Abstract
Gastrointestinal tumors are the main causes of death among the patients. These tumors are mainly diagnosed in the advanced stages and their response to therapy is unfavorable. In spite of the development of conventional therapeutics including surgery, chemotherapy, radiotherapy and immunotherapy, the treatment of these tumors is still challenging. As a result, the new therapeutics based on (nano)biotechnology have been introduced. Hydrogels are polymeric 3D networks capable of absorbing water to swell with favorable biocompatibility. In spite of application of hydrogels in the treatment of different human diseases, their wide application in cancer therapy has been improved because of their potential in drug and gene delivery, boosting chemotherapy and immunotherapy as well as development of vaccines. The current review focuses on the role of hydrogels in the treatment of gastrointestinal tumors. Hydrogels provide delivery of drugs (both natural or synthetic compounds and their co-delivery) along with gene delivery. Along with delivery, hydrogels stimulate phototherapy (photothermal and photodynamic therapy) in the suppression of these tumors. Besides, the ability of hydrogels for the induction of immune-related cells such as dendritic cells can boost cancer immunotherapy. For more specific cancer therapy, the stimuli-responsive types of hydrogels including thermo- and pH-sensitive hydrogels along with their self-healing ability have improved the site specific drug delivery. Moreover, hydrogels are promising for diagnosis, circulating tumor cell isolation and detection of biomarkers in the gastrointestinal tumors, highlighting their importance in clinic. Hence, hydrogels are diagnostic and therapeutic tools for the gastrointestimal tumors.
Keywords: Hydrogels, Gastrointestinal tumors, Drug and gene delivery, Cancer immunotherapy, Stimuli-responsive hydrogels
Introduction
Cancer is a prominent contributor to global mortality, with around 9.6 million deaths attributed to cancer in 2018, out of a total of 18.1 million of diagnosed cases. Furthermore, up to 43.8 million cancer cases suffer from relapse within five years or less [1–3]. The most common tumor types in men are lung, prostate, colorectal, gastric and liver cancers, while breast, colorectal, lung, cervical and thyroid tumors are common among the females. According to the data, one in five of the males and one in six of females are diagnosed with tumor during their life [2]. Among them, one-in-eight men and one-in-eleven women cannot overcome this disease in their longevity [4]. The incidence rate of cancer has shown an increase in most the countries. The survival rate of cancer patients is low because of the late diagnosis and restricted access to therapeutics, which are more prevalent in developing countries [4]. Currently, there are a number of therapeutics for human tumors, including surgical resection, immunotherapy, chemotherapy, radiotherapy, targeted therapy and hormonal therapy [2, 5]. However, these therapeutic modalities are associated with adverse impacts affecting the life quality of patients. Moreover, the resistance to therapeutics is another hurdle towards the anticancer activity of chemotherapy drugs [5]. Therefore, oncological research has been directed towards the development novel therapeutics for the cancer [6], and the development of biomedical materials is among them.
The biomedical application of nano- and micro-materials in disease therapy has been of importance. These materials can propose new insights for disease therapy, especially cancer treatment, including cancer diagnosis, phototherapy, immunotherapy, improving gene therapy, boosting chemotherapy and significant reduction in tumorigenesis [7–11]. The current issues in cancer therapy, including drug resistance and the side effects of therapeutics, can be solved by such materials through the specific delivery of drugs and genes as well as high accumulation at the tumor site. Moreover, there is a need for the encapsulation of cargoes, including genes, to protect against degradation by enzymes. Therefore, there are many reasons for the development of materials in cancer therapy. Hydrogels are three-dimensional physical or chemical polymeric networks possessing hydrophilic groups [12]. Although hydrogels are not soluble in water, they are can absorb and swell in water, providing a number of characteristics, including biocompatibility, viscoelasticity and specific mechanical strength [13–15]. Hydrogels have been significantly applied in drug delivery and tissue engineering [16–18]. Both natural and synthetic organic polymers can be used for the development of hydrogels. In the recent years, natural hydrogels have been mainly comprised of polymers, and an example is collagen, which is one of the most common natural hydrogel materials and has been applied for skin and blood vessel reconstruction [19, 20]. Furthermore, natural-based hydrogels (such as hydrogels developed from fibrin and alginate) have been popular as safe structures in wound healing and drug delivery. The application of natural hydrogels in drug delivery can be limited due to their elasticity and easy degradation in vivo [21, 22].
The application of hydrogels in cancer is exponentially growing regarding the promise provided by these structures in tumor elimination. Upon incomplete radiotherapy suppression, the injectable hydrogels containing OK-432 and doxorubicin can be administered to increase anticancer immunotherapy through STING upregulation [23]. Injectable hydrogels can also be prepared from ferritin and oxidized dextran to increase tumor penetration through active transcytosis and promote anticancer immunotherapy [24]. In addition to accelerating cancer immunotherapy [25–27] and vaccine development [28], hydrogels can be used for photothermal therapy [29] and drug delivery [30]. Moreover, stimuli-responsive hydrogels, including NIR- and pH-sensitive hydrogels, can be used in cancer therapy [31].
Gastrointestinal (GI) tumors are among the most malignant diseases posing major threat to human health and providing high mortality and morbidity. There are significant challenges in the treatment of GI tumors, requiring novel strategies in their suppression. First of all, there is a complicated and dense tumor microenvironment (TME) preventing the entrance of bioactive molecules and therapeutics to this region, reducing the penetration of drug. As a result, the nanostructures have been introduced for the drug delivery in GI tumor therapy [32]. Moreover, the conventional therapeutics used for GI tumors suffer from specific delivery and may cause damage to healthy tissues. Due to the malignant behavior of GI tumors, they have the potential to develop resistance to conventional therapeutics including radiotherapy [33], chemotherapy [34] and immunotherapy [35, 36]. Therefore, the new strategies should be introduced for GI tumor therapy that nanoarchitectures are among them [37]. In addition, there are challenges in the diagnosis of GI tumors that nanoparticles can aim in improving diagnosis of patients [38]. Regarding these challenges in the treatment of GI tumors and promises of hydrogels, the present review has been dedicated to understanding the application of hydrogels for the treatment of gastrointestinal cancers as a major threat to human health, and such hydrogels can mediate drug delivery and increase anticancer activity along with reversing drug resistance. The development of hydrogels and their stimuli-responsive kinds can improve the fight against digestive tumors. Figure 1 represents an overview of the application of hydrogels for the treatment of gastrointestinal tumors.
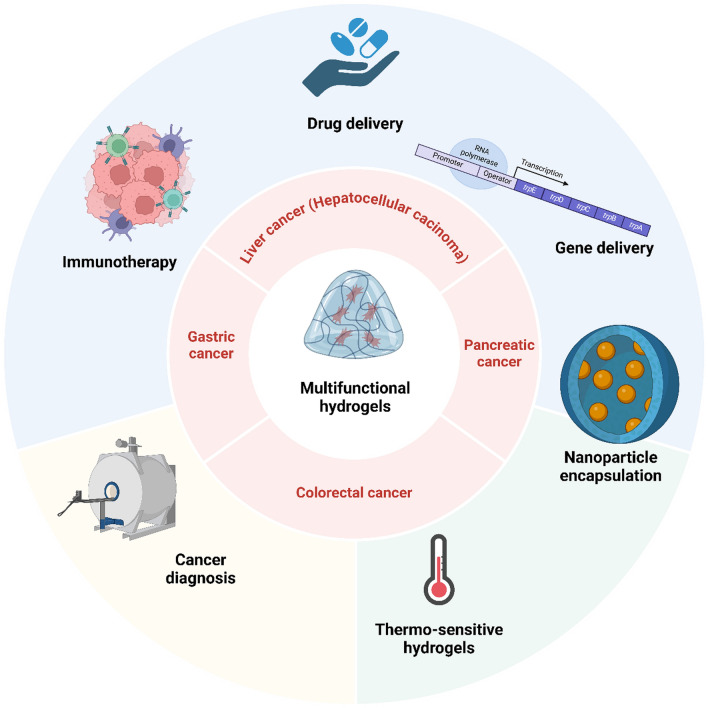
Fig. 1.
An overview of the application of hydrogels in the treatment of gastrointestinal tumors. Hydrogels have been utilized in the treatment different gastrointestinal tumors including liver cancer, gastric tumor, colorectal and pancreatic cancers. Hydrogels can deliver drugs including phytochemicals and synthetic compounds to accelerate tumor suppression. Moreover, they accelerate immunotherapy through induction of dendritic cells and subsequent induction of T cells. The tumor biomarkers can be detected by hydrogels to increase cancer diagnosis. Stimuli-responsive hydrogels including thermo-, pH- and enzyme-sensitive hydrogels have been designed to accelerate gastrointestinal cancer therapy. Other kinds of nanoparticles including liposomes, micelles and quantum dots can be loaded in hydrogels for the cancer diagnosis and therapy. Despite using genetic tools in gastrointestinal cancer therapy, they suffer from poor bioavailability and therefore, their loading in hydrogels can increase the potential in tumor suppression. (Created by Biorender.com)
Hydrogels: basics and oncological application
Wichterle and Lim, in 1960, used the word hydrogel for the first time to describe the material they developed from poly-2-hydroxyethylmethacrylate (PHEMA), which is a synthetic biocompatible material used in contact lens applications [39]. Then, the PHEMA lenses were distributed in Western Europe in 1962, despite poor availability and acceptance. However, PHEMA lens very soon received approval from FDA [40]. Until now, hydrogels have been used as contact lenses, particularly in soft lenses developed from silicon hydrogels. Furthermore, extensive applications in biomedicine, especially in tissue engineering and wound healing, have been observed [41, 42]. Their application in wound healing is due to their ability to provide a moist environment and prevent the spread of fluids to other regions of skin tissue. Currently, several of their commercial forms are available, including DermaFilm®, Kaltostat®, Condress®, and Sofargen® [43]. Moreover, a number of them have been embedded with bioactive compounds, including iodine or zinc ions, to improve their antimicrobial and cleansing features, respectively [40]. The clinical applications of hydrogels for the treatment of human diseases have also been followed. Perseris®, Sublocad® and Azasite® are several clinically used hydrogels for the treatment of diseases including schizophrenia, analgesia, and bacterial conjunctivitis, respectively [44]. Hydrogels can be categorized based on their composition, crosslinking, configuration, and source. For crosslinking, hydrogels can be developed through chemical or physical crosslinking. For composition, they can be developed from homopolymers, co-polymers, semi-IPN and IPN. From the source standpoint, hydrogels can be natural, synthetic, or hybrid. From the configuration standpoint, they can be amorphous, crystalline and semi-crystalline [45]. The common application of natural-based hydrogels is related to the delivery system development and tissue scaffolds because of their degradability and biocompatibility. The benefits of natural hydrogels include favorable biocompatibility, minimal toxicity, and natural degradation, while their cons include weak mechanical features and potential for immunogenicity. Synthetic hydrogels are mainly utilized in controlled drug release and regenerative medicine, offering precise control over physical and chemical properties. Their benefits include adjustable characteristics, including pore size and degradation rate, robust mechanical strength, while their cons include toxicity and less biocompatibility compared to natural hydrogels. Hybrid hydrogels possess the features of both natural and synthetic hydrogels using in drug delivery systems and tissue engineering. Their benefits include improved mechanical properties and biocompatibility, customizable features, whereas their cons include complicated synthesis and potential issues with batch-to-batch consistency. Amorphous hydrogels are promising in wound dressings and contact lenses due to their flexible and soft nature. Their benefits include favorable water content and conformability to various shapes, while they suffer from lower mechanical strength and structural integrity. Crystalline hydrogels are utilized in load-bearing applications, including cartilage replacements due to their structured networks. Their benefits include improved mechanical strength and stability, while their cons are decreased swelling capability and flexibility, which might limit their use in some biomedical applications. Semi-crystalline hydrogels are deployed for drug delivery systems that require a balance between mechanical properties and fluid dynamics. Their benefits include improved mechanical strength and water absorption capabilities, while their cons include complicated manufacturing processes. More information regarding the synthesis of hydrogels can be found in these reviews [46–50].
Until now, multiple studies have shown the different strategies utilized for the preparation of the hydrogels [14, 51–60]. The polymerization method is the first strategy for the development of hydrogels. A number of polymerization methods, including free-radical polymerization, can be utilized for hydrogel synthesis that is simple and efficient. Another strategy is the crosslinking method, which can occur as physical crosslinking using hydrogen bonding and ionic interactions, or chemical crosslinking using covalent bonding to improve the mechanical stability and functionality of hydrogels. Notably, the monomers used for the synthesis of hydrogels are of importance and can affect hydrogel characteristics. As an example, acrylic acid and its derivatives have been extensively used in the synthesis of hydrogels due to the high water absorbance ability and adjustable mechanical features. Both natural and synthetic polymers including alginate, chitosan, poly(vinyl alcohol) and polyacrylamide can be used for the synthesis of hydrogels. In order to develop biocompatible hydrogels, the synthesis of these materials using a green approach is suggested. Furthermore, stimuli-responsive hydrogels (sensitive to pH, temperature, light and magnetic field) and nanocomposite hydrogels (adding nanostructures into the structure of hydrogels) have been developed for biomedical applications.
The application of hydrogels in cancer therapy has opened a new gate for tumor suppression. Hydrogels have been shown to be beneficial for tumor microenvironment (TME) remodelling [61], immunotherapy [62, 63], vaccine development [64], drug delivery [65, 66], nanoparticle and drug co-delivery [67] and phototherapy [68]. Hydrogels can be developed from pectin and carboxymethyl to deliver silibinin, impairing the progression of lung tumor in vivo and impairing the function of TMEM16A ion channel [69]. Despite using oral vaccines, their clinical application due to the safety issues is under question. Therefore, chitosan-sodium alginate microcapsules have been developed to encapsulate engineered bacteria and protect against the conditions in the stomach. Moreover, the dissolution of the microcapsules can lead to the release of bacteria in the intestine periodically, improving cancer immunotherapy [70]. Another function of hydrogels is related to the stimulation of macrophages, increasing their phagocytic activity, and promoting systemic cancer immunotherapy [71]. Hydrogels can accelerate cancer phototherapy, and for this purpose, injectable nanocomposite hydrogels have been developed based on alginate-Ca2+ to provide melittin-assisted Ca2+ overload for increasing photothermal-mediated tumor ablation [72]. One of the interesting points of hydrogels in cancer chemotherapy is their potential for the slow release of drugs such as doxorubicin [73]. This can potentiate chemotherapy and prevent drug resistance in cancer cells. The presence of tumor cells at TME can cause cancer relapse, and therefore, immunomodulatory DNA hydrogels comprised of PD-L1 aptamers can be developed to sense cancer cells for monitoring postoperative tumor relapse [74].
Hydrogels in the treatment of gastrointestinal cancers
Colorectal cancer
One of the most common digestive tumors is colorectal cancer (CRC), where up to half of the CRC patients can develop metastasis into the liver and lung [75, 76]. The conventional therapeutics for CRC include surgical resection, radiation, and chemotherapy [77]. However, chemotherapy or surgery cannot significantly improve the survival of CRC patients. Therefore, the development of new strategies, mainly based on hydrogels, can improve the treatment of CRC. Hydrogels have been developed from the chemical crosslinking of hyaluronic acid (HA) and carboxymethyl cellulose sodium (CMCNa) and then loaded with oxaliplatin. These hydrogels can reduce intra-abdominal adhesion after chemotherapy. Moreover, the slow release of oxaliplatin from these hydrogels was observed to release drug via diffusion. The in vivo experiment was performed on an SD rat model, where the suppression of intra-peritoneal adhesion was provided by the hydrogels [78]. In this regard, hydrogels have been developed from HA and CMCNa through crosslinking, and then a double emulsion strategy was utilized to develop oxaliplatin-embedded Poly-(d,l-lactide-co-glycolide) (PLGA) microparticles. The PLGA microparticles had a size and zeta potential of 1100.4 nm and 77.9%, respectively. The microparticle-embedded hydrogels were able to release drug in a prolonged manner and they improved the pharmacokinetic profile of oxaliplatin. These hydrogels provided protection of damaged tissues against peritoneal adhestion [79]. However, the function of hydrogels is more than the regulation of abdominal adhesions; they can exert anticancer activities. Studies have shown that polysaccharides-based hydrogels are promising candidates for prolonged drug delivery because of their biocompatibility and high swelling [80–82]. Alginate (Alg) is one of the polysaccharides with common use capable of supporting the drugs against the gastric juice and providing intestinal delivery of drug [83]. In this regard, hydrogels have been prepared from Alg and CMC via crosslinking with Ca2+. Then, methotrexate (MTX)-loaded CaCO3 (CaCO3/MTX) and aspirin (Asp) are co-entrapped in the hydrogels. These hydrogels provide the protection of MTX in stomach and small intestine and deliver it to the colorectum [84]. Therefore, they are promising candidates in cancer therapy through the delivery of chemotherapy drugs.
One of the factors determining the cellular fate is the mechanical interactions occurring between the cells and TME, which are important in metastasis and migration. The type I collagen hydrogels have been shown to affect the migration of CRC cells, and this is determined by the stiffness of the hydrogels related to the collagen concentration and gelatin temperature [85]. However, this aspect has been ignored in most of the studies and they emphasized on the development of novel hydrogels and loading different drugs or other bioactive factors in the treatment of CRC. The physical cross-linked hydrogels have been developed from ABA triblock copolymers of vitamin D-functionalized polycarbonate and poly(ethylene glycol) to deliver Avastin in the treatment of CRC. Two models of CRC, including subcutaneous and intraperitoneal metastatic tumor models, were used. These hydrogels exerted more suppressive activity on subcutaneous tumors compared to intraperitoneal models, and they preferentially accumulated at the tumor site with low accumulation in the liver and kidney. These hydrogels improved the survival rate of animal models and they suppressed metastasis [86].
