Abstract
In a previous study, we found avian sarcoma and leukosis virus (ASLV) gag genes in 19 species of birds in the order Galliformes including all grouse and ptarmigan (Tetraoninae) surveyed. Our data suggested that retroviruses had been transmitted horizontally among some host species. To further investigate these elements, we sequenced a replication-defective retrovirus, here named tetraonine endogenous retrovirus (TERV), from Bonasa umbellus (ruffed grouse). This is the first report of a complete, replication-defective ASLV provirus sequence from any bird other than the domestic chicken. We found a replication-defective proviral sequence consisting of putative Gag and Env proteins flanked by long terminal repeats. Reverse transcription-PCR analysis showed that retroviral gag sequences closely related to TERV are transcribed, supporting the hypothesis that TERV is an active endogenous retrovirus. Phylogenetic analyses suggest that TERV may have arisen via recombination between different retroviral lineages infecting birds. Southern blotting using gag probes showed that TERV occurs in tetraonines but not in chickens or ducks, suggesting that integration occurred after the earliest phasianid divergences but prior to the radiation of tetraonine birds.
Endogenous retroviruses have been found in all vertebrate hosts examined. These viruses are integrated into host genomes at multiple locations and are usually transmitted vertically via the germline. The most extensively studied endogenous avian retroviruses are found in the genome of the domestic chicken and belong to the avian sarcoma and leukosis virus (ASLV), or alpharetrovirus, genus. Endogenous ASLVs include Rous associated virus-0 (RAV-0), endogenous avian retroviruses (EAVs), and avian retrotransposons from chickens (ART-CH). RAV-0 is thought to represent a recently integrated endogenous virus, because its provirus DNA sequence is highly conserved relative to those of exogenous ASLVs such as Rous sarcoma virus (RSV). EAV-0 elements are thought to be older integrations of avian retroviruses, because they are less similar to exogenous viruses and their phylogeny closely reflects host phylogeny (5, 19). The number of known EAVs is increasing and includes recently identified EAV-HP and ev/J, which are apparently the same endogenous virus described independently (20, 21). ART-CH elements have deletions in all retroviral genes, but they retain cis-acting sequences necessary for retrotransposition (11, 17).
Recently, we showed that endogenous ASLVs are found in three families of galliform (fowl-like) birds, and, in some cases, the phylogenetic patterns observed for virus genes were incongruent with host phylogeny (8). Our findings are beginning to elucidate the ancient evolutionary association between retroviruses and birds, and they suggest the possibility of more-recent horizontal transmission of endogenous viruses between avian hosts as well.
In this report we describe a new avian proviral genome, obtained from a genomic library of Bonasa umbellus (ruffed grouse), called tetraonine endogenous retrovirus (TERV). Tetraonines are a subfamily of galliform birds consisting of grouse and ptarmigan. This is the first report of a complete, replication-defective ASLV provirus sequence from a bird other than the domestic chicken. We compare the structure of TERV to those of published avian retroviruses in order to investigate its function and evolution. Southern blot and reverse transcription-PCR (RT-PCR) analyses are used to document the distribution and expression of TERV-related viruses in galliform birds. We hypothesize that TERV is an active, endogenous retrovirus formed through recombination between endogenous retroviral lineages.
Generation of grouse λ bacteriophage genomic library and characterization of provirus structure.
A B. umbellus lambda genomic library was constructed using a Lambda FIX II/XhoI partial-fill-in vector kit (Stratagene, La Jolla, Calif.). This library was screened by lifting plaques onto nylon membranes and probing with a 32P-labeled gag probe. This probe was amplified by PCR using GAG.F1 and GAG.R1 primers (Fig. 1) and standard PCR conditions as previously described (8). After gel purification of PCR products, ≈25 ng of probe DNA was radiolabeled using [α-32P]dATP (3,000 Ci/mmol; Amersham Pharmacia Biotech), 6 U of Klenow fragments, and random primers. Positive plaques were grown in liquid culture, and recombinant phage DNA was isolated using standard protocols (22). Provirus-positive phage DNA was randomly fragmented and subcloned into pZero vector (Stratagene). Plasmid DNA was isolated from positive colonies using a QIAprep spin miniprep kit (Qiagen) and sequenced using universal primers as previously described (8). The sequence was edited, and contigs were assembled, using Sequencher (Gene Codes Corp.). Published ASLV Sequences are listed with GenBank accession numbers in Table 1.
