Abstract
Background
Central obesity is a well-recognized risk factor for diabetes, yet the potential role of lipids in the diabetes risk associated with central obesity remains unclear. This study aimed to explore the possible mediating role of 11 lipid parameters [high-density lipoprotein cholesterol (HDL-C), total cholesterol (TC), non-high-density lipoprotein cholesterol (Non-HDL-C), low-density lipoprotein cholesterol (LDL-C), triglycerides (TG), remnant cholesterol (RC), and ratios of Non-HDL-C/HDL-C, RC/HDL-C, LDL/HDL-C, TG/HDL-C, TC/HDL-C] in the association of central obesity with diabetes risk.
Methods
We utilized data from 15,453 participants in the NAGALA longitudinal cohort to assess the association of baseline central obesity indicators [waist-height ratio (WHtR), waist circumference (WC)] and the 11 lipid parameters with diabetes risk. Mediation analysis models were constructed to explore the mediating role of lipid parameters in the association of WC/WHtR with diabetes.
Results
Confirmatory associative analysis using multivariable Cox regression showed that, except for Non-HDL-C, TC and LD-C, the remaining eight lipid parameters were significantly associated with WC/WHtR and diabetes risk. Mediation analysis indicated that TG, RC, HDL-C, and lipid ratios such as Non-HDL-C/HDL-C ratio, RC/HDL-C ratio, TG/HDL-C ratio, TC/HDL-C ratio and LDL/HDL-C ratio are potential lipids affecting the diabetes risk related to central obesity. Among these, the RC/HDL-C ratio seemed to contribute the most in the WC/WHtR-related diabetes risk association, with a mediation percentage of about 37%. Additionally, lipid ratio parameters appeared to play a more mediating role in the association of central obesity-related diabetes risk than individual lipids.
Conclusions
In central obesity-related diabetes risk, most lipids, especially lipid ratio parameters, play a significant mediating role. Given these findings, we advocate for increased efforts in multifactorial risk monitoring and joint management of diabetes. The evaluation of lipids, particularly lipid ratio parameters, may be holds substantial value in the prevention and management of diabetes risk under close monitoring of central obesity.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12902-024-01764-5.
Keywords: Lipids, RC/HDL-C ratio, Central obesity, Diabetes
Introduction
Diabetes is a major global public health concern, consistently ranking as one of the leading causes of death and disability [1]. Obesity is a critical factor in the onset and progression of diabetes [1–3], with reports suggesting that approximately 49.6% of diabetes cases are attributable to obesity [1]. Given the ongoing global obesity epidemic, it is projected that by the mid-21st century, around 1.3 billion people will be affected by diabetes [1], posing a substantial challenge to public health systems [4, 5]. Therefore, effectively controlling the prevalence of obesity [6, 7] and obesity-mediated metabolic abnormalities [8–10] plays a crucial role in reducing the future burden of diabetes.
It is well established that obesity is characterized by the accumulation of adipose tissue, with adipose tissue macrophages being the most abundant immune component in this state. In obesity, adipose tissue macrophages affect the synthesis of cholesterol and fatty acids by regulating sterol regulatory element-binding protein and the liver X receptor, which leads to further lipid accumulation and disruptions in glucose metabolism [9, 11]. Beyond the direct effects of fat accumulation, researchers have identified a phenomenon termed “adipose dysregulation” in obese patients, which promotes ectopic lipid overflow and deposition in non-adipose tissues (lipotoxicity), creating a “diabetogenic” environment [12]. Additionally, results based on Mendelian randomization studies suggest a causal role of lipid metabolism in obesity-related diabetes, while targeted lipidomics research [13] has further highlighted the primary role of lipid substances containing triacylglycerol 50:3 in the risk of obesity-related diabetes [14]. These findings provide compelling evidence for the potential role of lipids in the risk of obesity-related diabetes; thus, further exploring whether the clinical association of obesity with diabetes risk is mediated by lipids and identifying the most valuable lipid mediators can offer significant help in the prevention or intervention of diabetes. In our previous study assessing the efficacy of commonly used simple obesity indices to predict future diabetes, we found that central obesity indices such as WC and WHtR significantly outperformed the general obesity index body mass index (BMI) in predicting future diabetes through normalized Hazard Ratio (HR) analysis and time-dependent Receiver Operating Characteristic curve analysis [15]. This perspective is also highly recognized in multiple studies related to chronic diseases [16–18]. Against the backdrop of the obesity and diabetes pandemic, as a continuation of prior research, in the current analysis, we aimed to further assess the role of lipids in the risk of central obesity-related diabetes, providing new insights into the pathogenesis and risk management of central obesity-related diabetes.
Methods
Data source and study population
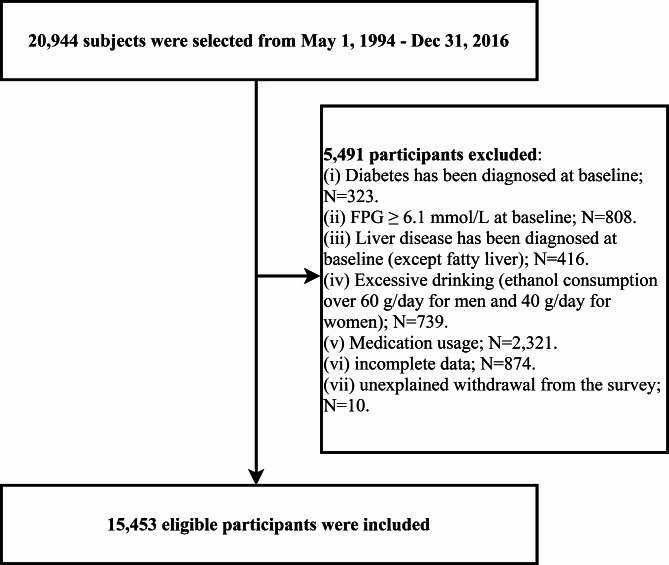
To investigate potential lipids influencing the risk of central obesity-related diabetes, a secondary analysis was conducted on 20,944 participants from the NAGALA cohort, enrolled between May 1, 1994, and December 31, 2016. Briefly, the NAGALA cohort study is a health examination program conducted in the Gifu area of Japan, targeting the general population. Its primary aim is to prospectively investigate risk factors influencing the incidence and progression of chronic diseases, specifically diabetes and non-alcoholic fatty liver disease. Details of the NAGALA cohort study design are extensively documented in a previous publication [19]. Furthermore, comprehensive data from the NAGALA study, collected by Professor Okamura and colleagues, has been shared on the Dryad public database for researchers to delve deeper into information beneficial for chronic disease risk management. For our study’s purposes, we excluded participants who, at baseline, had diabetes, impaired fasting glucose, liver diseases, or were using any medication (including but not limited to antidiabetic drugs, lipid-lowering drugs, antihypertensive drugs and hormones), those with excessive alcohol consumption (men/women > 60/40 g/day) [20], missing data, or who were lost to follow-up for unknown reasons. Ultimately, 15,453 eligible participants were included in our analysis (Fig. 1). The Murakami Hospital Ethics Committee granted ethical approval for the NAGALA cohort study (IRB 2018-09-01), and informed consent for the use of all participant data was obtained, adhering to the principles of the Helsinki Declaration. Clinical trial number: not applicable.
Fig. 1.
Flow chart of study participants
Data collection
All participants completed a questionnaire survey under the guidance of professional medical staff and underwent a physical examination, providing blood samples after fasting for 8 h. The questionnaire covered information such as sex, age, baseline medication usage, health conditions, smoking/drinking status, and habit of exercise. Smoking status was categorized as non, former, or current smoker. Drinking status was classified based on the participant’s alcohol intake in the previous month into none/little, moderate, or heavy [20]. Having a habit of exercise was defined as engaging in any form of sports more than once a week.
Anthropometric measures
Height and weight were measured on automated equipment without wearing shoes or thick clothing, and the measurements were accurate to 0.1 cm and 0.1 kg; BMI was calculated using the formula: weight (kg) / [height (m)]2. In addition, WC at the umbilicus was measured in the standing position using a flexible tape measure by a trained medical staff; WHtR was calculated as WC (cm) / height (cm).
Blood pressure was measured using a mercury sphygmomanometer. During the measurement, medical staff recorded systolic and diastolic blood pressure (S/DBP) based on the first and fifth Korotkoff sounds. Take a total of three blood pressure measurements, each time 2 min apart, and record the average of the last two measurements.
Diagnosis of fatty liver
Additionally, fatty liver was diagnosed by gastroenterology specialists following an abdominal ultrasound examination conducted by experienced ultrasonographers [21].
