Abstract
Background
Sudanese children with End-Stage Kidney Disease (ESKD) often show limited improvement in hemoglobin levels despite treatment with recombinant human erythropoietin (rHuEPO). This study aims to assess the response to rHuEPO therapy by analyzing β-globin mRNA expression and reticulocyte parameters. Additionally, it classifies anemia among Sudanese pediatric patients based on iron status, considering age and gender as biological markers for evaluating treatment response.
Methods
A prospective observational cohort study was conducted from January 2019 to February 2020 in Khartoum, Sudan, involving 45 anemic children aged 2 to 15 years diagnosed with ESKD. The treatment protocol included rHuEPO injections and maintenance hemodialysis. Laboratory assessments consisted of complete blood count (CBC), absolute reticulocyte count, ferritin, and transferrin measurements. β-globin mRNA expression was quantified using reverse transcription polymerase chain reaction (RT-PCR), and reticulocyte parameters, including Reticulocyte Hemoglobin Content (CHr), percentage of hypochromic reticulocytes (HYPO%), and Immature Reticulocyte Fraction (IRF), were measured via flow cytometry.
Results
Significant variations in hemoglobin levels were observed across different age groups (p = 0.011). Gender analysis revealed a significant association with IRF, showing a lower IRF in male patients (p = 0.017). However, there were no significant differences in hemoglobin levels between genders (p = 0.999). β-globin mRNA expression showed considerable variability, with a strong positive correlation with hemoglobin levels (r = 0.875, p < 0.0001).
Conclusion
Age and gender significantly influence treatment responses in children with ESKD, highlighting the need to consider growth physiology in anemia management. This study underscores the variability in β-globin mRNA expression and its association with Flow Cytometry parameters, demonstrating their effectiveness in evaluating iron status and guiding rHuEPO dosage.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12882-024-03806-5.
Keywords: β-globin mRNA expression, End-stage kidney disease (ESKD), Ferritin, Flow cytometry, Hypochromic cells (HYPO%), Immature reticulocyte fraction (IRF), Reticulocyte hemoglobin content (CHr), Sudanese children
Introduction
Chronic Kidney Disease (CKD) in Sudanese pediatric populations presents with varying degrees of anemia, which closely correlates with the progression of kidney damage, up to and including End-Stage Kidney Disease (ESKD). Research by Hussein R et al. identifies glomerulonephritis as the leading cause of ESKD in Sudanese individuals under 18, accounting for 36.8% of cases. Other causes include unknown etiologies (25.3%) and congenital anomalies of the kidney and urinary tract (CAKUT, 14.9%), highlighting a significant gap in epidemiological data regarding pediatric kidney disease in the region [1].
Sudan faces considerable socio-economic challenges that exacerbate these health issues. Despite its abundant natural resources, the country remains below the poverty threshold, struggling with a humanitarian crisis intensified by economic decline and a healthcare system that is unable to meet the growing demands [2]. The ongoing state of emergency, as reported by the United Nations International Children’s Emergency Fund (UNICEF), emphasizes the critical need for targeted child survival and development strategies as part of the Country Programme of Cooperation 2018–2021 [3].
Anemia in children with CKD has a multifactorial etiology, primarily resulting from erythropoietin deficiencies and disruptions in iron metabolism, including both absolute iron deficiency (AID) and functional iron deficiency (FID). AID is marked by depleted iron stores, while FID occurs when, despite adequate iron reserves, there is insufficient iron available for erythropoiesis. Additional factors such as inflammation, secondary hyperparathyroidism, and uremic toxins further complicate the condition. The response to Recombinant Human Erythropoietin (rHuEPO) treatment is variable, often reflecting the complexity of anemia in this context, where hypo-responsiveness is a frequent challenge [4].
Evaluating the early myeloid response to treatment is crucial for the effective management of anemia in Sudanese children with ESKD, as it has a direct impact on their growth and quality of life.
The primary objective of this study is to assess the myeloid expression of β-globin mRNA in blood reticulocytes as a marker of response to rHuEPO therapy in Sudanese children with ESKD. The Immature Reticulocyte Fraction (IRF) is utilized as a key indicator of erythropoietic activity, with values ≤ 0.23 indicating an inadequate bone marrow response to anemia, and values > 0.23 prompting further diagnostic investigation into the underlying causes of anemia [5]. Additionally, this study evaluates iron status through the measurement of Reticulocyte Hemoglobin Content (CHr) and the percentage of hypochromic red cells (%Hypo), potentially providing more nuanced insights compared to traditional markers like ferritin and transferrin saturation. By examining the relationships between the response to rHuEPO therapy, reticulocyte β-globin mRNA expression, IRF, CHr, and %Hypo, this study aims to better characterize the type of anemia affecting this population and to refine treatment strategies based on age- and gender-specific physiological responses.
Materials and methodology
Subject population
The study initially enrolled 51 anemic Sudanese children, of whom six withdrew for various reasons. The remaining 45 participants (21 males and 24 females, aged ≤ 15 years) completed six months of treatment and follow-up. All patients received Epoetin beta (Recormon® 4000 IU/ml) and intravenous iron sucrose (100 mg/ml) alongside maintenance hemodialysis. The severity of anemia was classified according to World Health Organization (WHO) hemoglobin concentration norms for age [6]. The Kidney Disease: Improving Global Outcomes (KDIGO) guidelines recommend monitoring iron status every three months and evaluating hemoglobin (Hb) levels monthly, targeting an Hb level of 12 g/dl [7].
Causes of withdrawal
Among the six withdrawals, four were males and two females. Reasons for withdrawal included two males being repatriated, one male succumbing to complications from dilated cardiomyopathy, and another being prepared for a kidney transplant. One female achieved stable hemoglobin levels before transplant preparation, while another, suffering from severe hepatitis and requiring recurrent transfusions, passed away.
Ethics
Ethical approval was obtained from the Research Ethics Committee (REC) of Karary University (IRB KU-2018-13), the Ministry of Health-Khartoum State, and Soba University Hospital. Permissions were also secured from the administrations of the participating hospitals, and informed consent was acquired from the guardians of all participants. Strict protocols for confidentiality and anonymity were maintained throughout the study.