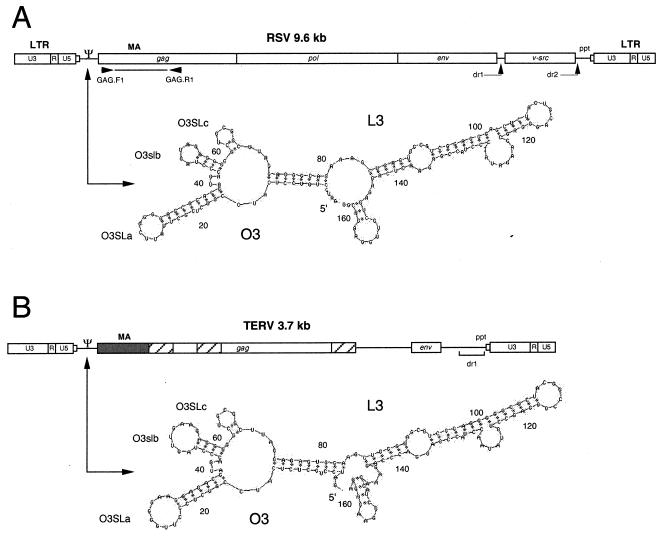
FIG. 1.
Comparison of RSV (A) and TERV (B) genome and packaging signal (Ψ) secondary structures (3, 23). The RSV genome is not drawn to scale. The first 342 nucleotides (shaded region) of TERV gag are highly conserved relative to published ASLVs. The nucleotide similarity ranges from 80 (EAV-HP) to 97% (B. umbellus ASLV). The matrix (MA) region of Gag is indicated above coding regions. Hatched boxes, three regions in TERV with the greatest similarity (29 to 59%) to those of EAV-HP (20, 21). The single line between TERV gag and env denotes an apparent noncoding sequence. Primers GAG.F1 and GAG.R1 (8), used to amplify the probe for genomic library screens, are shown below RSV. Predicted secondary structures of the retroviral packaging sequence were modeled using Mfold (4, 32). Nucleotide positions listed are relative to the 5′ end of the packaging sequence (Fig. 2, MΨ). Major stem-loop structures are identified using the notation of Banks et al. (3). The RSV secondary structure is based on a consensus of 20 previously published ASLV packaging sequences. This structure is identical to the structure published by Banks and Linial (2).
TABLE 1.
Virus names and GenBank accession numbers
| Virus | Accession no. |
|---|---|
| Gallus ASLVs | |
| RSV | D10652 |
| RAV-0 | M73497, J02295, and J02015 |
| Avian sarcoma virus Y73 | J02027 |
| Avian myeloblastosis virus | L10922 |
| Fujinami sarcoma virus | AF033810 |
| EAV-HP | J238125 and AJ238124 |
| EAV-0 | M31065 |
| ART-CH | L25262 |
| Gallus varius ASLV | AF225361 |
| Tetraonine/odontophorid ASLVs | |
| TERV | AF289082 |
| B. umbellus ASLV | AF225316 |
| Colinus virginianus ASLV | AF225336 |
| Lagopus lagopus ASLV | AF225366 |
| Phasianus colchicus ASLV | AF225384 |
| Mammalian retroviruses | |
| Porcine endogenous virus | Y12239 |
| Murine leukemia virus | AF064089 |
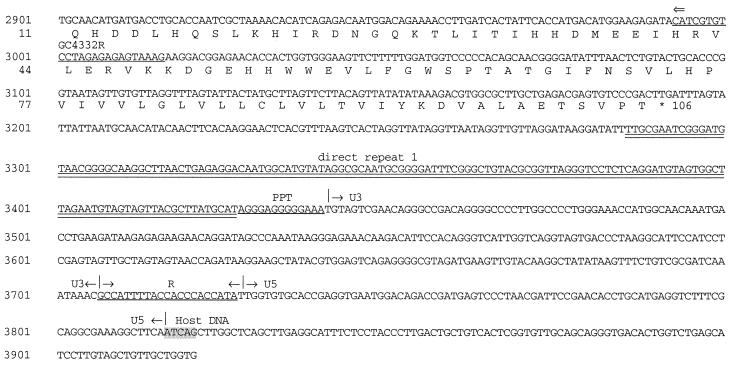
The DNA insert of one recombinant lambda was sequenced to reveal a replication-defective retroviral sequence of 3,711 bp, which we call TERV (Fig. 2). Host flanking sequences at the 5′ and 3′ ends of TERV (1,439 and 733 kb, respectively) were found to be not similar to any sequences in GenBank using BLAST (1). The TERV provirus genome structure was similar to those of other defective avian retroviruses such as Fujinami sarcoma virus, EAV-HP, and ART-CH (11, 20, 21, 24). The TERV protein coding sequence was flanked by long terminal repeats (LTRs), and these in turn were flanked by 5-bp direct repeats (5′-ATCAG-3′). The terminal nucleotides of the provirus were 5′-TG…CA-3′ and were part of imperfect indirect repeats (Fig. 2).
FIG. 2.