Biochemical parameter measurement
Venous blood samples were collected from subjects after fasting overnight for at least 8 h. Blood tests were performed using a modular analysis system (Hitachi High-Technologies Corp., Ltd., Tokyo, Japan), which is widely used in biochemical analysis in Japan. According to data provided by the manufacturer, the analytical coefficients of variation for GGT, aspartate transaminase (AST), alanine aminotransferase (ALT), plasma glucose, HbA1c, triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C) were 2%, 1.9%, 1.7%, 1.2%, 2.3%, 2.3%, 2.1%, and 2.5%, respectively.
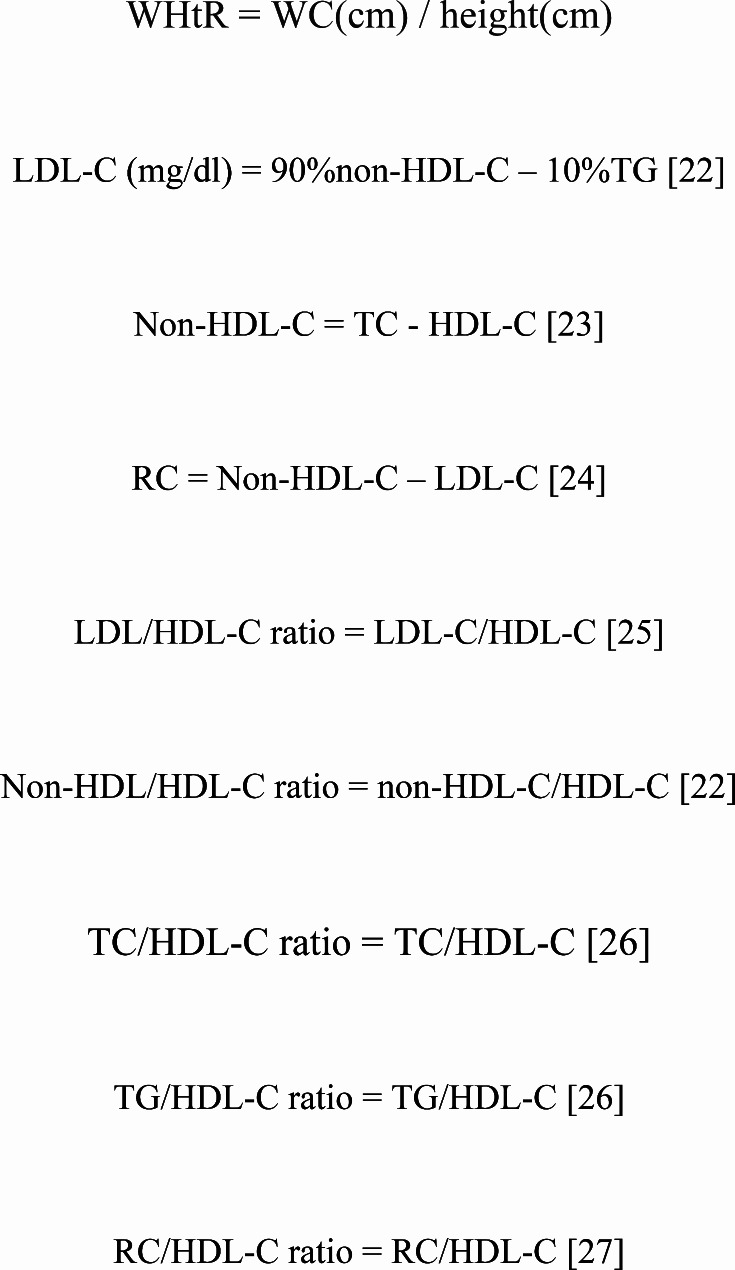
To comprehensively explore potential lipid mediators associated with central obesity-related diabetes risk, we further calculated the WHtR and various lipid parameters [22–27]. The detailed calculation process is illustrated in Fig. 2.
Fig. 2.
Formulas for calculating WHtR and lipid parameters
Diagnosis of diabetes
In the current study, diabetes was diagnosed according to the criteria set by the American Diabetes Association [28]. Diabetes is defined as having a HbA1c level of ≥ 6.5% or a FPG level of ≥ 7.0mmol/L or a self-reported history of diabetes.
Covariates
The covariates in this study were determined based on factors related to diabetes, central obesity, and dyslipidemia. These include height, weight, sex, age, BMI, GGT, FPG, ALT, SBP, AST, HbA1c, DBP, smoking status, fatty liver, habit of exercise, and drinking status [4–6, 19, 29–31].
Statistical analysis
Baseline characteristics were described as medians (interquartile range) or frequency (percentages) or means [standard deviation]. Before describing participant baseline characteristics, data with skewed distribution underwent a Box-Cox transformation to normalize. Standardized difference values were then calculated to quantify the magnitude of the difference between diabetes and non-diabetes subjects (> 10% as statistically significant) [32, 33].
Before mediation analysis, the degree of collinearity among lipid parameters, WC/WHtR, and other covariates was assessed by calculating the variance inflation factor for multiple linear equations [34]. Based on the results of collinearity diagnostic screening from Supplementary Tables 1–13, we noted high collinearity between DBP and WC, weight, BMI; and between WHtR and all lipid parameters with DBP and weight. Therefore, DBP, weight and BMI were excluded from subsequent analyses.
Next, mediation analysis was conducted following methods suggested by Professor VanderWeele [35, 36]. Initially, multivariable Cox regression models were used to confirm the associations between exposure variables (WC and WHtR), mediator variables (lipid parameters), and diabetes. To standardize HRs, the 11 lipid parameters were normalized to Z-scores before inclusion in the multivariable Cox regression models. Additionally, when verifying the associations of WC/WHtR with diabetes, each of the 11 lipid parameters was separately included in the Cox regression model to observe if the association of WC/WHtR with diabetes weakened, thus satisfying the precondition for mediation analysis [35, 36]. In addition, we also plotted the receiver operating characteristic curves of simply measuring obesity parameters BMI, WC and WHtR for predicting diabetes, and calculated the corresponding area under the curve. This was followed by checking the associations of WC/WHtR with the 11 lipid parameters using multiple linear regression. The stepwise adjustments in these confirmatory analyses followed the STROBE reporting guidelines [37], with results presented in the main text. Finally, mediation models were constructed to explore potential lipid mediators influencing central obesity-related diabetes risk. For comparing the mediation percentages (ratio of indirect to total effects) of different lipid parameters in the associations of WC/WHtR with diabetes, lipid parameters were Z-score transformed before mediation analysis. Additionally, Bootstrap sampling was used to test the significance of the mediation analysis, repeating sampling 1000 times in the original data for bias-corrected mediation analysis 95% confidence intervals (CIs) [38]. All analyses were performed using R language version 4.2.0 and Empower(R) version 4.1, and all P-value tests were two-sided, with statistically significance set at P < 0.05.
Results
Characteristics of study participants
In this cohort study based on the general population, we included 15,453 participants without baseline diabetes. During a mean follow-up period of 6.1 years, 373 individuals were diagnosed with incident diabetes. Table 1 displays the baseline characteristics of the participants, grouped by whether they developed diabetes in the future. Notably, all characteristics showed standardized differences greater than 10% between the diabetes and non-diabetes groups at baseline, indicating significant differences between the two groups. Among these, differences in blood glucose metabolism indicators, such as FPG and HbA1c, were particularly pronounced, with standardized differences of 121% and 107%, respectively. In obesity indicators, WC (92%) and WHtR (90%) were slightly higher than BMI (86%). Additionally, significant differences were observed in all lipid parameters between the two groups (35-88%), with the highest standardized difference observed in the RC/HDL-C ratio (88%).
Table 1.