Study design
This prospective observational cross-sectional study was conducted from January 2019 to February 2020 in three teaching hospitals in Khartoum State: Soba University Hospital, Gaafar Ibn Ouf Children’s Hospital, and Omdurman Children Hospital ‘Mohammed Elamin Hamid’. A control group was not included due to the ethical concerns and difficulties associated with blood sampling from healthy children. The absence of a control group is acknowledged, and results are presented as preliminary findings.
Inclusion criteria
Participants included were anemic Sudanese children aged ≤ 15 years with hemoglobin levels ≤ 11.0 g/dl, diagnosed with end-stage kidney disease and undergoing regular hemodialysis. These children were scheduled for routine administration of rHuEPO and intravenous iron.
Exclusion criteria
Children with hemoglobin levels ≥ 12.0 g/dl, those with congenital hemoglobinopathies, CKD associated with cancer, or those receiving treatments that interfere with iron absorption (such as ACE inhibitors and proton pump inhibitors) were excluded.
Sample size justification
A power analysis was conducted to ensure the sample size was adequate. Based on preliminary data, the study aimed for 80% power with a significance level of 0.05, allowing for the detection of statistically significant differences and correlations. The analysis confirmed that the current sample size was sufficient for reliable findings. Although a larger sample would improve generalizability, the rarity of pediatric ESKD in our setting and ethical considerations for recruiting vulnerable populations limited the ability to increase the sample size. Nevertheless, the cohort represents a comprehensive sample for robust analysis and meaningful conclusions.
Patient assessment and treatment protocol
Renal failure was confirmed through Renal Function Tests (RFT) and abdominal ultrasound, with a Glomerular Filtration Rate (GFR) of < 20–30 ml/min. Treatment involved Epoetin beta (Recormon® 4000 IU/ml) and intravenous iron sucrose (100 mg/ml), with dosage adjustments based on FDA guidelines and patient response. The initial dose was 150 units/kg/week, typically administered in one to three doses per week, depending on availability. According to FDA standards, the recommended intravenous iron sucrose dose is 0.5 mg/kg for children ≥ 2 years on hemodialysis and receiving erythropoietin therapy, not exceeding 100 mg per dose every two weeks [8, 9]. Hemodialysis was conducted three times weekly using the GAMBRO system, supervised by pediatric nephrologists, following KDIGO anemia guidelines for CKD [10].
Data collection
A total-coverage sampling strategy was used to recruit 51 children diagnosed with ESKD from several hospitals in Khartoum State. Following the withdrawal of six participants, 45 children completed all study requirements. The sample size was calculated to achieve 90% statistical power at a 5% significance level, with 48 participants required for robust findings.
Statistical analysis
Data analysis was conducted using SPSS version 16, employing Pearson correlation coefficients to identify associations between reticulocyte hemoglobin content (CHr), percentage of hypo chromic red blood cells (%Hypo), and other hematological indices. Regression analysis using R package software (version 4.1.3) was performed to express relationships between quantitative variables, such as manual reticulocyte count, flow cytometry reticulocyte count, and CHr IRF parameters, and β-globin expression results. Receiver Operator Characteristics (ROC) curves assessed the diagnostic performance of CHr and %Hypo for Iron Deficiency Anemia (IDA) and Functional Iron Deficiency (FID). The Marginal Homogeneity Test compared hemoglobin categories at 1st and 6th months, while the Monte Carlo test and Cramer’s V statistic elucidated associations between age, gender, and iron status. β-globin mRNA data were analyzed using the Fold Change Expression (FCE) method. Missing data were managed by excluding records with missing values and applying listwise deletion as needed.
Laboratory techniques
Hematology and iron profile analysis
Monthly hemoglobin monitoring was conducted using the Sysmex Automated Hematology Analyzer (KX-21 N). Spectrophotometric techniques were used for iron profile analyses, including serum ferritin, serum iron, total iron-binding capacity (TIBC), and transferrin saturation [11, 12].
Flow cytometry
Reticulocytes were analyzed using the Beckman Coulter 5-color Cytomics FC500. Thiazole Orange dye was employed to quantify reticulocytes and assess their maturation indicators, such as IRF and CHr [13].
Staining procedure
A stock solution of Thiazole Orange (1 mg/mL) was prepared in methanol and diluted in phosphate-buffered saline containing EDTA and sodium azide. EDTA blood from anemic and non-anemic children was stained, incubated, and analyzed using flow cytometry to assess reticulocyte maturation. More details are available in the supplementary files.
Quantification of β-globin mRNA expression
Quantitative Real-Time PCR (RT-PCR) analysis of β-globin mRNA expression was conducted using Maximas SYBR Green/Fluorescein qPCR Master Mix with β-globin-specific primers. Data collection and analysis were performed on the Rotor-Gene Q platform, following the 2-ΔΔCt quantitative method for precise gene expression measurement [14].
Results
Hematological profile and reticulocyte analysis
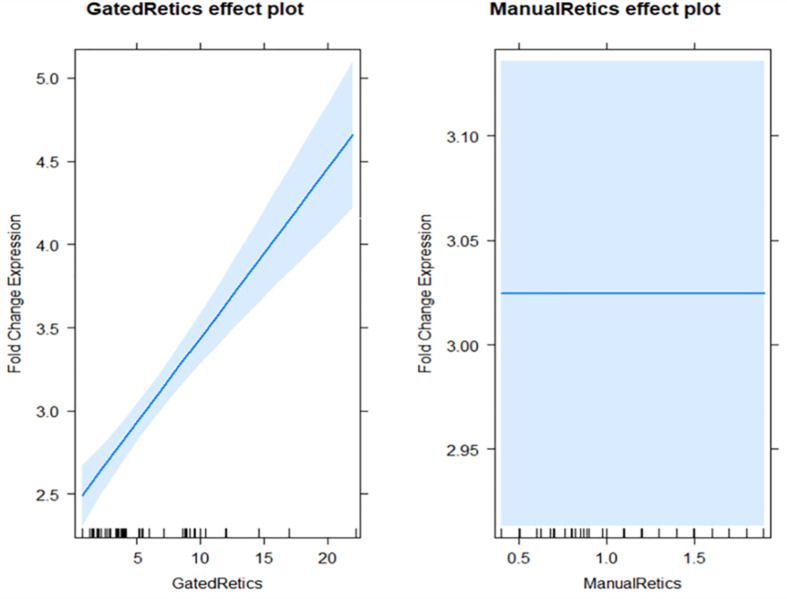
The complete blood count (CBC) across the study population revealed a normocytic, normochromic red cell morphology. Reticulocyte counts, corrected against 1,000 RBCs and gated via flow cytometry against 50,000 RBCs, showed a significant correlation with β-globin mRNA expression, indicating a robust marrow response (β = 0.10, p < 0.001). This correlation was more pronounced in flow cytometry than in manual reticulocyte counts, which demonstrated a negligible association (β = 4.721 × 10^-14, p = 1.0) (Fig. 1) (See Table 1).
