Abstract
Objective
The purpose of the current review is to identify the main problems of endotracheal intubation, which will serve as a basis for the design requirements for a novel endotracheal tube.
Methodology
A PICO systematic search was conducted in PubMed up to December 2021 to identify issues related both to the endotracheal intubation procedure and device-specific factors.
Results
Two primary categories of problems were identified during endotracheal intubation: a) Issues related to laryngotracheal symptoms such as cough, hoarseness, aphonia, dysphonia, dysphagia, swallowing difficulties and the risk of stenosis with long-term intubation. The underlying pressure, abrasion and/or decubitus phenomena should be considered in a new design approach. b) Issues related to the cuff sealing and microaspirations, where the risk of ventilator-associated pneumonia (VAP) highlights the need to improve the design.
Discussion & Conclusion
This review has yielded valuable input for rethinking the design of endotracheal tubes to ensure an efficient and safe airway. This new design should focus on the protection of anatomical structures, avoid or reduce the phenomena of laryngotracheal symptoms, and even reduce the risk of ventilator-associated-pneumonia (VAP) and/or prevent the need for certain tracheostomies.
Keywords: endotracheal intubation, laryngotracheal damage, mechanical ventilation, tracheal stenosis, ventilation associated pneumonia
Introduction
Hippocrates, 460–380 BC, described endotracheal intubation for ventilation1,2 and the Talmud, between 200 BC and 400 AD, or the Greek physicists Aesculapius, Aretaeus or the Roman anatomist Gallenus mentioned the use of the reed for this purpose. As might be expected, there are many and varied examples of the use of devices and methods to secure the airway throughout history to the present day2. Joseph O’Dwyer, an American paediatrician, used the first metal tube to secure the airway during the treatment of diphtheria around 1880. Sir William Macewen used the first endotracheal tube in 1879 to administer general anaesthesia with chloroform, in cases of oedema of the glottis and to prevent the passage of blood to the larynx.1,3 Originally, endotracheal tubes (ET) lacked a cuff for fixation, a feature added by Arthur Guedel and Ralph Water around 1932.1 The need for better positioning of the endotracheal tube would come with the development of laryngoscopy, initially blind and tactile systems. Manuel García (1805–1868), a Spanish singing teacher, was the first to use indirect techniques to observe the vocal cords by positioning small mirrors at different angles on an instrument.1
There are several types of devices used to ventilate patients. Face masks or nasal cannula used for oxygen therapy are less invasive devices. When invasive mechanical ventilation is required, endotracheal tubes become relevant in addition to the use of other ancillary devices such as laryngoscopes, videolaryngoscopes or optical stylets. In 1981, laryngeal masks were developed in an attempt to find an alternative for face masks aiming to avoid the need for intubation. In cases when it is not possible to ventilate or intubate a patient, emergency cricothyroid access is essential. However, where a prolonged airway is anticipated, tracheostomies and related devices are the preferred options.4
Endotracheal intubation is a common and routine practice used in different clinical settings and date back more than 100 years, with few design changes during this time.5
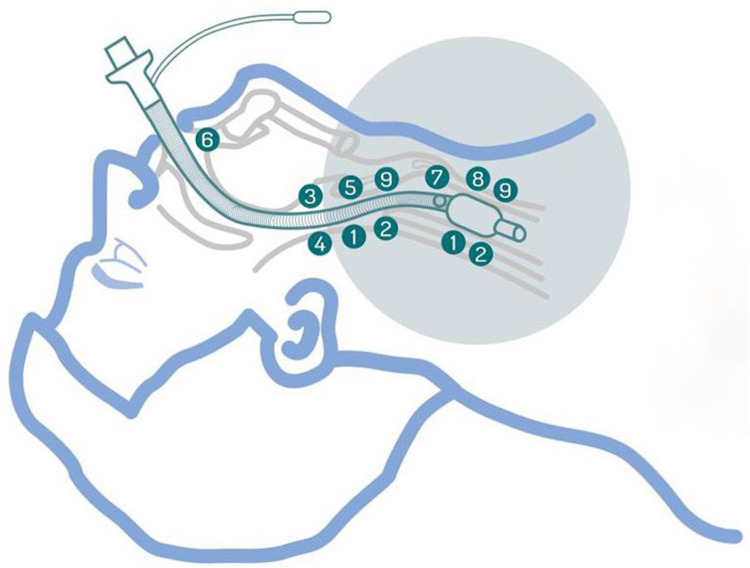
Between 13 and 20 million endotracheal intubations are performed in US hospitals each year,6,7 roughly 50 million in the world in the same period.8,9 However, the literature mentions several concerns related to laryngotracheal symptoms, ventilation associated pneumonia and stenosis10,11 that must be addressed from a clinical point of view through improvements in the design of endotracheal tubes. The main problems associated with pressure, abrasion, and decubitus phenomena exerted by the cuff and endotracheal tube over the laryngotracheal mucosa are summarized in Figure 1.
Figure 1.
Main problems associated with pressure, abrasion, and decubitus phenomena exerted by the cuff and endotracheal tube over the laryngotracheal mucosa. 1-Pressure exerted by the cuff 2-Cough, hoarseness, aphonia, dysphonia 3-Dysphagia and swallowing difficulties 4-Vocal cord paralysis 5-Arytenoid dislocation 6-Kinking of reinforced endotracheal tube 7-Subglottic suction 8-Microaspiration of contaminated fluids 9-Laryngeal tracheal stenosis.
During general anaesthesia and once the patient is asleep, it is usually necessary to insert a tube reaching the trachea through the mouth or nose, connecting to a ventilator to ensure the patient´s breathing. Although training and skills of the anaesthetists should be also considered, even if these endotracheal tubes are placed correctly, several risks and complications remain. These could be minimized or prevented if the anatomy and histology of the airway and trachea were better considered in the design of endotracheal tubes. Complications such as pressure, abrasion and decubitus phenomena can occur, even in short intubation, leading to airway injuries. Examples include post-operative vocal cord or laryngeal lesions,12 sore throat (odynophagia),13 voice changes (dysphonia due to irritation of the phonatory apparatus) or loss of voice (aphonia) and cough.14 All these lesions become more intense when intubation is prolonged. Even decubitus of the tube could affect specific parts of the vocal cords, such as the arytenoids, and could lead to vocal fatigue throughout the day. Prolonged intubation often leads to a high incidence of tracheal stenosis.11 In many cases, it is necessary to perform a tracheostomy typically recommended, based on consensus, from the 10th day of mechanical ventilation to prevent tracheal stenosis.15
In addition, active or passive bronchoaspiration may occur because the airway is not completely protected or sealed. As the supraglottic area is unprotected and the cuffs of the endotracheal tubes are short and therefore have little surface area to interact with the tracheal walls, there is a high risk of airway contamination.16–19 This risk is also increased in prolonged intubations.
The aim of this study is to identify the key challenges associated with endotracheal intubation in a hospital setting, as summarized in Figure 1. The results will provide essential information to determine the design requirements needed to develop a safer and more effective endotracheal intubation device.
Materials and Methods
On 2 December 2021, a PubMed National Center for Biotechnology Information (NBCI) repository search was conducted using PICO methodology. Terms included such as PATIENT (surgery, emergency, respiratory failure, cardiac insufficiency), INTERVENTION (endotracheal intubation, tracheotomy, airway control), COMPARATOR (laryngeal mask, balloon, reinforced tube), and OUTCOME (pneumonia, mortality, morbidity, sore throat, hoarseness, dysphonia, aphonia, hypoxia, stenosis, occlusion, intubation failure, duration of intubation, cough, odynophagia, selective intubation, tube position, subglottic damage, laryngeal damage, aspiration channel, extubation) were combined using Booleans AND/OR. Additional articles were provided by an expert with over 30 years of experience in anaesthesiology.
Articles written in English and Spanish were included. Articles without a summary or abstract were not included, and time limits were not used as exclusion criteria. Articles which did not meet criteria related to the subject of the review were not included. In addition, articles referring exclusively to medical devices other than endotracheal tubes were not included.
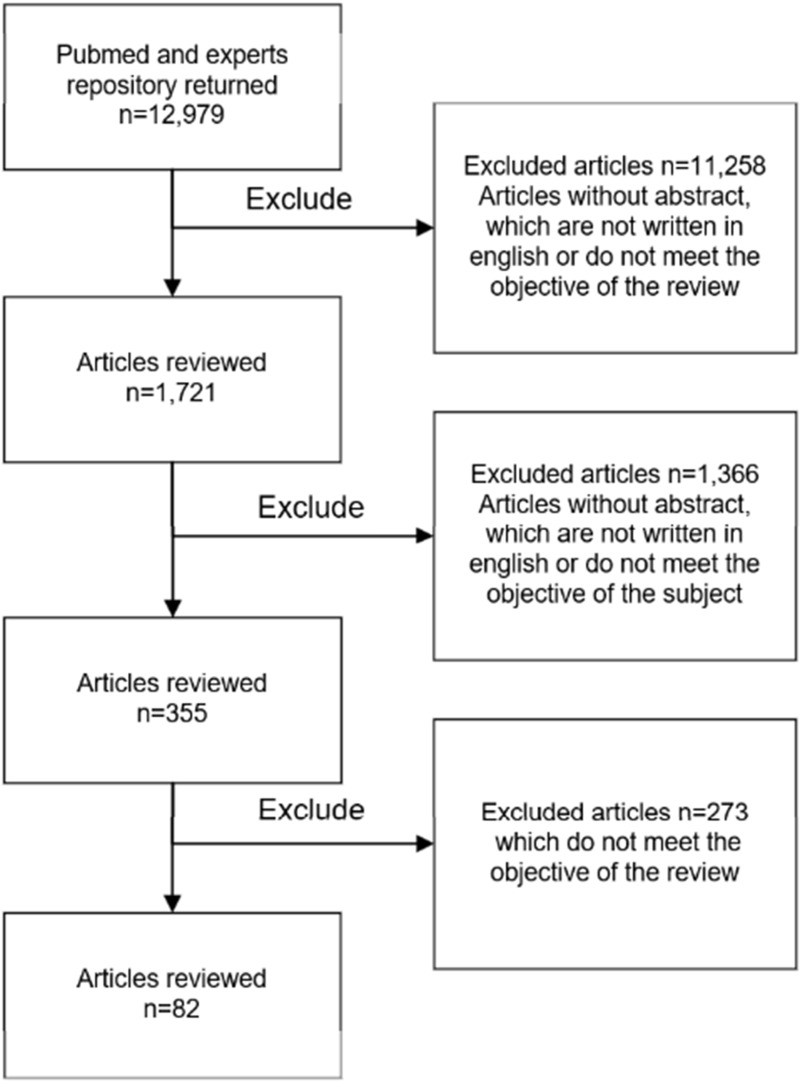
The repository and expert provisions included N=12,980 articles. After reviewing them and applying exclusion criteria, N=83 articles were included in the review. Figure 2 shows the selection flow chart.