Characteristics of the study subjects with and without diabetes
| Non-diabetes | Diabetes | Standardized difference (%) | |
|---|---|---|---|
| No of subjects | 15,080 | 373 | |
| Sex | 49 (39, 59) | ||
| Women | 6947 (46.07%) | 87 (23.32%) | |
| Men | 8133 (53.93%) | 286 (76.68%) | |
| Age, years | 42.00 (37.00–50.00) | 46.00 (41.00–53.00) | 40 (30, 51) |
| Weight, kg | 60.41 (11.48) | 69.84 (13.32) | 76 (66, 86) |
| Height, cm | 1.65 (0.08) | 1.67 (0.09) 1.68 | 19 (9, 29) |
| WHtR | 0.46 (0.05) | 0.51 (0.06) | 90 (80, 100) |
| WC, cm | 76.25 (8.97) | 85.08 (10.20) | 92 (82, 102) |
| BMI, kg/m2 | 22.04 (3.07) | 25.03 (3.82) | 86 (76, 97) |
| ALT, U/L | 17.00 (13.00–23.00) | 24.00 (18.00–39.00) | 67 (56, 77) |
| AST, U/L | 17.00 (14.00–21.00) | 20.00 (16.00–26.00) | 44 (34, 55) |
| GGT, U/L | 15.00 (11.00–22.00) | 24.00 (17.00–36.00) | 47 (37, 58) |
| TC, mmol/L | 5.12 (0.86) | 5.43 (0.90) | 35 (25, 46) |
| TG, mmol/L | 0.72 (0.49–1.11) | 1.21 (0.86–1.93) | 73 (62, 83) |
| HDL-C, mmol/L | 1.47 (0.40) | 1.19 (0.33) | 77 (66, 87) |
| LDL-C. mmol/L | 3.15 (2.63–3.69) | 3.63 (3.09–4.14) | 60 (50, 70) |
| Non-HDL-C, mmol/L | 3.59 (3.00-4.23) | 4.20 (3.57–4.82) | 65 (55, 75) |
| RC, mmol/L | 0.44 (0.36–0.53) | 0.55 (0.46–0.67) | 80 (70, 91) |
| TC/HDL-C ratio | 3.50 (2.86–4.39) | 4.71 (3.86–5.78) | 87 (77, 97) |
| TG/HDL-C ratio | 0.50 (0.30–0.89) | 1.09 (0.64–1.93) | 74 (63, 84) |
| LDL/HDL-C ratio | 2.19 (1.64–2.96) | 3.19 (2.50–4.11) | 86 (75, 96) |
| Non-HDL/HDL-C ratio | 2.50 (1.86–3.39) | 3.71 (2.86–4.78) | 87 (77, 97) |
| RC/HDL-C | 0.30 (0.22–0.43) | 0.48 (0.36–0.66) | 88 (78, 98) |
| FPG, mmol/L | 5.15 (0.41) | 5.61 (0.36) | 121 (111, 132) |
| HbA1c, % | 5.16 (0.32) | 5.53 (0.37) | 107 (97, 118) |
| SBP, mmHg | 114.31 (14.91) | 122.03 (15.59) | 51 (40, 61) |
| DBP, mmHg | 71.44 (10.47) | 77.18 (10.23) | 55 (45, 66) |
| Habit of exercise | 2655 (17.61%) | 51 (13.67%) | 11 (1, 21) |
| Fatty liver | 2514 (16.67%) | 223 (59.79%) | 99 (89, 109) |
| Drinking status | 21 (11, 31) | ||
| no or little | 11,536 (76.50%) | 266 (71.31%) | |
| light | 1714 (11.37%) | 40 (10.72%) | |
| moderate | 1320 (8.75%) | 37 (9.92%) | |
| heavy | 510 (3.38%) | 30 (8.04%) | |
| Smoking status | 45 (35, 55) | ||
| non | 8882 (58.90%) | 145 (38.87%) | |
| former | 2872 (19.05%) | 77 (20.64%) | |
| current | 3326 (22.06%) | 151 (40.48%) |
Values were expressed as mean (SD) or median (interquartile range) or n (%)
Abbreviations: WHtR: waist-to-height ratio; WC: waist circumference; BMI: body mass index; ALT: alanine aminotransferase; AST: aspartate aminotransferase; GGT: gamma-glutamyl transferase; HDL-C: high-density lipoprotein cholesterol; TC: total cholesterol; TG: triglyceride; LDL-C: low density lipoprotein cholesterol; Non-HDL-C: non-high-density lipoprotein cholesterol; RC: remnant cholesterol; HbA1c: hemoglobin A1c; FPG: fasting plasma glucose; SBP: systolic blood pressure; DBP: diastolic blood pressure
Relationship of lipid parameters with diabetes
Table 2 presents the results of the Cox regression analysis of lipid parameters (standardized to Z-scores) with the risk of diabetes. After adjusting for age, sex, height, fatty liver, and lifestyle factors, the associations of all 11 lipid parameters with diabetes risk remained statistically significant (Model 1). In contrast, after further adjustments in Model 2 for HbA1c, GGT, SBP, ALT, FPG, and AST, the associations of Non-HDL-C, LDL-C, TC with diabetes risk were no longer significant. Notably, combined lipid ratio parameters showed a superior capability in assessing diabetes risk compared to individual lipid parameters. Among these, the RC/HDL-C ratio emerged as the best lipid parameter for assessing diabetes risk (HR = 1.20, 95%CI: 1.09, 1.31).
Table 2.
Relationship between lipid parameters and diabetes
| HR (95%CI) | ||||
|---|---|---|---|---|
| Unadjusted Model | Model 1 | Model 2 | ||
| TC | 1.41 (1.29, 1.55) ** | 1.15 (1.04, 1.28) * | 0.95 (0.85, 1.06) | |
| TG | 1.47 (1.41, 1.54) ** | 1.26 (1.17, 1.34) ** | 1.14 (1.05, 1.23) * | |
| HDL-C | 0.44 (0.38, 0.50) ** | 0.66 (0.57, 0.77) ** | 0.74 (0.63, 0.86) ** | |
| LDL-C | 1.63 (1.51, 1.77) ** | 1.25 (1.12, 1.39) ** | 1.00 (0.90, 1.12) | |
| Non-HDL-C | 1.70 (1.57, 1.84) ** | 1.28 (1.15, 1.42) ** | 1.02 (0.91, 1.14) | |
| RC | 1.84 (1.71, 1.98) ** | 1.38 (1.25, 1.52) ** | 1.11 (1.01, 1.23) * | |
| TC/HDL-C ratio | 1.81 (1.69, 1.94) ** | 1.37 (1.25, 1.51) ** | 1.18 (1.07, 1.31) * | |
| TG/HDL-C ratio | 1.33 (1.29, 1.37) ** | 1.22 (1.16, 1.29) ** | 1.15 (1.07, 1.22) ** | |
| LDL/HDL-C ratio | 1.79 (1.67, 1.92) ** | 1.36 (1.24, 1.50) ** | 1.17 (1.06, 1.30) * | |
| Non-HDL/HDL-C ratio | 1.81 (1.69, 1.94) ** | 1.37 (1.25, 1.51) ** | 1.18 (1.07, 1.31) * | |
| RC/HDL-C ratio | 1.66 (1.58, 1.75) ** | 1.36 (1.25, 1.48) ** | 1.20 (1.09, 1.31) ** | |
Abbreviations: HDL-C: high-density lipoprotein cholesterol; TC: total cholesterol; TG: triglyceride; LDL-C: low density lipoprotein cholesterol; Non-HDL-C: non-high-density lipoprotein cholesterol; RC: remnant cholesterol
Model 1 adjusted sex, age, height, fatty liver, habit of exercise, smoking status and drinking status
Model 2 adjusted model 1 + SBP, FPG, HbA1c, ALT, AST and GGT
*P < 0.05, **P < 0.001
Relationship of WC/WHtR with diabetes
In examining the association of WC/WHtR with diabetes risk, 13 multivariable Cox regression models were run (Table 3). In Models 1 and 2, after stepwise adjustments for all non-collinear covariates except the mediator variables, a significant positive correlation was found between WC/WHtR and diabetes risk (WC: HR = 1.423; WHtR: HR = 1.380). Additionally, each of the 11 lipid parameters (mediator variables) was separately incorporated as covariates in the Cox regression models (Models 3–13), to observe changes and significance in the association of WC/WHtR with diabetes risk. It was observed that the association of WC/WHtR with diabetes risk intensified when lipid parameters such as TC, LDL-C, and Non-HDL-C were individually adjusted (Models 3, 6, 7). In contrast, the association weakened when adjusting for other lipid parameters (Table 3). This suggested that TC, LDL-C, and Non-HDL-C might not be potential mediators in the association of WC/WHtR with diabetes risk.
Table 3.
