Mol Med. 2020;24:8206–8220. doi: 10.1111/jcmm.15488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang Y, Yang P, Zhu Y, Su Y. LncRNA TUG1 contributes to ESCC progression via regulating miR-148a-3p/MCL-1/Wnt/β-catenin axis in vitro. Thorac Cancer. 2020;11:82–94. doi: 10.1111/1759-7714.13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang D, You D, Pan Y, Liu P. Downregulation of lncRNA-HEIH curbs esophageal squamous cell carcinoma progression by modulating miR-4458/PBX3. Thorac Cancer. 2020;11:1963–1971. doi: 10.1111/1759-7714.13489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang B, Zhao Y, Shi M, Song L, Wang Q, Qin Q, Song X, Wu S, Fang Z, Liu X. DNAJB6 Promotes Ferroptosis in Esophageal Squamous Cell Carcinoma. Dig Dis Sci. 2020;65:1999–2008. doi: 10.1007/s10620-019-05929-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang X, Liu C, Li H, Dai T, Luo G, Zhang C, Li T, Lü M. Hypoxia-responsive lncRNA G077640 promotes ESCC tumorigenesis via the H2AX-HIF1α-glycolysis axis. Carcinogenesis. 2023;44:383–393. doi: 10.1093/carcin/bgad036. [DOI] [PubMed] [Google Scholar]
- 23.Lu Z, Chen Z, Li Y, Wang J, Zhang Z, Che Y, Huang J, Sun S, Mao S, Lei Y, Gao Y, He J. TGF-β-induced NKILA inhibits ESCC cell migration and invasion through NF-κB/MMP14 signaling. J Mol Med (Berl) 2018;96:301–313. doi: 10.1007/s00109-018-1621-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sang C, Chao C, Wang M, Zhang Y, Luo G, Zhang X. Identification and validation of hub microRNAs dysregulated in esophageal squamous cell carcinoma. Aging (Albany NY) 2020;12:9807–9824. doi: 10.18632/aging.103245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He Z. LINC00473/miR-497-5p Regulates Esophageal Squamous Cell Carcinoma Progression Through Targeting PRKAA1. Cancer Biother Radiopharm. 2019;34:650–659. doi: 10.1089/cbr.2019.2875. [DOI] [PubMed] [Google Scholar]
- 26.Liu T, Liang X, Yang S, Sun Y. Long noncoding RNA PTCSC1 drives esophageal squamous cell carcinoma progression through activating Akt signaling. Exp Mol Pathol. 2020;117:104543. doi: 10.1016/j.yexmp.2020.104543. [DOI] [PubMed] [Google Scholar]
- 27.Eoh KJ, Paek J, Kim SW, Kim HJ, Lee HY, Lee SK, Kim YT. Long non-coding RNA, steroid receptor RNA activator (SRA), induces tumor proliferation and invasion through the NOTCH pathway in cervical cancer cell lines. Oncol Rep. 2017;38:3481–3488. doi: 10.3892/or.2017.6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park SA, Kim LK, Kim YT, Heo TH, Kim HJ. Long non-coding RNA steroid receptor activator promotes the progression of endometrial cancer via Wnt/ β-catenin signaling pathway. Int J Biol Sci. 2020;16:99–115. doi: 10.7150/ijbs.35643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cooper C, Guo J, Yan Y, Chooniedass-Kothari S, Hube F, Hamedani MK, Murphy LC, Myal Y, Leygue E. Increasing the relative expression of endogenous non-coding Steroid Receptor RNA Activator (SRA) in human breast cancer cells using modified oligonucleotides. Nucleic Acids Res. 2009;37:4518–4531. doi: 10.1093/nar/gkp441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luo P, Jing W, Zhu M, Li ND, Zhou H, Yu MX, Liang CZ, Tu JC. Decreased expression of LncRNA SRA1 in hepatocellular carcinoma and its clinical significance. Cancer Biomark. 2017;18:285–290. doi: 10.3233/CBM-160305. [DOI] [PubMed] [Google Scholar]
- 31.Zhang M, Wang F, Zhang X. miRNA-627 inhibits cell proliferation and cell migration, promotes cell apoptosis in prostate cancer cells through upregulating MAP3K1, PTPRK and SRA1. Int J Clin Exp Pathol. 2018;11:255–261. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Technical appendix, statistical code, and dataset available from the corresponding author at tzsry110120@163.com.