Fig. 1.
Comparison between Flow Cytometry and Manual Reticulocyte Counts in β-globin mRNA Expression
Table 1.
Diagnosis of anemia among Sudanese children with ESKD by age categories according to KDIGO
| Age categories | Hb < 11.0 g/dl | Hb < 11.5 g/dl | Hb < 12.0 g/dl | Total | Monte Carlo Sig. (2-sided) | Cramer’s V correlation |
|---|---|---|---|---|---|---|
| 0.5–5 years | 5 (100%) | 0 (0%) | 0 (0%) | 5 | 2.594 | 0.80 |
| 5–12 years | 12 (100%) | 0 (0%) | 0 (0%) | 12 | ||
| 12–15 years | 24 (85.7%) | 1 (3.6%) | 3 (10.7%) | 28 | ||
| Total | 41 | 1 | 3 | 45 |
Hemoglobin levels over time
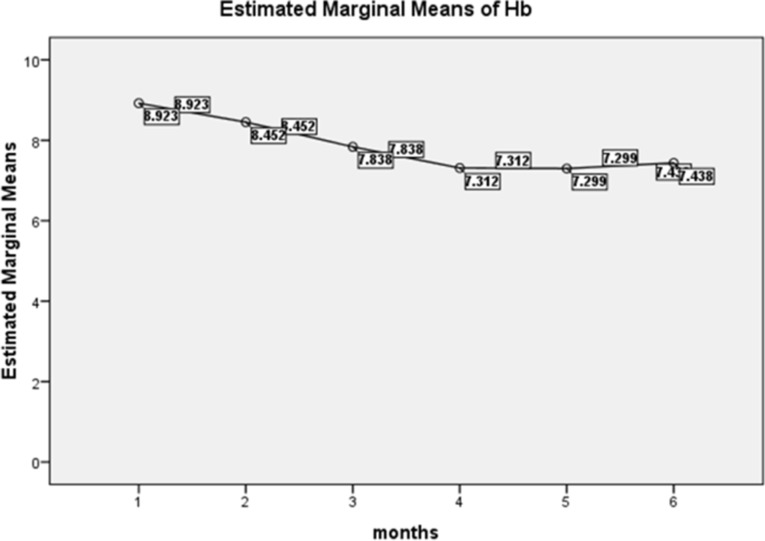
The estimated marginal means of hemoglobin (Hb) were analyzed over six months, categorizing the severity of anemia according to WHO age-specific guidelines (Fig. 2). According to KDIGO criteria, hypo-responsiveness to rHuEPO treatment was primarily observed in the 12–15-year age group, with Hb values consistently remaining below 11 g/dl (Table 2).
Fig. 2.
Estimated Marginal Means of Hemoglobin among Anemic Sudanese Children with ESKD
Table 2.
Marginal homogeneity test for hemoglobin categories at the first and sixth month
| Hb (1st month) | Hb (6th month) | Total | Marginal Homogeneity Test |
|---|---|---|---|
| < 11.0 g/dl | < 11.5 g/dl | < 12.0 g/dl | |
| < 11.0 g/dl | 30 (93.8%) | 1 (3.1%) | 1 (3.1%) |
| < 11.5 g/dl | 3 (100%) | 0 (0%) | 0 (0%) |
| < 12.0 g/dl | 8 (80.0%) | 0 (0%) | 2 (20.0%) |
| Total | 41 | 1 | 3 |
Longitudinal hemoglobin analysis
A significant shift in hemoglobin categories was observed from the first to the sixth month of treatment (p = 0.01), suggesting changes in anemia severity over time (Table 2).
Iron status classification
The anemia classification based on iron status was assessed at the third and sixth months. The findings showed a higher prevalence of anemia from other causes compared to functional iron deficiency, with no cases of absolute iron deficiency observed (Table 3).
Table 3.
Classification of Anemia at 3rd and 6th Month Follow-Up intervals based on Iron Status
| Anemia Classification (3rd month) | Anemia Classification (6th month) | Total | Marginal Homogeneity Test |
|---|---|---|---|
| Functional Iron Deficiency (FID) | Anemia of other causes | ||
| Absolute Iron Deficiency (AID) | 0 | 1 | 1 |
| Functional Iron Deficiency (FID) | 0 | 4 | 4 |
| Anemia of other causes | 2 | 38 | 40 |
| Total | 2 | 43 | 45 |
Impact of demographic factors on anemia
Age was a significant factor influencing iron status over the six-month period (p = 0.49), while gender did not show a significant impact on the anemia classification based on KDIGO criteria (Table 4).
Table 4.
Impact of gender on Anemia classification based on KDIGO categories
| Gender | Anemia Classification | Total | Fisher’s Exact Test | Contingency Coefficient |
|---|---|---|---|---|
| Functional Iron Deficiency (FID) | Anemia of other causes | |||
| Female | 2 (8.0%) | 23 (92.0%) | 25 | 0.49 |
| Male | 0 (0%) | 20 (100%) | 20 | |
| Total | 2 (4.4%) | 43 (95.6%) | 45 |
β-globin gene expression analysis
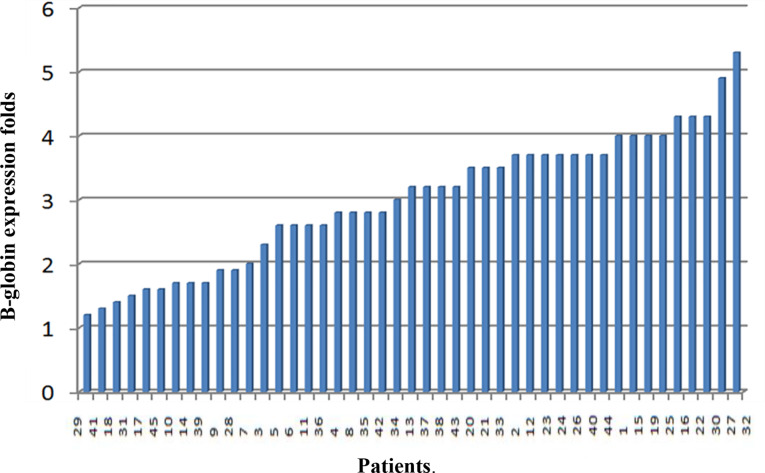
β-globin gene expression was evaluated in all 45 anemic Sudanese children using RT-PCR. Expression levels varied widely, ranging from 1.2 to 5.3-fold changes, and showed a strong correlation with hemoglobin levels (r = 0.87, p < 0.001) (Fig. 3).