One of the types of hydrogels is mucoadhesive hydrogels, which are capable of binding to mucin, as a glycoprotein generated by the cells in the mucosal tissues. The presence of functional groups, including carboxylic acids, amines, or thiols can affect the extent and strength of interaction with mucin [87, 88]. In line with this, mucoadhesive hydrogels have been developed by grafting poly(acrylic acid) (PAA) on cellulose nanocrystals (CNC) at 6, 9 and 12 CNC:PAA w/w ratios. These hydrogels possessed rheological behavior when there is mucin compared to CNC and other gels, and they can release cisplatin in a sustained way to increase suppression of CRC [89]. In another study, hydrogels were developed from chitosan and chondroitin sulfate. In order to improve chitosan solubility and hydrogel characteristics, the hydrogel synthesis was performed from ionic liquids. Then, curcumin and silver nanoparticles were loaded into hydrogels. These hydrogels, along with photodynamic therapy (PDT), demonstrated potential in the induction of cell death in CRC due to increased metal-mediated singlet oxygen. Moreover, their biocompatibility was high due to lack of impact on healthy tissues [90].
One of the strategies is loading nanostructures inside the hydrogels for CRC therapy. In this line, alginate nanostructures have been functionalized with folic acid and then embedded in hydrogels. The conjugation of folic acid to alginate nanoparticles was performed by NH2-linkage, and they can target the folate receptor overexpressed on the surface of cancer cells. The pH-sensitive hydrogels were developed via a free radical polymerization strategy to mediate the sustained release of diferourylmethane. The pH-mediated release of drug occurred at a pH level of 7.4, and it was internalized by the tumor cells through folate receptors [91]. This system can release the nanoparticles at the tumor site and then the nanoparticles provide the controlled release of drugs to increase the anticancer potential. In another work, hydrogels were developed based on inulin, and then they were loaded with hollow MnO2 nanocarriers containing oxaliplatin. Bacteria in colon tissue mediated the metabolism of these nanoparticles to degrade inulin and produce short-chain fatty acids to mediate immune responses and regulate microbiota, providing site-specific release of oxaliplatin and increasing ROS generation in the acidic TME [92]. It can be highlighted that hydrogels are promising factors for the delivery of chemotherapy drugs in CRC suppression. Hydrogels can also impair peritoneal carcinometastasis of the colorectal and decrease its growth [93–100]. Hydrogels are ideal candidates for the site-specific delivery of naringenin and tannic acid in CRC therapy [101, 102], emphasizing their function in natural product delivery. Moreover, hydrogels provide the prolonged release of drugs in CRC therapy [103].
Gastric cancer
Gastric cancer (GC) is another malignant of gastrointestinal tract, and it is a major public health problem [104]. GC has been mentioned as the most malignant tumor in China and the leading cause of death [105]. Surgical resection, along with chemotherapy and radiotherapy, are conventional therapies for GC [106]. However, the tumor cells can develop resistance to traditional therapies. Therefore, the application of nanoparticles for the treatment of GC has surged. Regarding this, hydrogels have been developed to deliver a number of chemotherapy drugs, including cisplatin, to improve the survival time of animal models bearing GC cells and impair proliferation and invasion [107]. Hydrogels can deliver several chemotherapy drugs to suppress GC. In line with this, biodegradable hydrogels have been developed from PDLLA-PEG-PDLLA to deliver cisplatin and 5-fluorouracil. The hydrogels provided the prolonged release of cargo, and stimulated apoptosis, while suppressing proliferation of cancer cells. On the other hand, the adverse impacts were reduced, and the in vivo experiment highlighted the reduction of growth in metastatic tumors [108].
Compared to systemic therapy, local therapy has been suggested to be more effective in cancer therapy [109–111]. The local intraperitoneal chemotherapy allows to preserve high levels of drug at peritoneal cavity for a prolonged period of time, and this can also decrease side effects [112, 113]. In line with this, hydrogels have been developed from polyethylene-glycol-modified bovine serum albumin (PEG-BSA). Then, red blood cell membrane biomimetic nanostructures containing paclitaxel were loaded into the hydrogels. The nanostructures had a size of 133 nm with a negative zeta potential. Moreover, their drug encapsulation and loading were 85% and 22%, respectively. These injectable hydrogels provided prolonged drug release with a cumulative release of paclitaxel of 30% after six days. In addition to being biocompatible and biodegradable, these hydrogels showed high anticancer activity and impaired tumorigenesis in vivo [114]. Regarding the high importance of intraperitoneal delivery of drugs in GC therapy, a similar strategy has also been used for the delivery of cisplatin. A biodegradable hydrogel has been developed from crosslinking of hyaluronic acid with adipic dihydrazide and aldehyde that can mediate in-situ gelation in peritoneum, and it released cisplatin for 4 days. This delivery system reduced the weight of peritoneal nodules [115]. It appears that the development of hydrogels from hyaluronic acid is beneficial in improving the sustained release of cargo. For instance, hyaluronic acid-based hydrogels can release cisplatin for 2 days [116]. Hybrid networks can be also developed, such as a network of gelatin microsphere-alginate hydrogel, which can provide prolonged release of 5-fluorouracil as cargo in GC therapy [117]. Hydrogels should be developed from sources and compounds to improve their erosion capacity. Notably, the development of hydrogels from poly(organophosphazene) (PPZ) containing α-amino-ω-methoxy-poly(ethylene glycol) (AMPEG) 750 instead of AMPEG 550 improves the erosion ability of the hydrogels, and they can deliver docetaxel in GC therapy [118]. As a result, hydrogels can provide co-delivery of drugs (paclitaxel and doxorubicin) [119] and mediate prolonged release of chemotherapy drugs [120]. Nanoparticles can be loaded in gelatin hydrogels to exert anti-tumor activity upon local implantation [121].
Liver cancer (hepatocellular carcinoma)
Liver cancer is the fifth leading cause of death in the USA, and its incidence rate has gradually increased [122]. Up to 80–90% of all liver cancers are hepatocellular carcinoma (HCC), and cholangiocarcinoma is responsible for the 10–15% of liver cancer cases [123]. Hepatoblastoma and HCC can affect both children and adolescents, responsible for 67–80% and 20–33% of cases, respectively [124]. Despite advances in the treatment of HCC and the development of new strategies for the diagnosis of this disease, it is still one of the leading causes of death. Regardless of the diagnosis, liver cancer cells can develop therapy resistance, urging scientists to develop new strategies for its treatment. Although chemotherapy is the main tool for liver cancer therapy, systemic chemotherapy shows poor efficacy, and it is associated with side effects. Hydrogels can provide new insights for the effective treatment of cancer through local chemotherapy [125]. The hydrogels utilized for the treatment liver cancer have been synthesized from different compounds including hyaluronic acid and tyramine [126], poloxamer 407 and alginate [127], PEG and gelatin [128], PCLA [129], cellulose [130], curdlan derivatives [131], k-carrageenan [132], PVA/CNCs [133] and PNIPAm-co-Polyacrylamide [134]. Each of these hydrogels demonstrates its own benefits, and they can be used for drug delivery, sustained release of cargo, and overall, improving the fight against tumors.
Surgery is one of the options in the treatment of HCC. In addition to the surgical hemorrhage, the relapse rate of HCC is also high. Hydrogels have been shown to overcome such challenges in the treatment of HCC and reduce its recurrence. In this regard, nanoparticle-embedded hydrogels have been suggested. A block copolymer containing PEG and polyglutamic acid has been utilized for the encapsulation of monoclonal antibodies, generating self-assembled micelles. However, such nanostructures suffer from poor stability, and therefore, magnesium calcium carbonate nanostructures encapsulating monoclonal antibodies were developed through a chemical precipitation strategy to improve stability. Finally, the hydrogels synthesized from fibrinogen and thrombin were combined with previously synthesized nanostructures. These hydrogels not only diminish surgical hemorrhage but also prevent cancer recurrence through inducing dendritic cell maturation and activation of T cells to impair tumorigenesis [135].
In addition to proliferation, metastasis and recurrence, another issue in HCC is the angiogenesis, which is the generation of new vessels from the pre-existing ones to increase the nutrition entrance into the tumor site and mediate their spread. HCC could recruit tumor-associated macrophages (TAMs) in revascularization and enhance tumorigenesis. In this regard, hydrogels have been developed from the co-assembly of PCN-Len nanoparticles and oxidized dextran, and then, the nanomodulators of TAMs-reprogramming polyTLR7/8a, known as (p(Man-IMDQ) nanoparticles, were embedded into the hydrogels. Each part has a function in the cancer therapy. PCL-Len nanoparticles can target the tyrosine kinases in vascular endothelial cells to impair VEGFR. Then, (p(Man-IMDQ) nanoparticles mediate the re-education of M2 polarized macrophages into M1 macrophages through mannose-binding receptors and diminish VEGF secretion by suppressing the invasion and growth of vascular endothelial cells. The in vivo experiment showed that single administration of such hydrogels can reduce tumor microvessel density and increase vascular network maturation [136].
In addition to the delivery of chemotherapy drugs, hydrogels have been used to deliver bioactive natural compounds with anticancer activity. Curcumin is a bioactive compound of Curcumin longa, and in addition to its antioxidant activity, this compound has shown high potential in cancer therapy and overcoming chemoresistance. The delivery of curcumin by hydrogels can potentiate its anticancer activity. The hydrogels were developed from glycyrrhetinic acid-modified curcumin supramolecular pro-gelators showing desirable water solubility. The formation of supramolecular gels is due to the disulphide bond reaction mediated by glutathione. The hydrogels can release curcumin in a prolonged manner, and due to the upregulation of GA on the surface of liver cancer cells, they show high internalization in tumor cells and impair tumorigenesis [137]. In addition, the hydrogels developed from PDLLA-PEG-PDLLA can be loaded with paclitaxel nanostructures to impair liver metastasis [138]. Therefore, hydrogels are promising candidates for the suppression of lymph node metastasis [139] and delivery of chemotherapy drugs [140] in cancer therapy.
Pancreatic cancer
Pancreatic cancer (PC) is one of the leading causes of death worldwide [141]. Although PC is accountable for 3% of all cancer cases, PC is responsible for 7% of all cancer deaths [122, 142]. The on-time diagnosis of PC is still challenging, and it is mainly diagnosed in advanced stages. Moreover, only 10% to 20% of patients are eligible for the surgical resection [143]. The aggressive nature of PC can result in drug resistance [144, 145]. Gemcitabine is commonly used for the treatment of PC; however, the tumor cells can develop resistance to therapy. Regarding this, hydrogels have been utilized for the prolonged release of gemcitabine in PC therapy. The hydrogels have been prepared from oxidized-carboxymethylcellulose (OCMC) and CMCS via a dopamine-functionalized method to mediate prolonged release of gemcitabine for impairing growth, increasing apoptosis and the treatment of PC [146]. In another experiment, PEG cross-linked HA hydrogels comprised of PEGylated TRAIL have been prepared. HA was conjugated to the 4-arm PEG(10 k)-amine as cross-linker using another cross-linker 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide hydrochloride, and then PEG-TRAIL was loaded within the hydrogels. The yield for the preparation of hydrogels at a 100:1 ratio was more than 88%, and they were moderately stiff. These hydrogels released PEG-TRAIL in a period of 14 days in vitro, while it lasted 7 days in vivo. They demonstrated high anticancer activity through PEG-TRAIL mediated apoptosis [147]. Since TRAIL is beneficial for increasing apoptosis in PC cells, another study focused on the development of albumin-crosslinked PEG hydrogels through a thiol-maleimide reaction. These hydrogels are comprised of the thiolated human serum albumin and 4-arm PEG20k-maleimide. The gelation time of such hydrogel is adjustable, and it is in the range of 15 s to 5 min. This hydrogel demonstrated a hard interaction with the TRAIL protein, showing favorable release profiles for increasing therapeutic potential. Such hydrogels are beneficial for the injectable, prolonged release of antitumor compounds [148]. Solid tumor treatment can be accelerated by the application of intratumoral radiotherapy. Recently, hydrogels have been applied to potentiate PC radiation therapy. Notably, hydrogels can mediate intratumoral radiotherapy. In this way, thermosensitive micellar nanoparticles were synthesized from an elastin-like polypeptide (ELP), and their labeling was performed by radionuclide (131)I to generate in situ hydrogel. The presence of body heat induces the ELP micelles to change into insoluble form. The high energy β-emissions of (131)I increase depot stability through mediating crosslinks within the ELP depot over 24 h. These ELP depots demonstrated 52% and 70% accumulation in prostate and pancreatic tumors, respectively [149]. However, the studies regarding the hydrogel application in PC therapy are poor in terms of the variety of the applied drugs, the impact on the underlying molecular pathways in cancer, and the lack of attention to overcoming chemoresistance. Figure 2 highlights the development of hydrogels for the treatment of GI tumors.
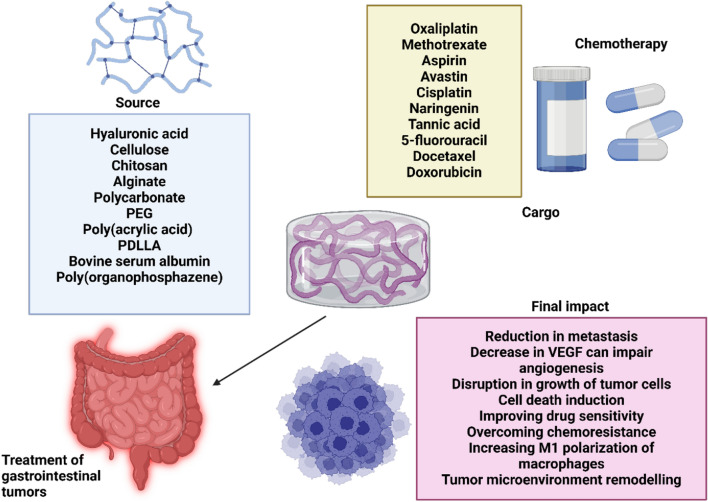
Fig. 2.
The development of hydrogels in the treatment of gastrointestinal cancers. There are multiple sources utilized for the development of hydrogels in gastrointestinal cancer therapy. Various kinds of polymers have been used, especially natural-based polymers such as cellulose, chitosan and alginate. These hydrogels have been mainly deployed for the delivery of chemotherapy drugs including oxaliplatin, doxorubicin, docetaxel and 5-fluorouracil. Their final impact on the tumor cells can be decreasing metastasis and proliferation of cancer cells, macrophage reprogramming to M1 phenotype, reversing drug resistance and reduction in angiogenesis through controlling VEGF levels. (Created by Biorender.com)
Stimuli-responsive hydrogels in the treatment of gastrointestinal tumors
Stimuli-responsive hydrogels respond to the endogenous or exogenous stimuli by changing their physical and chemical features. Such changes can occur in response to pH, temperature, light, magnetic or electric fields, and specific biochemicals. Overall, there are several kinds of stimuli-responsive hydrogels with potential applications in cancer therapy. The pH-sensitive hydrogels are able to respond to alterations in pH levels that are efficient for the drug delivery in TME. The temperature-sensitive hydrogels undergo a volume-phase transition in response to temperature changes that can be utilized for responding to body heat or external temperature stimuli. The light-responsive hydrogels can alter their structure or release the cargo in response to specific light wavelengths with spatio-temporal precision. The magnetic-sensitive hydrogels contain magnetic particles that can react to magnetic fields, thereby releasing cargo. The redox-sensitive hydrogels respond to the alterations in the levels of glutathione in healthy and cancerous tissues. Moreover, there are enzyme-responsive hydrogels capable of responding to the enzymes in TME, including matrix metalloproteinase. More information regarding stimuli-responsive hydrogels can be found in these reviews [150–153].
Stimuli-responsive hydrogels in colorectal cancer
The stimuli-responsive of nanomaterials are a novel class in which they respond to the specific stimulus in the TME to release the cargo. Currently, the TME has a number of distinct features compared to the normal tissues, including alterations in pH, redox status, temperature and enzymes. Therefore, the development of stimuli-responsive hydrogels can provide new insights in the treatment of cancer. The most common kind of hydrogels that have been used for CRC therapy is thermosensitive hydrogels. This kind of hydrogel can be synthesized from conjugated linoleic acid-coupled Pluronic F127 (Plu-CLA) to deliver 5-fluouracil. This thermosensitive hydrogel can impair proliferation and metastasis, while it induces apoptosis in CRC cells [154]. Thermosensitive hydrogels release the drug in demand, and they can also be used for the delivery of other chemotherapy drugs including doxorubicin [155]. Other compounds for the synthesis of thermosensitive hydrogels are poloxamers P407 and P188 as well as alginate that can deliver 5-fluorouracil. This injectable hydrogel demonstrates a free-flowing form in ambient temperature, while having a SOL–GEL transition at 26.0 °C ± 0.6 °C, providing an in situ non-flowing gel at the physiological temperature. This thermosensitive hydrogel showed an elastic behavior and responded to shear-thinning fluid. The presence of alginate delays the release of cargo, and the drug undergoes release mainly by diffusion [156]. Thermosensitive hydrogels can also be synthesized from methyl-cellulose to deliver oxaliplatin as a chemotherapy drug. Along with high biocompatibility and thermosensitive feature, these hydrogels were able to provide prolonged release of cargo, and they stimulated apoptosis through inhibition of the PI3K/Akt axis [157]. Other kinds of nanoparticles can be loaded into thermosensitive hydrogels to increase their potential in cancer therapy. Biodegradable thermosensitive hydrogels have been synthesized from chitosan, and 5-fluorouracil-embedded micellar nanoparticles and cisplatin were loaded in the hydrogels. These hydrogels can disrupt the proliferation and invasion of CRC cells, while improving the survival time of animal model. Moreover, hydrogels can impair the metastasis of CRC cells to liver and lung [158]. As a result, the thermosensitive hydrogels are a promising approach in CRC therapy [159, 160]. However, other kinds of hydrogels, especially pH-sensitive hydrogels, can also be developed for CRC treatment.