Figure 2.
Flow diagram for selection of articles up to December 2021 with data regarding problems associated to endotracheal intubation procedure and device-related factors.
The identified articles were classified according to their contribution to the different aspects addressed (laryngeal pharyngeal symptoms, effects of cuff pressure, types of endotracheal cuffs, ventilator-associated pneumonia and tracheostomies following short and long-term endotracheal intubation). Case reports, classical articles, clinical trials, clinical studies, comparative studies, evaluation studies, letters, meta-analyses, observational studies, randomised controlled trials, systematic reviews or technical reports were considered for inclusion in the current review.
Results
The basic structure of endotracheal tubes compromises a) a connector enabling connection to the mechanical ventilator, resuscitation bag, or anaesthesia device, b) the body of the tube through which a volume of air is delivered, and c) the tracheal cuff, located in the distal portion of the tube to seal the tracheal lumen around the tube preventing air leakage during mechanical ventilation and protecting against the passage of pharyngeal contents. The cuff is inflated by a small pilot balloon with a one-way valve and a standard syringe connector. The tubes often contain a metal coil to prevent kinking, and occasionally they include a guide or stylet made of a malleable material to facilitate insertion.4 During endotracheal intubation and after insertion of the tube with the aid of laryngoscopy, the tube passes through the oral cavity and vocal cords to position the cuff in the proximal trachea of the subglottic portion. Once correctly positioned, cuff inflation is required for fixation.
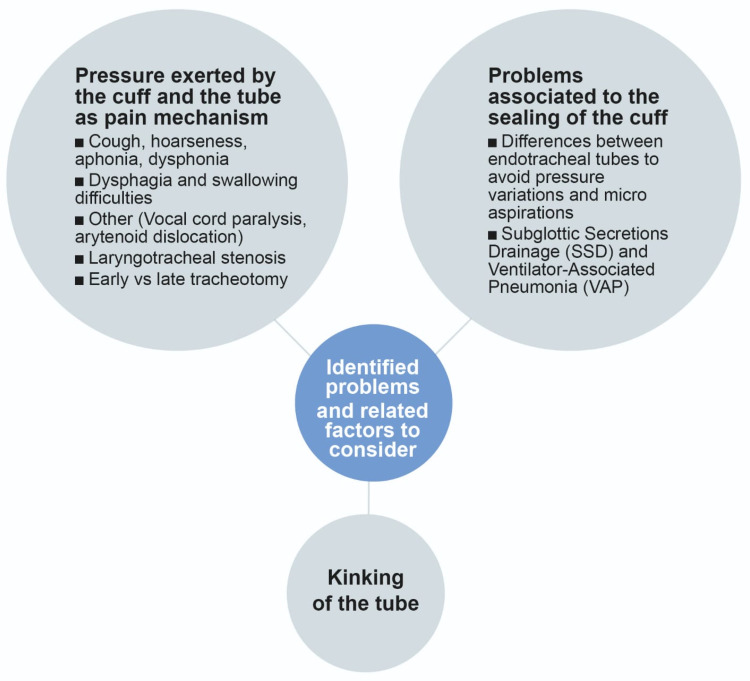
The following results, represented in Figure 3, have been considered as design requirement input in the process of the design of a new endotracheal tube.
Figure 3.
Main problems related to conventional endotracheal intubation process identified during the review.
The Pressure Exerted by the Cuff and the Tube as a Pain Mechanism
There is a clinically accepted consensus of maintaining the cuff pressure between 20–30 cmH2O pressure.20,21 Maintaining a correct cuff pressure is crucial for the cuff fixation and sealing effect and to avoid the risk of VAP. Pressure below these values increases the risk of microchannel generation favouring aspiration; conversely, overpressure increases the risk of tracheal capillary perfusion, localized ischemia, stenosis, necrosis, inflammation, ulceration, nerve damage or fistula, in addition to the previously mentioned effects such as cough, sore throat, hoarseness and bloody expectoration. In this regard, clinical protocols recommend pressure monitoring every 6–8 hours.22 However, ensuring control of the cuff pressure is not always guaranteed. In a prospective observational cohort study conducted by Nseir et al,23 they found that cuff pressure was normal (20–30 cmH2O) for 18% of the studied patients during 100% of the recording time. Cuff underinflation occurred for 54%, cuff overinflation for 73%, and both underinflation and overinflation for 44%. Also, underinflation or overinflation for more than 30 min was observed for 33% of the studied patients.
Several factors affect the cuff pressure: 1) design and materials used in the tube, 2) the use of nitrous gas, 3) patient position changes during anaesthesia; this can cause displacement of an ET in the trachea, and affect its pressure,20 and 4) environmental pressure changes. Body positioning and movements also affect the cuff pressure. Okgun et al22 studied this effect concluding that around 50% of the measurements were out of the recommended interval. Specifically, 2.5% of the results showed deflated cuffs, while 47.3% were overinflated. Finally, the change of positions during intubation was also analysed in this clinical trial conducted with 25 patients; 16 position changes were analysed and the authors concluded that the cuff pressure increased from 25 to 32 cmH2O. 22 Therefore, after changing a patient’s position, the pressure had to be adjusted.20,22 Finally, cuff pressure may also be affected by alterations in environmental air pressure. The volume of the gases and bubbles tend to increase in hypobaric conditions,24 resulting in substantially higher cuff pressures. Such changes are frequently seen during aeromedical evacuations.25 Applying the same reasoning, similar changes in gas volumes are also experienced during hyperbaric oxygen therapy. Therefore, in order to prevent the tracheal area from being severely damaged by the fluctuant cuff volumes, the cuff of an intubated patient should be inflated with saline.26
However, the overpressure on anatomical structures is not only determined by the pressure exerted by the cuff. The decubitus phenomena caused by direct exposure of the laryngotracheal structures to the tube, as shown in Figure 4, seems to be a determinant factor in the damage caused by both short-term exposure and long-term intubation.
Figure 4.
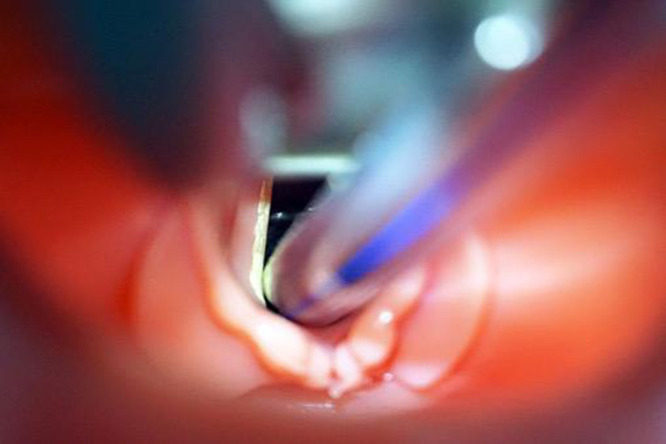
Decubitus of an endotracheal tube at the level of vocal cords. Courtesy of Dr Bravo P.L. Hospital Nuestra Señora de la Candelaria, HUNSC, Tenerife (Spain).
In 1988 Bishop27 suggested that pressure of the cuff is the most relevant factor contributing to tracheal injury. The curve of the endotracheal tube is corrected when it passes over the cricoid. The strength required to deflect the tubes has been measured in vitro28 and in a dog model.29 Pressure is the result from the division of the strength and the contact area and due to the small and tangential contact, pressure at the contact point exceeds several hundred millimetres of mercury.27 Steen et al28 measuring the force required to lift the tubes 0.5 mm, concluded that the best model tracheal tube for prolonged use should provide a minimization of tracheal and laryngeal force, minimization of tracheal mucosal damage due to cuff tracheal wall pressures, maintenance of no-leak ventilation and minimization of kinking.28 A very interesting result was obtained in 1983 in the study carried out by Weimuller et al.29 This study focused on the measurement of the pressure exerted by the tube where the author identified a higher pressure than capillary perfusion pressure in the lateral-posterior area of the larynx. Besides that, the measured pressure between 200–400 mmHg coexisted at the same point of injury.29 Also Li Bassi et al,30 in an in vitro study measuring the transmission of cuff pressure to the tracheal wall by using a pressure sensor system in a cylindrical tracheal model, attempted to show the potential unsafe tracheal pressure transmitted by HVLP cuffs, even in cases with clinically acceptable values. However, not only exerted pressure is related to the cuff or tube decubitus effect. Distally, Kastanos et al12 found in a study conducted in 1983 tracheal granulomas phenomena with pressure exerted at the tube tip level.
Cough, Hoarseness, Aphonia, Dysphonia
After extubation, laryngeal symptoms may begin to appear even if the period of endotracheal intubation has been a few hours, due to the problems associated with pressure and abrasion of the cuff and the pressure exerted by the tube itself. An example of the effect of the pressure and abrasion phenomena is recorded in Figure 5.
Figure 5.
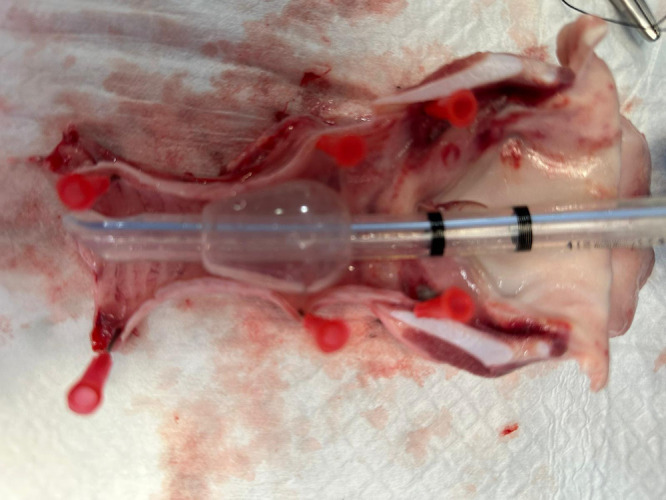
Effect of tracheal decubitus in an intubated pig when a conventional endotracheal tube was used during a preclinical trial of an innovative disposable endotracheal device versus a standard endotracheal tube. Courtesy of Dr. Centeno A. The Institute of Biomedical Research of A Coruña (Spain).