Relationship between WC/ WHtR and diabetes
| WC | WHtR | |||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| WC | WHtR | |||
| Model 1 | 1.757 (1.569, 1.967) | < 0.001 | 1.676 (1.512, 1.857) | < 0.001 |
| Model 2 | 1.455 (1.284, 1.649) | < 0.001 | 1.407 (1.257, 1.576) | < 0.001 |
| Model 3 | 1.465 (1.292, 1.661) | < 0.001 | 1.416 (1.264, 1.586) | < 0.001 |
| Model 4 | 1.440 (1.269, 1.635) | < 0.001 | 1.395 (1.244, 1.563) | < 0.001 |
| Model 5 | 1.421 (1.250, 1.614) | < 0.001 | 1.377 (1.227, 1.545) | < 0.001 |
| Model 6 | 1.461 (1.289, 1.658) | < 0.001 | 1.413 (1.261, 1.583) | < 0.001 |
| Model 7 | 1.458 (1.286, 1.654) | < 0.001 | 1.410 (1.259, 1.580) | < 0.001 |
| Model 8 | 1.441 (1.270, 1.636) | < 0.001 | 1.395 (1.245, 1.564) | < 0.001 |
| Model 9 | 1.426 (1.256, 1.620) | < 0.001 | 1.382 (1.232, 1.551) | < 0.001 |
| Model 10 | 1.437 (1.266, 1.631) | < 0.001 | 1.392 (1.241, 1.560) | < 0.001 |
| Model 11 | 1.428 (1.257, 1.623) | < 0.001 | 1.384 (1.234, 1.553) | < 0.001 |
| Model 12 | 1.426 (1.256, 1.620) | < 0.001 | 1.382 (1.232, 1.551) | < 0.001 |
| Model 13 | 1.423 (1.253, 1.617) | < 0.001 | 1.380 (1.230, 1.548) | < 0.001 |
Abbreviations: WHtR: waist-to-height ratio; WC: waist circumference; HR: Hazard ratios; CI: confidence interval; other abbreviations as in Table 1
All variables were calculated for 1SD change increasing of WC/WHtR
Model 1 adjusted sex, age, height, fatty liver, habit of exercise, smoking status and drinking status
Model 2 adjusted model 1 + SBP, FPG, HbA1c, ALT, AST and GGT
Model 3 adjusted model 2 + TC; Model 4 adjusted model 2 + TG; Model 5 adjusted Model 2 + HDL-C; Model 6 adjusted Model 2 + LDL-C; Model 7 adjusted Model 2 + Non-HDL-C; Model 8 adjusted Model 2 + RC; Model 9 adjusted Model 2 + TC/HDL-C ratio; Model 10 adjusted Model 2 + TG/HDL-C ratio; Model 11 adjusted Model 2 + LDL/HDL-C ratio; Model 12 adjusted Model 2 + non-HDL/HDL-C ratio; Model 13 adjusted Model 2 + RC/HDL-C ratio
Models 3–13 show the correlation between WC/WHtR and diabetes when lipid parameters are included in the regression model
Figure 3 shows the receiver operating characteristic curves of BMI, WC and WHtR to predict diabetes. Results showed that WC and WHtR were more accurate in predicting diabetes than BMI (Aure under the curve: WC 0.743 vs. WHtR 0.742 vs. BMI 0.733). This finding supported the important role of central adiposity in diabetes risk prediction.
Fig. 3.
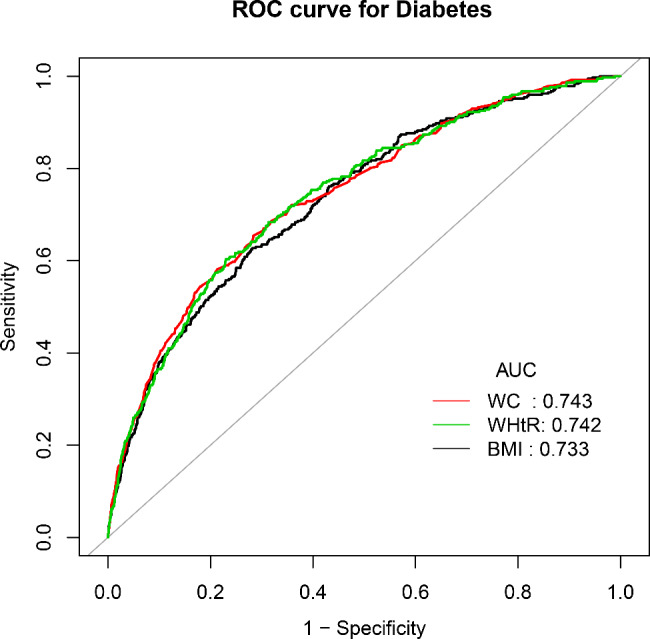
The receiver operating characteristic curves of BMI, WC and WHtR to predict diabetes
Association of WC/WHtR with lipid parameters
Subsequently, the relationship of WC/WHtR with lipid parameters was examined using linear regression (Table 4). After adjusting for confounders, all lipid parameters were found to be related to WC/WHtR. Apart from HDL-C, which was negatively correlated with WC/WHtR (WC: β= -0.09; WHtR: β= -0.14), all other lipid parameters were positively correlated. Notably, both WC and WHtR showed the strongest association with the TC/HDL-C ratio (WC: β = 0.15; WHtR: β = 0.22).
Table 4.
Association of WC/WHtR with lipid parameters
| β (95%CI) | P-value | β (95%CI) | P-value | |
|---|---|---|---|---|
| WC | WHtR | |||
| TC | 0.02 (0.02, 0.03) | < 0.001 | 0.04 (0.03, 0.05) | < 0.001 |
| TG | 0.03 (0.03, 0.03) | < 0.001 | 0.04 (0.04, 0.05) | < 0.001 |
| HDL-C | -0.09 (-0.10, -0.09) | < 0.001 | -0.14 (-0.14, -0.13) | < 0.001 |
| LDL-C | 0.05 (0.04, 0.05) | < 0.001 | 0.07 (0.06, 0.07) | < 0.001 |
| Non-HDL-C | 0.05 (0.04, 0.05) | < 0.001 | 0.07 (0.06, 0.07) | < 0.001 |
| RC | 0.07 (0.07, 0.08) | < 0.001 | 0.11 (0.10, 0.11) | < 0.001 |
| TC/HDL-C ratio | 0.15 (0.14, 0.16) | < 0.001 | 0.22 (0.21, 0.23) | < 0.001 |
| TG/HDL-C ratio | 0.03 (0.03, 0.03) | < 0.001 | 0.04 (0.04, 0.05) | < 0.001 |
| LDL/HDL-C ratio | 0.06 (0.06, 0.07) | < 0 0.001 | 0.10 (0.09, 0.10) | < 0.001 |
| Non-HDL/HDL-C ratio | 0.07 (0.06, 0.07) | < 0.001 | 0.10 (0.09, 0.10) | < 0.001 |
| RC/HDL-C ratio | 0.06 (0.05, 0.06) | < 0.001 | 0.08 (0.08, 0.09) | < 0.001 |
Abbreviations: β: regression coefficient; CI: confidence interval; other abbreviations as in Table 1
Adjusting variables: sex, age, height, fatty liver, habit of exercise, smoking status and drinking status, SBP, FPG, HbA1c, ALT, AST and GGT
Mediation analysis for WC/WHtR and diabetes via lipid parameters in the whole population
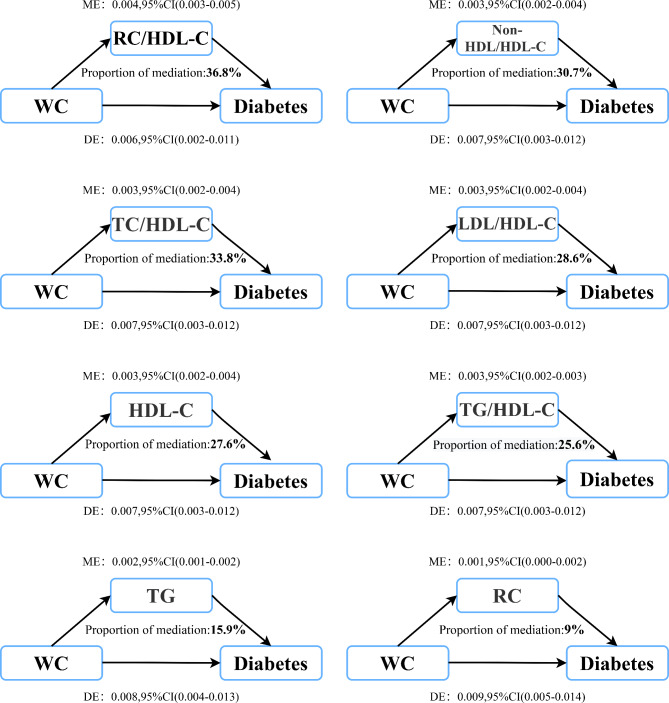
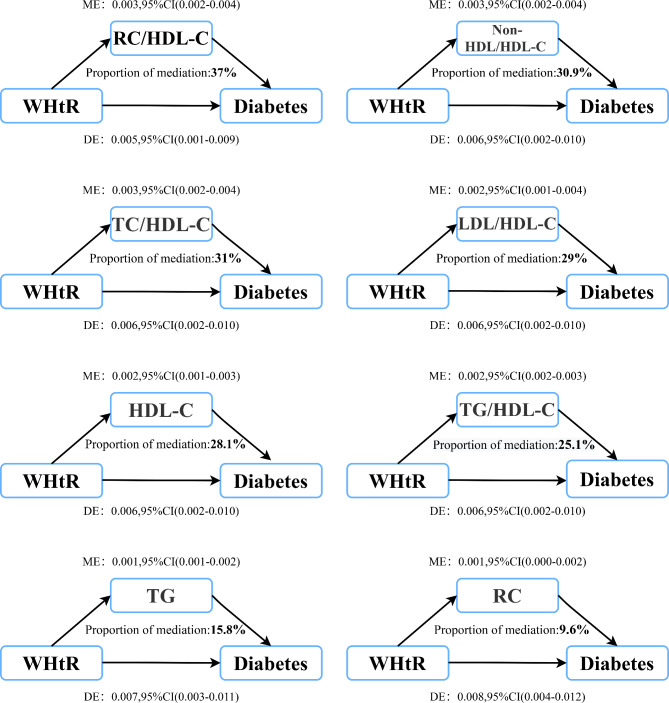
The mediation models constructed to explore lipid mediators in the association of WC/WHtR with diabetes risk revealed similar roles of lipid parameters as mediators in central obesity-related diabetes risk. The results of the mediation analysis are shown in Tables 5 and 6, with illustrative diagrams in Figs. 4 and 5. Most lipid parameters, except for Non-HDL-C, LDL-C and TC, mediated the association of WC/WHtR with diabetes risk, corroborating findings from the aforementioned associative analyses. Notably, the RC/HDL-C ratio was the most substantial mediator in both WC and WHtR associated diabetes risks, with mediation percentages of 36.8% and 37%, respectively (Tables 5 and 6). Moreover, combined lipid ratio parameters generally showed higher mediation effects in central obesity-related diabetes risk. The mediation percentages for TC/HDL-C ratio, TG/HDL-C ratio, LDL/HDL-C ratio, and Non-HDL/HDL-C ratio ranged between 25 and 31% (Tables 5 and 6). Additionally, among conventional lipid parameters, HDL-C also had a significant mediation role in the association of WC/WHtR with diabetes risk, with percentages of 27.6% and 28.1%, respectively.
