Abstract
It was previously demonstrated that Menta-FX, a mixture of Panax quinquefolius L. (PQE), Ginkgo biloba (GBE), and Hypericum perforatum extracts (HPE), enhances retinal ganglion cell survival after axotomy. However, the mechanisms of neuroprotection remain unknown. The aim of this study is to elucidate the neuroprotective mechanisms of Menta-FX. Since PQE, GBE and HPE have all been observed to display anti-oxidative property, the involvement of anti-oxidation in Menta-FX’s neuroprotective effect was investigated. Menta-FX lowered nitric oxide (NO) content in axotomized retinas without affecting nitric oxide synthase activity, suggesting that Menta-FX possibly exhibited a NO scavenging property. In addition, the effect of Menta-FX on the frequency of axotomy-induced nuclear fragmentation and caspase-3 activation was investigated. Menta-FX treatment significantly reduced nuclear fragmentation in axotomized retinas. Surprisingly, Menta-FX had no effect on caspase-3 activation, but selectively lowered caspase-3-independent nuclear fragmentation in axotomized retinal ganglion cells. In addition, inhibition of PI3K activity by intravitreal injection of wortmannin, a phosphoinositide-3 kinase (PI3K) inhibitor, completely abolished the neuroprotective effect of Menta-FX, indicating that Menta-FX’s neuroprotective effect was PI3K-dependent. Data here suggest that Menta-FX displayed a PI3K-dependent, selective inhibition on a caspase-3-independent apoptotic pathway in axotomized RGCs, thus, highlighting the potential use of herbal remedies as neuroprotective agents for other neurodegenerative diseases.
Keywords: Ganglion cells, Ginkgo biloba, Hypericum perforatum, Optic nerve, Panax quinquefolius L.
Introduction
Axotomy-induced retinal ganglion cell (RGC) death has been adopted as an animal model for investigating neuronal death in the central nervous system. Earlier studies aimed at elucidating the mechanisms of RGC death attributed the death of axotomized RGCs to the activation of apoptotic pathways. Caspase-3 and -9, for example, are activated in the damaged RGCs following axotomy (Kermer et al. 1999, 2000a; Cheung et al. 2004). Nonetheless, despite recent advances are made in understanding the apoptotic mechanisms of axotomized RGCs, effective neuroprotective agents for damaged RGCs remain unavailable. In a previous report, we have demonstrated that a mixture of extracts from Panax quinquefolius L. (American ginseng), Ginkgo biloba and Hypericum perforatum (St. John’s wort) (known as Menta-FX) significantly protected against axotomized RGC death (Cheung et al. 2002). While this suggests that herbal remedy may be used as neuroprotective agent, a little is known concerning Menta-FX’s mechanisms of neuroprotection. The aim of the current study is to elucidate the neuroprotective mechanisms of Menta-FX.
Earlier studies examining the biological actions of Panax quinquefolius L., Ginkgo biloba and Hypericum perforatum extracts showed that the three extracts all exhibit free radical scavenging capacity (Kitts et al. 2000; Baudouin et al. 1999; Wei et al. 2000; Bastianetto et al. 2000a; Tripathi and Pandey 1999). In addition, Ginkgo biloba and ginseng extracts have both been demonstrated to limit nitric oxide (NO) production (Kim et al. 1998; Marcocci et al. 1994). Furthermore, ginseng saponins have been shown to improve superoxide dismutase (SOD) activity following glutamate toxicity (Kim et al. 1998). These observations suggest that Menta-FX will likely display some anti-oxidative property. We are thus first interested to examine if Menta-FX exhibits anti-oxidative capacity, and whether anti-oxidation contributes to the neuroprotective effect of Menta-FX. The ability of Menta-FX to modulate endogenous content of NO, NOS, and SOD activity in axotomized retinas was investigated.