Fig. 3.
β-globin expression fold changes among studied patients
hemoglobin levels (r = 0.87, p < 0.00) (Fig. 3).
Regression and correlation analyses
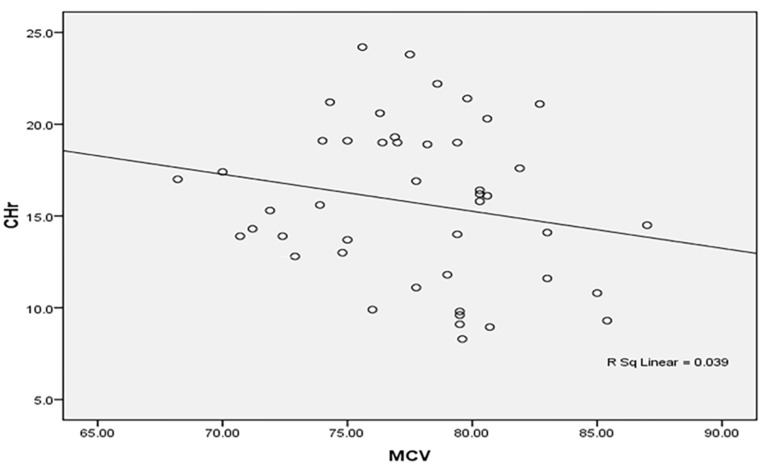
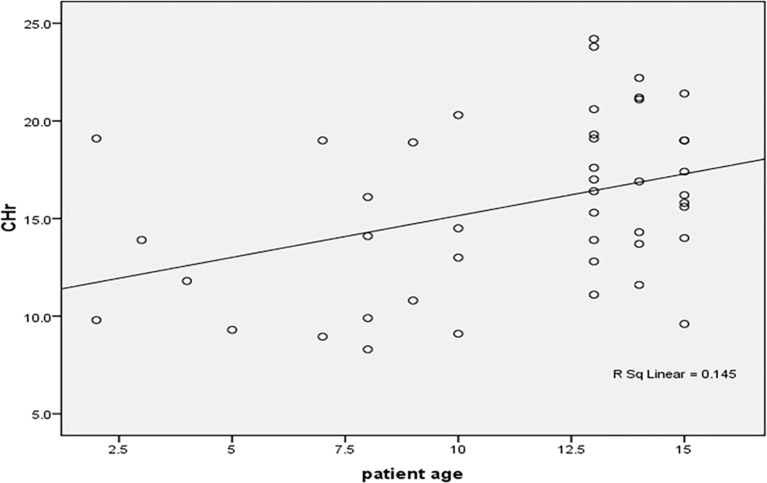
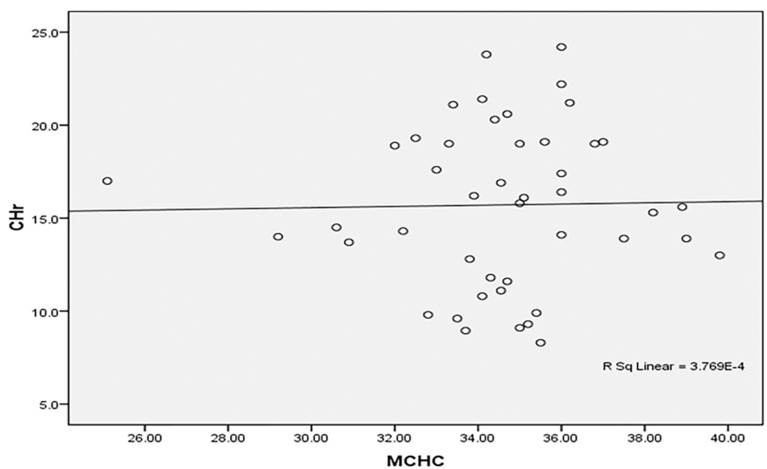
Regression analysis demonstrated an association between patient age and CHr (β = 0.39, p = 0.04). Correlation studies revealed that CHr was inversely related to mean corpuscular volume (MCV) and positively correlated with mean corpuscular hemoglobin concentration (MCHC). Figures 4, 5, 6, 7, 8, 9 and 10 depict various ROC curves and correlation graphs highlighting the relationships between hematological indices and anemia.
Fig. 4.
The area under curve ROC curve expression of anemic Sudanese gender effect on IRF
Fig. 5.
CHr shows an inverse correlation with MCV among the studied anemic Sudanese children with ESKD
Fig. 6.
Significant correlation between patient’s age and CHr among the studied anemic Sudanese children with ESKD
Fig. 7.
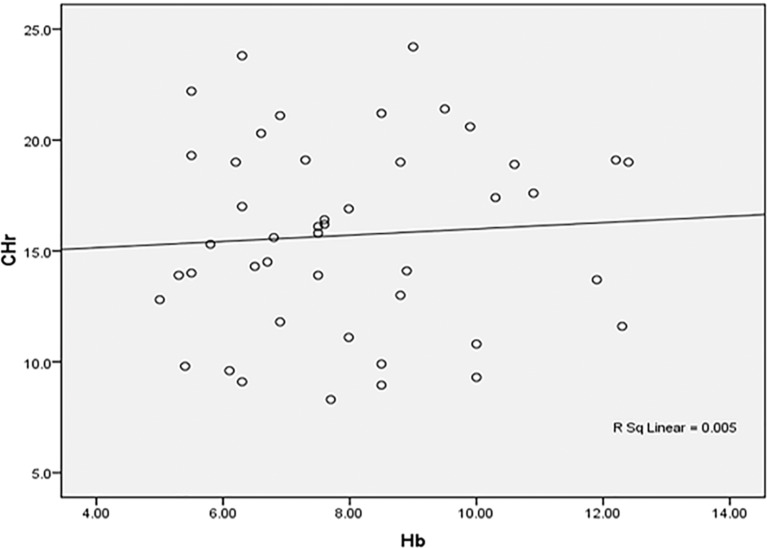
Positive insignificant correlation between Hb and CHr among the studied anemic Sudanese children with ESKD
Fig. 8.