Complete nucleotide sequence of a TERV provirus including the host flanking sequence. The complete provirus is 3,711 bp in length. Direct repeats (5 bp) flanking the provirus are shaded, and major LTR regions are indicated. The repeat region (R), tRNATrp PBS, and PPT are underlined. The TATA box and polyadenylation signal are boxed. Translations of Gag and Env proteins are below the nucleotide sequence. Downward arrow, junction between the highly conserved gag sequence (5′) and regions of low conservation (3′). The shaded region in Gag corresponds to a region possibly homologous to the L domain (PPPPY) in ASLVs. Double underlining, putative packaging sequences (MΨ and direct repeat 1). Only one copy of RSV-related direct repeat 1 was found in TERV. Primer sequences described in the text are underlined, and the primer names are indicated. ProbeMatrix and Probeenv, fragments representing probes used for Southern hybridization.
We compared TERV untranslated region (UTR) sequences to retrovirus UTR sequences of known function to identify potentially functionally homologous regions. The 3′ boundary of the 5′ LTR was distinguished by a sequence highly conserved relative to those of other avian retrovirus tRNATrp primer binding sites (PBS) (initiation of minus-strand synthesis). The 5′ boundary of the 3′ LTR was distinguished by the polypurine tract sequence (PPT) (initiation of plus-strand synthesis). Based on these boundaries, host flanking direct repeat sequences, and sequence conservation with other ASLV R regions, identical LTRs of 376 bp were defined. The typical proviral LTR structure (5′-U3-R-U5-3′) was found, and lengths for the U3, R, and U5 regions were 266, 21, and 89 bp, respectively. These LTRs were longer than LTRs from RSV (327 bp) (23), EAV-HP (315 bp) (20, 21), EAV-0 (243 bp) (5, 6), and RAV-0 (277) (13) but slightly shorter than ART-CH LTRs (388 bp) (17).
The TERV U3 sequence was most similar to that of RAV-0, particularly at conserved motifs that function in retrovirus transcription. Putative transcription factors were identified by searching on-line database TRANSFAC, specifying a threshold score of 85 (12), and by comparative sequence analysis with known transcriptional regulatory sequences from ASLVs. The 5′-most enhancer binding element was similar to an IK-2 element (position −221), which is bound by a transcription factor isolated from mice (10). The next sequence, a δEFI binding element, was located at −194 in relation to the transcription start site. It is thought that protein δEFI is an embryonic gene regulator in chickens (9). These two elements were not known from other avian retrovirus LTRs; thus further characterization is necessary as these elements show limited similarity to the consensus sequences. At position −165 there was a putative serum response element (SRE) (27, 28) that was highly conserved relative to the SRE found in RAV-0 (31). Within this element there was a CArG box, defined as 5′-CC(A/T)6GG-3′. This sequence was followed by an inverted CCAAT box at −132 also found in RAV-0 and RSV. The CCAAT box was in the middle of putative NF-Y and C/EBP binding sites. Finally, another SRE was located at −75.
The U3 region of TERV was highly conserved relative to those of other ASLVs, starting with the TATA box located at position −24, followed by a polyadenylation signal just upstream of the repeat region (R). The R and U5 regions of TERV had the greatest sequence similarity to those of RAV-0 at 91 and 89%, respectively, and had 82 and 86% similarity, respectively, to those of RSV (Table 2). The tRNATrp PBS were identical across all avian retroviruses mentioned in this report with the exception of that of EAV-0, which had an additional cytosine residue (Table 2).
TABLE 2.
Percentages of DNA sequence similarity among UTRs of TERV and other avian retroviruses
| Virusb | % Similaritya to TERV region:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| U3 | TATA box | TATA to R | R | U5 | PBS | MΨc | dr1d | |
| RSV | 37.42 | 85.71 | 67.74 | 81.82 | 85.57 | 100.00 | 80.40 | 88.65 |
| FuSV | 42.11 | 100.0 | 67.74 | 82.07 | 87.88 | 100.00 | 80.27 | 57.85 |
| AMV | 35.18 | 71.42 | 48.39 | 78.26 | 80.43 | 100.00 | 73.78 | 86.58 |
| Y73 | 42.12 | 85.71 | 64.52 | 86.36 | 81.59 | 100.00 | 79.59 | 88.49 |
| RAV-0 | 67.86b | 100.0 | 77.42 | 90.91 | 89.03 | 100.00 | NDe | 88.62 |
| ART-CH | 32.44 | 100.0 | 65.71 | 62.73 | 44.07 | 100.00 | 31.35 | 44.24 |
| EAV-HP | 35.22 | 85.71 | 45.16 | 62.73 | 44.06 | 100.00 | 33.68 | 47.78 |
| EAV-0 | 32.59 | 100.0 | 69.58 | 90.91 | 66.30 | 100.00 | ND | ND |
Percent similarities were calculated using uncorrected P distances ([1 − P]100). Boldface numbers indicate the greatest similarity to TERV.