Table 5.
Mediation analysis for waist circumference and diabetes via lipid parameters in the whole population
| Mediator | Total effect | Mediation effect | Direct effect | PM(%) | p-value of PM |
|---|---|---|---|---|---|
| TC | 0.010 (0.006, 0.015) | -0.001 (-0.001, 0.000) | 0.010 (0.006, 0.015) | - | - |
| TG | 0.010 (0.006, 0.015) | 0.002 (0.001, 0.002) | 0.008 (0.004, 0.013) | 15.9 | < 0.001 |
| HDL-C | 0.010 (0.006, 0.015) | 0.003 (0.002, 0.004) | 0.007 (0.003, 0.012) | 27.6 | < 0.001 |
| LDL-C | 0.010 (0.006, 0.015) | -0.000 (-0.001, 0.000) | 0.010 (0.006, 0.015) | - | - |
| Non-HDL-C | 0.010 (0.006, 0.015) | -0.000 (-0.001, 0.000) | 0.010 (0.006, 0.015) | - | - |
| RC | 0.010 (0.006, 0.015) | 0.001 (0.000, 0.002) | 0.009 (0.005, 0.014) | 9 | 0.024 |
| TC/HDL-C ratio | 0.010 (0.006, 0.015) | 0.003 (0.002, 0.004) | 0.007 (0.003, 0.012) | 30.7 | < 0.001 |
| TG/HDL-C ratio | 0.010 (0.006, 0.015) | 0.003 (0.002, 0.003) | 0.007 (0.003, 0.012) | 25.6 | < 0.001 |
| LDL/HDL-C ratio | 0.010 (0.006, 0.015) | 0.003 (0.002, 0.004) | 0.007 (0.003, 0.012) | 28.6 | < 0.001 |
| Non-HDL/HDL-C ratio | 0.010 (0.006, 0.015) | 0.003 (0.002, 0.004) | 0.007 (0.003, 0.012) | 30.7 | < 0.001 |
| RC/HDL-C ratio | 0.010 (0.006, 0.015) | 0.004 (0.003, 0.005) | 0.006 (0.002, 0.011) | 36.8 | < 0.001 |
Abbreviations: PM: propotion mediate; other abbreviations as in Table 1
Adjusting variables: sex, age, height, fatty liver, habit of exercise, smoking status and drinking status, SBP, FPG, HbA1c, ALT, AST and GGT
Table 6.
Mediation analysis for WHtR and diabetes via lipid parameters in the whole population
| Mediator | Total effect | Mediation effect | Direct effect | PM(%) | p-value of PM |
|---|---|---|---|---|---|
| TC | 0.009 (0.005, 0.012) | -0.001 (-0.001, 0.000) | 0.009 (0.005, 0.013) | - | - |
| TG | 0.009 (0.005, 0.012) | 0.001 (0.001, 0.002) | 0.007 (0.003, 0.011) | 15.8 | < 0.001 |
| HDL-C | 0.009 (0.005, 0.012) | 0.002 (0.001, 0.003) | 0.006 (0.002, 0.010) | 28.1 | < 0.001 |
| LDL-C | 0.009 (0.004, 0.013) | -0.001 (-0.001, 0.001) | 0.009 (0.005, 0.013) | - | - |
| Non-HDL-C | 0.009 (0.004, 0.013) | -0.001 (-0.001, 0.001) | 0.009 (0.005, 0.013) | - | - |
| RC | 0.009 (0.004, 0.013) | 0.001 (0.000, 0.002) | 0.008 (0.004, 0.012) | 9.6 | 0.030 |
| TC/HDL-C ratio | 0.009 (0.005, 0.013) | 0.003 (0.002, 0.004) | 0.006 (0.002, 0.010) | 31 | < 0.001 |
| TG/HDL-C ratio | 0.009 (0.005, 0.013) | 0.002 (0.002, 0.003) | 0.006 (0.002, 0.010) | 25.1 | < 0.001 |
| LDL/HDL-C ratio | 0.009 (0.005, 0.013) | 0.002 (0.001, 0.004) | 0.006 (0.002, 0.010) | 29 | < 0.001 |
| Non-HDL/HDL-C ratio | 0.009 (0.005, 0.013) | 0.003 (0.002, 0.004) | 0.006 (0.002, 0.010) | 30.9 | < 0.001 |
| RC/HDL-C ratio | 0.009 (0.005, 0.013) | 0.003 (0.002, 0.004) | 0.005 (0.001, 0.009) | 37 | < 0.001 |
Abbreviations: PM: propotion mediate; other abbreviations as in Table 1
Adjusting variables: sex, age, height, fatty liver, habit of exercise, smoking status and drinking status, SBP, FPG, HbA1c, ALT, AST and GGT
Fig. 4.
Lipid parameters mediation models of the relationship of WC with diabetes. ME: Mediation effect; DE: Direct effect; WC: waist circumference
Fig. 5.
Lipid parameters mediation models of the relationship of WHtR with diabetes. ME: Mediation effect; DE: Direct effect; WHtR: waist-to-height ratio
Discussion
In this large observational cohort study based on the general population, we used mediation analyses to validate that the risk of central obesity-associated diabetes was partially mediated by lipid parameters and further quantified the percentage of mediators of lipid parameters mediating the risk of WC/WHtR-associated diabetes. Key findings include: (1) Lipids parameters TG, HDL-C, RC, RC/HDL-C, non-HDL-C/HDL-C, TG/HDL-C, LDL-C/HDL-C, TC/HDL-C appeared to be potential lipids affecting the risk of WC/WHtR-related diabetes, with the exception of Non-HDL-C, LDL-C, TC. (2) Lipid ratio parameters appeared to play a more significant mediating role in the central obesity-related diabetes risk association compared to individual lipid parameters.
A large number of previous epidemiological studies have reported an association between obesity and diabetes risk. In general, the central obesity indices, WC and WHtR, are more strongly associated with the risk of developing diabetes than the general obesity index, BMI [15, 39, 40]; however, there is currently a lack of clarity regarding the mechanisms underlying the central obesity-related diabetes risk. Given that diabetes mellitus is a chronic metabolic disease with multifactorial and multi-pathway effects [3, 29–31]; in the current study, we aimed to explore the indirect impact of a common metabolic factor (lipid metabolism) on the association of central obesity with diabetes risk. Recent research evidence suggests that combined lipid ratio parameters have a stronger correlation with the risk of developing diabetes [23–27]. Combining lipid parameters significantly enhances their value in assessing diabetes risk, diagnosing the disease, and predicting its onset, aligning with the associative analysis results of this study. Furthermore, through mediation models, we identified eight lipid parameters that influenced the diabetes risk associated with WC/WHtR. Among them, the mediation percentages of lipid ratio parameters were between 25% and 37%, which was generally higher than that of individual lipid parameters. Based on findings from current research, we recommend focusing more on the RC/HDL-C ratio and other combined lipid ratio parameters in the risk assessment and management of diabetes to address the growing pandemic of this disease effectively.