These symptoms occur after a few hours of endotracheal intubation and worsen with prolonged intubation. Bishop et al27 described in 1988 that the two main mechanisms of laryngeal injury caused by an ET tube are tube movement with mucosal abrasion and pressure necrosis.27 The mucous membranes are highly sensitive tissues, and even simple flexion and extension movements of endotracheal tubes or cephalo-caudal movements caused by inspiration can result in significant signs of abrasion. According to the author and after radiographic visualization, these movements occur because the tube could move an average of 3.8 cm. Probably, the most relevant effect is the pressure exerted by the cuff.27 This data is consistent with the claims of Christensen et al13 who suggested the movement of the tube and cuff in the trachea increases the risk of postoperative throat complaints and that the tip of the tube may move 3–4 cm, because patients are positioned on the operating table with their head extended.
Mota et al14 described laryngeal symptoms as disturbances in speech or complaints related to the airways. Professionals express these speech disturbances as dysphonia or hoarseness, whispering, aphonia, fatigue or incapacity to maintain the speech or its volume. Also throat pain and the sensation of a foreign body in the throat can be included in this group. Most of these phonatory symptoms are related to an increase in disturbance parameters that lead to variation in frequency and intensity of vibration of the vocal folds. Pharyngo-laryngotracheal symptoms such as throat pain, difficulty in speaking, coughing, increased secretions, pain when swallowing, are also common post-surgery symptoms. Regarding phonatory symptoms, it is important to note that they are self-limiting and typically disappear within 24 to 48 hours. If they persist for more than 72 hours, vocal fold injury should be suspected by the anaesthesiologist. There are three types of risk factors after endotracheal intubations: 1) factors related to the patient, 2) those related to the technical requirements to achieve and maintain the intubation, and 3) factors related to the physician. Focusing on the second type, the following risk factors must be considered according to laryngotracheal symptoms: the duration of intubation, the size of endotracheal tube, agitation of the patient (especially if reintubation episodes are needed), improper positioning of the tube (placed too high or too far below the glottis, with a cuff located in the cricoid ring), poor humidification of inspired air and local infection.14
Regarding the tube size, Stout et al studied sore throat and hoarseness in 101 human adults after around 3 h of intubation. They used large tubes (9 mm internal diameter ID for men and 7.5 mm ID for women) and small tubes (7mm ID for men and 6.5 mm ID for women). The authors found that sore throat and hoarseness was lower in smaller tubes (22% vs 48%, and 18% vs 35%, respectively) and the lesions were less severe.31 The size of the endotracheal tube is a significant contributor to the patient’s comfort, as confirmed by Bishop et al,27 because the area of ischemia correlates with the size of the endotracheal tube. Ideally, the tube should be adapted to the pentagonal form of the glottis when it is opened. A relevant clinical report by Christensen et al13 included 1325 patients (890 women intubated with tube #7 and 433 men intubated with tube #8) who were interviewed after 6–24 hours. The cuffs were inflated with room air until no leak was observed. The incidence of sore throat in the study was 14.4%. The authors concluded that the incidence was higher in women than in men (17.9% vs 9.0%), and that there was no correlation between sore throat and duration of intubation (range 20–480 min) or number of intubation attempts (1–8 attempts). The incidence of dry throat after intubation was 70.5%, cough 18.5% and hoarseness 50.1%.
According to Jaensson M. et al,32 these effects increase when intubation is prolonged and when the cuff pressure is higher, with symptoms appearing after 1–2 hours post-intubation and remaining for 96 hours in 11% of patients. The severity seems to increase when tubes with a diameter of 7 mm or greater are used, and decreases with tubes with a smaller diameter, with fewer complications in tubes with diameters of 6 mm.32
In a study where 79 patients were intubated for more than 3 days, most of the patients reported some type of laryngeal injury, described as mild mucosal erythema (94%), ulceration (76%), granuloma formation (44%), or true vocal cord immobility (20%). Besides, the incidence was worst with longer intubations.33 According to Campbell et al, when the duration of intubation is more than 12 hours, the incidence of vocal cord immobility reached 7%, nevertheless the pressure of the cuff was maintained below 30 mmHg.34
In the study conducted by Kastanos et al,12 the incidences of long-term intubation of 19 patients using fiberoptic bronchoscopy were assessed for intubated patients for 2–14 days using endotracheal tubes size 7–9. Early laryngeal lesions were described in 12 (63%) patients and stridor and dyspnoea in 5% caused by established stenosis. Early tracheal lesions were detected in 6 patients (31%). Five patients (26%) presented tracheal granulomas at the tube tip level, localized at the anterior tracheal wall.
According to Lorente et al,35 in the 1970s, high-volume, low-pressure (HVLP) cuffs were designed to reduce the tracheal mucosal injury incidence associated with ischemia effects produced by low volume high-pressure (LVHP) cuffs. When HLVP cuffs are completely inflated, their diameter is 1.5–2 times the average diameter of the trachea in adults. HVLP and LVHP endotracheal tubes are commonly used on a daily level. Jensen et al36 identified this design feature was as contributing to a sore throat,3 with more than 50% of patients reporting this symptom, with the score being more severe in the case of high volume, low-pressure devices.
Different techniques are used during anaesthesia to determine the correct cuff pressure: minimum occlusive volume, minimum leak technique, auscultation, cuff pressure measurement or minimum leak palpation are prominent in this field. The first two often result in high cuff pressures.22 Also, the literature describes that direct palpation of the cuff generates pressure dispersions ranging from 6 to 60 cm H2O. 37 In real practice, cuff pressure is frequently not kept within clinical accepted intervals and low cuff pressures are very common, so adjusting the pressure is a recommended practice.21,37–39
According to Alzahrani et al,21 in 53% of cases, the cuffs were outside the established range. In this regard, clinical protocols recommend pressure monitoring every 6–8 h.22 However, studies suggest that this frequency of cuff pressure measurement is not sufficient to ensure and maintain the correct pressure during medical interventions, inviting the medical community to review clinical procedures and increase the frequency of monitoring, or even automate it if necessary.21
Dysphagia and Swallowing Difficulties
When any part of the swallowing mechanism fails, dysphagia appears as a symptom of swallowing reduction. Dysphagia may cause penetration of material into the larynx, but not the true vocals cords; this is known as aspiration.40 Different studies have found that dysphagia leads to longer hospital stays, rates of readmission, a higher mortality and increased use of the health care system.40–43
In a retrospective observational cohort study carried out by Macht et al42 with 446 patients, the documented prevalence of dysphagia was up to 84%, and the severity was moderate in 23% and severe in 17%. According to a systematic review carried out in 2010 including a total of 14 studies with a total of 3520 patients, they concluded that the incidence could range from 3 to 62%.44 In a recent larger study performed by Zuercher et al,41 including 1304 medical and surgical intensive care unit (ICU) patients with potential post-extubation dysphagia risk, an incidence rate of 12.4% was reported. The tremendous difference between the different studies in the incidence could be due to the study design, patient selection, and/or limited patient numbers.
Direct trauma appears to be the main mechanism of dysphagia in the intensive care unit.40,41,45 Any type of artificial tube (endotracheal tube, tracheotomy tubes, echocardiography probes or feeding tubes) could damage the anatomical structure. Traumas such as hematomas, oedema, compression, or arytenoid dislocation are caused at the tube-larynx interface. A decrease in the glottic reflex of occlusion generates a lack of airway protection. Atrophy in the laryngeal and extralaryngeal musculature generates a weakness of the lingual, pharyngeal, hypopharyngeal and laryngeal musculature. Even a lack of coordination between the physiological processes of breathing and swallowing increases the need for respiratory work. All this leads to aspiration pneumonia, one of the most serious complications associated with endotracheal intubation,46 where using fiber-optic endoscopic evaluation of swallowing (FEES) can help to determine which patients should be investigated further and those who are not at risk of aspiration.43,46
Other Symptoms Associated to Pressure and Decubitus
Vocal Cord Paralysis
Vocal cord paralysis also appears to be associated with nerve compression exerted by the endotracheal tube between the cuff and the underlying thyroid cartilage, causing cricoarytenoid dislocation or neuropraxia secondary to stretching during hyperextension of the neck.34 The most probable localization may be in the vocal process of the arytenoids and the membranous vocal cord, although this paralysis is temporary.47 When the cuff is inflated, it compresses the anterior branch of the recurrent nerve against the thyroid cartilage. This paralysis of the vocal cords can become dangerous because the resulting oedema can lead to dyspnoea.48 This immobility of the vocal folds, in addition to altering the respiratory process, reducing pulmonary clearance, also affects phonation and causes an increased risk of VAP, although studies establish that its incidence, risk factors and pathophysiology remain unclear.34
Shin et al48 suggested that the causes of vocal cord paralysis were related to articulation disorders and nerve damage. A violent insertion of the tube or arytenoids compression for a long time could lead to articulation disorders, such as cricoarytenoid dislocation or damage. This situation would show an abnormal arytenoid shape, bleeding, or inflammation.