Stimuli-responsive hydrogels in gastric cancer
The development of stimuli-responsive hydrogels can also offer help with the fight against GC. The majority of studies have focused on thermosensitive hydrogels in GC therapy. The thermosensitive hydrogels can be developed from poly(organophosphazene) (PPZ) to deliver docetaxel. These hydrogels can decrease tumor growth, improve the survival rate of animal models, and impair peritoneal metastasis in GC [161]. Previously, it was mentioned that Plu-CLA hydrogels can be developed for CRC therapy, and this has also been followed in the treatment of GC. The thermosensitive hydrogels based on Plu-CLA can deliver docetaxel, decreasing survival of GC cells. Moreover, these thermosensitive hydrogels stimulate apoptosis and decrease the number of peritoneal metastatic nodules [162]. The thermosensitive hydrogels are able to encapsulate gabbogic acid-loaded and iRGD-functionalized nanoparticles to exert anticancer activity [163]. The reason for functionalization with iRGD is to increase attachment to integrin receptors for increasing internalization by GC cells.
Stimuli-responsive hydrogels in liver cancer
Similar to other tumor types, stimuli-responsive hydrogels are also beneficial in the treatment of liver tumor. The most common type of hydrogels used for the treatment of liver cancer are thermosensitive hydrogels that can be prepared from PELG-PEG-PEGL to provide prolonged release of paclitaxel, and along with biocompatibility, they can suppress tumor xenograft [164]. In addition to paclitaxel, other kinds of chemotherapy drugs, such as doxorubicin, can be delivered by thermosensitive gels in cancer therapy [165]. One important potential is the co-delivery of chemotherapy drug and natural products in tumor suppression. Notably, thermosensitive Pluronic F127 hydrogels were utilized for the delivery of resveratrol microspheres and cisplatin to accelerate apoptosis and cell cycle arrest. In vivo experiments demonstrated the potential of hydrogels in reducing ascite number, decreasing proliferation, angiogenesis, and improving the survival rate of mice by increasing the potential of intraperitoneal chemotherapy [166]. Notably, the dual responsive hydrogels have been used in liver cancer therapy. The pH- and thermosensitive hydrogels can be developed from N-isopropyl acrylamide (NIPAm) and acrylamide (AAm) as comonomers. These hydrogels are known as PNIPAm-co-PAAm HG and they can deliver curcumin. The release of drug was performed in response to pH and temperature, and drug loading efficacy was 65%. The release of drug was performed at pH 5.5 and 40 °C and despite possessing favorable biocompatibility, they can reduce viability of tumor cells [167]. The hydrogels possessing self-healing and pH-sensitive features are of importance in cancer drug delivery. The changes in the TME (competition for proliferation and metabolism) can decrease the pH levels and put it at a mildly acidic level. Therefore, the pH-responsive hydrogels can release the drugs at the TME. Such hydrogels can be developed from N-carboxyethyl chitosan (CEC) through Michael reaction and dibenzaldehyde-terminated poly(ethylene glycol) (PEGDA). Doxorubicin was loaded in hydrogels for delivery, and the hydrogels possessed self-healing properties because of the dynamic covalent Schiff-base linkage between amine groups from CEC and benzaldehyde groups from PEGDA. In addition to biocompatibility and pH-sensitive release of drug, the hydrogels can impair the HCC [168]. Most studies have focused on the single drug delivery by hydrogels in cancer therapy. In order to accelerate loading bioactive compounds into hydrogel structures, porous hydrogels are suggested that can be developed from pNIPAM particles and deliver 5-fluorouracil and metformin. These hydrogels provide prolonged release of cargo in response to temperature, and due to encapsulation, they support the drug against resolving [169]. One of the challenges in the treatment of liver cancer is the presence of drug resistance. The upregulation of P-glycoprotein (P-gp) as a drug transporter on the surface of cancer cells can mediate drug efflux to decrease accumulation of drugs within the cells and mediate chemoresistance. To overcome such an issue, thermosensitive hydrogels have been developed from Pluronic PF127 and then modified with TPGS to suppress tumor growth and disrupt the resistance in P-gp-upregulating cancer cells [170]. Taking everything together, current studies highlight the importance of hydrogels in liver cancer therapy, and the thermosensitive hydrogels are more beneficial in this case due to responding to temperature. However, more attention should be paid to self-healing, thermosensitive hydrogels. The limitations have been mentioned in the conclusion of this paper.
Stimuli-responsive hydrogels in pancreatic cancer
The development of stimuli-responsive hydrogels is important in the treatment of PC due to their dense TME, requiring site-specific delivery systems to maximize the accumulation and internalization of drugs in PC cells. Paclitaxel-embedded liposomal gels are beneficial in the treatment of PC. The thermosensitive hydrogels demonstrate favorable sol-to-gel transition temperatures, and they can improve the drug stability [171]. A method for the preparation of such thermosensitive hydrogels is their development from PVLA-PEG-PVLA, and then paclitaxel- and gemcitabine-loaded liposomes functionalized with PR-b (targeting integrin α5β1) were loaded into hydrogels. These hydrogels can mediate prolonged delivery of cargo, and they can suppress the growth of PC [172]. Notably, pH- and enzyme-sensitive hydrogels have been also developed for the treatment of PC. These self-healing hydrogels have been developed from PLys- b-(PHIS- co-PBLG)-PLys- b-(PHIS- co-PBLG)- b-PLys that have been combined with the aqueous solution of gemcitabine. After injection at the tumor site, they are transformed into hydrogels, and their degradation occurs after being close to PC due to their pH-sensitive nature, improving their site-specific feature. The in vivo experiment showed that the efficacy of such hydrogels with embedding only 40% of the drug is similar to the peritumoral injection of 100% of the free drug [173]. Figure 3 demonstrates the application of stimuli-responsive hydrogels in GI cancer therapy.
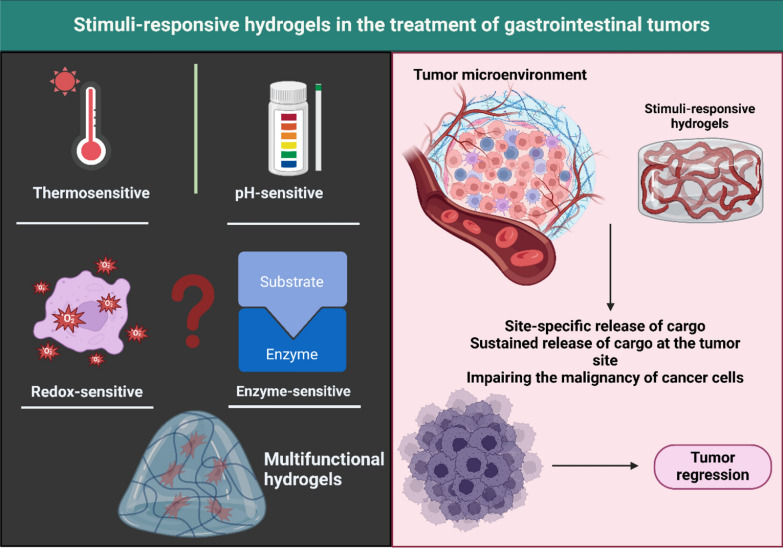
Fig. 3.
The role of stimuli-responsive hydrogels in gastrointestinal cancer therapy. Currently, most of the studies have focused on the application of pH- and thermo-sensitive hydrogels in GI cancer therapy. However, other kinds of hydrogels including redox- and enzyme-responsive are also present that future studies can focus on their application in GI tumor therapy. Such stimuli-sensitive hydrogels can release the cargo (drug or gene) at the tumor site to improve their internalization in reducing tumorigenesis. (Created by Biorender.com)
Hydrogels in the phototherapy of gastrointestinal tumors
Colorectal cancer phototherapy by hydrogels
Regarding the progresses in the field of nanobiotechnology, there has been a significant focus on the application of new methods, including immunotherapy, photothermal (PTT), and photodynamic (PDT) therapy, and their combination in tumor elimination [174–176]. PDT and PTT are becoming novel strategies in cancer therapy because of their non-invasive features, high specificity, and poor adverse impacts [177]. Indocyanine green (ICG) has been utilized as a photothermal compound, and through changing light into the singlet oxygen, it can cure tumor [178]. However, this agent has poor photostability [179]. According to this, ICG-embedded pH-sensitive bortezomib supramolecular hydrogels have been synthesized to improve photostability of ICG and increase the potential of PTT and PDT as well as their combination with chemotherapy [180]. In another work, the hydrogels have been developed from sonosensitizer protoporphyrin IX (PpIX)-conjugated manganese oxide (MnO2) nanoparticles and a glutathione (GSH) inhibitor. In this way, the MnO2 nanostructures can increase oxygen levels to reduce hypoxia and promote ROS generation through the action of sonodynamic therapy. Then, GSH depletion can suppress GSH synthesis, increasing the potential of sonodynamic therapy in CRC therapy [181]. The combination of immunotherapy and phototherapy for the treatment of CRC should be followed in the upcoming studies. Moreover, EGFR-conjugated fucoidan/alginate-embedded hydrogels have been demonstrated to stimulate the EGFR/Akt axis in increasing apoptosis, and they also enhance mtROS levels to induce mitochondrial dysfunction and enhance protein aggregates. Moreover, such hydrogels can mediate cell cycle arrest and increase ROS levels to mediate cell death in CRC cells [182].
Pancreatic cancer phototherapy by hydrogels
In the recent years, phototherapy has emerged as a potential therapeutic strategy for PC [183–185]. Hydrogels can increase phototherapy-mediated PC ablation. In this regard, a thermosensitive liposomal hydrogel was developed for the delivery of gemcitabine and NIR-II photothermal compound, known as DPP-BTz. The injectable hydrogels demonstrated a flowing solution at room temperature, while they demonstrated cross-linked gel structure at the physiological temperature. Exposure to 1064 nm laser irradiation can mediate heat to degrade the thermosensitive liposomes for releasing gemcitabine in the elimination of PC cells, highlighting the importance of phototherapy and chemotherapy combination [186].
Hydrogels in the immunotherapy of gastrointestinal tumors
Colorectal cancer immunotherapy by hydrogels
In the recent years, nanoparticles have been introduced as novel therapeutics for the regulation of tumors and improving cancer immunotherapy. The activation of alternative molecular pathways and other genomic changes in cancer can mediate immune evasion and the low response of patients to immunotherapy. Therefore, nanoparticles have been utilized for the delivery of immunomodulators and reversing the immune evasion in human cancers [10]. Increasing evidences have shown the application of hydrogels for the regulation of immune responses in CRC therapy. Regarding this, thermosensitive hydrogels have been developed based on the self-assembly of PDLLA-PEG-PDLLA to generate PLEL micelles and then loading R848 and oxaliplatin to develop PLDL hydrogels. The in vivo injection of these hydrogels leads to the in situ vaccination of tumor cells through the regulation of TAAs, CRT and HMGB1 to finally induce dendritic cell maturation. Then, dendritic cells mediate proliferation and activation of cytotoxic T lymphocytes in the lymph nodes to increase chemo-immunotherapy [187].
The immune checkpoint inhibitors have been widely utilized in the treatment of cancer, demonstrating better efficacy compared to small molecules and antibodies [188]. In addition, the anti-PD-L1 antibodies have been combined with other therapies, including radiotherapy and chemotherapy, to enhance their potential [189]. The introduction of nanomaterials can improve the efficacy of this therapy [190, 191]. In this regard, sodium alginate hydrogels have been synthesized that are comprised of elesclomol-Cu and galactose, inducing cuproptosis and reducing PD-L1 levels in cancer immunotherapy. The immobilization of elesclomol into a sodium alginate saccharide chain through the coordination with bivalent copper ions (Cu2 +), followed by the incorporation of galactose, can lead to the fabrication of hydrogels. The implantation of these hydrogels into the cancer site can lead to the crosslinking with Ca2+ in the generation of hydrogels. Such hydrogels mediate DLAT oligomerization and cuproptosis in CRC, and despite PD-L1 upregulation by radiotherapy, these hydrogels suppress PD-L1 to release ES-Cu and galactome, increasing the efficacy of immunotherapy and radiotherapy in CRC suppression [192]. Therefore, the immune checkpoint inhibition by hydrogels can accelerate immunotherapy [193].
Gastric cancer immunotherapy by hydrogels
In the recent years, immunotherapy has been introduced as a novel therapeutics for GC. Several factors are responsible for the immune evasion in GC, including IL-1β-associated NNT acetylation [194] and PD-1 [195]. Therefore, hydrogels have been introduced for the immunotherapy of GC. The immunosuppressive TME in GC can be mediated by tumor-associated macrophages (TMAs) that the M2 polarized macrophages have carcinogenic function. In this way, injectable hydrogels have been developed for the delivery of polyphyllin II (PP2) and resiquimod (R848) in localized immunotherapy of GC. The synthesis of hydrogels was based on the Schiff base reactions between aldehyde-functionalized polyethylene glycol and the amino group of polylysine. These hydrogels increase M1 polarization of macrophages and promote the generation of TNF-α and IL-6. Furthermore, they upregulate iNOS/CD206 ratio in TAMs, increase infiltration of CD8+ T cells in GC therapy, and reverse immunosuppressive TME [196].
Liver cancer immunotherapy by hydrogels
One of the most challenging issues in the immunotherapy of liver cancer is the presence of immune evasion. The researchers have revealed a number of mechanisms involved in the immune evasion including PRDM1/BLIMP1 [197], HERC2 [198], HKDC1 [199] and TP53/mTORC1 [200]. However, such advancements have not been sufficient to overcome immune evasion in cancer patients. Therefore, novel strategies should be used with the possibility of clinical translation, and hydrogels are among them. The abnormal expression of ABHD5 is observed in HCC, and it can mediate PD-L1 to induce immunosuppression. An experiment has focused on the delivery of ABHD5 using the host–guest interaction with branched polyethyleneimine-g-poly (ethylene glycol), poly (ethylene oxide) and poly (propylene oxide) block copolymers and α-CD (PPA/CD) for the prolonged release of cargo. These hydrogels possess high stability and elevate the gene transfection efficiency for increasing apoptosis in the HCC cells [201]. Navoximod, an Indoleamine 2, 3-dioxygenase 1 (IDO1) inhibitor, is able to enhance the herpes simplex virus type 1 (HSV-1) replication and HSV-1-induced oncolysis in cancer. Therefore, it is ideal for combination with HSV-1-mediated virotherapy. Both HSV-1 and Navoximod have been loaded in the injectable and biocompatible hydrogels known as V-Navo@gel to treat HCC. Such hydrogels provided a local delivery to increase the replication of virus (increasing anticancer immune responses) and were able to distribute in the tumor site upon one injection. These hydrogels improved the disease-free survival of mice and prevented cancer relapse. The mechanism of IDO1 upregulation is that virus induces Senser to mediate nuclear translocation of IRF1. Then, secretion of IFNα/β occurs, which mediates the STAT1-STAT2 complex to increase IDO1 levels in inhibition of virus replication. Therefore, its inhibition by hydrogels can increase virus-mediated anticancer immune responses [202]. Hydrogels can be harnessed for improving the cell death-accelerated cancer immunotherapy. Notably, chitosan hydrochloride and oxidized dextran (CH-OD) were used to develop injectable hydrogels for the delivery of sulfasalazine. Such hydrogels stimulate ferroptosis with immunogenic activity. Moreover, these hydrogels can induce M1 polarization of macrophages and enhance maturation of dendritic cells to accelerate immunotherapy. These hydrogels also demonstrate synergistic impact with PD-1 immunotherapy to regress up to 50% of ascites and mediate long-term immune memory [203].
Pancreatic cancer immunotherapy by hydrogels
Immunotherapy has emerged as a potent strategy in the elimination of PC. Regarding the fact that various factors and mechanisms, including CD155/TIGIT [204], autophagy-induced MHC-I degradation [205] and FAK-inhibited antigen processing [206], participate in immune evasion in PC, it would be difficult to target all of these factors to disrupt immune evasion. Therefore, current treatments have been directed towards potentiating the immunotherapy of PC using novel strategies, and hydrogels are among them. TME remodelling by hydrogels has been suggested to be beneficial in cancer immunotherapy. The injectable hydrogels were prepared from chitosan and then IRF5/CCL5-siRNA-embedded nanostructures were loaded into the hydrogels to mediate immune niche reprogramming. Such hydrogels can increase IRF5 levels and reduce CCL5 secretion to promote M1 polarization of macrophages, increasing T-cell mediated responses in PC immunotherapy [207]. The hydrogels can function as vaccines in PC immunotherapy. The CD103+ CD11b− cDC1-activated hydrogel microsphere vaccine was prepared for the PC ablation. For this purpose, Cas9 plasmid and CD40L were loaded into liposomes and CaCO3 nanostructures, respectively, and at the next step, their combination with FLT3L cytokines and HA was performed to generate a hydrogel microsphere vaccine. These hydrogels can increase cDC1-induced antigen cross-presentation and CD8+ T-cell induction to trigger PC transformation from “cold” to “hot” tumor. Moreover, such hydrogels increase systemic immunity and suppress distant metastasis that are valuable in improving the survival of animal models [25]. Notably, hydrogel-mediated immunotherapy can prevent PC recurrence after surgical resection [208]. Figure 4 highlights the application of hydrogels in the phototherapy and immunotherapy of GI tumors.