Positive insignificant correlation between MCHC and CHr among the studied anemic Sudanese children with ESKD
Fig. 9.
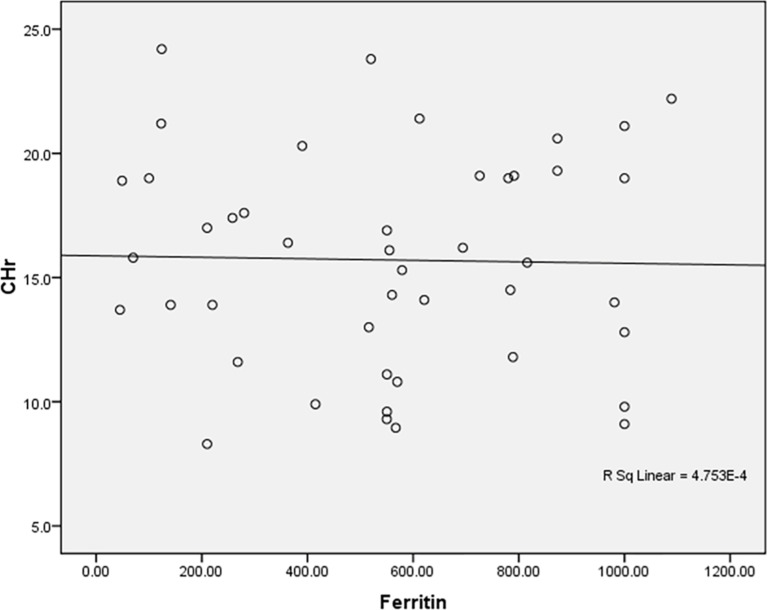
Positive insignificant correlation between Ferritin and CHr among the studied anemic Sudanese children with ESKD
Fig. 10.
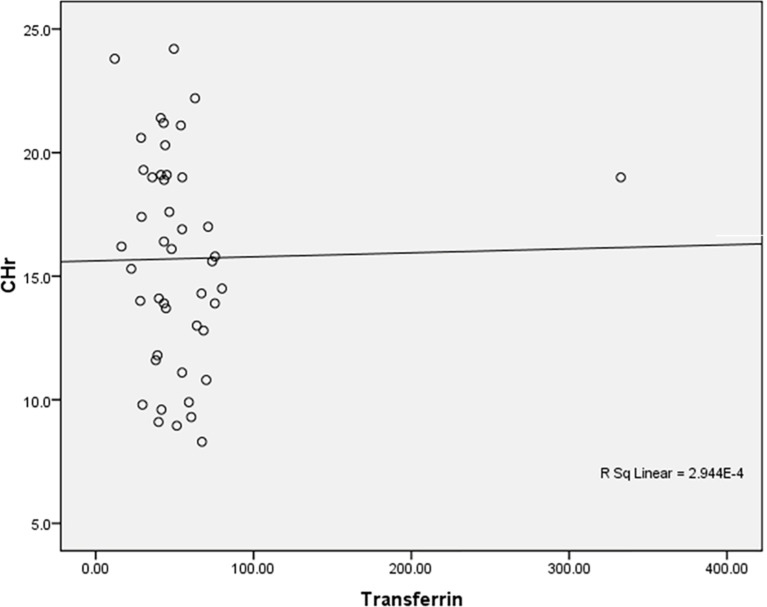
Negative insignificant correlation between Transferrin and CHr among the studied anemic Sudanese children with ESKD
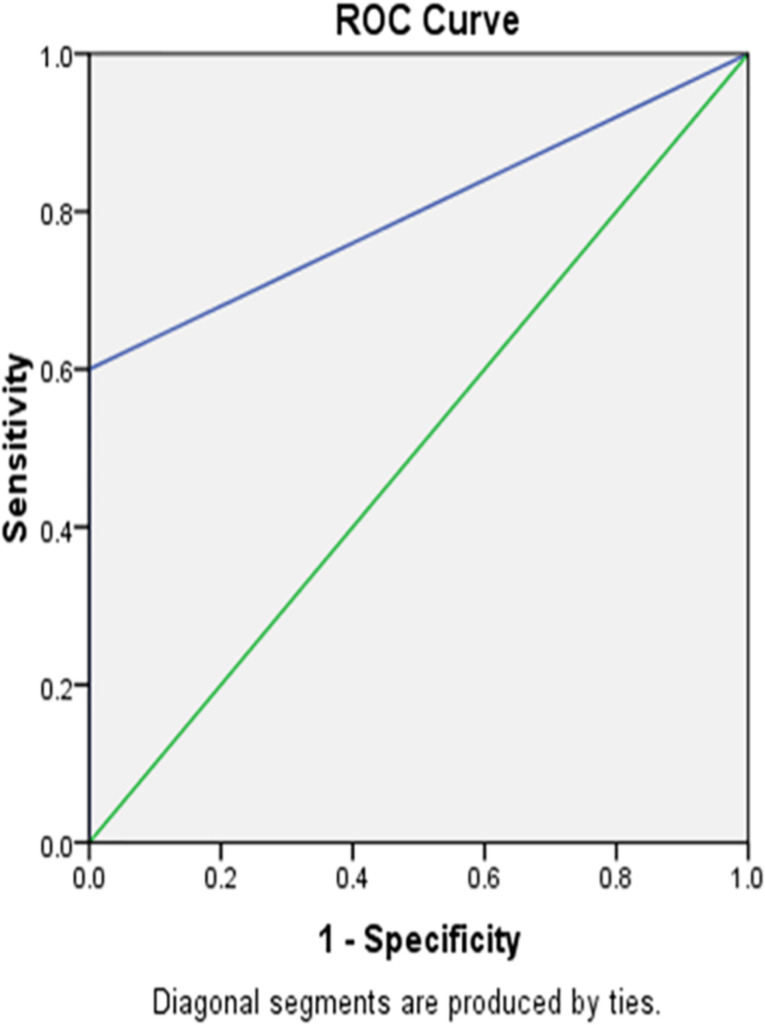
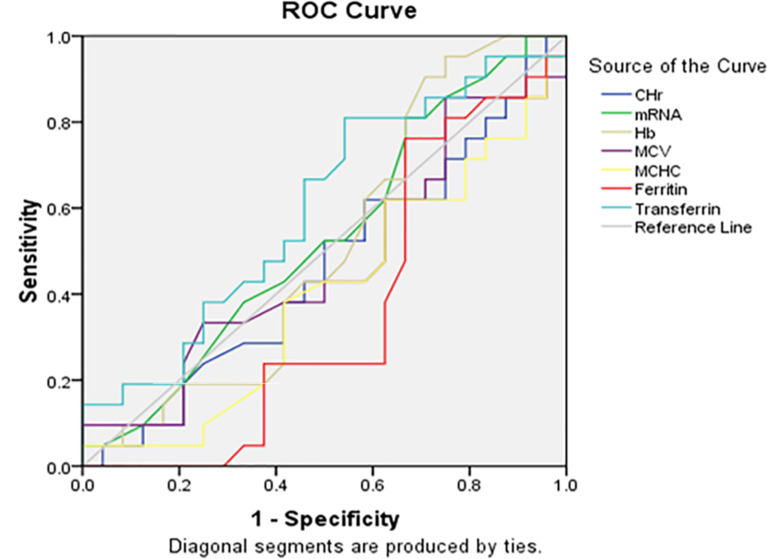
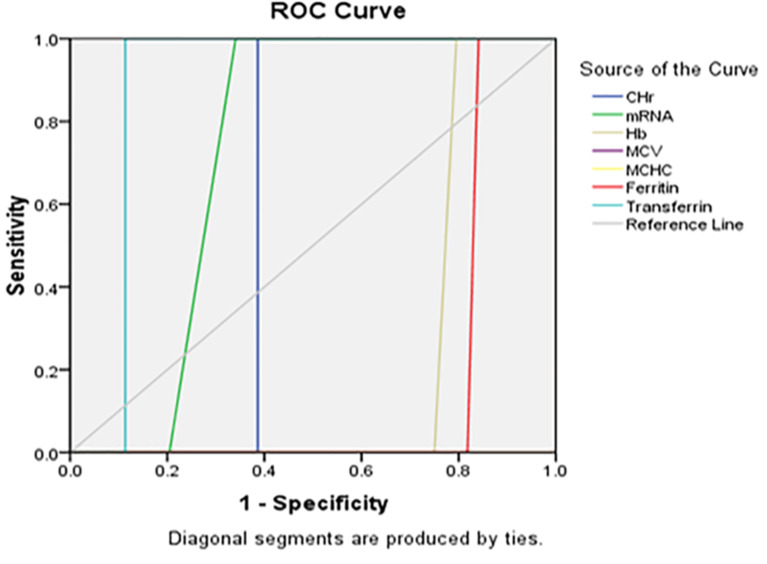
Receiver operator characteristics (ROC) analysis
ROC curves demonstrated the diagnostic performance of CHr and %Hypo in identifying IDA. Transferrin saturation was a significant predictor of iron status (Figs. 11 and 12).
Fig. 11.
The ROC curve of CHr, mRNA, and Hb versus gender among the studied anemic Sudanese children with ESKD
Fig. 12.
The ROC curve of CHr, mRNA, Hb versus Hypo% among the studied anemic Sudanese children with ESKD
Sensitivity and specificity of diagnostic tests
CHr showed a sensitivity of 50% and specificity of 4.65% in diagnosing IDA, highlighting the challenges of assessing iron status in pediatric ESKD (Table 5).
Table 5.
Sensitivity and specificity of CHr in diagnosing IDA among Studied Anemic Sudanese Children with ESKD
| Estimated Value | 95% Confidence Interval |
|---|---|
| Lower Limit | |
| Prevalence | 0.04 |
| Sensitivity | 0.50 |
| Specificity | 0.04 |
Discussion
This study explores the complexities of anemia in Sudanese children with End-Stage Kidney Disease (ESKD) undergoing hemodialysis, focusing on the diagnostic utility of β-globin mRNA expression and reticulocyte parameters. Anemia in pediatric ESKD is often due to insufficient erythropoietin (EPO) production, which is critical for red blood cell synthesis in the bone marrow. Our treatment approach, which combined Epoetin beta (Recormon® 4000 IU/ml) with intravenous iron sucrose (100 mg/ml), underscores the importance of combined therapy for effectively managing anemia in this population.