In addition to potentially exhibiting anti-oxidative property, the ability of Menta-FX to delay axotomized RGC death suggests that Menta-FX may also exert its neuroprotective effect by modulating nuclear fragmentation and/or caspase activity. None of the three herbal extracts examined has been suggested to affect the kinetics and/or expression of the apoptotic machinery. Examining the effect of Menta-FX on the apoptotic machinery will, therefore, not only elucidate the mechanisms of neuroprotection by Menta-FX on axotomized RGCs, but also expand our understanding on the biological actions of these herbs. The effects of Menta-FX on the kinetics of caspase-3 activation and nuclear fragmentation in axotomized retinas were thus examined. In addition, since the PI3K/Akt pathway has been observed to attenuate caspase activation (Cardone et al. 1998; Barber et al. 2001; Zhou et al. 2000), the involvement of the PI3K/Akt pathway in the neuroprotective effect of Menta-FX was also investigated.
Materials and Methods
Animals
Adult golden hamsters (Mesocricetus auratus; 6–8-week-old, weighing 60–80 g) were used in this study. All animals were anesthetized with intraperitoneal injection of sodium pentobarbitone (Nembutal, Rhone Merieux Australia Pty Ltd., Australia; 50 mg/kg) prior to operation. Operated animals were sacrificed with an overdose of anesthesia. All surgical procedures adhere to the ARVO statement for the use of animals in ophthalmic and vision research.
Extracts
Herbal extracts used in this study were obtained from CV Technologies (Edmonton, Alberta, Canada): Panax quinquefolius L. extract (PQE; standardized to contain 25% ginsenosides), Ginkgo biloba extract (GBE; standardized to contain 24% flavonoid glycosides and 6% terpenoids), and Hypericum perforatum extract (HPE; standardized to contain 0.3% hypericin) (Cheung et al. 2002). Menta-FX, a mixture of the three extracts, was prepared by combining 30.8% PQE, 61.5% HPE, and 7.7% GBE by weight.
Menta-FX was suspended in sterile 0.01 M phosphate-buffered saline (PBS; pH 7.4) at 60 mg/ml, stored at 4°C and administered to animals within 14 days (0.5 ml per animal daily). The extracts were delivered directly into the esophagus of the animals with a specialized feeding cannula.
Assessment of Endogenous NO Content and NOS Activity
In order to examine the effect of Menta-FX and various combinations of PQE, GBE, and HPE on the endogenous content of NO and NOS activity in axotomized retinas, anesthetized animals received optic nerve transection on their left optic nerves at 1.5 mm from the optic disc as described (Cheung et al. 2002). Animals were then treated daily with: (1) 0.01 M PBS for 3 or 7 days; (2) 30 mg Menta-FX for 3 or 7 days; (3) P + G for 7 days; (4) P + H for 7 days; and (5) G + H for 7 days. Operated animals were killed at 3 dpa (those receiving treatment for 3 days) or 7 dpa (those receiving treatment for 7 days) with an overdose of sodium pentobarbitone and their retinas were quickly dissected in normal saline. Six unoperated animals were also sacrificed to characterize the NO content and NOS activity in normal retinas. Due to the small volume of one retina, every two retinas were pooled up as one sample (n = 3–6 in each group). Each sample was homogenized in 150 μl of 20 mM Tris–HCl (pH 7.4) containing 15 μl protease inhibitor cocktail (Sigma, St Louis, MO). The homogenates were then centrifuged at 13,000 2 × g for 30 min at 4°C. Following centrifugation, the supernatants were collected for determination of NO content or NOS activity.
Endogenous NO content in the samples was determined by boiling the harvested supernatant at 100°C for 10 min. Boiled samples were centrifuged at 13,000 × g for 10 min; supernatants were collected and stored frozen until NO assay. In order to measure NOS activity, 50 μl of the homogenates were added with co-factors and substrates of NOS (50 μl of 20 mM Tris–HCl, pH 7.4, 10 mM l-arginine, 1 mM NADPH, 4 μM BH4, 4 μM FAD, 4 μM FMN, 1 mM CaCl2, and 5 units of calmodulin). Substrates-added homogenates were either boiled immediately for 10 min or incubated at 37°C for 4 h. At the end of the incubation period, the incubated samples were similarly boiled for 10 min to terminate all production of NO. After brief cooling on ice, all boiled samples will then be centrifuged at 13,000 × g for 10 min at room temperature. The supernatants were then collected and stored at −20°C until NO assay to determine the amount of nitrite in the samples. NOS activity was quantified as the amount of nitrite produced at 37°C during the 4-h incubation (Total nitrite content at the end of 4 h incubation—total nitrite content in the sample at time zero). Net NOS activity of the samples was determined without identifying the relative contribution of iNOS and nNOS in the measured NOS activity.