In the current study, we did not observe Non-HDL-C, LDL-C and TC mediating the association of WC/WHtR with diabetes risk. The main reason appears to be the insignificant association of Non-HDL-C, LDL-C, TC with diabetes risk in our study, which does not satisfy the precondition for mediation analysis. Similar reports are found in various population-based prospective cohort studies [41, 42]. For instance, a study conducted on 5201 Iranian participants from 1999 to 2008 by Hadaegh F and others found that the association between TC and Non-HDL-C with diabetes risk disappeared after adjusting for blood pressure, glucose metabolism, and obesity indicators [41]. This finding aligns with our study, where the association of TC, LDL-C, and Non-HDL-C with diabetes risk became non-significant post-adjustment for blood pressure, glucose metabolism, and liver enzymes (Table 2). Additionally, a cohort study from China involving 5,563 participants found similar results [43]. In this study, Chen and colleagues explored the mediating role of conventional lipid parameters TC, TG, LDL-C, and HDL-C, along with blood pressure and liver and kidney metabolic indicators in WHtR-related diabetes risk. The study revealed that TG mediated 11.2% of WHtR-related diabetes risk in men and HDL-C mediated 25.45% in women, with TC and LDL-C not serving as mediators. In our study, we assessed TG and HDL-C to mediate 15.8% and 28.1% of WHtR-related diabetes risk in the overall population, respectively, without finding a mediating role for TC and LDL-C. In summary, the role of TC, LDL-C, and Non-HDL-C in obesity-related diabetes risk remains unclear and needs further research for clarification.
The pathophysiological mechanisms through which lipid parameters potentially mediate the risk of diabetes related to central obesity are not yet fully clear, but some research evidence may offer partial explanations: Firstly, population-based studies have shown that abdominal fat is more susceptible to lipolysis than subcutaneous fat [44], as they have a lower antilipolytic response to insulin. Secondly, abdominal adipose tissue exhibits high expression of the Resistin gene, and excess Resistin can lead to decreased glucose tolerance and insulin resistance (IR) [45, 46]; thirdly, immune factors may also have an impact on this process. Studies have shown that approximately 40% of immune cells in visceral adipose tissue in obese states are macrophages, and that these macrophages in visceral adipose tissue are more inclined to polarise towards a pro-inflammatory phenotype (type M1) and secrete cytosolic inflammatory factors (TNF-α, MCP-1, IL-1, and IL-6) that affect insulin signaling pathways, thereby producing or exacerbating the state of IR [47, 48]. Fourthly, it should also be noted that adipose tissue is an endocrine organ, and when visceral fat accumulates it induces endocrine dysregulation in adipose tissue, in which imbalance of lipocalin secretion may be an important mechanism for lipid-mediated risk of diabetes associated with central obesity. It is well known that lipocalin is a constitutive adipocyte protein that reduces hepatic glycogen production and inhibits fatty acid synthesis by binding to cellular receptors such as AdipoR1 and AdipoR2 in vivo [49]; in addition, lipocalin can agonize hepatic insulin receptor substrate 2 (IRS-2) to enhance insulin sensitivity [49]. When excessive abdominal fat accumulates, lipocalin secretion is reduced and lipocalin is unable to perform its normal biological function, which can be detrimental to glucose metabolism [49, 50].
Although the specific pathophysiological mechanisms linking central obesity with diabetes risk remain unclear, the findings of the current study provided useful clues for exploring these mechanisms and clinical interventions. Whether using WC or WHtR as indicators of central obesity, our study observed a similar mediating role of lipid parameters. These insights offer new perspectives on the potential mechanisms underlying the association of central obesity with diabetes risk, and suggest novel approaches for preventing or managing diabetes risk in the general population by controlling metabolic factors such as dyslipidemia. Clinical evidence has already highlighted the effectiveness of combined risk factor management, significantly reducing the risk of diabetes and its complications. The Steno 2 study in Denmark found a roughly 60% reduction in cardiovascular disease risk and a significant decrease in diabetes-related microvascular complications (neuropathy, retinopathy) following intensive treatment targeting HbA1c, blood pressure, and lipids, compared to a control group [51]. The large-scale, multicenter J-DOIT3 study in Japan demonstrated a significant reduction in cerebrovascular events (HR: 0.42, 95%CI: 0.24–0.7, P = 0.002) in participants receiving intensive treatment for HbA1c, blood pressure, and lipids. Similarly, the Joint Asia Diabetes Evaluation (JADE) program implemented in Hong Kong, China, showed analogous findings. The JADE program, centered around the ABC targets [52] (HbA1c < 7%, blood pressure ≤ 130/80 mmHg, LDL-C ≤ 2.6 mmol/L) and weight management, aimed to reduce the risk of diabetes and its complications in the entire population through combined risk factor management [53]. The program has significantly reduced diabetes-related CVD risk (40%) and all-cause mortality (66%), and alleviated the healthcare burden associated with diabetes [53, 54]. Despite the substantial clinical benefits brought by combined risk factor management, the global attainment rate of diabetes ABC targets remains low, with regions like China and South Korea having rates as low as less than 5% [55, 56]; and in the United States, the rate is just under 10% when weight management is included in the combined management goals [57]. Therefore, in light of our study’s findings, we call on public health policymakers to focus more on multi-factorial risk monitoring and combined management of diabetes, particularly emphasizing lipid ratio parameters such as RC/HDL-C ratio, to enhance public awareness of managing diabetes risks through a multi-factorial approach. Furthermore, the development of multi-factorial risk models for diabetes, based on ABC targets and weight management, integrated into electronic medical record systems, is needed. This would alert frontline clinicians to patients not meeting targets and provide individualized recommendations based on the risk assessment model. Such an approach could improve precision medicine in diabetes, reducing the globally escalating risk of the disease.
Strengths and limitations
Strengths of the current study: Based on the NAGALA longitudinal cohort, we revealed for the first time that eight simple lipid parameters serve as potential mediators influencing central obesity-associated diabetes risk in the general population, and the results were relatively robust (cross-validated in the two central obesity indicators, WC and WHtR); furthermore, these novel findings provide new insights, as well as reference materials for integrated diabetes risk factor management, and thus have important clinical implications.
Some limitations of the current study need to be mentioned: (1) The current findings suggest that the association of central obesity with diabetes risk is only partially mediated by lipid parameters, but the specific pathophysiological mechanisms of this meditation are unknown and require additional basic research to elucidate. (2) The lack of data on hip circumference and intra-abdominal/visceral fat in the NAGALA dataset makes it unclear whether lipid parameters play a similarly mediating role in waist-to-hip ratio and visceral fat-associated diabetes risk. (3) The use of statin lipid-lowering drugs increases the risk of diabetes mellitus while affecting cholesterol and lipid metabolism [58]; the potential impact of lipid-lowering drugs on the results of the current study could not be assessed because the current study already excluded participants who were on medications at baseline from the original dataset. (4) The participants in the current study were from the general population of Japan, and therefore caution should be exercised when the results of the current study are referenced to other ethnic groups. (5) While we meticulously adjusted for covariates at each step of our mediation analysis, it’s important to acknowledge that our results are still subject to limitations due to unmeasured confounding factors. Conversely, to truly invalidate the current findings, unmeasured confounders would have to influence the central obesity-related diabetes risk association through a pathway that is independent of the covariates being measured, however, this occurs in a biological situation is virtually impossible [59]. (6) The current study primarily evaluates the mediating effect of baseline lipid parameters on the future risk of diabetes-related to early central obesity. It does not account for the dynamic changes in lipid parameters and obesity during follow-up, and further research is needed. (7) The current study is based on a secondary analysis of NAGALA public data. Since the public data set no longer provides data update services, we cannot further evaluate the history of other chronic diseases (such as kidney disease and heart disease) and various metabolism-related variables (Such as white blood cells, C-reactive protein, insulin, creatinine, and uric acid) potential impact on study results. (8) Although WC measurement is widely used, there is still no recommended unified measurement method. In the current study, WC was measured at the level of the umbilicus. According to previous research data, the variation in WC measurement values among different measurement methods is minimal in men, whereas there is a significant variation in WC measurement values in women [60, 61]. In contrast, WC values measured at the umbilical level in women are generally higher than those measured at other sites. This could result in more women being classified as abdominally obese and may exaggerate the critical role of central adiposity in women’s diabetes risk.