Influence of iron supplementation and erythropoiesis
Iron supplementation, when administered alongside rHuEPO, enhances erythropoiesis and decreases reliance on blood transfusions. Regular monitoring of iron status and hemoglobin (Hb) levels is crucial for the early detection and management of anemia, ultimately improving clinical outcomes. Our study demonstrates that age significantly impacts iron status, with variations observed in β-globin mRNA expression, CHr, IRF, and mean corpuscular indices. CHr emerged as a sensitive and specific diagnostic tool, particularly for assessing iron deficiency, with transferrin saturation providing more predictive value than ferritin. Interestingly, male patients exhibited a more hypo-responsive pattern to treatment, particularly evident in IRF measures.
Comparative analysis and literature review
Our findings align with Bamgbola et al., who identified age, gender, and other risk factors as determinants of erythropoietin resistance in pediatric dialysis cohorts, where factors like chronic inflammation play a significant role in influencing treatment outcomes [15]. Additionally, our results highlight the ongoing challenge of inadequate micronutrient supplementation, a common concern in adult populations with CKD.
Elevated transferrin saturation levels (≥ 20%) in our cohort suggested a potential risk of iron overload, classifying the anemia as likely driven by non-iron deficiency causes. This finding underscores the utility of CHr and %Hypo as more precise markers for evaluating iron status. Similar conclusions were drawn by Mohsen Saleh et al., who identified iron overload risks in pediatric CKD patients, advocating for cautious iron supplementation [16] .
