Abstract
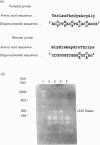
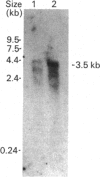
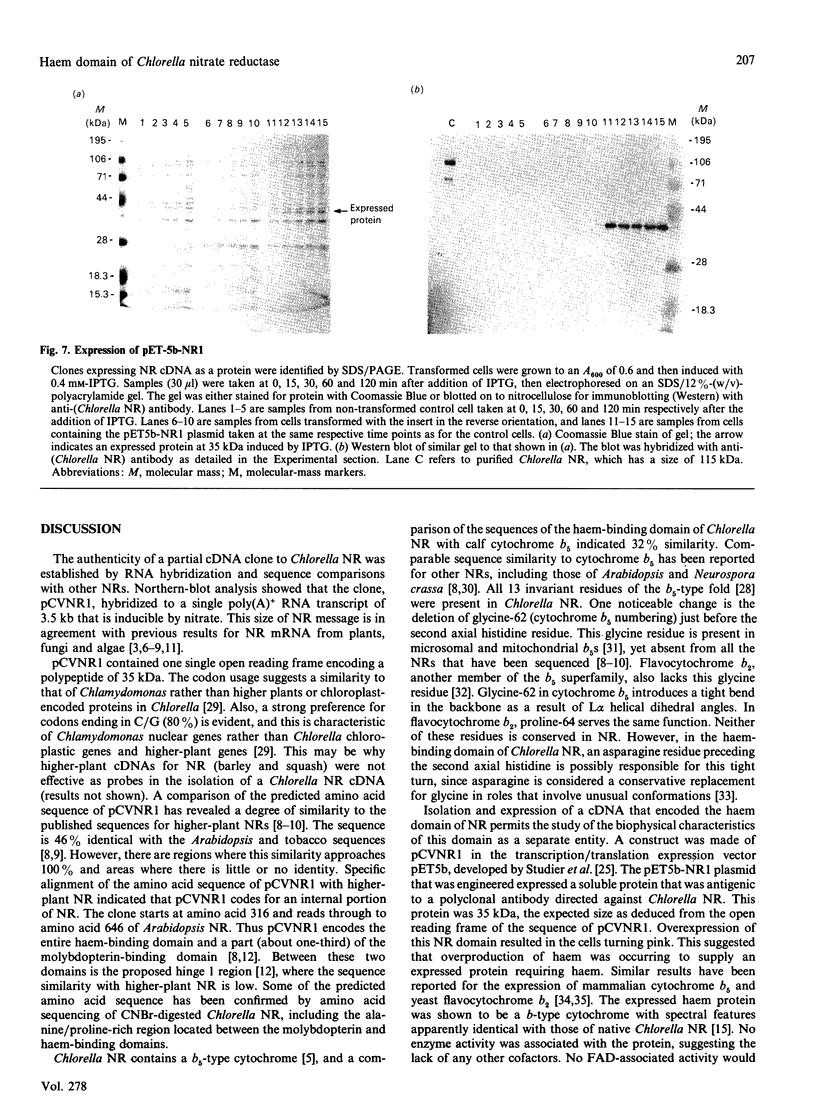
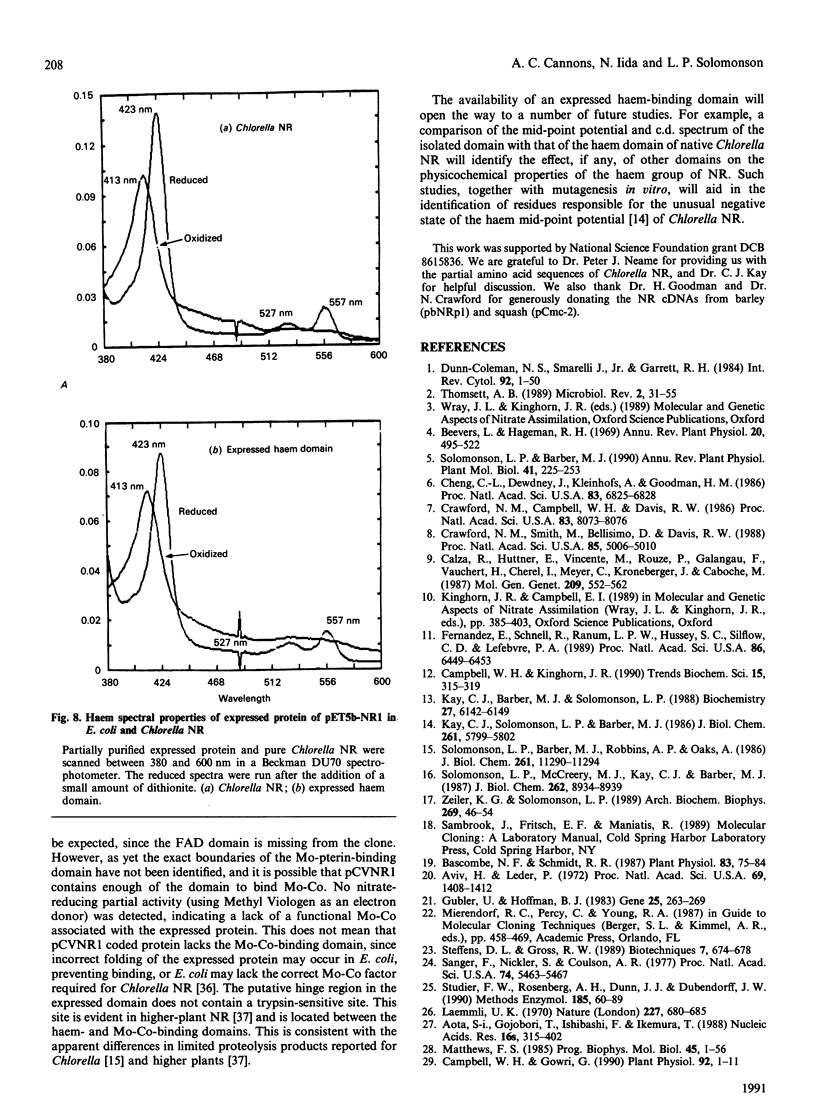
A partial cDNA clone coding for the haem-binding domain of NADH:nitrate reductase (EC 1.6.6.1) (NR) from the unicellular green alga Chlorella vulgaris has been isolated, sequenced and expressed. A 1.2 kb cDNA (pCVNR1) was isolated from a lambda gt11 expression library produced from polyadenylated RNA extracted from nitrate-grown Chlorella cells. pCVNR1 hybridized to a 3.5 kb mRNA transcript that was nitrate-inducible and absent from ammonium-grown cells. The entire sequence of pCVNR1 was obtained and found to have a single uninterrupted reading frame. The derived amino acid sequence of 318 amino acids has a 45-50% similarity to higher-plant NRs, including Arabidopsis thaliana, spinach (Spinacia oleracea) and tobacco (Nicotiana tabacum). A comparison with the putative domain structure of higher-plant nitrate reductases suggested that this sequence contains the complete haem-binding domain, approximately one-third of the Mo-pterin domain and no FAD-binding domain. A 32% sequence similarity is evident when comparing the Chlorella NR haem domain with that of calf cytochrome b5. Expression of pCVNR1 in a pET vector synthesized a 35 kDa protein that was antigenic to anti-(Chlorella NR) antibody. The spectral properties of this protein (reduced and oxidized) in the 400-600 nm region are identical with those of native Chlorella NR and indicate that haem is associated with the protein.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bascomb N. F., Schmidt R. R. Purification and Partial Kinetic and Physical Characterization of Two Chloroplast-Localized NADP-Specific Glutamate Dehydrogenase Isoenzymes and Their Preferential Accumulation in Chlorella sorokiniana Cells Cultured at Low or High Ammonium Levels. Plant Physiol. 1987 Jan;83(1):75–84. doi: 10.1104/pp.83.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck von Bodman S., Schuler M. A., Jollie D. R., Sligar S. G. Synthesis, bacterial expression, and mutagenesis of the gene coding for mammalian cytochrome b5. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9443–9447. doi: 10.1073/pnas.83.24.9443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black M. T., White S. A., Reid G. A., Chapman S. K. High-level expression of fully active yeast flavocytochrome b2 in Escherichia coli. Biochem J. 1989 Feb 15;258(1):255–259. doi: 10.1042/bj2580255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calza R, Huttner E, Vincentz M, Rouzé P, Galangau F, Vaucheret H, Chérel I, Meyer C, Kronenberger J, Caboche M. Cloning of DNA fragments complementary to tobacco nitrate reductase mRNA and encoding epitopes common to the nitrate reductases from higher plants. Mol Gen Genet. 1987 Oct;209(3):552–562. doi: 10.1007/BF00331162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell W. H., Gowri G. Codon usage in higher plants, green algae, and cyanobacteria. Plant Physiol. 1990 Jan;92(1):1–11. doi: 10.1104/pp.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell W. H., Kinghorn K. R. Functional domains of assimilatory nitrate reductases and nitrite reductases. Trends Biochem Sci. 1990 Aug;15(8):315–319. doi: 10.1016/0968-0004(90)90021-3. [DOI] [PubMed] [Google Scholar]
- Cheng C. L., Dewdney J., Kleinhofs A., Goodman H. M. Cloning and nitrate induction of nitrate reductase mRNA. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6825–6828. doi: 10.1073/pnas.83.18.6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford N. M., Campbell W. H., Davis R. W. Nitrate reductase from squash: cDNA cloning and nitrate regulation. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8073–8076. doi: 10.1073/pnas.83.21.8073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford N. M., Smith M., Bellissimo D., Davis R. W. Sequence and nitrate regulation of the Arabidopsis thaliana mRNA encoding nitrate reductase, a metalloflavoprotein with three functional domains. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5006–5010. doi: 10.1073/pnas.85.14.5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández E., Schnell R., Ranum L. P., Hussey S. C., Silflow C. D., Lefebvre P. A. Isolation and characterization of the nitrate reductase structural gene of Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6449–6453. doi: 10.1073/pnas.86.17.6449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Kay C. J., Barber M. J., Solomonson L. P. Circular dichroism and potentiometry of FAD, heme and Mo-pterin prosthetic groups of assimilatory nitrate reductase. Biochemistry. 1988 Aug 9;27(16):6142–6149. doi: 10.1021/bi00416a047. [DOI] [PubMed] [Google Scholar]