There are several medical record reviews that analyse vocal cord paralysis and sore throat in patients related to the use of endotracheal tubes.49–52 The incidence varied markedly among different studies: 12.1%,49 0.043%,50 0.03%51 and 0.077%.52
This significant difference could be due to the limitations in their studies: Higgins et al,49 only considered the 5264 patients who received a successful telephone interview out of the 17,877 patients who underwent ambulatory surgery, while other studies analysed all medical records: 100,291,50 23,010,51 and 31,54152 individuals. This apparently rare complication (<0.1%), besides being a serious problem in itself, can be a predictor of subsequent pulmonary complications and prolonged hospital stay.51
According to Kikura et al52 the risk of vocal cord paralysis was found to be increased three-fold in patients aged older than 50, two-fold in patients intubated between 3–6 hours, fifteen-fold in patients intubated 6 hours or more, and two-fold in patients with a history of diabetes mellitus or hypertension. A similar tendency was obtained by Lim et al.50
Arytenoid Dislocation
Arytenoid subluxation is a partial displacement of the arytenoid cartilage within the cricoarytenoid joint. This is not a common complication related to damage in the cricoarytenoid junction during intubation or laryngoscope insertion, upper airway instrumentation and external laryngeal damages. Its incidence has been calculated to range from 0.01–0,1%.53 According to Quick et al,54 dislocation of an arytenoid cartilage has generally not been included in the list of complications of endotracheal intubation since the reported incidence is rare, although the true incidence may be higher. Rudert et al55 reported a much higher incidence of 30% in their case series of patients referred with persistent hoarseness after laryngeal instrumentation; 80% to 90% of cases were related to intubation trauma. Subluxation results in hypomobility of the true vocal cords and incomplete closure of the glottis, mimicking vocal cord paralysis. Violent insertion of the tube or prolonged compression of the arytenoids can cause joint disorders described as dislocation or cricoarytenoid damage. The cricoid pressure exerted by the cuff causes mucosal damage and inflammation, worsening the view of the larynx.48
Laryngotracheal Stenosis
Prolonged intubation often leads to a high incidence of tracheal stenosis.11 This is due to several factors: 1) the cuff pressure on the tracheal walls is exerted on a very short segment of the trachea; 2) the cuff inflation pressures exceeding the perfusion capillary pressure at which the trachea is perfused and 3) the location in the trachea where the endotracheal tube rests, causing stenosis in the decubitus zone.56
Laryngotracheal stenosis, represented in Figure 6, is the narrowing of the airway caused by an intrinsic or extrinsic mass, with intubation-associated ischemia being the most common cause. The physiopathology is characterised by elevated cuff pressures, exceeding 30 mmHg, which compress and impair perfusion, resulting in ischemia, ulceration, and tracheal chondritis. These lesions heal through fibrosis, resulting in progressive stenosis typically occurring early after intubation. The initial lesion often manifests in the posterior cricoid and is commonly associated with prolonged intubation. Apart from cuff pressure, various factors contribute to its development, including endotracheal tube size, gastroesophageal reflux, bacterial colonization, underlying disease, and patient motion. Laryngotracheal stenosis can be classified as supraglottic, glottic and subglottic, or a combination of these.4
Figure 6.
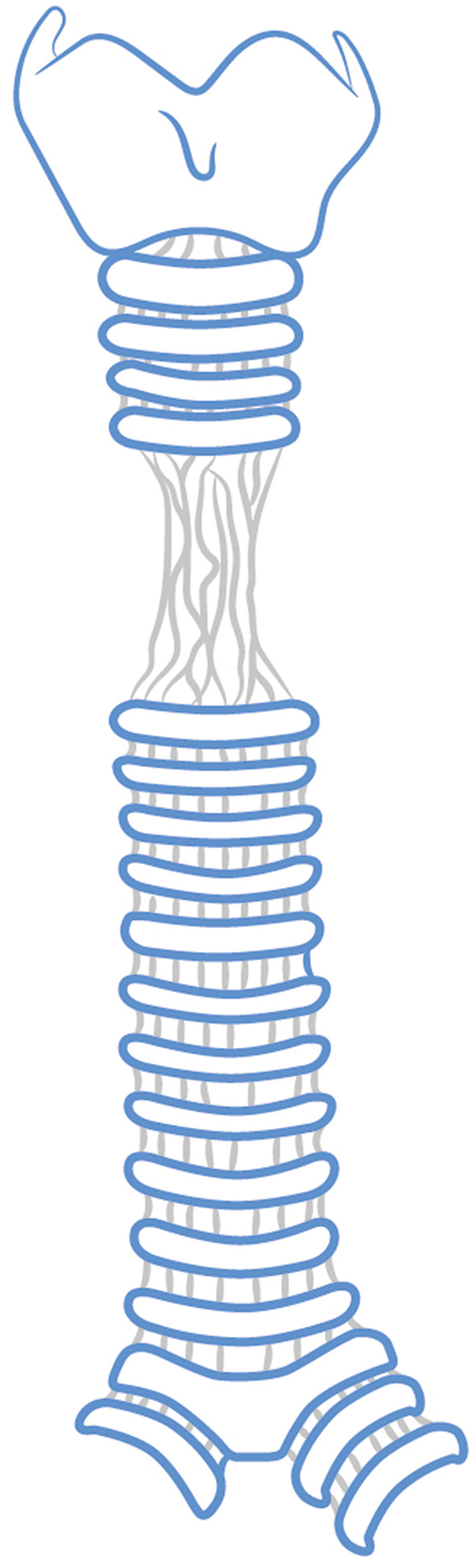
Example of tracheal stenosis.
According to Dutoit-Marco and Schwander,56 laryngeal stenosis resulting from prolonged intubation begins with laryngeal granuloma. This lesion typically occurs either in the cartilaginous glottis or on the process of the arytenoid cartilage, but it can be located anywhere on, above or below the vocal cord. Granulomas are secondary effects resulting from either the pressure exerted by the tube on the glottal contour or direct trauma during intubation. They can be induced by laryngeal movement, eg due to repeated swallowing during anaesthesia, the curvature of the tube, the position of the patient, or one or more vocal cord contusions due to screaming or coughing after extubation. Among other complications, vocal cord synechiae, whether partial or total, single or multiple have also been observed with usually posterior orientation. It may connect the two vocal processes. Behind it, there is a second triangular opening corresponding to the posterior commissural region. Fixation of the cricoarytenoid joint in a subluxation position with secondary arthropathy may also be observed. The vocal cord progressively becomes immobilised. The most severe observed lesions are glottosubglottic lesions, which include tubular or annular stenosis, chondral cricoid stenosis, parietal dyskinetic elements, massive subglottic synechia and aerodigestive fistula. These glottic and subglottic strictures are often associated with tracheal lesions, either due to the cuff or the tracheotomy required to manage subglottic stenosis.
Donnelly et al10 conducted a study in the sixties the assess the extent of laryngotracheal damage relative to the duration of endotracheal intubation through detailed clinical and histopathologic observations of 99 autopsy specimens from adult patients intubated between 15 minutes and 176 hours during the 30-day period preceding their death. A relationship was found between laryngeal damage and the duration of intubation but not with respect to age and sex. Histologic studies demonstrated focal or complete loss of mucosal epithelium in contact with the tube even after 1 hour, the ischemic nature of the process, exudative inflammatory response in the ulcers to extubation, increased frequency of perichondritis of the vocal process after 48 hours of intubation and common infestation of the ulcer by microorganism after 24 hours. Prolonged intubation leads to continued abrasion, compression of tissues between the tube and underlying cartilage or bone, followed by ischemia and necrosis, compromising the reparative ability of the tissues. Intubation beyond 96 hours resulted in severe damage to vocal processes and the subglottic area, with a higher incidence of vocal fold ulceration.
Whited et al,11 in a prospective study of 200 patients in 1984, found that the incidence of laryngotracheal stenosis was related to the duration of intubation. The incidence of temporary airway compromise was 6% in patients intubated between 2 and 5 days, 5% in those intubated for 6 to 10 days, and 12% in patients intubated for more than 11 days and up to 24 days with the stenosis having a chronic component.
Early Tracheostomy vs Late Tracheostomy
In many cases, it is necessary to perform a tracheostomy when there is a risk of stenosis. This is a surgical procedure in which an artificial and invasive airway is created by opening the trachea and draining it from the outside. This procedure is typically recommended by consensus from the 10th day of mechanical ventilation to prevent tracheal stenosis.15 However, tracheostomy can also lead to complications such as tracheal ulcers, false trachea, haemorrhage, infection, distortion of soft tissues, airway obstruction, etc. Nevertheless, performing this technique does not prevent a high incidence of post-tracheostomy stenosis.
Tracheostomy is a procedure that has been used by the Egyptians and Indians for more than 3000 years.57 The diphtheria epidemic of the 19th century increased its use and improved outcomes in patient’s upper airway obstruction. In the 20th century, further improvements to the devices designed by Chevalier-Jackson consolidated its use in the medical community, despite the associated complications and risks of the procedure. With the advent of mechanical ventilation, tracheostomy became one of the most commonly performed procedures on patients admitted to intensive care units. However, despite its favourable historical evolution and widespread use, there is still a debate and controversy regarding patient selection and the indications for its use. The main indications for tracheostomy in ICU are similar to those in other medical specialties, although underlying diseases may vary. These indications include relief of upper airway obstruction, assistance with pulmonary toilet, and airway control for long-term mechanical ventilation. Patients with upper airway obstruction usually have neoplasia, infectious, or functional disorder that compromise ventilation. Patients are typically stabilized initially with translaryngeal intubation and subsequently converted to tracheostomy if the airway obstruction is not remedied within 10–14 days.57
The aim of a Cochrane review on Early versus late tracheostomy for critically ill patients15 was to assess the effectiveness and safety of early (≤ 10 days after tracheal intubation) versus late (> 10 days after tracheal intubation) tracheostomy in critically ill adults with different clinical conditions who were expected to require prolonged mechanical ventilation. At the longest follow-up available in these trials, moderate-quality evidence showed lower mortality in the early tracheostomy group compared with the late group. Different results were reported for time spent on mechanical ventilation, and no differences were found in the incidence for pneumonia. However the odds of discharge from the ICU at day 28 were higher in the early tracheostomy group. Benefits in terms of safety and efficacy have been reported in favour of early tracheostomy. Factors such as fewer days on mechanical ventilation, lower incidence of ventilator-associated pneumonia and reduced mortality have been cited. There is little evidence to support the current standard of 10–14 days for tracheostomy. The reason clinicians are reluctant to change to early tracheostomy is concern about accepting a practice that is not based on sound research. However, the evidence seems to favour early rather than late tracheostomy.58 But controversy remains in this regard. A systematic review and meta-analysis by Meng et al,59 comparing the outcomes of early tracheostomy with late tracheostomy in critically ill patients on prolonged mechanical ventilation, which included 9 studies with 2040 patients, suggested that tracheostomy performed within 10 days of translaryngeal intubation may reduce the duration of sedation. However, compared with late tracheostomy, early tracheostomy did not reduce mortality, the incidence of VAP, the duration of mechanical ventilation, or the length of stay in intensive care, similar to the results obtained by Andriolo et al15 in 2015. In 2004 Rumbak et al60 evaluated the effects of early percutaneous dilatation tracheotomy compared with delayed tracheotomy in critically ill medical patients requiring prolonged mechanical ventilation, in an attempt to obtain results in favour of early tracheotomy.