Limitations of traditional iron markers and role of CHr and HYPO%
Traditional serum markers like ferritin and transferrin saturation are sensitive to inflammatory states and may not reliably indicate iron status [17]. Inflammatory conditions can lead to decreased transferrin levels and, consequently, falsely elevated transferrin saturation. Ferritin is similarly affected by inflammation, malnutrition, and chronic disease, with significant fluctuations in levels (17–70%) [18]. Iron deficiency can manifest before anemia becomes clinically apparent, highlighting the need for more accurate and early indicators like CHr and %Hypo, especially as bone marrow biopsy—the gold standard for assessing iron deficiency—is invasive and not feasible for pediatric patients [19].
CHr provides an early snapshot of iron status and hemoglobin production, reflecting bone marrow activity and response to iron therapy. %Hypo, which measures the percentage of red blood cells with low hemoglobin content (< 28 g/dl), serves as a marker of functional iron status, often indicating iron-restricted erythropoiesis before clinical anemia is detectable. Research by Mäkelä et al. demonstrated CHr’s superiority over serum ferritin and TSAT in assessing iron deficiency during acute infections, while Dignass et al. found that CHr was a more reliable marker than serum ferritin in anemia associated with chronic disease [20]. Our study corroborates these findings, showing a strong correlation between CHr and age, and confirming its utility in diagnosing iron deficiency anemia (IDA), as supported by Badr et al. [21].
Emerging diagnostic tools
Interestingly, our findings contrast with those of Tessitore et al., who reported that %Hypo was a better predictor of IDA than ferritin, TSAT, and CHr in hemodialysis patients [22]. Such discrepancies highlight the need for population-specific diagnostic strategies, as marker efficacy may vary across different stages of kidney disease and patient demographics. Our results are also in line with Mehta et al., who evaluated CHr and IRF as early indicators of iron therapy response, finding that CHr was the most timely marker for iron supply to developing RBCs [23].
Potential of β-globin mRNA and novel therapeutics
Our analysis further underscores the potential of β-globin mRNA as a biomarker for erythropoiesis and as an early indicator of erythroid response to rHuEPO treatment. This finding is consistent with Ferrer et al., who observed increased β-globin mRNA levels following rHuEPO administration [24]. Furthermore, emerging therapies like hypoxia-inducible factor-prolyl hydroxylase inhibitors (HIF-PHI) offer promising alternatives for managing anemia in CKD, as discussed by Badura et al. [25].
Novelty and incremental value of study findings
This study highlighted the challenges in anemia management among pediatric ESKD patients, notably the poor response to treatment over the follow-up period, necessitating the incorporation of blood transfusion into the treatment regimen. The study identified diverse causes of anemia, including age-related physiological factors, and reported a case of significant menstrual blood loss in a female patient on hemodialysis, prompting the need for coagulation factor assessments.
The study also documented anemia complications, such as fatigue, lethargy, depression, and cardiovascular disorders as the disease advanced.
Importantly, the study introduced novel diagnostic approaches for anemia associated with ESKD, utilizing advanced technologies like flow cytometry and PCR to evaluate bone marrow activity post-treatment. These methods provided an accurate and in-depth assessment, offering an alternative to the painful bone marrow aspiration, which requires supplementary laboratory diagnostics for comprehensive evaluation.
Limitations and future directions
While our study provides a valuable baseline for understanding anemia in pediatric ESKD, certain limitations should be noted. The relatively small sample size and limited follow-up duration may affect the generalizability of our findings. Additionally, the lack of a control group restricts the ability to draw broader comparisons. The variability in clinical characteristics and treatment responses necessitates further research with larger cohorts and longer observation periods to validate these results. Addressing these limitations will enhance our understanding of anemia management in pediatric ESKD and support the development of tailored, effective interventions based on comprehensive physiological assessments.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Gratitude is extended to all contributors and participants of the study.
Author contributions
1- AA Basic researcher and PhD student 2-AM RT-PCR technical supervisor and training techniques with submitting theoretical Molecular biology courses 3-OA Flowcytomery technical supervision with submitting theoretical Flowcytometry Introduction course 4-EF Flowcytomery technical supervision 5- MO Statistical analysis with multi-regression between Flowctometry and RT-PCR 6- NA Medical clinician supervisor of study cases for Pediatric Kidney Disease and Kidney Transplant 7- NM Professor of Hematology and Molecular Hematology as a Secondary Supervisor of the research 8- NH Professor of Pathology as a main supervisor of the research.
Funding
No funding was received for this research.
Data availability
Availability of Data and Materials: Data is available from the corresponding author upon reasonable request.
Declarations
Ethics approval
Ethical approval for this study was obtained from the appropriate ethics committee, and informed consent was secured from all participants’ guardians.
Consent to participate
The study objectives and protocols were thoroughly explained to the parents and guardians of the children involved. This included information on laboratory follow-up procedures, contact details of the patients’ guardians, and collection of socio-demographic, genetic, and medical histories. It was ensured that all participants’ guardians were well-informed and agreed to the study terms, with the promise of transparency in communicating results throughout and after the study period. Participation was voluntary, with no coercion or financial incentives provided. The study benefited participants significantly, offering close clinical follow-up and comprehensive monthly laboratory evaluations, including CBC, RFT, CRP, bleeding profile, and PTH assessments. Blood samples were obtained by nurses from either a peripheral vein or fistula before hemodialysis sessions. Beyond the six-month study period, follow-up was extended to a full year for each patient, depending on their health status. This facilitated enhanced monitoring and informed medical decision-making. The study established a foundation of trust and communication between the patients’ parents, the researcher, and the treating physicians. Parents were regularly updated on their child’s health and psychological status. In cases where a patient passed away, the family informed the researcher, ensuring transparency about the time, circumstances, and nature of the event.
Ethical considerations for participant withdrawals
For participants who withdrew from the study, psychological support was provided by specialists, and financial assistance came from charitable organizations dedicated to supporting pediatric kidney patients. These considerations were embedded in the treatment protocol, particularly for those returning to their place of origin outside Khartoum State. Risks associated with discontinuation included lack of regular dialysis, inadequate treatment, and insufficient nutritional support. Charitable organizations contributed to patient welfare by offering entertainment and nutritious meals during dialysis, containing energy and protein sources. Medical staff actively advised guardians on proper nutritional practices and emphasized limiting water intake and foods that could exacerbate osmotic imbalances or contribute to high blood pressure. Government agencies provided free access to essential treatments, including calcium supplements, diuretics, and antihypertensive medications, along with the meals during dialysis sessions, reflecting the support available within the local context.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hussein R, Ye X, Ellidir R, Ali E, Bakhiet Y, Karar M, et al. # 4285 causes of pediatric end stage kidney disease in sudan. Nephrol Dial Transplant. 2023;38(Supplement_1):gfad063c_4285.