Quantification of NO Content
Due to the ephemeral nature of NO as a free radical gas, accurate measurements of NO content in tissues have been difficult. The amount of nitrate and nitrite, stable breakdown products of NO, has, therefore, been conventionally used as an estimate for the NO content in the sample. Since the ratio of nitrate/nitrite produced can vary greatly, nitrate is converted to nitrite to most accurately quantify the NO content in the samples. The amount of nitrite in the sample was determined by a change in absorbance at 540 nm after incubation with the Griess reagent. NO assays were performed according to the manufacturer’s menu of the Nitrate/Nitrite Colorimetric Assay Kit from Cayman Chemical (Alexis Corporation, Nottingham, UK).
Measurement of SOD Activity
Adult male hamsters received unilateral optic nerve transection at 1.5 mm from the optic disc (Cheung et al. 2002). Starting on the day of operation, operated animals received PBS or 30 mg Menta-FX per day until the day before euthanasia (n = 5 per group). Animals were killed at 3 or 7 days post axotomy with an overdose of sodium pentobarbitone and perfused transcardially with normal saline (0.9% NaCl) containing 0.38% sodium citrate to prevent clotting of blood vessels. The retinas were then dissected in normal saline and placed immediately into 20 mM Tris–HCl containing 10% protease inhibitor cocktail (Sigma, St Louis, MO). The retinas were then homogenized and centrifuged at 13,000 × g at 4°C for 30 min. The supernatants were collected and placed on ice until assay.
An aliquot of 20 μl of the homogenates were used for assay. The assays were performed according to the manufacturer’s instructions of the Superoxide Dismutase Assay Kit from Calbiochem (San Diego, CA), measuring absorbance at 525 nm.
Effects of Menta-FX on Caspase-3 Activation and Nuclear Fragmentation
Optic nerve transection at 1.5 mm from the optic disc was performed on adult hamsters, as described (Cheung et al. 2002). Immediately following axotomy, a piece of gelfoam (Upjohn, Kalamazoo, MI) soaked with 6% Fluoro-Gold (FG; Fluorochrome, Denver, CO) was placed at the ocular stump to label all RGCs. Operated animals then received oral administration of 0.01 M PBS or 30 mg Menta-FX daily starting on the day of axotomy. Animals were killed at 1, 3, 5, and 7 dpa (n = 3–4 per group). Treatment with vehicle or Menta-FX continued until the day before euthanasia. The left eyes of the euthanized animals were enucleated after transcardial perfusion with 4% PFA. The retinal-scleral cups were post-fixed in the same fixative for 4 h and were then prepared as 10 μm thick frozen sections and stored at −20°C until immunohistochemical analysis. Only retinal sections containing the optic nerve stump were used to ensure comparable length of all retinal sections.
Retinal sections were subjected to immunohistochemical analysis against activated caspase-3 (1:150; Cell Signaling Technology, Beverly, MA). Activated caspase-3 staining was then visualized by texas red-conjugated goat anti-rabbit secondary antibody at 1:50 (Vector laboratories). All slides were counterstained with 4,6-diaminido-2-phenylindole (DAPI; Sigma, St. Louis, MO), coverslipped and examined under fluorescence microscope. Negative controls were also prepared in parallel with the experimental slides in the current study. The total numbers of FG-labeled RGCs, texas red-positive cells (for activated caspase-3) and fragmented nuclei on three retinal sections were counted per animal. Nuclear fragmentation representing a late stage of apoptosis was identified as previously described (Darzynkiewics and Traganos 1998). Since Menta-FX treatment enhances the number of total RGCs, comparisons between the PBS-treated and Menta-FX-treated group at the same time-point were validated by converting the numbers counted into percentages of total RGCs (surviving RGCs plus RGCs displaying fragmented nuclei) exhibiting activated caspase-3 immunoreactivity or nuclear fragmentation.