FuSV, Fujinami sarcoma virus; AMV, avian myeloblastosis virus; Y73, avian sarcoma virus Y73.
MΨ comparisons for RSV, FuSV, AMV, and Y73 are based on the alignment presented in Banks and Linial (2).
The dr1 sequence (117 bp) is located adjacent to the 5′ end of the PPT.
ND, no data were available for comparison.
Analysis of putative Gag and Env proteins.
Two open reading frames (ORFs) that had sequence similarity to those of published ASLVs were found. The N-terminal 123 amino acids encoded by the first ORF had 88% identity to RSV and 95 to 97% identity to previously published B. umbellus Gag sequences corresponding to the M domain of the matrix region (Fig. 1). The remaining 460 amino acids had 29 to 59% identity with those of ASLV Gag proteins in three regions, each separated by sequences that showed no significant sequence similarity to published ASLV amino acid sequences in GenBank (Fig. 1). The regions of EAV-HP had the highest similarity to these regions, slightly higher than published regions of B. umbellus ASLV. One other region of amino acid similarity to Gag begins at P240 and is a proline-rich region (PSAPSAPPPAP) possibly homologous to the L domain (PPPPY) found in all ASLVs, which functions in virus assembly (30).
A second ORF, located 360 bp downstream of the gag gene, encoded 106 amino acids that had 29 to 40% identity to Gag of ASLV and murine leukemia virus and porcine endogenous retrovirus Env based on BLAST search results (Fig. 1). The putative ORF corresponded to the carboxyl terminus of the transmembrane region of Env. This Env-related ORF was just upstream of a 110-bp sequence with 89% identity to those of exogenous ASLVs, which corresponds to the direct repeat 1 sequence found in all ASLV genomes studied, including replication-defective transforming avian retroviruses.
Analysis of gag gene transcription.
Sequence analysis suggests that TERV is capable of transcription. To determine if gag sequences were transcribed, total RNA was extracted from heart muscle of one adult B. umbellus animal and whole 8-day-old Phasianus colchicus and Colinus virginianus embryos using Trizol reagent (Gibco Life Technologies) according to the manufacturer's protocol. RNA extract was treated with 5 U of DNase I, amplification grade (Gibco Life Technologies), to eliminate DNA contamination according to the manufacturer's protocol. RT-PCR was performed on DNase-treated total RNA using a Titan one-tube RT-PCR kit (Roche) and two sets of primers at an annealing temperature of 55°C. Primers GAG.F1 and GAG.R1 (8) are general gag primers, while GC2128F and GC2604R (Fig. 2) are specific to TERV gag. Chicken β-actin primers were used as a positive control for RNA. These primers (β-actinF [5′-AATGAGAGGTTCAGGTGCCC-3′] and β-actinR [5′-ATCACAGGGGTGTGGGTGTT-3′]) amplify a 410-bp fragment. RT-PCR products were verified by DNA sequence analysis as described previously (8). PCR using GAG.F1 and GAG.R1 primers was performed on DNase-treated and untreated samples to verify that DNA contamination was eliminated.
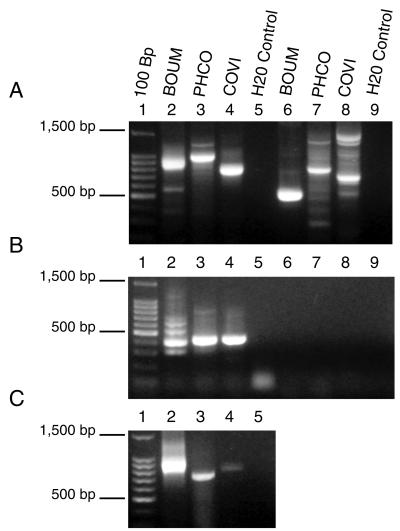
RT-PCR using GAG.F1 and GAG.R1 primers on DNase-treated samples resulted in products from all three birds as did PCR analysis of non-DNase-treated samples (Fig. 3). Size variation in amplicons is consistent with results obtained in our previous study of avian retroviral gag sequences (8). Sequence analysis of the RT-PCR product from Phasianus suggested that these primers amplified nontarget transcripts, whereas sequence analysis confirmed that gag transcripts were amplified from Bonasa and Colinus. RT-PCR using TERV-specific primers (GC2128F and GC2604R) resulted in amplification in all three birds. The product from B. umbellus was 475 bp, the size predicted from TERV, while the predominant products from Phasianus and Colinus were 200 to 300 bp larger (Fig. 3). These RT-PCR products were sequenced, and all were verified as gag sequences.
FIG. 3.