- 2.Wharton G, Ali OE, Khalil S, Yagoub H, Mossialos E. Rebuilding Sudan’s health system: opportunities and challenges. Lancet. 2020;395(10219):171–3. [DOI] [PubMed] [Google Scholar]
- 3.UNICEF Sudan. Consolidated Strategy Note for the Country Programme of Cooperation 2018–2021 in Sudan.
- 4.Atkinson MA, Furth SL. Anemia in children with chronic kidney disease. Nat Rev Nephrol. 2011;7(11):635–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang CC, Kass L. Clinical significance of immature reticulocyte fraction determined by automated reticulocyte counting. Am J Clin Pathol. 1997;108(1):69–73. [PubMed] [Google Scholar]
- 6.WHO multicentre growth reference study group. WHO child growth standards based on length/height, weight, and age. Acta Paediatr. 2006;450:76–85. [DOI] [PubMed] [Google Scholar]
- 7.Levin A, Ahmed SB, Carrero JJ, Foster B, Francis A, Hall RK, Herrington WG, Hill G, Inker LA, Kazancıoğlu R, Lamb E. Executive summary of the KDIGO 2024 Clinical Practice Guideline for the evaluation and management of chronic kidney disease: known knowns and known unknowns. Kidney Int. 2024;105(4):684–701. [DOI] [PubMed] [Google Scholar]
- 8.Greenbaum LA. Anemia in chronic renal disease. In: Geary DF, Schaefer F, editors. Pediatric kidney disease. Cham: Springer; 2023. pp. 1603–30. [Google Scholar]
- 9.Cohen CT, Powers JM. Intravenous iron therapy in pediatrics: who should get it and when is the right time? Hematol Am Soc Hematol Educ Program. 2023;2023(1):630–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McMurray J, Parfrey P, Adamson JW, Aljama P, Berns JS, Bohlius J, Drüeke TB, Finkelstein FO, Fishbane S, Ganz T, MacDougall IC, Kidney Disease: Improving Global Outcomes (KDIGO) anemia work group. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl. 2012;2:279–335. [Google Scholar]
- 11.Jabbar HK, Hassan MK, Al-Naama LM. Hematology, Transfusion and Cell Therapy. 2022. [DOI] [PMC free article] [PubMed]
- 12.Total iron measurement. In human serum with a novel smartphone-based assay. IEEE J Transl Eng Health Med. 2020;8:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tilva SH, UR P, Goswami HM. Reticulocyte Hemoglobin Content (CHr): A Reliable Indicator in Iron Deficiency Anemia.
- 14.Irenge LM, Robert A, Gala JL. Quantitative assessment of human β-globin gene expression in vitro by TaqMan real-time reverse transcription-PCR: comparison with competitive reverse transcription-PCR and application to mutations or deletions in noncoding regions. Clin Chem. 2005;51(12):2395–6. [DOI] [PubMed] [Google Scholar]
- 15.Bamgbola OF, Kaskel FJ, Coco M. Analyses of age, gender, and other risk factors of erythropoietin resistance in pediatric and adult dialysis cohorts. Pediatr Nephrol. 2009;24:571–9. [DOI] [PubMed] [Google Scholar]
- 16.Elalfy MS, Aloraby MF, Elshimy A, Sallam DE. Iron status in chronic kidney Disease Pediatric patients on Hemodialysis. GEGET. 2023;18(1):43–56. [Google Scholar]
- 17.Kadatane SP, Satariano M, Massey M, Mongan K, Raina R. The role of inflammation in CKD. Cells. 2023;12(12):1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ryan AM, Cushen S, Bhuachalla EN, Dwyer F, Power DG. The role of inflammatory biomarkers in the Assessment of Nutritional Status and Disease States. Top Clin Nutr. 2015;30(1):3–15. [Google Scholar]
- 19.Dinh NH, CheanhBeaupha SM, Tran LT. The validity of reticulocyte hemoglobin content and percentage of hypochromic red blood cells for screening iron-deficiency anemia among patients with end-stage renal disease: a retrospective analysis. BMC Nephrol. 2020;21:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Almashjary MN. Reticulocyte hemoglobin content: advancing the frontiers in Iron-deficiency Anemia diagnosis and management. J Appl Hematol. 2024;15(1):1–8. [Google Scholar]
- 21.Badr MA, Elashkar SS, Ismail WA, Abozid AA, Hanna D. Role of reticulocyte hemoglobin content in diagnosis of iron deficiency anemia. Alex J Pediatr. 2023;36(2):105–12. [Google Scholar]
- 22.Tessitore N, Solero GP, Lippi G, Bassi A, Faccini GB, Bedogna V, et al. The role of iron status markers in predicting response to intravenous iron in hemodialysis patients on maintenance erythropoietin. Nephrol Dial Transpl. 2001;16(7):1416–23. [DOI] [PubMed] [Google Scholar]
- 23.Mehta S, Goyal L, Kaushik D, Gulati S, Sharma N, Harshvardhan L, Gupta N. Reticulocyte hemoglobin vis-a-vis immature reticulocyte fraction, as the earliest indicator of response to therapy in iron deficiency anemia. J Assoc Physicians India. 2017;65(12):14–7. [PubMed] [Google Scholar]
- 24.Ferrer M, Ortega O, Colomina J, Mulet C, Martínez A, Esteban MJ, Soriano JB. Reticulocyte parameters as early markers of response to recombinant human erythropoietin in children with chronic renal failure. Nefrología. 2018;38(1):89–94. 10.1016/j.nefro.2017.06.007. [Google Scholar]
- 25.Badura K, Janc J, Wąsik J, Gnitecki S, Skwira S, Młynarska E, Rysz J, Franczyk B. Anemia of chronic kidney Disease—A narrative review of its pathophysiology, diagnosis, and management. Biomedicines. 2024;12(6):1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Availability of Data and Materials: Data is available from the corresponding author upon reasonable request.