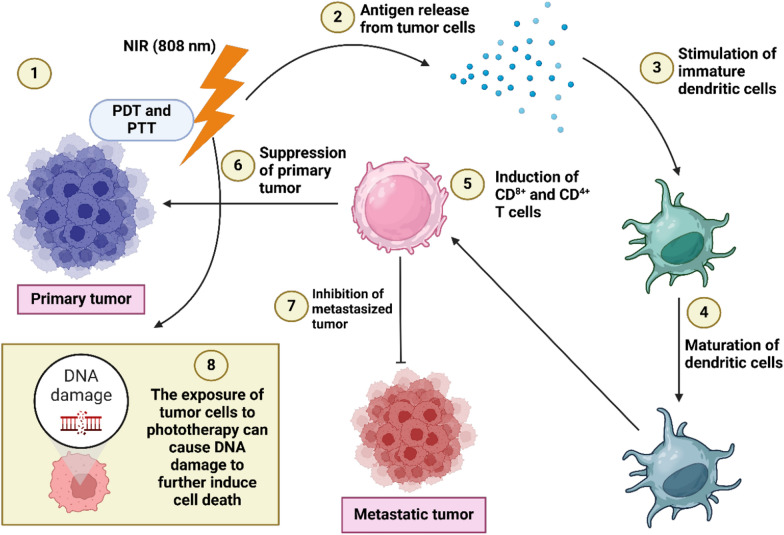
Fig. 4.
The potential of hydrogels in accelerating phototherapy and immunotherapy. The exposure of primary tumor cells to NIR irradiation can cause photothermal and photodynamic therapy (1), resulting in the release of antigens from tumor cells (2). Then, the dendritic cells are stimulated (3) and they are matured (4) to induce CD8+ and CD4+ T cells, (5) impairing progression of primary tumor (6) and reducing tumorigenesis of metastatic tumor (7). Moreover, phototherapy can stimulate DNA damage to mediate cell death in disrupting progression of cancer cells (8). (Created with Biorender.com)
Hydrogels in the diagnosis and detection of gastrointestinal tumors
Colorectal cancer
One of the simple methods for cancer diagnosis is the extraction of exosomes from interstitial fluid. Hydrogel microneedles are promising in this way, and they are able to be expanded in the skin tissue for interstitial fluid absorption. Then, exosomes can bind to the glypican-1 antibodies within the structure of hydrogels. Upon the removal of hydrogels, it is possible to purify the exosomes from interstitial fluid for the investigation of tumor-associated biomarkers [209]. Another method for the diagnosis of CRC is based on the determination of serological signatures [210]. In this way, the hemispherical hydrogels comprised of immobolized proteins and oligosaccharides (glycohip) can be utilized. The compounds immobilized on the glycochip are tumor-associated glycans (SiaTn, Tn, TF, Le(C), Le(Y), SiaLe(A), and Manβ1-4GlcNAcβ) and antibodies against human immunoglobulins IgG, IgA, and IgM. This glycochip has demonstrated potential in the detection of antibodies against the tumor-associated glycans in patient’s sera. Another important source for the detection of CRC is based on the circulating tumor markers [211]. Regarding this, chemically stable and instantly degradable (CSID) hydrogel immunospheres have been developed to isolate circulating tumor cells (CTCs) and circulating tumor exosomes (CTXs). The synthesis of CSID hydrogels has been performed by the hybridization of alginate and poly(vinyl alcohol). These hydrogels demonstrate pH-responsive feature, and they are stable in the biological buffer (no sign of dissociation). The chelating agents mediate gradual degradation of hydrogel. In the next step, the CSID hydrogels were functionalized with anti-EpCAM antibody and anti-CD63 antibody for the isolation of circulating markers in liquid biopsy, including CTCs and CTXs. Hydrogels can even be used for the evaluation of gene expression in CRC [212]. In this way, a new system has been developed known as MDHB (Multiplexed Digital-PCR coupled with Hydrogel Bead-array). In order to prepare a template for bead-based emulsion PCR (emPCR), reverse transcription using sequence-tagged primers was used. The immobilization of emPCR was performed by hydrogels to generate a single-bead layer on a chip. This system was decoded by gene-specific probe hybridization and Cy3-dUTP based primer extension reaction. The presence of positive beads is in parallel with the abundance of expressed genes. The MDHB system demonstrated a discrimination limit of 0.1%, and the sensitivity was below 100 cells upon application of β-actin gene as the detection target. The MDHB system has been utilized to detect the genes for CRC progression, including c-myc, COX-2, MMP7, and DPEP1, with high detection scores. Therefore, hydrogels are promising candidates for the diagnosis of CRC in the future.
Gastric cancer
One experiment has evaluated the application of hydrogels in the detection of biomarkers in GC. Since GC is a malignant disease, it is of importance to detect it in early stages, and the development of materials for its diagnosis is also valuable. Regarding this, supramolecular hydrogels have been developed from Cy5.5-conjugated polyethyleneimine/hyaluronic acid polyplexes that have CD44-targeting ability and are sensitive to near-infrared (NIR) light. These hydrogels demonstrated high water stability, biocompatibility, and selective targeting of CD44 on the stem-like GC cells. The NIR-sensitive feature provided the ability of these nanoparticles for the imaging of these stem cells [213]. Since the stem cells are responsible for the carcinogenesis and cancer relapse, such a system can provide new insights towards diagnosis and treatment of GC.
Liver cancer (hepatocellular carcinoma)
Similar to other gastrointestinal tumors, the diagnosis of liver cancer can occur through CTCs. However, the use of CTCs for the diagnosis of liver cancer has been faced with issues in clinics due to the low abundance of CTCs in the blood environment, making it hard for their capture. Therefore, a new strategy has been developed to recognize the sialyated glycans on the surface of CTCs using an ultrastrong ligand, l-histidine (HH). The integration of HH and a cell-imprinted polymer was performed to develop a hydrogel for CTC imprinting, providing high elasticity, hemocompatibility and anti-interference abilities. The capture efficiency of hydrogels was suggested to be more than 95% in the peripheral blood, and they can release CTCs in a controlled way. This strategy has been beneficial in distinguishing between the liver cancer, cirrhosis, and healthy groups. The diagnostic accuracy was estimated to be 94%, and this method was affordable [214].
Pancreatic cancer
The diagnosis of PC can also be accelerated by hydrogels. PC cells release carbohydrate antigen 19–9 (CA 19–9) as a glycosylated protein biomarker that has low levels in healthy patients (< 37 U/ml), but its levels increase in cancer patients [215]. Evaluating Ca19-9 levels is beneficial after surgical resection to monitor the disease [216, 217]. An experiment has focused on the development of hydrogels from cellulose for encapsulating quantum dots and molecularly imprinted materials (MIPs) in PC diagnosis. The MIPs function as biorecognition elements, and their conjugation to cadmium telluride quantum dots occurs as a sensing system. The excitation wavelength and emission range were 477 nm and 530–780 nm, respectively. The enhancement in the levels of CA19-9 can mediate fluorescent quenching of cellulose hydrogels, displaying linearity in the range of 2.76 × 10 −2—5.23 × 10 2 U/ml. Notably, these hydrogels have a linear response lower than the cut-off values for the diagnosis of PC (< 23 U/ml) along with detection limit and imprinting factor of 1.58 × 10 −3 U/ml and 1.76, respectively. The hydrogels have high specificity towards CA19-9, and they possess good stability, highlighting their importance in the diagnosis of PC [218].
Hydrogels in gene delivery for the treatment of gastrointestinal tumors
Colorectal cancer
In recent years, gene therapy has emerged as a promising tool in tumor suppression. However, gene therapy suffers from degradation of genes by enzymes and their poor selectivity towards tumor cells. Therefore, the application of nanoparticles for gene delivery has increased. Although the focus of current studies has been on drug delivery using hydrogels, several experiments have investigated the potential of hydrogels in gene delivery. The presence of ulcerative colitis can result in the development of CRC. Therefore, the alleviation of colitis can prevent the CRC. The downregulation of TNF-α by siRNA is beneficial for the alleviation of colitis. Notably, a hybrid structure of sodium alginate hydrogel and metal–organic framework (MOF) has been developed that can accumulate in the local colon tissues to downregulate TNF-α for the alleviation of colitis, lacking impact on the weight, blood parameters and diarrhea in mice [219].
Gastric cancer
For the delivery of siRNA, lentiviral transfection is used, but there are some safety issues in vivo. Therefore, biomaterials and non-viral vectors have been utilized for the delivery of therapeuticscompounds including carbon nanotubes and chitosan [220, 221]. Collagen has also been of importance in the development of drug delivery systems, and it is a natural component of connective tissue. The collagen-based biomaterials demonstrate a number of features, including biocompatibility, high safety, and unique characteristics [222]. Collagen has been used for the siRNA delivery to improve its local retention and release of cargo in a prolonged manner [223]. In line with this, hydrogels have been developed from collagen to deliver id1-siRNA. For improving the siRNA delivery, cationic polyethylenimine (PEI) has been loaded into the scaffold. These structures can deliver Id-1-siRNA and due to adding PEI to collagen, the intracellular accumulation in the GC cells increases to suppress tumorigenesis [224].
Liver cancer (hepatocellular carcinoma)
RNA interference (RNAi) has become a promising tool in the treatment of human diseases since 1998 [225]. The inhibition of expression in specific genes can be provided by siRNA application. Despite the potential of siRNA as a powerful gene modulator, the degradation of this tool in biological fluids and the physico-chemical characteristics, including high molecular weight, negative charge, and rigid structure, can reduce the intracellular accumulation of siRNA. Moreover, siRNA has an issue in endoplasmic reticulum escape and release in cytoplasm [226]. The application of siRNA in liver cancer has shown a significant increase, and one of the reasons is the dysregulation of molecular pathways in its progression. Hydrogels have been shown to be promising for siRNA delivery in HCC therapy. Injectable hydrogels have been utilized for the delivery of siRNA-loaded nanoparticles. In order to increase specific cancer delivery, siRNA was conjugated to GalNAc. This complex was then grafted to DP7-C nanoparticles and finally loaded into hydrogels. These hydrogels can improve the stability and enhance the potential of siRNA in downregulating Pin1, disrupting HCC progression [227]. However, studies related to the gene delivery should be increased to better understand the concept of hydrogels in gene therapy. Notably, hydrogels have been utilized in the delivery of microRNAs (miRNAs) with dysregulation in HCC. In this line, miR-192 was embedded in hydrogels and through binding to WNT10B, it impairs the Wnt/β-catenin axis for the inhibition of tumorigenesis. Moreover, upregulation of miR-192 through the hydrogel delivery diminished the levels of CD90, EpCAM, and CD133. The delivery of miR-192 by hydrogels can stimulate apoptosis while it suppressing growth and metastasis of HCC cells [228]. Table 1 provides a summary of the application of hydrogels in the treatment of GI tumors.
Table 1.
The introduction of hydrogels in the treatment of gastrointestinal tumors
| Hydrogel | Cancer | Remark | References |
|---|---|---|---|
| Hydrogel microsphere vaccine | Pancreatic cancer | Release of FLT3L and CD40L at acidic pH to enhance migration of dendritic cells to lymph nodes for enhancing anti-cancer immunity | [25] |
| PLGA-PEG-PLGA hydrogel | Pancreatic cancer | Injectable and biocompatible hydrogels for the delivery of gemcitabine in enhancing apoptosis an dreducing proliferation | [229] |
| sulfhydryl-hyaluronic acid-dopamine hydrogel | Pancreatic cancer |
Loaded with polydopamine-cloaked cytokine interleukin-15 and platelets conjugated with anti-TIGIT Increasing number of CD8 + and NK cells to boost anti-cancer immunity |
[208] |
| PVLA-PEG-PVLA hydrogel | Pancreatic cancer | Delivery of liposomes consisting of paclitaxel and gemcitabine to suppress progression | [172] |
| GelMA/SilMA hydrogel | Colorectal cancer |
Loading curcumin-shellac nanoparticles within the structure of hydrogels Controlled release through the swelling and degradation of matrix Increasing cellular uptake of curcumin |
[230] |
| mPEG-luteolin-BTZ@ICG hydrogels | Colorectal cancer |
Delivery of indocyanine green and bortezomib to exert combination chemotherapy and photodynamic therapy Favorable tumor suppression activity |
[180] |
| PEG-PCL-PEG hydrogel | Colorectal cancer | Sustained delivery of 5-fluorouracil to suppress growth and dissemination of tumor cells | [93] |
| pH-responsive nanohydrogels | Colorectal cancer |
Delivery of naringenin Prolonged and targeted release of bioactive compound in response to pH |
[101] |
| Methyl-cellulose-based injectable hydrogel | Colorectal cancer |
Thermo-sensitive feature Delivery of oxaliplatin Impairing peritoneal metastasis |
[157] |
| Solid lipid nanoparticle-loaded hydrogels | Colorectal cancer |
Loading topotecan in solid lipid nanostructures to embed inside hydrogels for the colorectal delivery Improved anti-cancer activity |
[94] |
| Magnetic-driven hydrogel microrobots | Colorectal cancer | Improving anti-cancer activity of lycorine hydrochloride to reduce proliferation and mobility along with apoptosis induction | [95] |
| Thermosensitive hydrogels | Colorectal cancer |
Loading 5-fluorouracil-embedded micelles and cisplatin inside the hydrogels Reduction in proliferation and invasion Improving survival rate |
[158] |
| Injectable hydrogel | Hepatocellular carcinoma |
Development of hydrogels from branched polyethyleneimine-g-poly (ethylene glycol), poly (ethylene oxide) and poly (propylene oxide) block copolymers and α-CD (PPA/CD) Delivery of ABHD5 siRNA to improve gene transfection efficiency and mediate apoptosis |
[201] |
| Injectable hydrogel | Hepatocellular carcinoma |
Development of hydrogels from dextran and chitosan Stimulation of ferroptosis and increasing M1 polarization of macrophages to boost immunotehrapy |
[203] |
| Magnetic hydrogels | Hepatocellular carcinoma | Thermo-sensitive feature and ability in decreasing postoperative recurrence rate | [231] |
| Nanohydrogel | Hepatocellular carcinoma |
The development of hydrogels from algin/polyethyleneimine Delivery of miR-192 to downregulate Wnt expression |
[228] |
| Injectable thermosensitive hydrogel | Hepatocellular carcinoma |
Delivery of GalNAc-siRNA and DP7-C nanostructures by hydrogels Endosomal escape in hepatocytes Suppressing tumor expression and controlling Pin1 expression |
[227] |
| Thermosensitive hydrogel | Hepatocellular carcinoma | Loading norcantharidin nanoparticles and oxaliplatin within the hydrogels to disrupt angiogenesis and proliferation and improve the survival of animal model | [232] |
| Thermosensitive hydrogel | Hepatocellular carcinoma | Loading norcantharidin nanostructures and doxorubicin inside the hydrogels to reduce proliferation and angiogenesis | [233] |
| GelMA/PVA hydrogel | Hepatocellular carcinoma | Suppressing β-klotho/HDAC3 axis to reduce progression of cancers | [234] |
| Alginate hydrogel | Hepatocellular carcinoma |
Loading MSA-2 as STING agonist inside the hydrogels to enhance M1 polarization of macrophages and enhance dendritic cell maturation Promoting the infiltration of lymphocytes in cancer immunotherapy |
[235] |
| Injectable nanocomposite-hydrogel | Hepatocellular carcinoma |
Gradual release of lactate oxidase (LOX)-loaded hollow mesoporous MnO2 nanostructures to reduce levels of lactate Reversing immunosuppressive tumor microenvironment and exerting combination with immunotherapy |
[236] |
| Magnetic colloidal hydrogel | Hepatocellular carcinoma |
The development of hydrogels based on a binary system comprising super-paramagnetic Fe3O4 nanoparticles and gelatin nanoparticles Extruding through percutaneous needle and self-healing activity The generation of heat |
[237] |
| 4armPEGDA and N-carboxyethyl chitosan hydrogels | Hepatocellular carcinoma |
pH-sensitive feature an self-healing property Loading doxorubicin on hydrogels to impair cancer growth |
[238] |
| Thermo-sensitive hydrogel | Hepatocellular carcinoma |
Development of hydrogels from F127 and loading with doxorubicin and Au-MnO-L nanostructures Exerting photothermal activity Injectable feature Sustained drug release Combination of chemotherapy and photothermal therapy |
[239] |
| Acrylate-based hydrogels | Hepatocellular carcinoma | High biocompatibility and delivery of doxorubicin to exert anti-cancer activity | [240] |
| Nanocomposite hydrogel | Hepatocellular carcinoma | Development of hydrogels from PDLLA-PEG-PDLLA to induce toxicity of dendritic cells and lymphocytes | [241] |
| PDLLA-PEG-PDLLA hydrogels | Hepatocellular carcinoma |
Thermo-sensitive feature and loading norcantharidin inside the structure of hydrogels Improving retention time of drug |
[242] |
| Thermo-sensitive hydrogel | Hepatocellular carcinoma |
Development of hydrogels from PCL-PEG-PCL copolymer Improving residence time of drug in tumor site Loading liposomal doxorubicin inside the hydrogels Reducing tumor growth |