Conclusion
In summary, most lipid parameters serve as potential mediators affecting the risk of diabetes associated with central obesity. It is worth noting that, compared with individual lipid parameters, lipid ratio parameters appear to have a greater mediating effect on central obesity-related diabetes. These findings offer new insights into the potential mechanisms linking central obesity and diabetes risk and suggest novel approaches for the general population to prevent or manage the risk of diabetes-related to central obesity by controlling metabolic factors like dyslipidemia.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Thanks to Professor Okamura’s team for their great efforts in data collection and collation.
Abbreviations
- DBP
Diastolic blood pressure
- Non-HDL-C
Non-high-density lipoprotein cholesterol
- WC
Waist circumference
- FPG
Fasting plasma glucose
- ALT
Alanine aminotransferase
- RC
Remnant cholesterol
- CI
Confidence intervals
- HbA1c
Hemoglobin A1c
- HDL-C
High-density lipoprotein cholesterol
- WHtR
Waist-to-height ratio
- AST
Aspartate aminotransferase
- BMI
Body mass index
- TG
Triglyceride
- SBP
Systolic blood pressure
- HR
Hazard ratio
- TC
Total cholesterol
- GGT
Gamma-glutamyl transferase
- LDL-C
Low density lipoprotein cholesterol
- ALT
Alanine aminotransferase
Author contributions
YZ and XP-P: Project administration supervision and conceptualization.YZ and GT-S: Methodology.SL: Writing-original draft preparation.YZ, MZ, WJ-L, MB-K, JJ-Q, GT-S and XP-P: Writing-reviewing and editing.SL, JJ-Q and MB-K: Software.SL and MB-K: Validation and formal analysisSL, JJ-Q and MB-K: Data curation.All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China [82060067, 81660359], the Natural Science Foundation of Jiangxi Province [20232BAB216004, 20203BBGL73233], the Science Foundation of Jiangxi Provincial Department of Education [No: GJJ200190].
Data availability
The datasets analysed during the current study are available in the Dryad repository. [https://datadryad.org/stash/dataset/doi:10.5061/dryad.8q0p192]
Declarations
Ethics approval and consent to participate
The Murakami Hospital Ethics Committee granted ethical approval for the NAGALA cohort study (IRB 2018-09-01), and informed consent for the use of all participant data was obtained, adhering to the principles of the Helsinki Declaration. Clinical trial number: not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yang Zou, Email: jxyxyzy@163.com.
Xiaoping Peng, Email: ndyfypxp@163.com.
References
- 1.GBD 2021 Diabetes Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the global burden of Disease Study 2021. Lancet. 2023;402:203–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sidik SM. Diabetes and obesity are rising globally - but some nations are hit harder. Nature. 2023. [DOI] [PubMed]
- 3.Gregg EW, Cadwell BL, Cheng YJ, Cowie CC, Williams DE, Geiss L, et al. Trends in the prevalence and ratio of diagnosed to undiagnosed diabetes according to obesity levels in the U.S. Diabetes Care. 2004;27:2806–12. [DOI] [PubMed] [Google Scholar]
- 4.Bommer C, Sagalova V, Heesemann E, Manne-Goehler J, Atun R, Bärnighausen T, et al. Global Economic Burden of Diabetes in adults: projections from 2015 to 2030. Diabetes Care. 2018;41:963–70. [DOI] [PubMed] [Google Scholar]
- 5.Liu J, Liu M, Chai Z, Li C, Wang Y, Shen M, et al. Projected rapid growth in diabetes disease burden and economic burden in China: a spatio-temporal study from 2020 to 2030. Lancet Reg Health West Pac. 2023;33:100700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang C, Cifu AS, Sam S. Obesity and Weight Management for Prevention and Treatment of Type 2 diabetes. JAMA. 2022;328:389–90. [DOI] [PubMed] [Google Scholar]
- 7.ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. Obesity and Weight Management for the Prevention and Treatment of type 2 diabetes: standards of Care in Diabetes-2023. Diabetes Care. 2023;46:S128–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Yu F, Zheng X, Li J, Zhang Z, Zhang Q, et al. Balancing adipocyte production and lipid metabolism to treat obesity-induced diabetes with a novel proteoglycan from Ganoderma Lucidum. Lipids Health Dis. 2023;22:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dahik VD, Frisdal E, Le Goff W. Rewiring of lipid metabolism in adipose tissue macrophages in obesity: impact on insulin resistance and type 2 diabetes. Int J Mol Sci. 2020;21:5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park CY, Kim D, Seo MK, Kim J, Choe H, Kim JH, et al. Dysregulation of Lipid Droplet Protein Expression in Adipose Tissues and Association with metabolic risk factors in adult females with obesity and type 2 diabetes. J Nutr. 2023;153:691–702. [DOI] [PubMed] [Google Scholar]
- 11.Muir LA, Kiridena S, Griffin C, DelProposto JB, Geletka L, Martinez-Santibañez G, et al. Frontline Science: Rapid adipose tissue expansion triggers unique proliferation and lipid accumulation profiles in adipose tissue macrophages. J Leukoc Biol. 2018;103:615–28. 10.1002/JLB.3HI1017-422R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murdolo G, Piroddi M, Luchetti F, Tortoioli C, Canonico B, Zerbinati C, et al. Oxidative stress and lipid peroxidation by-products at the crossroad between adipose organ dysregulation and obesity-linked insulin resistance. Biochimie. 2013;95:585–94. 10.1016/j.biochi.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 13.de Klerk JA, Beulens JWJ, Mei H, Bijkerk R, van Zonneveld AJ, Koivula RW, et al. Altered blood gene expression in the obesity-related type 2 diabetes cluster may be causally involved in lipid metabolism: a mendelian randomisation study. Diabetologia. 2023;66:1057–70. 10.1007/s00125-023-05886-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qiu G, Wang H, Yan Q, Ma H, Niu R, Lei Y, et al. A lipid signature with perturbed Triacylglycerol Co-regulation, identified from targeted Lipidomics, predicts risk for type 2 diabetes and mediates the risk from Adiposity in two prospective cohorts of Chinese adults. Clin Chem. 2022;68:1094–107. [DOI] [PubMed] [Google Scholar]
- 15.Sheng G, Qiu J, Kuang M, Peng N, Xie G, Chen Y, et al. Assessing temporal differences of baseline body mass index, waist circumference, and waist-height ratio in predicting future diabetes. Front Endocrinol (Lausanne). 2023;13:1020253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tian T, Zhang J, Zhu Q, Xie W, Wang Y, Dai Y. Predicting value of five anthropometric measures in metabolic syndrome among Jiangsu Province, China. BMC Public Health. 2020;20:1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang S, Li M, Chen Y, Zhao X, Chen X, Wang H, et al. Comparison of the correlates between Body Mass Index, Waist circumference, Waist-to-height ratio, and chronic kidney disease in a Rural Chinese Adult Population. J Ren Nutr. 2019;29:302–e3091. [DOI] [PubMed] [Google Scholar]
- 18.Lee X, Gao Y, Zhang Y, Feng Y, Gao L, Wang A, et al. Comparison of 10 obesity-related indices for predicting hypertension based on ROC analysis in Chinese adults. Front Public Health. 2022;10:1042236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okamura T, Hashimoto Y, Hamaguchi M, Obora A, Kojima T, Fukui M. Ectopic fat obesity presents the greatest risk for incident type 2 diabetes: a population-based longitudinal study. Int J Obes (Lond). 2019;43:139–48. [DOI] [PubMed] [Google Scholar]
- 20.Hashimoto Y, Hamaguchi M, Kojima T, Ohshima Y, Ohbora A, Kato T, et al. Modest alcohol consumption reduces the incidence of fatty liver in men: a population-based large-scale cohort study. J Gastroenterol Hepatol. 2015;30:546–52. [DOI] [PubMed] [Google Scholar]
- 21.Hamaguchi M, Kojima T, Itoh Y, Harano Y, Fujii K, Nakajima T, et al. The severity of ultrasonographic findings in nonalcoholic fatty liver disease reflects the metabolic syndrome and visceral fat accumulation. Am J Gastroenterol. 2007;102:2708–15. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y, Zhang X, Pan B, Jin X, Yao H, Chen B, et al. A modified formula for calculating low-density lipoprotein cholesterol values. Lipids Health Dis. 2010;9:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kachekouche Y, Dali-Sahi M, Bendaoud R, Dennouni-Medjati N, Abderahim M. Predictive value of non-HDL cholesterol for cardiovascular disease in a population in far western Algeria with type 2 diabetes. Diabetes Metab Syndr. 2019;13:826–9. [DOI] [PubMed] [Google Scholar]