RT-PCR analysis of total RNA isolated from heart muscle for an adult B. umbellus animal (BOUM) and 8-day-old embryos of P. colchicus (PHCO) and C. virginianus (COVI). (A) RT-PCR on total RNA. Lane 1, 100-bp ladder (Promega); lanes 2 to 5, primers GAG.F1 and GAG.R1; lanes 6 to 9, primers GC2128F and GC2604R. (B) RT-PCR using β-actin primers (lanes 2 to 5) and PCR using primers GAG.F1 and GAG.R1 on DNase-treated RNA extracts (lanes 6 to 9). (C) PCR on non-DNase-treated samples using primers GAG.F1 and GAG.R1.
Normally, following transcription, two copies of the retroviral RNA genome are incorporated into viral particles. This packaging process involves the recognition and binding of sequence Ψ on the RNA genome by viral proteins. In ASLVs, a 160-nucleotide sequence has been identified as the minimal packaging signal (MΨ) in the leader region between the PBS and the Gag initiation codon (3). TERV contained a sequence highly conserved relative to avian retrovirus MΨs located between the primer binding site and the gag initiation codon. TERV MΨ had a similarity of 78.2% to a consensus alignment (2) of 20 exogenous and endogenous avian retrovirus packaging sequences. EAV-HP and ART-CH did not have the same level of conservation in the packaging signal. The sequences between the PBS and the gag initiation codon in ART-CH and EAV-HP are about 100 bp shorter than those in TERV and other ASLVs.
It appears that the secondary structure of MΨ plays a significant role in efficient packaging (2). TERV and the ASLV consensus packaging sequence were analyzed using the Mfold program (version 3.0) to model the most-stable secondary structures (4, 16). The lowest free energies were −61.02 kcal/mol for the secondary structure of TERV and −56.82 kcal/mol for the folding of a consensus sequence of 20 ASLV packaging signals (Fig. 1). The two modeled structures were remarkably similar. TERV MΨ had the two major stem-loop regions O3 and L3 and the three minor stem-loops O3SLa, O3SLb, and O3SLc previously identified by Banks and Linial (2). In ART-CH and EAV-HP the most-stable secondary structures for sequences that correspond to MΨ were not similar to those of RSV, the consensus of ASLVs, or TERV when folded with Mfold (not shown).
Our previous work showing that some ASLV phylogenetic relationships reflect overlapping host species ranges rather than host species phylogeny (8) suggests that horizontal transmission of ASLVs has occurred in the past. Here we have demonstrated that TERV is transcribed and contains sequences required for packaging and retrotransposition. We have no direct evidence regarding the means by which TERV or other tetraonine ASLVs move between host species; however, one possible mechanism is hybridization among host species, which is known to occur naturally between some tetraonines (14). Alternatively, TERVs could have been transmitted horizontally if they were transcribed and packaged with replication-competent retroviruses.
Phylogeny and recombination.
To explore the relationship of TERV and other endogenous and exogenous ASLVs, we conducted phylogenetic analyses using three regions of TERV. Sequence alignments corresponding to various regions of TERV and ASLVs were performed using Clustal X (26). Phylogenetic analyses were performed using maximum parsimony (MP) as implemented in PAUP* (25). Branch-and-bound MP analyses were conducted, and bootstrap values were determined using 100 replicate searches.
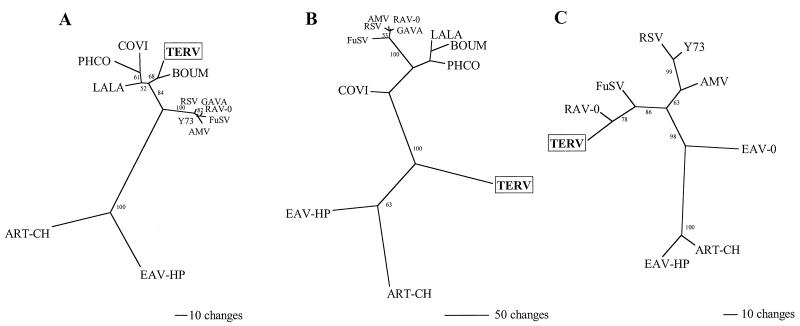
The first region examined corresponds to the matrix gene of gag. MP analysis using this region (342 nucleotides) yielded one tree composed of three major groups (Fig. 4A). The first group consisted of endogenous and exogenous viruses isolated from birds in the genus Gallus, including the domestic chicken (Gallus gallus). The second group consisted of presumably endogenous proviruses from grouse and ptarmigan (Tetraoninae), and the third group consisted of endogenous viruses EAV-HP and ART-CH. TERV was the sister taxon to ASLV, whose gag gene was previously sequenced from B. umbellus.
FIG. 4.