[243] |
| Thermosensitive injectable hydrogel | Hepatocellular carcinoma |
Development of hydrogels based on PECT Increased anti-cancer activity of embelin |
[244] |
| Thermosensitive injectable hydrogel | Colorectal cancer | The OxP/R848@PLEL hydrogels can provide synergistic anti-cancer activity of oxaliplatin and resiquimod (R848_ to enhance dendritic cells maturation and promote the expansion of T lymphocytes | [187] |
| Natural hydrogels | Colorectal cancer | Development of hydrogels from alginate and sodium carboxymethyl cellulose with pH-sensitive feature to deliver aspitin and methotrexate | [84] |
| Thermosensitive hydrogel | Colorectal cancer | The thermosensitive hydrogels were prepared based on the features of poloxamers P407 and P188 to deliver 5-fluorouracil in impairing cancer growth | [156] |
| Injectable biodegradable hydrogels | Colorectal cancer |
Targeted delivery of avastin Development of hydrogels from vitamin D-functionalized polycarbonates |
[86] |
| HP-β-CD/agarose-g-poly(MAA) hydrogel | Colorectal cancer |
pH-sensitive feature Favorable biocompatibility Delivery of capecitabine in colorectal cancer therapy |
[245] |
| Cisplatin hydrogel | Gastric cancer |
Improving survival time of animal model Reducing tumor growth and metastasis |
[107] |
| Thermosensitive hydrogel | Gastric cancer | Co-delivery of 5-fluorouracil and cis-platinum to exert synergistic impact in suppressing recurrence and growth of metastatic tumors | [108] |
| Albumin hydrogel | Gastric cancer |
Loading paclitaxel in red blood cell membrane nanostructures and their inclusion in hydrogels Favorable biocompatibility and biodegradability Induction of chemotherapy |
[114] |
| Thermosensitive hydrogel | Gastric cancer |
Development of hydrogels from poly (organophosphazene) (PPZ) to deliver docetaxel Reduction in tumor growth Impairing peritoneal metastasis |
[161] |
| Thermosensitive hydrogel | Gastric cancer | The linoleic acid-incorporated poloxamer hydrogels have been loaded with docetaxel to impair peritoneal metastasis | [162] |
| Natural hydrogel | Gastric cancer | Intraperitoneal delivery of cisplatin using hyaluronan-based nanogels to suppress peritoneal dissemination of tumor cells | [116] |
| Thermosensitive hydrogel | Gastric cancer |
Loading gambogic acid nanostructures and iRGD as peptide inside the hydrogels High anti-cancer activity |
[163] |
| Injectable hydrogels | Gastric cancer | The polylysine hydrogels can deliver polyphyllin II (PP2) and resiquimod (R848) (PR-Gel) to induce M1 polarization of macrophages in cancer immunotherapy | [196] |
| In-situ forming hydrogels | Gastric cancer | The prolonged release of siRNA/PEI complex from hydrogels can reduce the levels of id1 to suppress cancer progression | [224] |
| Natural hydrogels | Colorectal cancer |
Development of hydrogels from hyaluronic acid and carboxymethyl cellulose sodium Prolonged release of oxaliplatin Providing intraperitoneal chemotherapy |
[78] |
| Composite hydrogels | Colorectal cancer |
Development of composite hydrogels from sonosensitizer protoporphyrin IX-conjugated manganese oxide (MnO2) nanoparticles and a glutathione (GSH) inhibitor after Ca2+ induced in situ gelation in the tumor site Amelioration of hypoxia through generation of oxygen Enhancing the levels of ROS |
[181] |
| PLGA microparticle-loaded hydrogels | Colorectal cancer |
Delivery of oxaliplatin and improving pharmacokinetic profile Sustained drug release |
[79] |
Clinical considerations
The clinical application of hydrogels is advantageous in the treatment of gastrointestinal tumors. These structures demonstrate desirable biocompatibility, and they are promising candidates for the sustained delivery of drugs in cancer therapy. Moreover, hydrogels can accelerate immunotherapy and phototherapy for tumor elimination. However, several criteria should be considered for their clinical application. The accumulation of hydrogels at the tumor site occurs in a passive manner, and their accumulation reduces with time and reaches baseline within 24 h. Despite low accumulation in liver tissue, there is no detection of hydrogels in the kidney or bladder, demonstrating specific accumulation of these structures in the tumor site. However, this depends on the composition of hydrogels and their size, stiffness, charge, and shape. Accordingly, adding quantum dots as a hard material to the structure of hydrogels can increase their accumulation at the tumor site [246]. Since the clinical application of hydrogels depends on their selective accumulation at the tumor site, it is suggested to add such hard materials into the structure of hydrogels. However, caution should be directed towards the biocompatibility of the added hard material, its metabolism, and clearance in the body. Regarding the importance of biocompatibility, a number of studies have focused on this issue. The HP-β-CD/agarose-g-poly(MAA) hydrogels have been demonstrated to be effective structures in drug delivery (capecitabine), and the evaluation of their toxicity has shown that they are biosafe and biocompatible, lacking toxicity on oral, dermal, or ocular organ tissues, along with no changes in the blood parameters and histological profile of vital organs [245]. According to this, even adding other structures into the hydrogel network cannot significantly change its biocompatibility. However, a rational investigation of metabolism and clearance in the body is required. Moreover, studies have evaluated the biocompatibility of hydrogels for a short time, while long-term studies are required to further highlight their biocompatibility and safety profile. Although hydrogels have shown significant impact in the treatment of gastrointestinal tumors, some cautions should also be considered. Depending on the structure and network of hydrogels, these materials can affect cancer hallmarks. For instance, biomimetic hydrogels grafted with GRGDS peptide and comprised of polyisocyanides can affect liver cancer progression. Hydrogels, comprised of long polymers with high critical stress, can increase the migration of liver cancer cells and promote actin stress fibers, along with inducing nuclear transfer of YAP [247]. Two points should be considered: similar experiments should be performed on other gastrointestinal tumors to confirm such function. Moreover, this impact of hydrogels on the cancer migration may aggravate the situation in clinical studies since the preparation of hydrogels on a large-scale is quite complex, and therefore, such adverse impacts on cancer patients should be considered and investigated to provide a better treatment of tumor. Despite the ideal promise of hydrogels for biomedical applications, the poor mechanical characteristics of hydrogels are a challenging issue. These challenges can reduce the therapeutic importance of hydrogels, especially for the treatment of gastrointestinal tumors, and provide challenges in the prolonged release of cargo. To overcome this, special attention should be directed towards the synthesis and compounds utilized for hydrogel preparation. The preparation of nanocomposite hydrogels from quaternized cellulose and rigid rod-like cationic cellulose nanocrystals is promising, and such hydrogels show gelation with temperature increase. These nanocomposite hydrogels demonstrate favorable mechanical strength along with high extension in degradation and prolonged release of cargo. Such unique features are because of the strong interaction between cellulose and nanocrystals through the cross-linking agent (β-glycerophosphate) [248]. This aspect is of importance in clinical studies, because hydrogels may follow the way of the gastrointestinal tract and are exposed to harsh conditions such as acidic stomach juice. Therefore, improving the mechanical strength and degradation features of hydrogels can provide sustained drug release in cancer therapy.
Conclusion and limitations: directions to the future studies
Hydrogels are promising candidates for the treatment of human cancers. One of the main reasons for the application of hydrogels in the treatment of cancer is the poor efficacy of the conventional therapeutics in cancer removal. Surgical resection has faced challenges due to the spread of tumor cells to other parts of body; chemotherapy and radiotherapy suffer from resistance and side effects; and immunotherapy has been challenged by the development of immune evasion. As a result, new kinds of therapies should be introduced for the suppression of tumors. Hydrogels are ideal candidates in this scenario, as they have demonstrated numerous benefits in the treatment of human cancers by facilitating drug delivery and addressing the aforementioned issues. In case of gastrointestinal tumors, increasing evidences has shown that hydrogels can improve the accumulation of drugs at the tumor site, and their stimuli-responsive types, including thermo- and pH-sensitive kinds, have been developed. Moreover, hydrogels can deliver phytochemicals with anticancer activity, synthetic drugs or their co-delivery in potentiating cancer suppression. Despite the introduction of gene therapy for cancer suppression, the precise delivery and protection of these molecules are needed. Therefore, hydrogels have been explored for the delivery of siRNA in the suppression of gastrointestinal tumors through encapsulation, site specific delivery, and protection against enzyme degradation. PDT and PTT are major therapeutics for gastrointestinal tumors, but the pharmacokinetic profile of these compounds is poor. Hydrogels can deliver such compounds for improving phototherapy-mediated tumor ablation. In addition, hydrogels can improve gastrointestinal cancer immunotherapy through the activation of immune-related cells, such as dendritic cells, to induce their migration into lymph nodes and increase T cell activity. Furthermore, hydrogels with immunogenic activity can improve ferroptosis. Finally, increasing evidence has shown that hydrogels are promising in the diagnosis of gastrointestinal tumors through isolation of circulating tumor cells, detection of biomarkers, and recognition of cancer stem cells, which are beneficial in on-time diagnosis of tumors. Despite such advances, there are still a number of limitations, as follows:
-
A)
Stimuli-responsive hydrogels have been synthesized for gastrointestinal tumor therapy. However, the major focus has been on the thermosensitive hydrogels, and a few studies have mentioned the application of pH-sensitive hydrogels. There are also other unique features in the TME, including redox status and enzymes that can be used for the development of stimuli-responsive hydrogels,
-
B)
Different kinds of nanoparticles and structures have been included in hydrogels for the gastrointestinal cancer therapy. One aspect that has been ignored is the inclusion of β-cyclodextrin in the hydrogels. β-cyclodextrin has been significantly applied in the cancer therapy [249–251], and this structure is able to provide the prolonged release of drugs and other cargoes. Therefore, its inclusion can promote the sustained release feature of hydrogels in potentiating gastrointestinal cancer therapy,
-
C)
The major emphasis has been on the diagnosis and therapy of gastrointestinal tumors by hydrogels, while their internalization mechanism has been ignored. Moreover, the stiffness of hydrogels may increase the migration of these tumors, and for biosafety issues, the degradation, clearance from the body, long-term safety, and mechanical strength in the treatment of gastrointestinal tumors should be evaluated in more experiments,
-
D)
Regarding GC, the application of hydrogels has increased the fight for their treatment. However, there is no experiment using hydrogels in the phototherapy of GC,
-
E)
Hydrogels have been used for TME remodeling, such as reprogramming TAMs, in the alleviation of immunosuppressive TME. However, a number of aspects have been ignored, including the function of hydrogels in affecting TAMs and manipulating their exosome section (macrophages can secrete exosomes to transfer cargo in tumorigenesis) and their ability to regulate cancer-associated fibroblasts as other factors involved in tumorigenesis,
-
F)
The potential of hydrogels in boosting cancer immunotherapy has been evaluated. The major focus has been on the activation of dendritic cells and the induction of ferroptosis-mediated immunotherapy. The immunogenic cell death is another factor for cancer therapy [252, 253] that potential of hydrogels for its regulation in gastrointestinal tumors should be evaluated. Moreover, the induction of natural killer cells and direct activation of CD8+ and CD4+ T cells require investigation,
-
G)
The application of hydrogels for the delivery of siRNA has been explored in gastrointestinal tumors. Hydrogels can protect the siRNA against enzymatic degradation, prolong blood circulation time, and increase tumor accumulation. However, there is no evidence regarding the application of hydrogels for the delivery of shRNA, CRISPR/Cas9, plasmids, and non-coding RNAs,
-
H)
An experiment has focused on the role of hydrogels for the delivery of miR-192 in HCC therapy to increase apoptosis and reduce proliferation, metastasis, and stemness. However, the impact on the regulation of chemoresistance should be investigated,
-
I)
Despite significant efforts to understand the function of hydrogels in gastrointestinal cancer therapy, there is an ignorance towards the regulation of cancer drug resistance that should be further elucidated,
-
J)
PC has a dense TME, and it impairs the entrance of drugs and other therapeutics for internalization in the tumor cells. Adding some agents for the degradation of TME in hydrogels can improve the accumulation and internalization of drugs in PC cells,
-
K)
Hydrogels have been used for the CTC detection in liver cancer. However, there are also other biomarkers in GC, including non-coding RNAs and exosomes, that can be identified for the diagnosis of GC. Furthermore, hydrogels can also be utilized for the diagnosis and therapy of GC simultaneously, known as theranostics, so that future studies can focus on it. One possibility is loading quantum dots into hydrogels for therapy and imaging,
-
L)
Various types of nanoparticles have been loaded into hydrogels for gastrointestinal cancer therapy. However, there is no experiment about loading metal–organic frameworks (MOFs) in hydrogels that can improve drug and gene delivery as well as phototherapy to accelerate gastrointestinal tumor suppression,
-
M)
An interesting point regarding the hydrogels is their synthesis from natural sources with high anticancer activity and favorable biocompatibility,
-
N)
One of the recent advances is the combination of exosomes and hydrogels to create a hybrid system. This can improve a number of characteristics of hydrogels, especially biocompatibility. However, this strategy has been ignored in the treatment and diagnosis of gastrointestinal tumors,
-
O)
Exosomes are potential factors in cancer therapy. Regarding the high importance of exosomes in gastrointestinal tumors, hydrogels are promising for the detection of exosomes in the blood to accelerate diagnosis of these tumors,
-
P)
Hydrogels have been used for the PTT of PC. However, PDT has been also shown to be beneficial in impairing PC progression [254, 255]. Loading PDT compounds in hydrogels and evaluating their potential in PDT-mediated PC ablation requires investigation,
-
Q)
Esophageal cancer is another gastrointestinal tumor, but hydrogels have been applied for the applications other than esophageal cancer therapy and reducing esophageal stricture, among others [256–258]. Therefore, the application of hydrogels for increasing immunotherapy, diagnosis of this tumor and its suppression through gene and drug delivery should be evaluated.
Acknowledgements
Some of the figures have been prepared by Biorender.com.
Author contributions
Min Tang, Junzhou Song, Shuyi Zhang, Xiaolei Shu, Shuang Liu, Writing-original first draft, Yavuz Nuri Ertas, Supervision, Validation, English Editing, Milad Ashrafizadeh, Ya Zhou, Ming Lei, Conceptualization, Writing-review editing.
Funding
None.
Availability of data and materials
This is review paper and no data has been produced.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Min Tang, Junzhou Song, and Shuyi Zhang have contributed equally to this work.
Contributor Information
Milad Ashrafizadeh, Email: dvm.milad73@yahoo.com.
Ya Zhou, Email: 15823063152@163.com.
Ming Lei, Email: leiming2024666@163.com.