- Kay C. J., Solomonson L. P., Barber M. J. Thermodynamic properties of the heme prosthetic group in assimilatory nitrate reductase. J Biol Chem. 1986 May 5;261(13):5799–5802. [PubMed] [Google Scholar]
- Kubo Y., Ogura N., Nakagawa H. Limited proteolysis of the nitrate reductase from spinach leaves. J Biol Chem. 1988 Dec 25;263(36):19684–19689. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lederer F., Cortial S., Becam A. M., Haumont P. Y., Perez L. Complete amino acid sequence of flavocytochrome b2 from baker's yeast. Eur J Biochem. 1985 Oct 15;152(2):419–428. doi: 10.1111/j.1432-1033.1985.tb09213.x. [DOI] [PubMed] [Google Scholar]
- Lederer F., Ghrir R., Guiard B., Cortial S., Ito A. Two homologous cytochromes b5 in a single cell. Eur J Biochem. 1983 Apr 15;132(1):95–102. doi: 10.1111/j.1432-1033.1983.tb07330.x. [DOI] [PubMed] [Google Scholar]
- Lê K. H., Lederer F. On the presence of a heme-binding domain homologous to cytochrome b(5) in Neurospora crassa assimilatory nitrate reductase. EMBO J. 1983;2(11):1909–1914. doi: 10.1002/j.1460-2075.1983.tb01678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews F. S. The structure, function and evolution of cytochromes. Prog Biophys Mol Biol. 1985;45(1):1–56. doi: 10.1016/0079-6107(85)90004-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomonson L. P., Barber M. J., Robbins A. P., Oaks A. Functional domains of assimilatory NADH:nitrate reductase from Chlorella. J Biol Chem. 1986 Aug 25;261(24):11290–11294. [PubMed] [Google Scholar]
- Solomonson L. P., McCreery M. J., Kay C. J., Barber M. J. Radiation inactivation analysis of assimilatory NADH:nitrate reductase. Apparent functional sizes of partial activities associated with intact and proteolytically modified enzyme. J Biol Chem. 1987 Jun 25;262(18):8934–8939. [PubMed] [Google Scholar]
- Steffens D. L., Gross R. W. Sequencing of cloned DNA using bacteriophage lambda gt11 templates. Biotechniques. 1989 Jul-Aug;7(7):674–680. [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Zeiler K. G., Solomonson L. P. Regulation of Chlorella nitrate reductase: control of enzyme activity and immunoreactive protein levels by ammonia. Arch Biochem Biophys. 1989 Feb 15;269(1):46–54. doi: 10.1016/0003-9861(89)90085-4. [DOI] [PubMed] [Google Scholar]