- 24.Hu X, Liu Q, Guo X, Wang W, Yu B, Liang B, et al. The role of remnant cholesterol beyond low-density lipoprotein cholesterol in diabetes mellitus. Cardiovasc Diabetol. 2022;21:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wei L, Wei M, Chen L, Liang S, Gao F, Cheng X, et al. Low-density lipoprotein cholesterol: high-density lipoprotein cholesterol ratio is associated with incident diabetes in Chinese adults: a retrospective cohort study. J Diabetes Investig. 2021;12:91–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khaloo P, Hasheminia M, Tohidi M, Abdi H, Mansournia MA, Azizi F, et al. Impact of 3-year changes in lipid parameters and their ratios on incident type 2 diabetes: Tehran lipid and glucose study. Nutr Metab (Lond). 2018;15:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheng G, Kuang M, Yang R, Zhong Y, Zhang S, Zou Y. Evaluation of the value of conventional and unconventional lipid parameters for predicting the risk of diabetes in a non-diabetic population. J Transl Med. 2022;20:266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.American Diabetes Association. Standards of medical care in diabetes–2011. Diabetes Care. 2011;34(Suppl 1):S11–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Milić S, Lulić D, Štimac D. Non-alcoholic fatty liver disease and obesity: biochemical, metabolic and clinical presentations. World J Gastroenterol. 2014;20:9330–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu X, Bragg F, Yang L, Kartsonaki C, Guo Y, Du H, et al. Smoking and smoking cessation in relation to risk of diabetes in Chinese men and women: a 9-year prospective study of 0·5 million people. Lancet Public Health. 2018;3:e167–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li MJ, Ren J, Zhang WS, Jiang CQ, Jin YL, Lam TH, et al. Association of alcohol drinking with incident type 2 diabetes and pre-diabetes: the Guangzhou Biobank Cohort Study. Diabetes Metab Res Rev. 2022;38:e3548. [DOI] [PubMed] [Google Scholar]
- 32.El-Chami MF, Bockstedt L, Longacre C, Higuera L, Stromberg K, Crossley G, et al. Transvenous single-chamber ventricular pacing in the Micra CED study: 2-year follow-up. Eur Heart J. 2022;43:1207–15. 10.1093/eurheartj/ehab767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muanda FT, Weir MA, Bathini L, Blake PG, Chauvin K, Dixon SN, et al. Association of Baclofen with Encephalopathy in patients with chronic kidney disease. JAMA. 2019;322:1987–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim JH. Multicollinearity and misleading statistical results. Korean J Anesthesiol. 2019;72:558–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee H, Cashin AG, Lamb SE, Hopewell S, Vansteelandt S, VanderWeele TJ, et al. A Guideline for reporting mediation analyses of randomized trials and observational studies: the AGReMA Statement. JAMA. 2021;326:1045–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.VanderWeele TJ. Mediation analysis: a practitioner’s guide. Annu Rev Public Health. 2016;37:17–32. [DOI] [PubMed] [Google Scholar]
- 37.Fitchett EJA, Seale AC, Vergnano S, Sharland M, Heath PT, Saha SK, et al. Strengthening the reporting of Observational studies in Epidemiology for Newborn infection (STROBE-NI): an extension of the STROBE statement for neonatal infection research. Lancet Infect Dis. 2016;16:e202–13. [DOI] [PubMed] [Google Scholar]
- 38.Efron B. Better Bootstrap Confidence Intervals. J Am Stat Assoc. 1987;82(397):171–85. [Google Scholar]
- 39.Boonpor J, Parra-Soto S, Talebi A, Zhou Z, Carrasco-Marin F, Petermann-Rocha F, et al. Associations and predictive performance of 11 anthropometric measures with incident type 2 diabetes: a prospective cohort study from the UK Biobank. Obes (Silver Spring). 2023;31:2648–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mirzaei M, Khajeh M. Comparison of anthropometric indices (body mass index, waist circumference, waist to hip ratio and waist to height ratio) in predicting risk of type II diabetes in the population of Yazd, Iran. Diabetes Metab Syndr. 2018;12:677–82. [DOI] [PubMed] [Google Scholar]
- 41.Hadaegh F, Hatami M, Tohidi M, Sarbakhsh P, Saadat N, Azizi F. Lipid ratios and appropriate cut off values for prediction of diabetes: a cohort of Iranian men and women. Lipids Health Dis. 2010;9:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feskens EJ, Kromhout D. Cardiovascular risk factors and the 25-year incidence of diabetes mellitus in middle-aged men. The Zutphen Study. Am J Epidemiol. 1989;130:1101–8. [DOI] [PubMed] [Google Scholar]
- 43.Chen N, Hu LK, Sun Y, Dong J, Chu X, Lu YK, et al. Associations of waist-to-height ratio with the incidence of type 2 diabetes and mediation analysis: two independent cohort studies. Obes Res Clin Pract. 2023;17:9–15. [DOI] [PubMed] [Google Scholar]
- 44.Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004;145:2273–82. [DOI] [PubMed] [Google Scholar]
- 45.McTernan CL, McTernan PG, Harte AL, Levick PL, Barnett AH, Kumar S. Resistin, central obesity, and type 2 diabetes. Lancet. 2002;359:46–7. [DOI] [PubMed] [Google Scholar]
- 46.Ye R, Onodera T, Scherer PE. Lipotoxicity and β Cell Maintenance in Obesity and type 2 diabetes. J Endocr Soc. 2019;3:617–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fisman EZ, Tenenbaum A. Adiponectin: a manifold therapeutic target for metabolic syndrome, diabetes, and coronary disease? Cardiovasc Diabetol. 2014;13:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Medina-Urrutia A, Posadas-Romero C, Posadas-Sánchez R, Jorge-Galarza E, Villarreal-Molina T, González-Salazar Mdel C, et al. Role of adiponectin and free fatty acids on the association between abdominal visceral fat and insulin resistance. Cardiovasc Diabetol. 2015;14:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vaag AA. Glycemic control and prevention of microvascular and macrovascular disease in the Steno 2 study. Endocr Pract. 2006;12(Suppl 1):89–92. [DOI] [PubMed] [Google Scholar]
- 52.International Diabetes Federation Guideline Development Group. Global guideline for type 2 diabetes. Diabetes Res Clin Pract. 2014;104:1–52. [DOI] [PubMed] [Google Scholar]
- 53.Ng IHY, Cheung KKT, Yau TTL, Chow E, Ozaki R, Chan JCN. Evolution of Diabetes Care in Hong Kong: from the Hong Kong Diabetes Register to JADE-PEARL program to RAMP and PEP program. Endocrinol Metab (Seoul). 2018;33:17–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jiao FF, Fung CSC, Wan EYF, Chan AKC, McGhee SM, Kwok RLP, et al. Five-year cost-effectiveness of the Multidisciplinary Risk Assessment and Management Programme-Diabetes Mellitus (RAMP-DM). Diabetes Care. 2018;41:250–7. [DOI] [PubMed] [Google Scholar]
- 55.Zhong VW, Yu D, Zhao L, Yang Y, Li X, Li Y, et al. Achievement of Guideline-recommended targets in Diabetes Care in China: a nationwide cross-sectional study. Ann Intern Med. 2023;176:1037–46. [DOI] [PubMed] [Google Scholar]
- 56.Kim BY, Won JC, Lee JH, Kim HS, Park JH, Ha KH, et al. Diabetes fact sheets in Korea, 2018: an Appraisal of current status. Diabetes Metab J. 2019;43:487–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Andary R, Fan W, Wong ND. Control of Cardiovascular Risk factors among US adults with type 2 diabetes with and without Cardiovascular Disease. Am J Cardiol. 2019;124:522–7. [DOI] [PubMed] [Google Scholar]
- 58.Casula M, Mozzanica F, Scotti L, Tragni E, Pirillo A, Corrao G, et al. Statin use and risk of new-onset diabetes: a meta-analysis of observational studies. Nutr Metab Cardiovasc Dis. 2017;27:396–406. [DOI] [PubMed] [Google Scholar]
- 59.VanderWeele TJ, Ding P. Sensitivity analysis in Observational Research: introducing the E-Value. Ann Intern Med. 2017;167:268–74. [DOI] [PubMed] [Google Scholar]
- 60.Mason C, Katzmarzyk PT. Variability in waist circumference measurements according to anatomic measurement site. Obes (Silver Spring). 2009;17:1789–95. 10.1038/oby.2009.87. [DOI] [PubMed] [Google Scholar]
- 61.Willis LH, Slentz CA, Houmard JA, Johnson JL, Duscha BD, Aiken LB, et al. Minimal versus umbilical waist circumference measures as indicators of cardiovascular disease risk. Obes (Silver Spring). 2007;15:753–9. 10.1038/oby.2007.612. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analysed during the current study are available in the Dryad repository. [https://datadryad.org/stash/dataset/doi:10.5061/dryad.8q0p192]