Problems Related to the Sealing of the Cuff
Differences Between Endotracheal Tubes to Avoid Pressure Variations and Microaspirations
Some medical device manufacturers have modified the composition and design of the cuffs, using different materials such as polyurethane (PU), with a thinner wall than polyvinylchloride (PVC) to avoid the formation of microchannels and thus reduce the risk of microaspiration. Conical shapes also seemed to improve the seal, with the same advantages of the low-pressure, high-volume tubes.4
Regarding the pressure exerted on the trachea, some relevant conclusions have been found, where it seems that polyurethane materials have a better safety profile. Nseir et al23 measured this effect and concluded that the main results of the study were that PU cuffs have less effect on tracheal pressure variations during the intubation. Tapered-conical cuffs were associated with an increased cuff pressure when it was compared with cylindrical or standard cuffs. However, variation in the cuff pressure is frequent with PVC cuffs. Regarding gastric fluid microaspiration, it was less frequent with PU cuffs compared with PVC cuffs. There was no significant difference between cylindrical PU and tapered-conical PVC cuffs. Something similar was concluded in a systematic review carried out by Blot et al61 evidencing that compared with PVC-cuffed tubes, PU-cuffed tubes protect more efficiently against microaspiration, or at least postpone substantial leakage of secretions. Nevertheless, in the vivo experiment the difference was not significant in the aspirated microspheres and the evidence that PU-cuffed ETs prevent pneumonia is less robust, because, according to the authors, microaspiration is probably delayed rather than eliminated with the use of PU cuffs.
One aspect of air leakage generated by endotracheal tubes that needs to be considered is the PEEP (Positive End Expiratory Pressure). According to the results obtained in a benchtop study conducted by Zanella et al,62 among all PVC cuffs, conical shape polyurethane cuffs ensured the highest sealing properties. Ouanes et al63 analysed in 2011 an evaluation of potential effects of positive end expiratory pressure (PEEP), inspiratory effort intensity, and tube characteristics on fluid leakage past the cuff during an in vitro benchtop study. It was found that the occurrence of leakage increased proportionally to inspiratory effort intensity. The reason could be that this high effort decreases the intrathoracic pressure above the cuff, leading to the leakage. This leakage was higher with PVC cuffs than with PU cuffs.
Subglottic Secretions Drainage (SSD) and Ventilator-Associated Pneumonia (VAP)
VAP is a problem widely described in the published scientific literature and of concern to the medical community. This collateral damage associated with the lack of tightness of traditional ETs causes oropharyngeal and bronchial tree colonization. Inflating the endotracheal cuff is very useful in preventing aspiration of contaminated material into the lungs past the endotracheal tube and gas leakage during positive pressure ventilation. Aspiration of microbiologically contaminated fluids that accumulate above the cuff is the main mechanism of bacterial entry into the lower respiratory tract. When this occurs repeatedly, and due to the formation of longitudinal folds in the cuff, it is known as subglottic secretions. Several countries, including USA, Canada and China have suggested subglottic secretion drainage for preventing VAP.16–18 Rello et al19 described in 2010 that VAP is a serious healthcare-associated infection that occurs in up to approximately 30% of mechanically ventilated patients. VAP is defined as pneumonia occurring more than 48 hours after the initiation of mechanical ventilation. The occurrence of VAP increases patient mortality to an estimated 20–55% and prolongs hospital stay by approximately 6 days, with average hospital costs estimated at over $40,000 in per patient.19,64,65 Therefore, some endotracheal tubes where mechanical ventilation may be prolonged, included in their design an additional suction tube to reduce microaspiration and thus the risk of VAP. Figure 7 illustrates an endotracheal tube with suction tube and suction port above the cuff.
Figure 7.
Endotracheal tube with conventional suction port for Subglottic Suction Drainage (SDD).
Between 2003 and 2006, Lacherade et al66 conducted a multicentre trial to evaluate whether SDD decreased the global incidence of microbiological VAP. This team led a randomized clinical trial in 4 French centres on 333 adult patients with more than 48 hours of mechanical ventilation. Patients were randomized to receive intermittent SSD (n=169) or not (n=164). In the SSD group, subglottic secretions were manually aspirated with a 10-mL syringe at a planned frequency of one aspiration per hour. The control group did not receive SSD. The study concluded that subglottic secretion drainage during mechanical ventilation resulted in a significant reduction in VAP, including late-onset VAP. In addition, the absolute risk reduction in the patients suggested that for every 100 patients treated with SSD, 11 cases of VAP could be avoided.
New randomized controlled trials have recently been published.67–69 In two of them, there is evidence that the reduction in the risk of developing VAP with the SSD ET group compared to the conventional ET group is close to 50%.67,69 There was no significant difference in median ICU stay between patients who had developed VAP69 and the mortality during the first 28 days among patients who had developed VAP is 42% in the conventional group vs 24% in the SSD group.69 A recent systematic review and updated meta-analysis70,71 found that SSD could dramatically reduce VAP. With regard to ICU length of stay, there is still some controversy. Dewi et al70 found a significant reduction in ICU length of stay when comparing usual care with the SSD group, but no significant reduction in mortality. Conversely, the results of Pozuelo-Carrascosa et al71 observed just the opposite.
A final note should be made regarding the characteristics of cuff materials and their use in conjunction with subglottic suction in the prevention of VAP. According to Lorente et al35 in the 1970s, HLVP cuffs were designed to reduce the incidence of mucosa ischemia that was observed with the use of LVHP tubes. When they are completely inflated, their diameter is 1.5–2 times the average diameter of an adult trachea.35 But excess of material creates folds and subsequent channels through subglottic secretions accumulated above the cuff may pass to the lower respiratory tree. This phenomenon is much more likely to occur with HVLP than with LVHP cuffs, so that the risk of VAP increases. Besides that, they concluded that the use of an endotracheal tube with polyurethane cuff and intermittent subglottic secretion drainage helps prevent early-and late-onset VAP when compared to the incidence of VAP of a conventional endotracheal tube with PVC cuff, without subglottic secretion drainage.35
Two techniques have been identified in the implementation of subglottic suctioning: continuous and intermittent aspiration. In the former, the negative pressure applied for secretion aspiration remains constant, while in the latter, periodic aspiration cycles are established. However, according to the damage caused to the subglottic mucosa, the choice of continuous subglottic suctioning or intermittent suctioning is not without controversy. Berra and colleagues72 reported the histological changes that occur as a result of subglottic suctioning in an experimental sheep model. The main endpoint of their Randomized Clinical Trial (RCT) was to assess the safety of the technique. They clearly expressed that subglottic aspiration was harmful. All of the sheep showed macroscopic and microscopic damage in the posterior tracheal mucosa at the suction port site. From a histological point of view, the injury varied from erosion and oedema to haemorrhage and inflammation at the mentioned level. On the other hand, sheep intubated with standard endotracheal tubes did not show these effects after 3 days of mechanical ventilation. In short, this finding challenged the claim that there were no adverse effects when subglottic suction drainage is applied. This fact was also confirmed by Spapen et al73 regarding mucosal damage during subglottic aspiration.
According to the evidences on the safety and efficacy of continuous versus intermittent aspiration, Lorente et al35 suggested that although this assertion has not been studied, it is possible that intermittent subglottic drainage is less harmful, but also less effective than the continuous technique in preventing the leakage of fluids above the cuff, and subsequently, in prevention of the risk of VAP, although this fact has not been studied either. In the study by Dewi et al70 analysing the effectiveness of intermittent and continuous SSD in preventing VAP, although there was a reduction in risk in both groups, the difference between the two groups (intermittent and continuous) was not statistically significant. For all these reasons, including the fact that the cost of specialized SSD is about seven times higher than that of conventional SSD, the controversy remains, and the authors conclude that further high-quality studies are needed to clarify the potential contribution of SSD in medical care. Seguin et al74 came to similar conclusions when comparing the effect of continuous suctioning of subglottic secretions versus intermittent suctioning of subglottic secretions on the tracheal mucosa anterior to the suction port of the endotracheal tube. The occurrence or worsening of tracheal mucosal damage did not differ between the two groups, although the average daily volume of secretions aspirated was higher in the intermittent group.
Other Design Input Related to Kinking of the Tube
In head and neck surgery and prolonged prone intubation, reinforced tubes are often used.75–79 Although their design is intended to prevent kinking, several cases have been reported where the internal lumen was obstructed by the intubated patient’s bite during passage through the oral cavity, resulting in irreversible deformations that could complicate the intubated patient’s artificial airway.75–82
Discussion
Endotracheal intubation is a widely used clinical procedure during anaesthesia and in patients admitted to intensive care units, which is not without associated problems. After surgical procedures and during short-term intubation, laryngo-tracheal symptoms (sore throat, cough, dysphonia or aphonia, hoarseness, dysphagia, etc.) occur with an incidence of 30–60%. This symptomatology could be related to the intubation procedure during anaesthesia or be associated to other factors related to the tube itself, excessive pressure of the cuff, the size of the tube, the patient’s movements and agitation, or the reintubations required. Sometimes, the pressure exerted by the fixation cuffs above 30 cmH2O or the pressure of hundreds of mmHg exerted by the tubes themselves causes this symptomatology. With prolonged surgical procedures or in cases where the cuff pressure is not properly monitored, this symptomatology may be aggravated when intubation is extended. Additional symptoms such as vocal cord paralysis or arytenoid dislocation may also be observed, although a low incidence has been reported.
A new design should ensure an efficient and safe airway and should be designed to protect the anatomical structures against elements that may damage them, in which physical phenomena such as pressure, abrasion and decubitus generated from the cuff and the tube are underlying phenomena in standard endotracheal tubes. The effects of pressure, abrasion and decubitus caused by the cuff and tube due to direct glottic exposure should be considered.