Phylogenetic analyses of gag and UTR sequences. Unrooted MP trees were constructed using the branch-and-bound option in PAUP∗ (25). Bootstrap values along branches were calculated using 100 random-addition replicate searches. COVI, C. virginianus ASLV; PHCO, P. colchicus ASLV; LALA, Lagopus lagopus ASLV; BOUM, B. umbellus ASLV; GAVA, G. varius ASLV; FuSV, Fujinami sarcoma virus; AMV, avian myeloblastosis virus; Y73, avian sarcoma virus Y73. Branch lengths are proportional to inferred amounts of evolutionary change. (A) Relationship of 10 previously published ASLV and TERV sequences based on 342 nucleotide sites from the matrix region of the gag gene. (B) One of two equally parsimonious trees for the relationship of 11 published ASLVs and TERV based on the more-divergent amino acid region of Gag downstream from the region analyzed in panel A. The two equally parsimonious trees differ only in the placement of GAVA. (C) Phylogeny of eight previously published UTR sequences and TERV.
The second region corresponded to the remaining sequence in TERV Gag adjacent to the highly conserved matrix region. Only short stretches of amino acids could be aligned to other ASLV Gag proteins, resulting in a data set with 125 parsimony-informative characters. Two equally parsimonious trees that differed only in the placement of Gallus varius ASLV were found (Fig. 4B). Interestingly, TERV and the other B. umbellus ASLV sequences were not sister taxa based on this portion of the gag gene. Instead, TERV is located between an EAV-HP/ART-CH group and a group containing the remaining ASLVs.
The third region analyzed was aligned with eight published avian retrovirus UTR sequences. This phylogeny (Fig. 4C) showed relationships similar to those from the tree shown in Fig. 4A. We found that EAV-HP and ART-CH formed a group that was sister to EAV-0 (EAV-0 was not included in gag phylogeny). TERV was most closely related to endogenous avian retrovirus RAV-0. No UTR sequences from other tetraonine retroviruses were available for comparison.
Recombination within the gag gene of RSV can occur with a relatively high frequency (15). Incongruent trees from our phylogenetic analyses suggest the possibility that TERV was formed by recombination between retroviruses. One recombination point may occur near amino acid 123, where the similarity of TERV Gag to B. umbellus Gag drops drastically from 97 to around 40%. Phylogenetic analysis illustrates that this downstream region of TERV Gag is not sister to those of other tetraonine ASLVs, RAV-0, or exogenous ASLVs (Fig. 4B). Phylogenetic analysis and amino acid identity of a short region of Env (transmembrane region) suggest a relationship that is also incongruent with UTR and 5′ gag phylogenies (not shown). These findings are compatible with the interpretation that TERV was formed by recombination, although the parental sequences for divergent regions of TERV have yet to be discovered. An alternative explanation to recombination is that highly divergent regions of TERV result from differing selective pressures on the viral genome. We are examining additional complete tetraonine retrovirus genomes to investigate this possibility.
Distribution of TERV-related sequences.
To determine the distribution of TERV-related sequences, genomic DNA was isolated from six galliform species and one anseriform (Aythya americana) using standard protocols. Roughly 3 μg of genomic DNA was digested to completion with HindIII, electrophoresed in a 1.0% agarose gel, and blotted overnight onto a positively charged nylon membrane (Hybond-XL; Amersham Pharmacia Biotech). Two different probes, probematrix and probeenv, were amplified by PCR from TERV-lambda DNA. These probes corresponded to a region of matrix (GC2128F and GC2604R) and a divergent region similar to that in which env is located (GC3877F and GC4332R) (Fig. 2). Hybridization was performed at 65°C in standard buffer overnight with probematrix first, followed by probeenv. The nylon membrane was washed in several steps with decreasing salt and sodium dodecyl sulfate (SDS) concentrations, down to 0.5× SSPE (1× SSPE is 0.18 M NaCl, 10 mM NaH2 PO4, and 1 mM EDTA [pH 7.7]) and 0.1% SDS, and increasing temperature to 65°C. Prior to being probed with probeenv, the membrane was stripped by washing with 0.1% SDS at 100°C for 30 min and exposed to film for 1 week to verify that all of probematrix was removed.
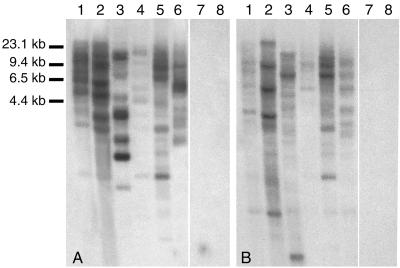
Results of Southern analysis at high stringency using both probes were positive for the five grouse and ptarmigan tested and negative for a domestic chicken and a redhead (Fig. 5). Genomic DNA from two B. umbellus individuals was analyzed and had almost identical banding patterns. Both probes showed complex hybridization patterns consistent with the idea that TERV integrated in multiple locations within the bird genome.