Effects of Wortmannin on the Neuroprotective Effect of Menta-FX
The involvement of PI3K in the neuroprotective effect of Menta-FX was investigated. Optic nerve transection was performed at 1.5 mm from the optic disc on adult hamsters as described (Cheung et al. 2002). All RGCs were labeled with retrogradely transported FG by placing a piece of gelfoam (Upjohn) soaked with 6% FG (Fluorochrome) at the ocular stump immediately after transection. Operated animals were then divided into four groups (n = 4 per group): (1) daily oral administration of 0.01 M PBS for 7 days and intravitreal injections of 10% DMSO (4 μl) at 0, 3 and 6 dpa; (2) daily oral administration of 0.01 M PBS for 7 days and intravitreal injections of 0.1 mM wortmannin (WM, 4 μl) at 0, 3, and 6 dpa; (3) daily oral administration of 30 mg Menta-FX for 7 days and intravitreal injections of 10% DMSO (4 μl) at 0, 3 and 6 dpa; (4) daily oral administration of 30 mg Menta-FX for 7 days and intravitreal injections of 0.1 mM WM (4 μl) at 0, 3 and 6 dpa. Injections were made at the superior scleral-conjunctival junction of the operated eye using heat-pulled glass micropippettes connected to a Hamilton syringe filled with 30% glycerol for slow injection of fluids into the vitreous body of the eye. All operated animals were sacrificed on day 7 post-axotomy. The operated eyeballs were then enucleated and the retinas dissected. The dissected retina was divided into four quadrants and sampling of the number of labeled RGCs in the four quadrants was performed to determine the density of labeled RGCs in the retina (Cheung et al. 2002).
Effects of Menta-FX on Akt Phosphorylation in Axotomized Retinas
In order to examine if Menta-FX protects axotomized RGCs by enhancing Akt phosphorylation following axotomy, optic nerve transection was performed at 1.5 mm from the optic disc in adult hamsters as previously described (Cheung et al. 2002). Starting on the day of operation, operated animals received daily oral administration of 0.01 M PBS or 30 mg Menta-FX for 3 or 7 days (n = 3 per group). Operated animals were sacrificed at 3 or 7 dpa with an overdose of anesthesia. The retinas of the operated eyes were then quickly dissected, snap-frozen with liquid nitrogen and stored at −80°C until Western blotting.
Procedures for Western blot analysis used in this study were adopted from Klocker et al. (2000) with slight modifications. Retinas were homogenized in lysis buffer (50 mM Tris, 150 mM NaCl, 1% Triton X-100, 10% protease inhibitor cocktail from Sigma and 1% phosphatase inhibitor cocktail from Sigma) and lysed on ice for 20 min. Following centrifugation at 13,000 rpm for 15 min to pellet cell debris, the protein concentration of the supernatant was measured using BCA reagent (Pierce, Rockford, IL). An aliquot of 80 μg of retinal lysates were then separated by reducing SDS-PAGE (12.5% gel) and transferred onto nitrocellulose membrane. The membranes were briefly washed and blocked with 5% skim milk in Tris-buffered-saline containing 0.05% Tween 20 (TBST) for 2 h in room temperature. Incubation with a polyclonal antibody against phospho-Akt (phosphorylation at Ser473, 1:500; Cell Signaling Technology) in 5% skim milk in TBST was performed overnight at 4°C. Subsequently, the membranes were again washed with TBST and incubated with HRP-conjugated secondary antibody for 1 h in room temperature, followed by detection using the SuperSignal® West Pico Chemiluminescent Substrate kit (Pierce). The chemiluminescence was captured by the UVP-Chemi system and the intensity of each band was analyzed using the LabWorks™ Image acquisition and Analysis Software (UVP Inc., Upland, CA). All experiments for Western blotting were performed with three animals in each group and repeated twice. Selected blots were directly reprobed with anti-β-actin antibody (1:5000; Sigma) as previously described (Liao et al. 2000) to ensure even loading and transfer of samples.