This review has provided several elements that suggest a rethinking of the design of endotracheal tubes. In this sense, Figure 8 shows the elements that should be included in the design of a new endotracheal device in order to solve the problems associated with the conventional device.
Figure 8.
A design approach for an innovative endotracheal device. WIPO International patent application WO2021/234183 A1. a) Viscoelastic self-expandable endotracheal device subglottic level b) Viscoelastic self-expandable glottic and vocal cord level c) Viscoelastic self-expandable supraglottic level d) Combination of reinforced and non-reinforced zones e) Supraglottic suctioning tube f) Shorter distal tip g) Viscoelastic compression tube h) Balloon i) 3-way stopcock j) suction connector.
Elements a), b) and c) in Figure 8 show a viscoelastic self-expandable piece for fixation in the supraglottic, glottic through vocal cords and subglottic and tracheal walls. The design includes d) reinforced and non-reinforced zones. While reinforced zones avoid kinking phenomena, the non-reinforced zone offers the possibility to recover its internal lumen diameter if the patient bites the tube. Additionally, the tube includes e) supraglottic big lumen aspiration tube, f) shorter distal tip to reduce the abrasion and g), h), i) viscoelastic compression and self-expandable system for insertion and extraction of the novel endotracheal tube.
Figure 9 shows the new design with a viscoelastic non-pneumatic and self-expanding material that avoids direct contact with the tube, conforms to the anatomical areas, and exposes the patient to significantly lower pressures, ie less than 10 cmH20, in the laryngotracheal axis and keeps this pressure stable for the duration of the clinical intervention. In addition, this tube adapts to flexion, extension, and lateralization movements during the intervention or by the patient’s own movements and agitation, absorbs them and keeps exerted low pressure constant, eliminating the need to monitor inflation pressures during surgery or in the ICU.
Figure 9.
A novel endotracheal tube with compressed viscoelastic piece for insertion and expanded viscoelastic for fixation. The viscoelastic piece protects against pressure, abrasion and decubitus at supraglottic, glottic and subglottic level.
According to the results, the prolonged duration of mechanical ventilation and ICU stay seems to increase the risk of developing VAP, with 30% of ventilated patients appearing to develop VAP, prolonging ICU stay by approximately 6 additional days compared to patients who do not develop VAP, with a reported mortality rate that can vary between 20 and 50%. Although there are several causes, the lack of airtightness exerted by the endotracheal tube cuff, either due to pressure loss, inefficient pressure monitoring or inadequate selection of the tube size or even the polymeric materials of the cuff (PU cuffs seem to perform better than PVC ones), creates microchannels that allow the passage of fluids from the oropharynx or gastrointestinal tract, generating microaspirations of contaminated fluids into the lower airways. Therefore, and following the above-mentioned string, a self-expandable viscoelastic material designed for the glottic axis would longitudinally increase the sealing surface and reduce microchannels. This would avoid the low sealing surface of current devices and the need for pressure monitoring. All the above should be guaranteed with the commitment to maintain adequate airtightness to avoid the risk of VAP, even during long term intubations, and thus improve the duration of mechanical ventilation, ICU stay, mortality rates and therefore the associated hospital costs.
One of the strategies identified to manage VAP is subglottic suctioning, which reduces the incidence of VAP, although there is no clear evidence regarding the duration of mechanical ventilation, length of stay in the ICU, or reduction in mortality. As for subglottic suctioning, two trends have been identified: continuous and intermittent. However, there seems to be no consensus on the benefits of one over the other. What seems clear is that subglottic suctioning causes lesions in the tracheal mucosa near the site of suctioning, where histologic signs such as erosion, oedema, haemorrhage, inflammation, or necrosis may be seen. It seems that suctioning and its positioning should be considered in a novel endotracheal disposable device.
Figure 10 represents the incorporation of a high-flow suction tube at supraglottic level, increasing the contaminated fluids suction flow rate and reducing the VAP risk due to microaspiration. However, the novelty may lie in the location of the suction point, where it remains to be demonstrated whether this aspiration should be performed at the subglottic level or whether a could be applied at supraglottic level within more available anatomical space. This location could reduce suction injuries, such as erosion, oedema, haemorrhage and inflammation.
Figure 10.
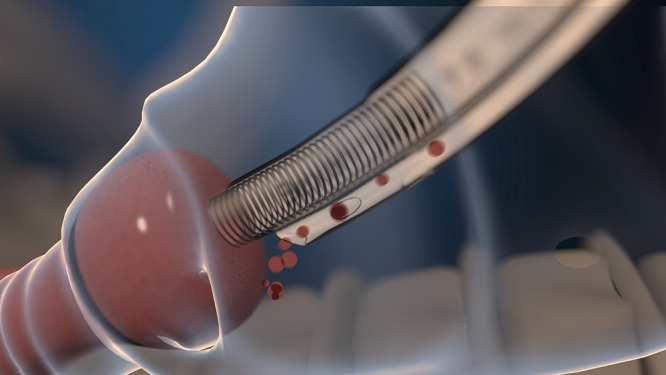
A novel endotracheal tube with high-flow suction tube at supraglottic level.
Prolonged intubation, and for patients admitted to the ICU because of this excessive pressure, also brings with it another set of problems identified during the review. Prolonged pressure on the laryngotracheal walls can cause a narrowing of the airway, leading to difficulties in ventilation due to laryngotracheal stenosis, which requires to take additional measures such as performing tracheotomies to ensure the patient’s airway. In this sense, and when prolonged intubation is foreseen, early tracheostomies are performed. However, the evidence of the benefits between early tracheostomy and late tracheostomy in terms of mortality, ICU stay, duration of mechanical ventilation does not seem to be clear.
The viscoelastic non-pneumatic and self-expanding design represented in Figure 8 aims to reduce lateral damage and provide a new solution for prolonged intubation, as it appears that in many cases it is the abrasion, decubitus and pressure exerted by the tube or cuff are responsible for narrowing the airway leading to stenosis. In the absence of high exerted pressure levels, a new intubation device that protects against laryngotracheal damage could suggest a reduction in the incidence of both supraglottic and subglottic stenosis, thereby promoting longer and safer intubation. Additionally, it may delay the decision to tracheostomise patients at a very early stage and foster an endotracheal intubation practice that mitigates the collateral symptoms until the 10th day of intubation.
However, all these novel design inputs will necessarily require relevant clinical evidence to support and confirm the conclusions drawn in this review.
Conclusions
As the essential design of endotracheal tubes has not changed in the last 100 years, this scientific review has identified and listed design input requirements for a new and innovative endotracheal intubation device, that enable effective and safer procedures with less collateral symptomology during laryngotracheal intubations. Undoubtedly, the aspects identified could be of great interest to the medical device development industry, the health system and the patient care.
Acknowledgments
We would like to express our gratitude to Dr Pedro Luis Bravo, whose design of the device has greatly inspired the writing of this article.
Author Contributions
All authors made a significant contribution to the work reported, be it in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas. Furthermore, they took part in the drafting, revising or critically reviewing the article and gave their final approval of the version to be published. They also agreed on the journal to which the article has been submitted, and agree to be accountable for all aspects of the work.
Disclosure
Mr Gorka Ramirez reports grants from Basque Government, outside the submitted work. The authors report no other conflicts of interest in this work.