References
- 1.Bray F, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 2.Ammendola M, et al. Stability and anti-proliferative properties of biologically active compounds extracted from Cistus L. after sterilization treatments. Sci Rep. 2020;10(1):6521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reig-Vano B, et al. Alginate-based hydrogels for cancer therapy and research. Int J Biol Macromol. 2021;170:424–36. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Latest global cancer data: Cancer burden rises to 18.1 million new cases and 9.6 million cancer deaths in 2018. International agency for research on cancer. Geneva: World Health Organization; 2018. p. 1–4. [Google Scholar]
- 5.Pucci C, Martinelli C, Ciofani G. Innovative approaches for cancer treatment: Current perspectives and new challenges. Ecancermedicalscience. 2019; 13. [DOI] [PMC free article] [PubMed]
- 6.Montané X, et al. Encapsulation for cancer therapy. Molecules. 2020;25(7):1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li B, Ashrafizadeh M, Jiao T. Biomedical application of metal-organic frameworks (MOFs) in cancer therapy: stimuli-responsive and biomimetic nanocomposites in targeted delivery, phototherapy and diagnosis. Int J Biol Macromol. 2024;260:129391. [DOI] [PubMed] [Google Scholar]
- 8.Wang Z, et al. Chitosan-and hyaluronic acid-based nanoarchitectures in phototherapy: combination cancer chemotherapy, immunotherapy and gene therapy. Int J Biol Macromol. 2024;273:132579. [DOI] [PubMed] [Google Scholar]
- 9.Dai J, et al. Peptide-functionalized,-assembled and-loaded nanoparticles in cancer therapy. Drug Discov Today. 2024;29:103981. [DOI] [PubMed] [Google Scholar]
- 10.Lu Q, et al. Nanoparticles in tumor microenvironment remodeling and cancer immunotherapy. J Hematol Oncol. 2024;17(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li T, et al. Chitosan-functionalized bioplatforms and hydrogels in breast cancer: immunotherapy, phototherapy and clinical perspectives. Drug Discov Today. 2024;29(1): 103851. [DOI] [PubMed] [Google Scholar]
- 12.Li J, Song W, Li F. Polymeric DNA hydrogels and their applications in drug delivery for cancer therapy. Gels. 2023;9(3):239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamidi M, Azadi A, Rafiei P. Hydrogel nanoparticles in drug delivery. Adv Drug Deliv Rev. 2008;60(15):1638–49. [DOI] [PubMed] [Google Scholar]
- 14.Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chem Rev. 2001;101(7):1869–79. [DOI] [PubMed] [Google Scholar]
- 15.Peppas NA, et al. Hydrogels in biology and medicine: from molecular principles to bionanotechnology. Adv Mater. 2006;18(11):1345–60. [Google Scholar]
- 16.Jen AC, Wake MC, Mikos AG. Review: hydrogels for cell immobilization. Biotechnol Bioeng. 1996;50(4):357–64. [DOI] [PubMed] [Google Scholar]
- 17.Langer R, Peppas NA. Advances in biomaterials, drug delivery, and bionanotechnology. AIChE J. 2003;49(12):2990–3006. [Google Scholar]
- 18.Del Valle LJ, Díaz A, Puiggalí J. Hydrogels for biomedical applications: cellulose, chitosan, and protein/peptide derivatives. Gels. 2017;3(3):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seliktar D, et al. Dynamic mechanical conditioning of collagen-gel blood vessel constructs induces remodeling in vitro. Ann Biomed Eng. 2000;28(4):351–62. [DOI] [PubMed] [Google Scholar]
- 20.Auger FA, et al. Tissue-engineered human skin substitutes developed from collagen-populated hydrated gels: clinical and fundamental applications. Med Biol Eng Comput. 1998;36(6):801–12. [DOI] [PubMed] [Google Scholar]
- 21.Bao Z, et al. Natural polymer-based hydrogels with enhanced mechanical performances: preparation, structure, and property. Adv Healthc Mater. 2019;8(17):e1900670. [DOI] [PubMed] [Google Scholar]
- 22.Sanmartin-Masia E, Poveda-Reyes S, Gallego Ferrer G. Extracellular matrix–inspired gelatin/hyaluronic acid injectable hydrogels. Int J Polym Mater Polym Biomater. 2017;66(6):280–8. [Google Scholar]
- 23.Cao Y, et al. Injectable hydrogel loaded with lysed OK-432 and doxorubicin for residual liver cancer after incomplete radiofrequency ablation. J Nanobiotechnology. 2023;21(1):404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu R, et al. Ferritin-based nanocomposite hydrogel promotes tumor penetration and enhances cancer chemoimmunotherapy. Adv Sci (Weinh). 2024;11(3):e2305217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu X, et al. Interventional hydrogel microsphere vaccine as an immune amplifier for activated antitumour immunity after ablation therapy. Nat Commun. 2023;14(1):4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fang T, et al. Injectable cold atmospheric plasma-activated immunotherapeutic hydrogel for enhanced cancer treatment. Biomaterials. 2023;300: 122189. [DOI] [PubMed] [Google Scholar]
- 27.Li Q, et al. Genetically engineered artificial exosome-constructed hydrogel for ovarian cancer therapy. ACS Nano. 2023;17(11):10376–92. [DOI] [PubMed] [Google Scholar]
- 28.He T, et al. Antigenicity and adjuvanticity co-reinforced personalized cell vaccines based on self-adjuvanted hydrogel for post-surgical cancer vaccination. Biomaterials. 2023;301:122218. [DOI] [PubMed] [Google Scholar]
- 29.Wang T, et al. Photothermal hyaluronic acid composite hydrogel targeting cancer stem cells for inhibiting recurrence and metastasis of breast cancer. Int J Biol Macromol. 2023;252:126358. [DOI] [PubMed] [Google Scholar]
- 30.Cao J, et al. Sequentially degradable hydrogel-microsphere loaded with doxorubicin and pioglitazone synergistically inhibits cancer stemness of osteosarcoma. Biomed Pharmacother. 2023;165:115096. [DOI] [PubMed] [Google Scholar]
- 31.Li P, et al. NIR- and pH-responsive injectable nanocomposite alginate-graft-dopamine hydrogel for melanoma suppression and wound repair. Carbohydr Polym. 2023;314:120899. [DOI] [PubMed] [Google Scholar]
- 32.Salapa J, et al. Nano drug delivery systems in upper gastrointestinal cancer therapy. Nano Convergence. 2020;7:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buckley AM, et al. Targeting hallmarks of cancer to enhance radiosensitivity in gastrointestinal cancers. Nat Rev Gastroenterol Hepatol. 2020;17(5):298–313. [DOI] [PubMed] [Google Scholar]
- 34.Wang M, et al. The emerging roles of circular RNAs in the chemoresistance of gastrointestinal cancer. Front Cell Dev Biol. 2022;10:821609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma E-S, et al. Immune evasion mechanisms and therapeutic strategies in gastric cancer. World J Gastrointest Oncol. 2022;14(1):216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferrone C, Dranoff G. Dual roles for immunity in gastrointestinal cancers. J Clin Oncol. 2010;28(26):4045–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang Z, et al. The applications of gold nanoparticles in the diagnosis and treatment of gastrointestinal cancer. Front Oncol. 2022;11: 819329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou J, et al. Emerging role of nanoparticles in the diagnostic imaging of gastrointestinal cancer. Semin Cancer Biol. 2022. 10.1016/j.semcancer.2022.04.009. [DOI] [PubMed] [Google Scholar]
- 39.Andrade F, et al. Stimuli-responsive hydrogels for cancer treatment: the role of pH, light, ionic strength and magnetic field. Cancers. 2021;13(5):1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caló E, Khutoryanskiy VV. Biomedical applications of hydrogels: a review of patents and commercial products. Eur Polymer J. 2015;65:252–67. [Google Scholar]
- 41.Peña B, et al. Injectable hydrogels for cardiac tissue engineering. Macromol Biosci. 2018;18(6):e1800079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vega SL, Kwon MY, Burdick JA. Recent advances in hydrogels for cartilage tissue engineering. Eur Cell Mater. 2017;33:59–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tavakoli S, Klar AS. Advanced hydrogels as wound dressings. Biomolecules. 2020;10(8):1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wei W, et al. Research progress in the application of in situ hydrogel system in tumor treatment. Drug Deliv. 2020;27(1):460–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dattilo M, et al. Polysaccharide-based hydrogels and their application as drug delivery systems in cancer treatment: a review. J Funct Biomater. 2023;14(2):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bashir S, et al. Fundamental concepts of hydrogels: synthesis, properties, and their applications. Polymers. 2020;12(11):2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Madduma-Bandarage US, Madihally SV. Synthetic hydrogels: synthesis, novel trends, and applications. J Appl Polym Sci. 2021;138(19):50376. [Google Scholar]
- 48.Akhtar MF, Hanif M, Ranjha NM. Methods of synthesis of hydrogels… A review. Saudi Pharm J. 2016;24(5):554–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mathur AM, Moorjani SK, Scranton AB. Methods for synthesis of hydrogel networks: a review. J Macromol Sci Part C Polym Rev. 1996;36(2):405–30. [Google Scholar]
- 50.Uliniuc A, et al. New approaches in hydrogel synthesis—click chemistry: a review. Cellul Chem Technol. 2012;46(1):1. [Google Scholar]
- 51.Haraguchi K. Synthesis and properties of soft nanocomposite materials with novel organic/inorganic network structures. Polym J. 2011;43(3):223–41. [Google Scholar]
- 52.Ottenbrite RM, Huang SJ, Park K. Hydrogels and biodegradable polymers for bioapplications. Washington, DC: ACS Publications; 1996. [Google Scholar]
- 53.Kopecek J. Hydrogels: from soft contact lenses and implants to self-assembled nanomaterials. J Polym Sci Part A Polym Chem. 2009;47(22):5929–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hoare TR, Kohane DS. Hydrogels in drug delivery: progress and challenges. Polymer. 2008;49(8):1993–2007. [Google Scholar]
- 55.Lin-Gibson S, et al. Synthesis and characterization of poly (ethylene glycol) dimethacrylate hydrogels. Macromol Symp. 2005. 10.1002/masy.200550924. [Google Scholar]
- 56.Peppas NA, et al. Hydrogels in pharmaceutical formulations. Eur J Pharm Biopharm. 2000;50(1):27–46. [DOI] [PubMed] [Google Scholar]
- 57.Klouda L, Mikos AG. Thermoresponsive hydrogels in biomedical applications. Eur J Pharm Biopharm. 2008;68(1):34–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen G, Hoffman AS. Graft copolymers that exhibit temperature-induced phase transitions over a wide range of pH. Nature. 1995;373(6509):49–52. [DOI] [PubMed] [Google Scholar]
- 59.Ahmed EM. Hydrogel: Preparation, characterization, and applications: a review. J Adv Res. 2015;6(2):105–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Flory PJ. Principles of polymer chemistry. Ithaca: Cornell University Press; 1953. [Google Scholar]
- 61.Zhang H, et al. An injectable, in situ forming and NIR-responsive hydrogel persistently reshaping tumor microenvironment for efficient melanoma therapy. Biomater Res. 2023;27(1):118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Seo HS, et al. Enhanced postsurgical cancer treatment using methacrylated glycol chitosan hydrogel for sustained DNA/doxorubicin delivery and immunotherapy. Biomater Res. 2024;28:0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen J, et al. Engineered elastin-like polypeptide-based hydrogel delivering chemotherapeutics and PD-L1 antibodies for potentiated cancer immunotherapy. J Mater Chem B. 2023;11(43):10355–61. [DOI] [PubMed] [Google Scholar]
- 64.Gao T, et al. In situ hydrogel modulates cDC1-based antigen presentation and cancer stemness to enhance cancer vaccine efficiency. Adv Sci. 2024;11:e2305832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ostovar S, Pourmadadi M, Zaker MA. Co-biopolymer of chitosan/carboxymethyl cellulose hydrogel improved by zinc oxide and graphene quantum dots nanoparticles as pH-sensitive nanocomposite for quercetin delivery to brain cancer treatment. Int J Biol Macromol. 2023;253(Pt 4):127091. [DOI] [PubMed] [Google Scholar]
- 66.Di L, et al. A new Co(II)-coordination polymer: fluorescence performances, loaded with paclitaxel-hydrogel on breast cancer and molecular docking study. J Fluoresc. 2024. 10.1007/s10895-024-03670-4. [DOI] [PubMed] [Google Scholar]
- 67.Maher S, et al. Alginate-based hydrogel platform embedding silver nanoparticles and cisplatin: characterization of the synergistic effect on a breast cancer cell line. Front Mol Biosci. 2023;10:1242838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jeong DI, et al. Hydrogel design to overcome thermal resistance and ROS detoxification in photothermal and photodynamic therapy of cancer. J Control Release. 2024;366:142–59. [DOI] [PubMed] [Google Scholar]
- 69.Yin L, et al. Biodegradable hydrogel from pectin and carboxymethyl cellulose with Silibinin loading for lung tumor therapy. Int J Biol Macromol. 2023;243:125128. [DOI] [PubMed] [Google Scholar]
- 70.Zhang Y, et al. A programmable oral bacterial hydrogel for controllable production and release of nanovaccine for tumor immunotherapy. Biomaterials. 2023;299:122147. [DOI] [PubMed] [Google Scholar]
- 71.Liang JL, et al. Immunostimulant hydrogel-guided tumor microenvironment reprogramming to efficiently potentiate macrophage-mediated cellular phagocytosis for systemic cancer immunotherapy. ACS Nano. 2023;17(17):17217–32. [DOI] [PubMed] [Google Scholar]
- 72.Yang WJ, et al. An injectable nanocomposite alginate-Ca(2+) hydrogel for melittin-assisted Ca(2+)-overload and photothermal cancer therapy. Chem Commun. 2023;59(55):8568–71. [DOI] [PubMed] [Google Scholar]
- 73.Chen J, et al. Multifunctional hydrogel for synergistic reoxygenation and chemo/photothermal therapy in metastatic breast cancer recurrence and wound infection. J Control Release. 2024;365:74–88. [DOI] [PubMed] [Google Scholar]
- 74.Wang D, et al. Enrichment and sensing tumor cells by embedded immunomodulatory DNA hydrogel to inhibit postoperative tumor recurrence. Nat Commun. 2023;14(1):4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baize N, et al. Long-term survival of patients downstaged by oxaliplatin and 5-fluorouracil combination followed by rescue surgery for unresectable colorectal liver metastases. Gastroenterol Clin Biol. 2006;30(12):1349–53. [DOI] [PubMed] [Google Scholar]
- 76.Steele G Jr, Ravikumar T. Resection of hepatic metastases from colorectal cancer. Biologic perspective. Ann Surg. 1989;210(2):127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shitara K, et al. Prolonged survival of patients with metastatic colorectal cancer following first-line oxaliplatin-based chemotherapy with molecular targeting agents and curative surgery. Oncology. 2011;81(3–4):167–74. [DOI] [PubMed] [Google Scholar]
- 78.Lee JE, et al. Oxaliplatin-loaded chemically cross-linked hydrogels for prevention of postoperative abdominal adhesion and colorectal cancer therapy. Int J Pharm. 2019;565:50–8. [DOI] [PubMed] [Google Scholar]
- 79.Abuzar SM, et al. Pharmacokinetic profile and anti-adhesive effect of oxaliplatin-PLGA microparticle-loaded hydrogels in rats for colorectal cancer treatment. Pharmaceutics. 2019;11(8):392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pooresmaeil M, Nia SB, Namazi H. Green encapsulation of LDH (Zn/Al)-5-Fu with carboxymethyl cellulose biopolymer; new nanovehicle for oral colorectal cancer treatment. Int J Biol Macromol. 2019;139:994–1001. [DOI] [PubMed] [Google Scholar]
- 81.Taghizadeh MT, et al. Cross-linked chitosan in nano and bead scales as drug carriers for betamethasone and tetracycline. Int J Biol Macromol. 2019;131:581–8. [DOI] [PubMed] [Google Scholar]
- 82.Zhang K, et al. A redox and pH dual-triggered drug delivery platform based on chitosan grafted tubular mesoporous silica. Ceram Int. 2019;45(17):22603–9. [Google Scholar]
- 83.Pongjanyakul T, Rongthong T. Enhanced entrapment efficiency and modulated drug release of alginate beads loaded with drug–clay intercalated complexes as microreservoirs. Carbohyd Polym. 2010;81(2):409–19. [Google Scholar]
- 84.Sheng Y, et al. Dual-drug delivery system based on the hydrogels of alginate and sodium carboxymethyl cellulose for colorectal cancer treatment. Carbohydr Polym. 2021;269:118325. [DOI] [PubMed] [Google Scholar]
- 85.Castro-Abril H, et al. The role of mechanical properties and structure of type I collagen hydrogels on colorectal cancer cell migration. Macromol Biosci. 2023;23(10):e2300108. [DOI] [PubMed] [Google Scholar]
- 86.Lee AL, et al. Injectable biodegradable hydrogels from vitamin D-functionalized polycarbonates for the delivery of avastin with enhanced therapeutic efficiency against metastatic colorectal cancer. Biomacromol. 2015;16(2):465–75. [DOI] [PubMed] [Google Scholar]
- 87.Khutoryanskiy VV. Advances in mucoadhesion and mucoadhesive polymers. Macromol Biosci. 2011;11(6):748–64. [DOI] [PubMed] [Google Scholar]
- 88.Madsen F, Eberth K, Smart JD. A rheological assessment of the nature of interactions between mucoadhesive polymers and a homogenised mucus gel. Biomaterials. 1998;19(11–12):1083–92. [DOI] [PubMed] [Google Scholar]
- 89.Vakili MR, et al. Development of mucoadhesive hydrogels based on polyacrylic acid grafted cellulose nanocrystals for local cisplatin delivery. Carbohydr Polym. 2021;255:117332. [DOI] [PubMed] [Google Scholar]
- 90.de Freitas CF, et al. Curcumin and silver nanoparticles carried out from polysaccharide-based hydrogels improved the photodynamic properties of curcumin through metal-enhanced singlet oxygen effect. Mater Sci Eng C Mater Biol Appl. 2020;112:110853. [DOI] [PubMed] [Google Scholar]
- 91.Abbasi M, et al. Folic acid-decorated alginate nanoparticles loaded hydrogel for the oral delivery of diferourylmethane in colorectal cancer. Int J Biol Macromol. 2023;233:123585. [DOI] [PubMed] [Google Scholar]