FIG. 5.
Southern blot analysis of HindIII-digested avian genomic DNA using TERV probematrix and probeenv. Hybridization was performed at 65°C, and the filter was washed with salt-SDS solution at concentrations as low as 0.5× SSPE–0.5% SDS at 65°C. (A) Probematrix. (B) Probeenv. Lanes 1 and 2, B. umbellus (ruffed grouse); lanes 3, Bonasa bonasia (hazel grouse); lanes 4, Lagopus lagopus (willow ptarmigan); lanes 5, Lagopus leucurus (white-tailed ptarmigan); lanes 6, Dendragapus falcipennis (Siberian grouse); lanes 7, Anseriformes (Aythya americana [redhead]); lanes 8, G. gallus (domestic chicken). This shows the presence of TERV or closely related elements in all tetraonine birds surveyed.
Divergence date estimates from fossils suggest that tetraonines separated from their putative closest extant relative (Meleagris gallopavo [turkey]) in the mid-Miocene (15 to 20 million years ago), while modern tetraonines seem to have been present for at least 1 million years (7, 29). If our limited sampling of birds is representative, TERV could have integrated into the genome of a tetraonine sometime during the past 15 million years. Future surveys, including more Galliformes as well as birds from additional avian orders, are needed for more-reliable estimates of TERV age and relative timing of integration into host species genomes. The 100% identity between TERV LTRs suggests that TERV was active quite recently, although this activity may have been restricted within the genome.
Nucleotide sequence accession number.
The TERV sequence obtained in this study has been assigned GenBank accession no. AF289082.
Acknowledgments
This work was supported by University of Michigan graduate student block grant funds to D.E.D. and M.K.
We thank Michael Frohlich, David Parker, and Vici Blanc for excellent technical assistance and Sergei Drovetski of the University of Washington Burke Museum for providing some tissue samples used in this study.
REFERENCES
- 1.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banks J D, Linial M L. Secondary structure analysis of a minimal avian leukosis-sarcoma virus packaging signal. J Virol. 2000;74:456–464. doi: 10.1128/jvi.74.1.456-464.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banks J D, Yeo A, Green K, Cepeda F, Linial M L. A minimal avian retroviral packaging sequence has a complex structure. J Virol. 1998;72:6190–6194. doi: 10.1128/jvi.72.7.6190-6194.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barciszewski J, Clark B F C, editors. RNA biochemistry and biotechnology. Dordrecht, The Netherlands: Kluwer; 1999. [Google Scholar]
- 5.Boyce-Jacino M T, O'Donoghue K, Faras A J. Multiple complex families of endogenous retroviruses are highly conserved in the genus Gallus. J Virol. 1992;66:4919–4929. doi: 10.1128/jvi.66.8.4919-4929.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyce-Jacino M T, Resnick R, Faras A J. Structural and functional characterization of the unusually short long terminal repeats and their adjacent regions of a novel endogenous avian retrovirus. Virology. 1989;173:157–166. doi: 10.1016/0042-6822(89)90231-6. [DOI] [PubMed] [Google Scholar]
- 7.del Hoyo J, Elliott A, Sargatal J, Cabot J. Handbook of the birds of the world. New World vultures to guineafowl. Vol. 2. Barcelona, Spain: Lynx Edicions; 1992. [Google Scholar]
- 8.Dimcheff D E, Drovetski S V, Krishnan M, Mindell D P. Cospeciation and horizontal transmission of avian sarcoma and leukosis virus gag genes in galliform birds. J Virol. 2000;74:3984–3995. doi: 10.1128/jvi.74.9.3984-3995.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Funahashi J, Sekido R, Murai K, Kamachi Y, Kondoh H. Delta-crystallin enhancer binding protein delta EF1 is a zinc finger-homeodomain protein implicated in postgastrulation embryogenesis. Development. 1993;119:433–446. doi: 10.1242/dev.119.2.433. [DOI] [PubMed] [Google Scholar]
- 10.Georgopoulos K, Moore D D, Derfler B. Ikaros, an early lymphoid-specific transcription factor and a putative mediator for T cell commitment. Science. 1992;258:808–812. doi: 10.1126/science.1439790. [DOI] [PubMed] [Google Scholar]