References
- 1.Robinson DH, Toledo AH. Historical development of modern anesthesia. J Investig Surg off J Acad Surg Res. 2012;25(3):141–149. [DOI] [PubMed] [Google Scholar]
- 2.Szmuk P, Ezri T, Evron S, Roth Y, Katz J. A brief history of tracheostomy and tracheal intubation, from the bronze age to the space age. Intensive Care Med. 2008;34(2):222–228. doi: 10.1007/s00134-007-0931-5 [DOI] [PubMed] [Google Scholar]
- 3.Goksu S, Sen E. History of intubation. Journal of Academic Emergency Medicine. 2015;14(1):35–36. doi: 10.5152/jaem.2015.96720 [DOI] [Google Scholar]
- 4.Comité de Vía. Aérea e Interfaces de la Sociedad Argentina de Terapia Intensiva. Via Aérea. Manejo y Control Integral. 2a edición. Panamericana; 2010. [Google Scholar]
- 5.Guedel AE, Waters RM. A new intratracheal catheter. Anesthesia Analg. 1928;7(4):238–239. doi: 10.1213/00000539-192801000-00089 [DOI] [Google Scholar]
- 6.Nadeem AUR, Gazmuri RJ, Waheed I, et al. Adherence to evidence-base endotracheal intubation practice patterns by intensivists and emergency department physicians. J Acute Med. 2017;7(2):47–53. doi: 10.6705/j.jacme.2017.0702.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turner JS, Bucca AW, Propst SL, et al. Association of Checklist Use in Endotracheal Intubation With Clinically Important Outcomes: a Systematic Review and Meta-analysis. JAMA Network Open. 2020;3(7):e209278. doi: 10.1001/jamanetworkopen.2020.9278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kitzmann N. In adults with difficult intubations, what is a gold standard method for success? [master’s thesis]; Augsburg University; 2020. [Google Scholar]
- 9.Whitten CE. 10 Rules for approaching difficult intubation. Always prepare for failure. 2013. [Google Scholar]
- 10.Donelly WH. Histopathology of endotracheal intubation. An autopsy study of 99 cases. Arch Pathol. 1969;88:511–520. [PubMed] [Google Scholar]
- 11.Whited RE. A prospective study of laryngotracheal sequelae in long term intubation. Laryngoscope. 1984;94(3):367–377. doi: 10.1288/00005537-198403000-00014 [DOI] [PubMed] [Google Scholar]
- 12.Kastanos N, Estopá Miró R, Marín Perez A, Xaubet Mir A, Agustí-Vidal A. Laryngotracheal injury due to endotracheal intubation: incidence, evolution, and predisposing factors. A prospective long-term study. Crit Care Med. 1983;11(5):362–367. doi: 10.1097/00003246-198305000-00009 [DOI] [PubMed] [Google Scholar]
- 13.Christensen AM, Willemoes-Larsen H, Lundby L, Jakobsen KB. Postoperative throat complaints after tracheal intubation. Br J Anaesth. 1994;73(6):786–787. doi: 10.1093/bja/73.6.786 [DOI] [PubMed] [Google Scholar]
- 14.Mota LAA, de Cavalho GB, Brito VA. Laryngeal complications by orotracheal intubation: literature review. Int Arch Otorhinolaryngol. 2012;16(2):236–245. doi: 10.7162/S1809-97772012000200014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andriolo BN, Andriolo RB, Saconato H, Atallah ÁN, Valente O. Early versus late tracheostomy for critically ill patients. Cochrane emergency and critical care group, editor. Cochrane Database Syst Rev. 2015;2018(12). doi: 10.1002/14651858.CD007271.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klompas M, Branson R, Cawcutt K, et al. Strategies to prevent ventilator-associated pneumonia, ventilator-associated events, and nonventilator hospital-acquired pneumonia in acute-care hospitals: 2022 update. Infect Control Hosp Epidemiol. 2022;43(6):687–713. doi: 10.1017/ice.2022.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi Y, Huang Y, Zhang TT, et al. Chinese guidelines for the diagnosis and treatment of hospital-acquired pneumonia and ventilator-associated pneumonia in adults (2018 edition). J Thorac Dis. 2019;11(6):2581–2616. doi: 10.21037/jtd.2019.06.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muscedere J, Dodek P, Keenan S, Fowler R, Cook D, Heyland D. Comprehensive evidence-based clinical practice guidelines for ventilator-associated pneumonia: prevention. J Crit Care. 2008;23(1):126–137. doi: 10.1016/j.jcrc.2007.11.014 [DOI] [PubMed] [Google Scholar]
- 19.Rello J, Lode H, Cornaglia G, Masterton R. VAP care bundle contributors. a European care bundle for prevention of ventilator-associated pneumonia. Intensive Care Med. 2010;36(5):773–780. doi: 10.1007/s00134-010-1841-5 [DOI] [PubMed] [Google Scholar]
- 20.Choi E, Park Y, Jeon Y. Comparison of the cuff pressure of a TaperGuard endotracheal tube and a cylindrical endotracheal tube after lateral rotation of head during middle ear surgery. Medicine (Baltimore). 2017;96(10):e6257. doi: 10.1097/MD.0000000000006257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alzahrani AR, Al Abbasi S, Abahoussin OK, et al. Prevalence and predictors of out-of-range cuff pressure of endotracheal and tracheostomy tubes: a prospective cohort study in mechanically ventilated patients. BMC Anesthesiol. 2015;15(1):147. doi: 10.1186/s12871-015-0132-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okgun Alcan A, Yavuz van Giersbergen M, Dincarslan G, Hepcivici Z, Kaya E, Uyar M. Effect of patient position on endotracheal cuff pressure in mechanically ventilated critically ill patients. Aust Crit Care off J Confed Aust Crit Care Nurses. 2017;30(5):267–272. [DOI] [PubMed] [Google Scholar]
- 23.Nseir S, Brisson H, Marquette CH, et al. Variations in endotracheal cuff pressure in intubated critically ill patients: prevalence and risk factors. Eur J Anaesthesiol. 2009;26(3):229–234. doi: 10.1097/EJA.0b013e3283222b6e [DOI] [PubMed] [Google Scholar]
- 24.Department of Aerospace Medicine, Faculty of Medicine, Health Science University Turkey, Eskisehir, Turkey Ercan E, Demir E Department of Aerospace Medicine, Faculty of Medicine, Health Science University Turkey, Eskisehir, Turkey, Sabaner E, Department of Aerospace Medicine, Faculty of Medicine, Health Science University Turkey, Eskisehir, Turkey, et al. Incidence of decompression sickness in hypobaric hypoxia training.Undersea Hyperb Med.2020;Vol. 471:203–210 [PubMed] [Google Scholar]
- 25.Mann C, Parkinson N, Bleetman A. Endotracheal tube and laryngeal mask airway cuff volume changes with altitude: a rule of thumb for aeromedical transport. Emerg Med J. 2007;24(3):165–167. doi: 10.1136/emj.2006.039933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Intensive Care Unit and Hyperbaric Center, Lille University Hospital, Lille, France Benzidi Y, Duburcq T Intensive Care Unit and Hyperbaric Center, Lille University Hospital, Lille, France, Mathieu D, Intensive Care Unit and Hyperbaric Center, Lille University Hospital, Lille, France, et al. Evaluation of pressure in water-filled endotracheal tube cuffs in intubated patients undergoing hyperbaric oxygen treatment.Diving Hyperb Med J.2020;Vol. 503:230–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bishop MJ. Mechanisms of laryngotracheal injury following prolonged tracheal intubation. Chest. 1989;96(1):185–186. doi: 10.1378/chest.96.1.185 [DOI] [PubMed] [Google Scholar]
- 28.Steen JA, Lindholm CE, Brdlik GC, Foster CA. Tracheal tube forces on the posterior larynx: index of laryngeal loading. Crit Care Med. 1982;10(3):186–189. doi: 10.1097/00003246-198203000-00009 [DOI] [PubMed] [Google Scholar]
- 29.Weymuller EA, Bishop MJ, Fink BR, Hibbard AW, Spelman FA. Quantification of intralaryngeal pressure exerted by endotracheal tubes. Ann Otol Rhinol Laryngol. 1983;92(5 Pt 1):444–447. doi: 10.1177/000348948309200506 [DOI] [PubMed] [Google Scholar]
- 30.Li Bassi G, Ranzani OT, Marti JD, et al. An in vitro study to assess determinant features associated with fluid sealing in the design of endotracheal tube cuffs and exerted tracheal pressures*. Crit Care Med. 2013;41(2):518–526. doi: 10.1097/CCM.0b013e31826a4804 [DOI] [PubMed] [Google Scholar]
- 31.Stout DM, Bishop MJ, Dwersteg JF, Cullen BF. Correlation of endotracheal tube size with sore throat and hoarseness following general anesthesia. Anesthesiology. 1987;67(3):419–421. doi: 10.1097/00000542-198709000-00025 [DOI] [PubMed] [Google Scholar]
- 32.Jaensson M, Olowsson LL, Nilsson U. Endotracheal tube size and sore throat following surgery: a randomized-controlled study. Acta Anaesthesiol Scand. 2010;54(2):147–153. doi: 10.1111/j.1399-6576.2009.02166.x [DOI] [PubMed] [Google Scholar]
- 33.Santos PM, Afrassiabi A, Weymuller EA Jr. Risk factors associated with prolonged intubation and laryngeal injury. Otolaryngol Neck Surg. 1994;111(4):453–459. doi: 10.1177/019459989411100411 [DOI] [PubMed] [Google Scholar]
- 34.Campbell BR, Shinn JR, Kimura KS, et al. Unilateral vocal fold immobility after prolonged endotracheal intubation. JAMA Otolaryngol-- Head Neck Surg. 2020;146(2):160–167. doi: 10.1001/jamaoto.2019.3969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lorente L, Lecuona M, Jiménez A, Mora ML, Sierra A. Influence of an endotracheal tube with polyurethane cuff and subglottic secretion drainage on pneumonia. Am J Respir Crit Care Med. 2007;176(11):1079–1083. doi: 10.1164/rccm.200705-761OC [DOI] [PubMed] [Google Scholar]
- 36.Jensen PJ, Hommelgaard P, Søndergaard P, Eriksen S. Sore throat after operation: influence of tracheal intubation, intracuff pressure and type of cuff. Br J Anaesth. 1982;54(4):453–457. doi: 10.1093/bja/54.4.453 [DOI] [PubMed] [Google Scholar]
- 37.Wettstein RW, Gardner DD, Wiatrek S, Ramirez KE, Restrepo RD. Endotracheal cuff pressures in the PICU: incidence of underinflation and overinflation. Can J Respir Ther CJRT Rev Can Ther Respir RCTR. 2019;56:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nseir S, Zerimech F, De Jonckheere J, Alves I, Balduyck M, Durocher A. Impact of polyurethane on variations in tracheal cuff pressure in critically ill patients: a prospective observational study. Intensive Care Med. 2010;36(7):1156–1163. doi: 10.1007/s00134-010-1892-7 [DOI] [PubMed] [Google Scholar]
- 39.Valencia M, Ferrer M, Farre R, et al. Automatic control of tracheal tube cuff pressure in ventilated patients in semirecumbent position: a randomized trial. Crit Care Med. 2007;35(6):1543–1549. doi: 10.1097/01.CCM.0000266686.95843.7D [DOI] [PubMed] [Google Scholar]
- 40.McCarty EB, Chao TN. Dysphagia and swallowing disorders. Med Clin North Am. 2021;105(5):939–954. doi: 10.1016/j.mcna.2021.05.013 [DOI] [PubMed] [Google Scholar]