- 92.Li L, et al. Orally administrated hydrogel harnessing intratumoral microbiome and microbiota-related immune responses for potentiated colorectal cancer treatment. Research (Wash D C). 2024;7:0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang Y, et al. 5-FU-hydrogel inhibits colorectal peritoneal carcinomatosis and tumor growth in mice. BMC Cancer. 2010;10:402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xing R, et al. Development, characterization, and evaluation of SLN-loaded thermoresponsive hydrogel system of topotecan as biological macromolecule for colorectal delivery. Biomed Res Int. 2021;2021:9968602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jiang F, et al. Magnetic propelled hydrogel microrobots for actively enhancing the efficiency of lycorine hydrochloride to suppress colorectal cancer. Front Bioeng Biotechnol. 2024;12:1361617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Abdel-Bar HM, et al. Tunable biodegradable nanocomposite hydrogel for improved cisplatin efficacy on hct-116 colorectal cancer cells and decreased toxicity in rats. Biomacromol. 2016;17(2):407–14. [DOI] [PubMed] [Google Scholar]
- 97.Wintjens A, et al. Treating colorectal peritoneal metastases with an injectable cytostatic loaded supramolecular hydrogel in a rodent animal model. Clin Exp Metastasis. 2023;40(3):243–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Luo J, et al. Intraperitoneal administration of biocompatible hyaluronic acid hydrogel containing multi-chemotherapeutic agents for treatment of colorectal peritoneal carcinomatosis. Int J Biol Macromol. 2020;152:718–26. [DOI] [PubMed] [Google Scholar]
- 99.Yan Y, et al. Uptake and intracellular fate of disulfide-bonded polymer hydrogel capsules for Doxorubicin delivery to colorectal cancer cells. ACS Nano. 2010;4(5):2928–36. [DOI] [PubMed] [Google Scholar]
- 100.Dinh L, et al. A novel thermosensitive poloxamer-hyaluronic acid- kappa-carrageenan-based hydrogel anti-adhesive agent loaded with 5-fluorouracil: a preclinical study in Sprague-Dawley rats. Int J Pharm. 2022;621:121771. [DOI] [PubMed] [Google Scholar]
- 101.Md S, et al. Smart oral pH-responsive dual layer nano-hydrogel for dissolution enhancement and targeted delivery of naringenin using protein-polysaccharides complexation against colorectal cancer. J Pharm Sci. 2022;111(11):3155–64. [DOI] [PubMed] [Google Scholar]
- 102.Ren Y, et al. Improved anti-colorectal carcinomatosis effect of tannic acid co-loaded with oxaliplatin in nanoparticles encapsulated in thermosensitive hydrogel. Eur J Pharm Sci. 2019;128:279–89. [DOI] [PubMed] [Google Scholar]
- 103.Batool N, et al. Orally administered, biodegradable and biocompatible hydroxypropyl-β-cyclodextrin grafted poly(methacrylic acid) hydrogel for pH sensitive sustained anticancer drug delivery. Gels. 2022;8(3):190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Karimi P, et al. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomark Prev. 2014;23(5):700–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chen W, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–32. [DOI] [PubMed] [Google Scholar]
- 106.Zhang Y, et al. MiR-99a and MiR-491 regulate cisplatin resistance in human gastric cancer cells by targeting CAPNS1. Int J Biol Sci. 2016;12(12):1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Qian K, et al. Evaluation of cisplatin-hydrogel for improving localized antitumor efficacy in gastric cancer. Pathol Res Pract. 2019;215(4):755–60. [DOI] [PubMed] [Google Scholar]
- 108.Chen W, et al. Sustained co-delivery of 5-fluorouracil and cis-platinum via biodegradable thermo-sensitive hydrogel for intraoperative synergistic combination chemotherapy of gastric cancer. Bioact Mater. 2023;23:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Glehen O, et al. Peritoneal carcinomatosis from gastric cancer: a multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann Surg Oncol. 2010;17:2370–7. [DOI] [PubMed] [Google Scholar]
- 110.Rudloff U, et al. Impact of maximal cytoreductive surgery plus regional heated intraperitoneal chemotherapy (HIPEC) on outcome of patients with peritoneal carcinomatosis of gastric origin: results of the GYMSSA trial. J Surg Oncol. 2014;110(3):275–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gill RS, et al. Treatment of gastric cancer with peritoneal carcinomatosis by cytoreductive surgery and HIPEC: a systematic review of survival, mortality, and morbidity. J Surg Oncol. 2011;104(6):692–8. [DOI] [PubMed] [Google Scholar]
- 112.Jacquet P, Sugarbaker PH. Peritoneal-plasma barrier. In: Sugarbaker PH, editor. Peritoneal carcinomatosis: principles of management. Boston: Springer; 1996. p. 53–63. [Google Scholar]
- 113.Van Driel WJ, et al. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N Engl J Med. 2018;378(3):230–40. [DOI] [PubMed] [Google Scholar]
- 114.Qian H, et al. Therapy for gastric cancer with peritoneal metastasis using injectable albumin hydrogel hybridized with paclitaxel-loaded red blood cell membrane nanoparticles. ACS Biomater Sci Eng. 2019;5(2):1100–12. [DOI] [PubMed] [Google Scholar]
- 115.Emoto S, et al. Intraperitoneal administration of cisplatin via an in situ cross-linkable hyaluronic acid-based hydrogel for peritoneal dissemination of gastric cancer. Surg Today. 2014;44(5):919–26. [DOI] [PubMed] [Google Scholar]
- 116.Ohta S, et al. Intraperitoneal delivery of cisplatin via a hyaluronan-based nanogel/in situ cross-linkable hydrogel hybrid system for peritoneal dissemination of gastric cancer. Mol Pharm. 2017;14(9):3105–13. [DOI] [PubMed] [Google Scholar]
- 117.Aycan D, et al. Gelatin microsphere-alginate hydrogel combined system for sustained and gastric targeted delivery of 5-fluorouracil. Int J Biol Macromol. 2024;255:128022. [DOI] [PubMed] [Google Scholar]
- 118.Cho JK, et al. Injectable and biodegradable poly(organophosphazene) hydrogel as a delivery system of docetaxel for cancer treatment. J Drug Target. 2013;21(6):564–73. [DOI] [PubMed] [Google Scholar]
- 119.Cho JK, Kuh HJ, Song SC. Injectable poly(organophosphazene) hydrogel system for effective paclitaxel and doxorubicin combination therapy. J Drug Target. 2014;22(8):761–7. [DOI] [PubMed] [Google Scholar]
- 120.Suzuki T, et al. A simple preparation method of gelatin hydrogels incorporating cisplatin for sustained release. Pharmaceutics. 2022;14(12):2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang H, et al. Efficient antitumor effect of co-drug-loaded nanoparticles with gelatin hydrogel by local implantation. Sci Rep. 2016;6:26546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Miller KD, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69(5):363–85. [DOI] [PubMed] [Google Scholar]
- 123.Ringelhan M, et al. The immunology of hepatocellular carcinoma. Nat Immunol. 2018;19(3):222–32. [DOI] [PubMed] [Google Scholar]
- 124.Sharma R. Descriptive epidemiology of incidence and mortality of primary liver cancer in 185 countries: evidence from GLOBOCAN 2018. Jpn J Clin Oncol. 2020;50(12):1370–9. [DOI] [PubMed] [Google Scholar]
- 125.Ma J, et al. Hydrogels for localized chemotherapy of liver cancer: a possible strategy for improved and safe liver cancer treatment. Drug Deliv. 2022;29(1):1457–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Xu K, et al. Injectable hyaluronic acid-tyramine hydrogels incorporating interferon-α2a for liver cancer therapy. J Control Release. 2013;166(3):203–10. [DOI] [PubMed] [Google Scholar]
- 127.Huang L, et al. Thermo-sensitive composite hydrogels based on poloxamer 407 and alginate and their therapeutic effect in embolization in rabbit VX2 liver tumors. Oncotarget. 2016;7(45):73280–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Guerra AD, et al. The anti-tumor effects of M1 macrophage-loaded poly (ethylene glycol) and gelatin-based hydrogels on hepatocellular carcinoma. Theranostics. 2017;7(15):3732–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jung JM, et al. Therapeutic effects of boronate ester cross-linked injectable hydrogels for the treatment of hepatocellular carcinoma. Biomater Sci. 2021;9(21):7275–86. [DOI] [PubMed] [Google Scholar]
- 130.Ma F, et al. Colorimetric immunosensor based on Au@g-C(3)N(4)-doped Spongelike 3D network cellulose hydrogels for detecting α-fetoprotein. ACS Appl Mater Interfaces. 2019;11(22):19902–12. [DOI] [PubMed] [Google Scholar]
- 131.Na K, et al. Self-assembled hydrogel nanoparticles from curdlan derivatives: characterization, anti-cancer drug release and interaction with a hepatoma cell line (HepG2). J Control Release. 2000;69(2):225–36. [DOI] [PubMed] [Google Scholar]
- 132.Santhamoorthy M, et al. k-Carrageenan based magnetic@polyelectrolyte complex composite hydrogel for pH and temperature-responsive curcumin delivery. Int J Biol Macromol. 2023;244:125467. [DOI] [PubMed] [Google Scholar]
- 133.Hussein Y, et al. Enhanced anti-cancer activity by localized delivery of curcumin form PVA/CNCs hydrogel membranes: preparation and in vitro bioevaluation. Int J Biol Macromol. 2021;170:107–22. [DOI] [PubMed] [Google Scholar]
- 134.Thirupathi K, et al. pH and thermoresponsive PNIPAm-co-polyacrylamide hydrogel for dual stimuli-responsive controlled drug delivery. Polymers. 2022;15(1):167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhu JQ, et al. Hydrogel crosslinked with nanoparticles for prevention of surgical hemorrhage and recurrence of hepatocellular carcinoma. Adv Sci. 2024;11(9):e2305508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Liu X, et al. Supramolecular polymer-nanomedicine hydrogel loaded with tumor associated macrophage-reprogramming polyTLR7/8a nanoregulator for enhanced anti-angiogenesis therapy of orthotopic hepatocellular carcinoma. Adv Sci (Weinh). 2023;10(22):e2300637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chen G, et al. A glycyrrhetinic acid-modified curcumin supramolecular hydrogel for liver tumor targeting therapy. Sci Rep. 2017;7:44210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Du C, et al. Efficient suppression of liver metastasis cancers by paclitaxel loaded nanoparticles in PDLLA-PEG-PDLLA thermosensitive hydrogel composites. J Biomed Nanotechnol. 2017;13(11):1545–56. [DOI] [PubMed] [Google Scholar]
- 139.Zhang ZJ, et al. Programmable DNA hydrogel assisting microcrystal formulations for sustained locoregional drug delivery in surgical residual tumor lesions and lymph node metastasis. Adv Healthc Mater. 2024;13(11):e2303762. [DOI] [PubMed] [Google Scholar]
- 140.Yang X, et al. Development of cisplatin-loaded hydrogels for trans-portal vein chemoembolization in an orthotopic liver cancer mouse model. Drug Deliv. 2021;28(1):520–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nguyen HD, Lin C-C. Viscoelastic stiffening of gelatin hydrogels for dynamic culture of pancreatic cancer spheroids. Acta Biomater. 2024;177:203–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Orth M, et al. Pancreatic ductal adenocarcinoma: biological hallmarks, current status, and future perspectives of combined modality treatment approaches. Radiat Oncol. 2019;14(1):1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Werner J, et al. Advanced-stage pancreatic cancer: therapy options. Nat Rev Clin Oncol. 2013;10(6):323–33. [DOI] [PubMed] [Google Scholar]
- 144.Brahmer JR, et al. Safety and activity of anti–PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Royal RE, et al. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother. 2010;33(8):828–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Xu L, et al. Sustained delivery of gemcitabine via in situ injectable mussel-inspired hydrogels for the local therapy of pancreatic cancer. J Mater Chem B. 2022;10(33):6338–50. [DOI] [PubMed] [Google Scholar]
- 147.Byeon HJ, et al. Four-arm PEG cross-linked hyaluronic acid hydrogels containing PEGylated apoptotic TRAIL protein for treating pancreatic cancer. Acta Biomater. 2014;10(1):142–50. [DOI] [PubMed] [Google Scholar]
- 148.Kim I, et al. In situ facile-forming PEG cross-linked albumin hydrogels loaded with an apoptotic TRAIL protein. J Control Release. 2015;214:30–9. [DOI] [PubMed] [Google Scholar]
- 149.Schaal JL, et al. Injectable polypeptide micelles that form radiation crosslinked hydrogels in situ for intratumoral radiotherapy. J Control Release. 2016;228:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sood N, et al. Stimuli-responsive hydrogels in drug delivery and tissue engineering. Drug Deliv. 2016;23(3):748–70. [DOI] [PubMed] [Google Scholar]
- 151.Chatterjee S, Hui PCI. Stimuli-responsive hydrogels: an interdisciplinary overview. In: Popa L, Ghica MV, Dinu-Pîrvu C-E, editors. Hydrogels—smart materials for biomedical applications. London: Intechopen; 2018. p. 1–23. [Google Scholar]
- 152.Ding M, et al. Multifunctional soft machines based on stimuli-responsive hydrogels: from freestanding hydrogels to smart integrated systems. Mater Today Adv. 2020;8:100088. [Google Scholar]
- 153.Jiang Y, et al. Natural polymer-based stimuli-responsive hydrogels. Curr Med Chem. 2020;27(16):2631–57. [DOI] [PubMed] [Google Scholar]
- 154.Bae WK, et al. Enhanced anti-cancer effect of 5-fluorouracil loaded into thermo-responsive conjugated linoleic acid-incorporated poloxamer hydrogel on metastatic colon cancer models. J Nanosci Nanotechnol. 2011;11(2):1425–8. [DOI] [PubMed] [Google Scholar]
- 155.Carreño G, et al. Development of “on-demand” thermo-responsive hydrogels for anti-cancer drugs sustained release: Rational design, in silico prediction and in vitro validation in colon cancer models. Mater Sci Eng C Mater Biol Appl. 2021;131:112483. [DOI] [PubMed] [Google Scholar]
- 156.Al Sabbagh C, et al. Thermosensitive hydrogels for local delivery of 5-fluorouracil as neoadjuvant or adjuvant therapy in colorectal cancer. Eur J Pharm Biopharm. 2020;157:154–64. [DOI] [PubMed] [Google Scholar]
- 157.Yang J, et al. Thermosensitive methyl-cellulose-based injectable hydrogel carrying oxaliplatin for the treatment of peritoneal metastasis in colorectal cancer. J Mater Chem B. 2024. 10.1039/d4tb00210e. [DOI] [PubMed] [Google Scholar]
- 158.Yun Q, et al. Use of 5-fluorouracil loaded micelles and cisplatin in thermosensitive chitosan hydrogel as an efficient therapy against colorectal peritoneal carcinomatosis. Macromol Biosci. 2017. 10.1002/mabi.201600262. [DOI] [PubMed] [Google Scholar]
- 159.Fan R, et al. Enhanced antitumor effects by docetaxel/LL37-loaded thermosensitive hydrogel nanoparticles in peritoneal carcinomatosis of colorectal cancer. Int J Nanomed. 2015;10:7291–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Morello G, et al. A thermo-sensitive chitosan/pectin hydrogel for long-term tumor spheroid culture. Carbohydr Polym. 2021;274:118633. [DOI] [PubMed] [Google Scholar]
- 161.Han TS, et al. Improvement of anti-cancer drug efficacy via thermosensitive hydrogel in peritoneal carcinomatosis in gastric cancer. Oncotarget. 2017;8(65):108848–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bae WK, et al. Docetaxel-loaded thermoresponsive conjugated linoleic acid-incorporated poloxamer hydrogel for the suppression of peritoneal metastasis of gastric cancer. Biomaterials. 2013;34(4):1433–41. [DOI] [PubMed] [Google Scholar]
- 163.Zhang D, et al. Antitumor activity of thermosensitive hydrogels packaging gambogic acid nanoparticles and tumor-penetrating peptide iRGD against gastric cancer. Int J Nanomed. 2020;15:735–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cheng Y, et al. Thermosensitive hydrogels based on polypeptides for localized and sustained delivery of anticancer drugs. Biomaterials. 2013;34(38):10338–47. [DOI] [PubMed] [Google Scholar]
- 165.Wan J, et al. Doxorubicin-induced co-assembling nanomedicines with temperature-sensitive acidic polymer and their in-situ-forming hydrogels for intratumoral administration. J Control Release. 2016;235:328–36. [DOI] [PubMed] [Google Scholar]
- 166.Wen Q, et al. Therapeutic efficacy of thermosensitive Pluronic hydrogel for codelivery of resveratrol microspheres and cisplatin in the treatment of liver cancer ascites. Int J Pharm. 2020;582:119334. [DOI] [PubMed] [Google Scholar]
- 167.Santhamoorthy M, et al. Thermo-sensitive poly (N-isopropylacrylamide-co-polyacrylamide) hydrogel for pH-responsive therapeutic delivery. Polymers. 2022;14(19):4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Qu J, et al. pH-responsive self-healing injectable hydrogel based on N-carboxyethyl chitosan for hepatocellular carcinoma therapy. Acta Biomater. 2017;58:168–80. [DOI] [PubMed] [Google Scholar]
- 169.Wen Y, et al. A responsive porous hydrogel particle-based delivery system for oncotherapy. Nanoscale. 2019;11(6):2687–93. [DOI] [PubMed] [Google Scholar]
- 170.Gao L, et al. Evaluation of TPGS-modified thermo-sensitive Pluronic PF127 hydrogel as a potential carrier to reverse the resistance of P-gp-overexpressing SMMC-7721 cell lines. Colloids Surf B Biointerfaces. 2016;140:307–16. [DOI] [PubMed] [Google Scholar]
- 171.Mao Y, et al. Thermosensitive hydrogel system with paclitaxel liposomes used in localized drug delivery system for in situ treatment of tumor: better antitumor efficacy and lower toxicity. J Pharm Sci. 2016;105(1):194–204. [DOI] [PubMed] [Google Scholar]
- 172.Shabana AM, et al. Thermosensitive and biodegradable hydrogel encapsulating targeted nanoparticles for the sustained co-delivery of gemcitabine and paclitaxel to pancreatic cancer cells. Int J Pharm. 2021;593:120139. [DOI] [PubMed] [Google Scholar]
- 173.Bilalis P, et al. Self-healing pH- and enzyme stimuli-responsive hydrogels for targeted delivery of gemcitabine to treat pancreatic cancer. Biomacromol. 2018;19(9):3840–52. [DOI] [PubMed] [Google Scholar]
- 174.Liu C, et al. A water-soluble, NIR-absorbing quaterrylenediimide chromophore for photoacoustic imaging and efficient photothermal cancer therapy. Angew Chem Int Ed. 2019;58(6):1638–42. [DOI] [PubMed] [Google Scholar]