- 11.Gudkov A V, Komarova E A, Nikiforov M A, Zaitsevskaya T E. ART-CH, a new chicken retroviruslike element. J Virol. 1992;66:1726–1736. doi: 10.1128/jvi.66.3.1726-1736.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heinemeyer T, Chen X, Karas H, Kel A E, Kel O V, Liebich I, Meinhardt T, Reuter I, Schacherer F, Wingender E. Expanding the TRANSFAC database towards an expert system of regulatory molecular mechanisms. Nucleic Acids Res. 1999;27:318–322. doi: 10.1093/nar/27.1.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hughes S H. Sequence of the long terminal repeat and adjacent segments of the endogenous avian virus Rous-associated virus O. J Virol. 1982;43:191–200. doi: 10.1128/jvi.43.1.191-200.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnsgard P A. The grouse of the world. Lincoln: University of Nebraska Press; 1983. [Google Scholar]
- 15.Linial M, Brown S. High-frequency recombination within the gag gene of Rous sarcoma virus. J Virol. 1979;31:257–260. doi: 10.1128/jvi.31.1.257-260.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mathews D H, Sabina J, Zuker M, Turner D H. Expanded sequence dependence of thermodynamic parameters improves prediction of RNA secondary structure. J Mol Biol. 1999;288:911–940. doi: 10.1006/jmbi.1999.2700. [DOI] [PubMed] [Google Scholar]
- 17.Nikiforov M A, Gudkov A V. ART-CH: a VL30 in chickens? J Virol. 1994;68:846–853. doi: 10.1128/jvi.68.2.846-853.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oh W K, Yoo J C, Jo D, Song Y H, Kim M G, Park D. Cloning of a SH3 domain-containing proline-rich protein, p85SPR, and its localization in focal adhesion. Biochem Biophys Res Commun. 1997;235:794–798. doi: 10.1006/bbrc.1997.6875. [DOI] [PubMed] [Google Scholar]
- 19.Resnick R M, Boyce-Jacino M T, Fu Q, Faras A J. Phylogenetic distribution of the novel avian endogenous provirus family EAV-0. J Virol. 1990;64:4640–4653. doi: 10.1128/jvi.64.10.4640-4653.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruis B L, Benson S J, Conklin K F. Genome structure and expression of the ev/J family of avian endogenous viruses. J Virol. 1999;73:5345–5355. doi: 10.1128/jvi.73.7.5345-5355.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sacco M A, Flannery D M, Howes K, Venugopal K. Avian endogenous retrovirus EAV-HP shares regions of identity with avian leukosis virus subgroup J and the avian retrotransposon ART-CH. J Virol. 2000;74:1296–1306. doi: 10.1128/jvi.74.3.1296-1306.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sambrook J, Maniatis T, Fritsch E F. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 23.Schwartz D E, Tizard R, Gilbert W. Nucleotide sequence of Rous sarcoma virus. Cell. 1983;32:853–869. doi: 10.1016/0092-8674(83)90071-5. [DOI] [PubMed] [Google Scholar]
- 24.Shibuya M, Hanafusa H. Nucleotide sequence of Fujinami sarcoma virus: evolutionary relationship of its transforming gene with transforming genes of other sarcoma viruses. Cell. 1982;30:787–795. doi: 10.1016/0092-8674(82)90283-5. [DOI] [PubMed] [Google Scholar]
- 25.Swofford D L. PAUP∗: phylogenetic analysis using parsimony (∗and other methods), 4.0 ed. Sunderland, Mass: Sinauer; 1999. [Google Scholar]
- 26.Thompson J D, Gibson T J, Plewniak F, Jeanmougin F, Higgins D G. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Treisman R. Identification of a protein-binding site that mediates transcriptional response of the c-fos gene to serum factors. Cell. 1986;46:567–574. doi: 10.1016/0092-8674(86)90882-2. [DOI] [PubMed] [Google Scholar]
- 28.Treisman R. Transient accumulation of c-fos RNA following serum stimulation requires a conserved 5′ element and c-fos 3′ sequences. Cell. 1985;42:889–902. doi: 10.1016/0092-8674(85)90285-5. [DOI] [PubMed] [Google Scholar]
- 29.Unwin D M. The fossil record 2. In: Benton M J, editor. Aves. New York, N.Y: Chapman and Hall; 1993. pp. 717–737. [Google Scholar]
- 30.Wills J W, Cameron C E, Wilson C B, Xiang Y, Bennett R P, Leis J. An assembly domain of the Rous sarcoma virus Gag protein required late in budding. J Virol. 1994;68:6605–6618. doi: 10.1128/jvi.68.10.6605-6618.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zachow K R, Conklin K F. CArG, CCAAT, and CCAAT-like protein binding sites in avian retrovirus long terminal repeat enhancers. J Virol. 1992;66:1959–1970. doi: 10.1128/jvi.66.4.1959-1970.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zuker M, Mathews D H, Turner D H. Algorithms and thermodynamics for RNA secondary structure prediction: a practical guide. In: Barciszewski J, Clark B F C, editors. RNA biochemistry and biotechnology. Dordrecht, The Netherlands: Kluwer; 1999. pp. 11–43. [Google Scholar]