- 41.Zuercher P, Moret CS, Dziewas R, Schefold JC. Dysphagia in the intensive care unit: epidemiology, mechanisms, and clinical management. Crit Care. 2019;23(1):103. doi: 10.1186/s13054-019-2400-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Macht M, Wimbish T, Clark BJ, et al. Postextubation dysphagia is persistent and associated with poor outcomes in survivors of critical illness. Crit Care Lond Engl. 2011;15(5):R231. doi: 10.1186/cc10472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ajemian MS, Nirmul GB, Anderson MT, Zirlen DM, Kwasnik EM. Routine fiberoptic endoscopic evaluation of swallowing following prolonged intubation: implications for management. Arch Surg Chic Ill 1960. 2001;136(4):434–437. [DOI] [PubMed] [Google Scholar]
- 44.Skoretz SA, Flowers HL, Martino R. The incidence of dysphagia following endotracheal intubation. Chest. 2010;137(3):665–673. doi: 10.1378/chest.09-1823 [DOI] [PubMed] [Google Scholar]
- 45.Macht M, Wimbish T, Bodine C, Moss M. ICU-acquired swallowing disorders. Crit Care Med. 2013;41(10):2396–2405. doi: 10.1097/CCM.0b013e31829caf33 [DOI] [PubMed] [Google Scholar]
- 46.Christensen M, Trapl M. Development of a modified swallowing screening tool to manage post-extubation dysphagia. Nurs Crit Care. 2018;23(2):102–107. doi: 10.1111/nicc.12333 [DOI] [PubMed] [Google Scholar]
- 47.Cavo JW. True vocal cord paralysis following intubation. Laryngoscope. 1985;95(11):1352–1359. doi: 10.1288/00005537-198511000-00012 [DOI] [PubMed] [Google Scholar]
- 48.Shin YH, An DA, Choi WJ, Kim YH. Unilateral vocal cord paralysis following a short period of endotracheal intubation anesthesia. Korean J Anesthesiol. 2013;65(4):357–358. doi: 10.4097/kjae.2013.65.4.357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Higgins PP, Chung F, Mezei G. Postoperative sore throat after ambulatory surgery. Br J Anaesth. 2002;88(4):582–584. doi: 10.1093/bja/88.4.582 [DOI] [PubMed] [Google Scholar]
- 50.Lim S, Kim DC, Cho K, et al. Vocal cord paralysis following general anesthesia with endotracheal intubation: a clinical review on 43 cases. Anesth Pain Med. 2020;15(2):226–232. doi: 10.17085/apm.2020.15.2.226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sariego J. Vocal fold hypomobility secondary to elective endotracheal intubation: a general surgeon’s perspective. J Voice off J Voice Found. 2010;24(1):110–112. doi: 10.1016/j.jvoice.2008.05.001 [DOI] [PubMed] [Google Scholar]
- 52.Kikura M, Suzuki K, Itagaki T, Takada T, Sato S. Age and comorbidity as risk factors for vocal cord paralysis associated with tracheal intubation. Br J Anaesth. 2007;98(4):524–530. doi: 10.1093/bja/aem005 [DOI] [PubMed] [Google Scholar]
- 53.Lombardi RA, Arthur ME. Arytenoid subluxation. En: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023. Available from: http://www.ncbi.nlm.nih.gov/books/NBK544264/. Accessed 29, May, 2023. [Google Scholar]
- 54.Quick CA, Merwin GE. Arytenoid Dislocation. Arch Otolaryngol. 1978;104(5):267–270. doi: 10.1001/archotol.1978.00790050033007 [DOI] [PubMed] [Google Scholar]
- 55.Rudert H. Uncommon injuries of the larynx following intubation. Recurrent paralysis, torsion and luxation of the cricoarytenoid joints. HNO. 1984;32(9):393–398. [PubMed] [Google Scholar]
- 56.Dutoit-Marco ML, Schwander D. Las complicaciones laríngeas de la intubación endotraqueal y su tratamiento foniátrico [Laryngeal complications of endotracheal intubation and their phoniatric management]. Rev Logop Foniatría Audiol. 1987;7(3):144–152. doi: 10.1016/S0214-4603(87)75407-2 [DOI] [Google Scholar]
- 57.Heffner JE. Medical Indications for Tracheotomy. Chest. 1989;96(1):186–190. doi: 10.1378/chest.96.1.186 [DOI] [PubMed] [Google Scholar]
- 58.Ahrens T, Kollef MH. Early tracheostomy--has its time arrived? Crit Care Med. 2004;32(8):1796–1797. doi: 10.1097/01.CCM.0000133329.71596.9A [DOI] [PubMed] [Google Scholar]
- 59.Meng L, Wang C, Li J, Zhang J. Early vs late tracheostomy in critically ill patients: a systematic review and meta-analysis. Clin Respir J. 2016;10(6):684–692. doi: 10.1111/crj.12286 [DOI] [PubMed] [Google Scholar]
- 60.Rumbak MJ, Newton M, Truncale T, Schwartz SW, Adams JW, Hazard PB. A prospective, randomized, study comparing early percutaneous dilational tracheotomy to prolonged translaryngeal intubation (delayed tracheotomy) in critically ill medical patients. Crit Care Med. 2004;32(8):1689–1694. doi: 10.1097/01.CCM.0000134835.05161.B6 [DOI] [PubMed] [Google Scholar]
- 61.Blot SI, Rello J, Koulenti D. The value of polyurethane-cuffed endotracheal tubes to reduce microaspiration and intubation-related pneumonia: a systematic review of laboratory and clinical studies. Crit Care. 2016;20(1):203. doi: 10.1186/s13054-016-1380-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zanella A, Scaravilli V, Isgrò S, et al. Fluid leakage across tracheal tube cuff, effect of different cuff material, shape, and positive expiratory pressure: a bench-top study. Intensive Care Med. 2011;37(2):343–347. doi: 10.1007/s00134-010-2106-z [DOI] [PubMed] [Google Scholar]
- 63.Ouanes I, Lyazidi A, Danin PE, et al. Mechanical influences on fluid leakage past the tracheal tube cuff in a benchtop model. Intensive Care Med. 2011;37(4):695–700. doi: 10.1007/s00134-011-2145-0 [DOI] [PubMed] [Google Scholar]
- 64.Rello J, Ollendorf DA, Oster G, et al. Epidemiology and outcomes of ventilator-associated pneumonia in a large US database. Chest. 2002;122(6):2115–2121. doi: 10.1378/chest.122.6.2115 [DOI] [PubMed] [Google Scholar]
- 65.Baker AM, Meredith JW, Haponik EF. Pneumonia in intubated trauma patients. Microbiology and outcomes. Am J Respir Crit Care Med. 1996;153(1):343–349. doi: 10.1164/ajrccm.153.1.8542141 [DOI] [PubMed] [Google Scholar]
- 66.Lacherade JC, De Jonghe B, Guezennec P, et al. Intermittent subglottic secretion drainage and ventilator-associated pneumonia: a multicenter trial. Am J Respir Crit Care Med. 2010;182(7):910–917. doi: 10.1164/rccm.200906-0838OC [DOI] [PubMed] [Google Scholar]
- 67.Sheikh MdZ A, Qumrul Huda AK, Mondal MK, et al. Ventilator associated pneumonia in patients using endotracheal tube with intermittent subglottic secretion drainage and using endotracheal tube without drainage. Sch J Appl Med Sci. 2021;9(4):506–511. doi: 10.36347/sjams.2021.v09i04.002 [DOI] [Google Scholar]
- 68.Li Y, Yuan X, Sun B, et al. Rapid-flow expulsion maneuver in subglottic secretion clearance to prevent ventilator-associated pneumonia: a randomized controlled study. Ann Intensive Care. 2021;11(1):98. doi: 10.1186/s13613-021-00887-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tomaszek L, Pawlik J, Mazurek H, Mędrzycka-Dąbrowska W. Automatic continuous control of cuff pressure and subglottic secretion suction used together to prevent pneumonia in ventilated patients-a retrospective and prospective cohort study. J Clin Med. 2021;10(21):4952. doi: 10.3390/jcm10214952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dewi YS, Arifin H, Pradipta RO, et al. Efficacy of intermittent and continuous subglottic secretion drainage in preventing the risk of ventilator-associated pneumonia: a meta-analysis of randomized control trials. Medicina. 2023;59(2):283. doi: 10.3390/medicina59020283 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 71.Pozuelo-Carrascosa DP, Herráiz-Adillo Á, Alvarez-Bueno C, Añón JM, Martínez-Vizcaíno V, Cavero-Redondo I. Subglottic secretion drainage for preventing ventilator-associated pneumonia: an overview of systematic reviews and an updated meta-analysis. Eur Respir Rev. 2020;29(155):190107. doi: 10.1183/16000617.0107-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Berra L, Sampson J, Fumagalli J, Panigada M, Kolobow T. Alternative approaches to ventilator-associated pneumonia prevention. Minerva Anestesiol. 2011;77(3):323–333. [PubMed] [Google Scholar]
- 73.Spapen H, Suys E, Nieboer K, Stiers W, De Regt J. Automated intermittent aspiration of subglottic secretions and tracheal mucosa damage. Minerva Anestesiol. 2013;79(3):316–317. [PubMed] [Google Scholar]
- 74.Seguin P, Perrichet H, Pabic EL, et al. Effect of continuous versus intermittent subglottic suctioning on tracheal mucosa by the Mallinckrodt taperguard evac oral tracheal tube in intensive care unit ventilated patients: a prospective randomized study. Indian J Crit Care Med Peer-Rev off Publ Indian Soc Crit Care Med. 2018;22(1):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Malhotra D, Rafiq M, Qazi S, Gupta SD. Ventilatory Obstruction with spiral embedded tube – are they as safe? Indian J Anaesth. 2007;51(5):432–433. [Google Scholar]
- 76.Nikandish R, Farbood A, Amini A, Tarkesh F, Gharache S. A rare case of kinked reinforced endotracheal tube in an intensive care unit: a case report. Colomb J Anesthesiology. 2020;48(1):50–52. doi: 10.1097/CJ9.0000000000000127 [DOI] [Google Scholar]
- 77.Martens P. Persistent narrowing of an armoured tube. Anaesthesia. 1992;47(8):716–717. doi: 10.1111/j.1365-2044.1992.tb02411.x [DOI] [PubMed] [Google Scholar]
- 78.Spiess BD, Rothenberg DM, Buckley S. Complete airway obstruction of armored endotracheal tubes. Anesth Analg. 1991;73(1):95–96. doi: 10.1213/00000539-199107000-00020 [DOI] [PubMed] [Google Scholar]
- 79.Eipe N, Choudhrie A, Pillai AD, Choudhrie R. Neck contracture release and reinforced tracheal tube obstruction. Anesth Analg. 2006;102(6):1911–1912. doi: 10.1213/01.ANE.0000215149.69993.1B [DOI] [PubMed] [Google Scholar]
- 80.Wadhwa R, Dhakate G, Chilkoti G. Reinforced endotracheal tube: a life threatening experience in intensive care unit. Saudi J Anaesth. 2013;7(3):358. doi: 10.4103/1658-354X.115348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vijayakumar V, Ganesamoorthi A. Armored endotracheal tube: concerns in intensive care unit. Indian J Crit Care Med. 2017;21(1):60–61. doi: 10.4103/0972-5229.198331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Szekely SM, Webb RK, Williamson JA, Russell WJ. Problems related to the endotracheal tube: an analysis of 2000 incident reports. Anaesth Intensive Care. 1993;21(5):611–616. doi: 10.1177/0310057X9302100520 [DOI] [PubMed] [Google Scholar]