- 175.Jin X, et al. Molecular engineering of diketopyrrolopyrrole-conjugated polymer nanoparticles by chalcogenide variation for photoacoustic imaging guided photothermal therapy. J Mater Chem B. 2021;9(14):3153–60. [DOI] [PubMed] [Google Scholar]
- 176.Liu F, et al. Synergistic non-bonding interactions based on diketopyrrolo-pyrrole for elevated photoacoustic imaging-guided photothermal therapy. Biomater Sci. 2021;9(3):908–16. [DOI] [PubMed] [Google Scholar]
- 177.Liu Z, et al. Regioisomer-manipulating thio-perylenediimide nanoagents for photothermal/photodynamic theranostics. J Mater Chem B. 2020;8(25):5535–44. [DOI] [PubMed] [Google Scholar]
- 178.Saneja A, et al. Recent advances in near-infrared light-responsive nanocarriers for cancer therapy. Drug Discovery Today. 2018;23(5):1115–25. [DOI] [PubMed] [Google Scholar]
- 179.Yang R, et al. Indocyanine green-modified hollow mesoporous Prussian blue nanoparticles loading doxorubicin for fluorescence-guided tri-modal combination therapy of cancer. Nanoscale. 2019;11(12):5717–31. [DOI] [PubMed] [Google Scholar]
- 180.Qing W, et al. Indocyanine green loaded pH-responsive bortezomib supramolecular hydrogel for synergistic chemo-photothermal/photodynamic colorectal cancer therapy. Photodiagn Photodyn Ther. 2021;36:102521. [DOI] [PubMed] [Google Scholar]
- 181.Chen W, et al. Tumor redox microenvironment modulating composite hydrogels for enhanced sonodynamic therapy of colorectal cancer. J Mater Chem B. 2022;10(12):1960–8. [DOI] [PubMed] [Google Scholar]
- 182.Shanmugapriya K, Kim H, Kang HW. Epidermal growth factor receptor conjugated fucoidan/alginates loaded hydrogel for activating EGFR/AKT signaling pathways in colon cancer cells during targeted photodynamic therapy. Int J Biol Macromol. 2020. 10.1016/j.ijbiomac.2020.05.008. [DOI] [PubMed] [Google Scholar]
- 183.Tao W, et al. Enhanced ROS-boosted phototherapy against pancreatic cancer via Nrf2-mediated stress-defense pathway suppression and ferroptosis induction. ACS Appl Mater Interfaces. 2022;14(5):6404–16. [DOI] [PubMed] [Google Scholar]
- 184.Liu L, et al. Aptamer and peptide-engineered polydopamine nanospheres for target delivery and tumor perfusion in synergistic chemo-phototherapy of pancreatic cancer. ACS Appl Mater Interfaces. 2023;15(13):16539–51. [DOI] [PubMed] [Google Scholar]
- 185.Kang S, et al. Nonrecurring circuit nanozymatic enhancement of hypoxic pancreatic cancer phototherapy using speckled Ru-Te hollow nanorods. ACS Nano. 2020;14(4):4383–94. [DOI] [PubMed] [Google Scholar]
- 186.Kong Y, et al. Injectable and thermosensitive liposomal hydrogels for NIR-II light-triggered photothermal-chemo therapy of pancreatic cancer. ACS Appl Bio Mater. 2021;4(10):7595–604. [DOI] [PubMed] [Google Scholar]
- 187.Wang M, et al. Enhanced chemo-immunotherapy strategy utilizing injectable thermosensitive hydrogel for the treatment of diffuse peritoneal metastasis in advanced colorectal cancer. Adv Sci (Weinh). 2023;10(35):e2303819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Dong H, et al. B7–H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5(12):1365–9. [DOI] [PubMed] [Google Scholar]
- 189.Li J, Luo Y, Pu K. Electromagnetic nanomedicines for combinational cancer immunotherapy. Angew Chem Int Ed. 2021;60(23):12682–705. [DOI] [PubMed] [Google Scholar]
- 190.Zhang C, Pu K. Molecular and nanoengineering approaches towards activatable cancer immunotherapy. Chem Soc Rev. 2020;49(13):4234–53. [DOI] [PubMed] [Google Scholar]
- 191.Huang J, et al. Molecular radio afterglow probes for cancer radiodynamic theranostics. Nat Mater. 2023;22(11):1421–9. [DOI] [PubMed] [Google Scholar]
- 192.Shen W, et al. A polymeric hydrogel to eliminate programmed death-ligand 1 for enhanced tumor radio-immunotherapy. ACS Nano. 2023;17(23):23998–4011. [DOI] [PubMed] [Google Scholar]
- 193.Kim S, et al. Alum-tuned hyaluronic acid-based hydrogel with immune checkpoint inhibition for immunophoto therapy of cancer. J Control Release. 2023;362:1–18. [DOI] [PubMed] [Google Scholar]
- 194.Han Y, et al. IL-1β-associated NNT acetylation orchestrates iron-sulfur cluster maintenance and cancer immunotherapy resistance. Mol Cell. 2023;83(11):1887-1902.e8. [DOI] [PubMed] [Google Scholar]
- 195.Luo Q, et al. Apatinib remodels the immunosuppressive tumor ecosystem of gastric cancer enhancing anti-PD-1 immunotherapy. Cell Rep. 2023;42(5): 112437. [DOI] [PubMed] [Google Scholar]
- 196.Yang Y, et al. Injectable shear-thinning polylysine hydrogels for localized immunotherapy of gastric cancer through repolarization of tumor-associated macrophages. Biomater Sci. 2021;9(19):6597–608. [DOI] [PubMed] [Google Scholar]
- 197.Li Q, et al. PRDM1/BLIMP1 induces cancer immune evasion by modulating the USP22-SPI1-PD-L1 axis in hepatocellular carcinoma cells. Nat Commun. 2022;13(1):7677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Liu Y, et al. HERC2 promotes inflammation-driven cancer stemness and immune evasion in hepatocellular carcinoma by activating STAT3 pathway. J Exp Clin Cancer Res. 2023;42(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zhang Y, et al. HKDC1 promotes tumor immune evasion in hepatocellular carcinoma by coupling cytoskeleton to STAT1 activation and PD-L1 expression. Nat Commun. 2024;15(1):1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Yu J, et al. TP53/mTORC1-mediated bidirectional regulation of PD-L1 modulates immune evasion in hepatocellular carcinoma. J Immunother Cancer. 2023;11(11):e007479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Xu S, et al. Sustained release of therapeutic gene by injectable hydrogel for hepatocellular carcinoma. Int J Pharm X. 2023;6:100195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zhuang Q, et al. Navoximod modulates local HSV-1 replication to reshape tumor immune microenvironment for enhanced immunotherapy via an injectable hydrogel. Commun Biol. 2023;6(1):621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Meng J, et al. Ferroptosis-enhanced immunotherapy with an injectable dextran-chitosan hydrogel for the treatment of malignant ascites in hepatocellular carcinoma. Adv Sci (Weinh). 2023;10(20):e2300517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Freed-Pastor WA, et al. The CD155/TIGIT axis promotes and maintains immune evasion in neoantigen-expressing pancreatic cancer. Cancer Cell. 2021;39(10):1342-1360.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Yamamoto K, et al. Autophagy promotes immune evasion of pancreatic cancer by degrading MHC-I. Nature. 2020;581(7806):100–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Canel M, et al. FAK suppresses antigen processing and presentation to promote immune evasion in pancreatic cancer. Gut. 2023;73(1):131–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Gao C, et al. Injectable immunotherapeutic hydrogel containing RNA-loaded lipid nanoparticles reshapes tumor microenvironment for pancreatic cancer therapy. Nano Lett. 2022;22(22):8801–9. [DOI] [PubMed] [Google Scholar]
- 208.Zhao R, et al. Dual-crosslinking immunostimulatory hydrogel synchronously suppresses pancreatic fistula and pancreatic cancer relapse post-resection. Biomaterials. 2024;305:122453. [DOI] [PubMed] [Google Scholar]
- 209.Park W, et al. Hydrogel microneedles extracting exosomes for early detection of colorectal cancer. Biomacromol. 2023;24(3):1445–52. [DOI] [PubMed] [Google Scholar]
- 210.Butvilovskaya VI, et al. Multiplex determination of serological signatures in the sera of colorectal cancer patients using hydrogel biochips. Cancer Med. 2016;5(7):1361–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kim YJ, et al. Circulating tumor marker isolation with the chemically stable and instantly degradable (CSID) hydrogel immunospheres. Anal Chem. 2021;93(2):1100–9. [DOI] [PubMed] [Google Scholar]
- 212.Qi Z, et al. Digital analysis of the expression levels of multiple colorectal cancer-related genes by multiplexed digital-PCR coupled with hydrogel bead-array. Analyst. 2011;136(11):2252–9. [DOI] [PubMed] [Google Scholar]
- 213.Park J, et al. CD44-specific supramolecular hydrogels for fluorescence molecular imaging of stem-like gastric cancer cells. Integr Biol (Camb). 2013;5(4):669–72. [DOI] [PubMed] [Google Scholar]
- 214.Sun W, et al. Precise capture and dynamic release of circulating liver cancer cells with dual-histidine-based cell imprinted hydrogels. Adv Mater. 2024;36:e2402379. [DOI] [PubMed] [Google Scholar]
- 215.Azizian A, et al. CA19-9 for detecting recurrence of pancreatic cancer. Sci Rep. 2020;10(1):1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Jimenez-Luna C, et al. Proteomic biomarkers in body fluids associated with pancreatic cancer. Oncotarget. 2018;9(23):16573–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Goh SK, et al. Serum carbohydrate antigen 19–9 in pancreatic adenocarcinoma: a mini review for surgeons. ANZ J Surg. 2017;87(12):987–92. [DOI] [PubMed] [Google Scholar]
- 218.Piloto AML, et al. Cellulose-based hydrogel on quantum dots with molecularly imprinted polymers for the detection of CA19-9 protein cancer biomarker. Mikrochim Acta. 2022;189(4):134. [DOI] [PubMed] [Google Scholar]
- 219.Gao M, et al. Hydrogel-metal-organic-framework hybrids mediated efficient oral delivery of siRNA for the treatment of ulcerative colitis. J Nanobiotechnol. 2022;20(1):404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Liu Z, et al. The influence of chitosan hydrogel on stem cell engraftment, survival and homing in the ischemic myocardial microenvironment. Biomaterials. 2012;33(11):3093–106. [DOI] [PubMed] [Google Scholar]
- 221.Siu KS, et al. Non-covalently functionalized single-walled carbon nanotube for topical siRNA delivery into melanoma. Biomaterials. 2014;35(10):3435–42. [DOI] [PubMed] [Google Scholar]
- 222.Ruszczak Z, Friess W. Collagen as a carrier for on-site delivery of antibacterial drugs. Adv Drug Deliv Rev. 2003;55(12):1679–98. [DOI] [PubMed] [Google Scholar]
- 223.Krebs MD, Jeon O, Alsberg E. Localized and sustained delivery of silencing RNA from macroscopic biopolymer hydrogels. J Am Chem Soc. 2009;131(26):9204–6. [DOI] [PubMed] [Google Scholar]
- 224.Peng H, et al. Sustained delivery of siRNA/PEI complex from in situ forming hydrogels potently inhibits the proliferation of gastric cancer. J Exp Clin Cancer Res. 2016;35:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Peer D, et al. Systemic leukocyte-directed siRNA delivery revealing cyclin D1 as an anti-inflammatory target. Science. 2008;319(5863):627–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Whitehead KA, Langer R, Anderson DG. Knocking down barriers: advances in siRNA delivery. Nat Rev Drug Discov. 2009;8(2):129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Zhao B, et al. Sustained and targeted delivery of siRNA/DP7-C nanoparticles from injectable thermosensitive hydrogel for hepatocellular carcinoma therapy. Cancer Sci. 2021;112(6):2481–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Yang Q, et al. Hydrogel-based miR-192 delivery inhibits the development of hepatocellular carcinoma by suppressing the GSK3β/Wnt/β-catenin pathway. Neoplasma. 2023;70(4):555–65. [DOI] [PubMed] [Google Scholar]
- 229.Yang D, et al. Local sustained chemotherapy of pancreatic cancer using endoscopic ultrasound-guided injection of biodegradable thermo-sensitive hydrogel. Int J Nanomedicine. 2023;18:3989–4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Zhang B, et al. Curcumin-shellac nanoparticle-loaded GelMA/SilMA hydrogel for colorectal cancer therapy. Eur J Pharm Biopharm. 2024;202:114409. [DOI] [PubMed] [Google Scholar]
- 231.Yan X, et al. In situ thermal-responsive magnetic hydrogel for multidisciplinary therapy of hepatocellular carcinoma. Nano Lett. 2022;22(6):2251–60. [DOI] [PubMed] [Google Scholar]
- 232.Xiao S, et al. Intraperitoneal administration of thermosensitive hydrogel Co-loaded with norcantharidin nanoparticles and oxaliplatin inhibits malignant ascites of hepatocellular carcinoma. Drug Deliv. 2022;29(1):2713–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Gao B, et al. Intratumoral administration of thermosensitive hydrogel Co-loaded with norcantharidin nanoparticles and doxorubicin for the treatment of hepatocellular carcinoma. Int J Nanomedicine. 2021;16:4073–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Wen G, et al. Biohybrid hydrogel inhibiting β-klotho/HDAC3 axis for hepatocellular carcinoma treatment. Int J Biol Macromol. 2024;277:134369. [DOI] [PubMed] [Google Scholar]
- 235.Ao F, et al. STING agonist-based hydrogel enhances immune activation in synergy with radiofrequency ablation for hepatocellular carcinoma treatment. J Control Release. 2024;369:296–308. [DOI] [PubMed] [Google Scholar]
- 236.Chen Y, et al. Intratumoral lactate depletion based on injectable nanoparticles-hydrogel composite system synergizes with immunotherapy against postablative hepatocellular carcinoma recurrence. Adv Healthc Mater. 2024;13(6):e2303031. [DOI] [PubMed] [Google Scholar]
- 237.Wu WS, et al. Minimally invasive delivery of percutaneous ablation agent via magnetic colloidal hydrogel injection for treatment of hepatocellular carcinoma. Adv Mater. 2024;36(26):e2309770. [DOI] [PubMed] [Google Scholar]
- 238.Zhan J, et al. An injectable hydrogel with pH-sensitive and self-healing properties based on 4armPEGDA and N-carboxyethyl chitosan for local treatment of hepatocellular carcinoma. Int J Biol Macromol. 2020;163:1208–22. [DOI] [PubMed] [Google Scholar]
- 239.Huang S, et al. An injectable thermosensitive hydrogel loaded with a theranostic nanoprobe for synergistic chemo-photothermal therapy for multidrug-resistant hepatocellular carcinoma. J Mater Chem B. 2022;10(15):2828–43. [DOI] [PubMed] [Google Scholar]
- 240.Bayramoglu G, et al. Examination of fabrication conditions of acrylate-based hydrogel formulations for doxorubicin release and efficacy test for hepatocellular carcinoma cell. J Biomater Sci Polym Ed. 2014;25(7):657–78. [DOI] [PubMed] [Google Scholar]
- 241.Liu J, et al. Targeting SUMOylation with an injectable nanocomposite hydrogel to optimize radiofrequency ablation therapy for hepatocellular carcinoma. J Nanobiotechnol. 2024;22(1):338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Xue B, et al. Intratumoral injection of norcantharidin-loaded Poly(D, L-lactide)-b-Poly(ethylene glycol)-b-Poly(D, L-lactide) thermosensitive hydrogel for the treatment of primary hepatocellular carcinoma. J Biomed Nanotechnol. 2019;15(10):2025–44. [DOI] [PubMed] [Google Scholar]
- 243.Peng CL, et al. Development of in situ forming thermosensitive hydrogel for radiotherapy combined with chemotherapy in a mouse model of hepatocellular carcinoma. Mol Pharm. 2013;10(5):1854–64. [DOI] [PubMed] [Google Scholar]
- 244.Peng M, et al. Thermosensitive injectable hydrogel enhances the antitumor effect of embelin in mouse hepatocellular carcinoma. J Pharm Sci. 2014;103(3):965–73. [DOI] [PubMed] [Google Scholar]
- 245.Rehman U, et al. Smart pH-responsive co-polymeric hydrogels for controlled delivery of capecitabine: fabrication, optimization and in vivo toxicology screening. Curr Drug Deliv. 2021;18(9):1256–71. [DOI] [PubMed] [Google Scholar]
- 246.Bakalova R, et al. Passive and electro-assisted delivery of hydrogel nanoparticles in solid tumors, visualized by optical and magnetic resonance imaging in vivo. Anal Bioanal Chem. 2016;408(3):905–14. [DOI] [PubMed] [Google Scholar]
- 247.Liu Z, et al. Polyisocyanide hydrogels with tunable nonlinear elasticity mediate liver carcinoma cell functional response. Acta Biomater. 2022;148:152–62. [DOI] [PubMed] [Google Scholar]
- 248.You J, et al. Improved mechanical properties and sustained release behavior of cationic cellulose nanocrystals reinforeced cationic cellulose injectable hydrogels. Biomacromol. 2016;17(9):2839–48. [DOI] [PubMed] [Google Scholar]
- 249.Baek MJ, et al. Tailoring renal-clearable zwitterionic cyclodextrin for colorectal cancer-selective drug delivery. Nat Nanotechnol. 2023;18(8):945–56. [DOI] [PubMed] [Google Scholar]
- 250.Sun Y, et al. Sialic acid-targeted cyclodextrin-based nanoparticles deliver CSF-1R siRNA and reprogram tumour-associated macrophages for immunotherapy of prostate cancer. Eur J Pharm Sci. 2023;185:106427. [DOI] [PubMed] [Google Scholar]
- 251.Bose R, et al. Cyclodextrin nanoparticles in targeted cancer theranostics. Front Pharmacol. 2023;14:1218867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Kroemer G, et al. Immunogenic cell stress and death. Nat Immunol. 2022;23(4):487–500. [DOI] [PubMed] [Google Scholar]
- 253.Galluzzi L, et al. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17(2):97–111. [DOI] [PubMed] [Google Scholar]
- 254.Wang Y, et al. Photodynamic therapy of pancreatic cancer: where have we come from and where are we going? Photodiagn Photodyn Ther. 2020;31:101876. [DOI] [PubMed] [Google Scholar]
- 255.Yang H, et al. Photosensitizer nanoparticles boost photodynamic therapy for pancreatic cancer treatment. Nanomicro Lett. 2021;13(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Fan C, et al. Viscosity and degradation controlled injectable hydrogel for esophageal endoscopic submucosal dissection. Bioact Mater. 2021;6(4):1150–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Wei Y, et al. A novel tetra-PEG based hydrogel for prevention of esophageal stricture after ESD in a porcine model. Colloids Surf B Biointerfaces. 2023;226:113321. [DOI] [PubMed] [Google Scholar]
- 258.Wang Y, et al. Research on triamcinolone-loaded thermosensitive chitosan hydrogels for preventing esophageal stricture induced by endoscopic submucosal dissection. Int J Biol Macromol. 2024;261(Pt 1):129679. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This is review paper and no data has been produced.