Statistics
Data are expressed as mean ± standard error of mean (SEM). Statistical significance was evaluated by student’s t-test for comparisons between two groups; or by one-way ANOVA, followed by Tukey-Kramer post-hoc test for comparisons among three or more groups. Differences were considered significant for P < 0.05.
Results
Menta-FX Significantly Lowered Endogenous NO Content in Axotomized Retinas
We have previously shown that 30 mg of Menta-FX significantly enhances RGC survival at 7 dpa (Cheung et al. 2002). We subsequently studied the neuroprotective effect of Menta-FX at 14 dpa. We have found that while 30 mg of Menta-FX had no effect on axotomized RGC survival (6009 ± 933 vs. control: 5948 ± 512), 60 mg of Menta-FX significantly augmented RGC survival at 14 dpa (7974 ± 724; P < 0.05 vs. control). This observation further established the role of Menta-FX as a neuroprotective agent. However, since our attempts to explicate the neuroprotective mechanisms focus mainly on the molecular events happening prior to 7 dpa, the dose–response relationship of Menta-FX was investigated at 7 dpa. We found that the RGC-rescuing effect of Menta-FX plateaued at 30 mg at 7 dpa (data not shown). An aliquot of 30 mg of Menta-FX has thus been adopted as the optimum dose for examining its mechanisms of neuroprotection in the current study.
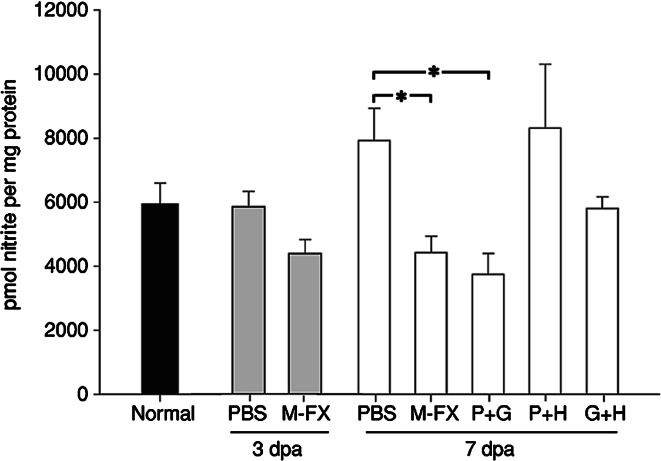
In order to examine if free radical scavenging contributes to Menta-FX’s neuroprotective property, the efficacy of Menta-FX and other combinations of PQE, GBE, and HPE on lowering NO content was investigated. The amount of nitrite, a stable break down product of NO, was used as an estimate for the NO content in the retinas. Transection of the optic nerve in PBS-treated animals did not affect nitrite content in axotomized retinas at 3 dpa compared to that found in normal, undamaged retinas. Treatment with 30 mg of Menta-FX for 3 days similarly had no effect on nitrite content. By 7 dpa, PBS-treated retinas displayed a slight increase in nitrite content compared to normal retinas, but the difference fell short of statistical significance. Treatment with 30 mg of Menta-FX significantly lowered the amount of nitrite in axotomized retinas (P < 0.05 vs. PBS-treated group at 7 dpa). Since 30 mg Menta-FX decreased the endogenous NO content of axotomized retinas, it is of interest to examine if other combinations of PQE, GBE, and HPE exerts similar effects. We found that while the combination P + G significantly diminished nitrite content in axotomized retinas at 7 dpa (P < 0.05 vs. PBS-treated group at 7 dpa), combinations P + H and G + H failed to significantly affect nitrite content at 7 dpa (Fig. 1).
Fig. 1.
The effect of Menta-FX (M-FX) and mixtures of Panax quinquefolius L. extract (P), Ginkgo biloba extract (G) and Hypericum perforatum extract (H) treatments on the endogenous nitrite content of axotomized retinas. Axotomy did not significantly affect the endogenous nitrite content in the PBS treated group at 3 and 7 days post-axotomy (dpa), although the endogenous nitrite content increased slightly at 7 dpa. Treatment with Menta-FX slightly lowered the endogenous content at 3 dpa, and provided significant reduction in the endogenous content in axotomized retinas at 7 dpa. Treatment with a mixture of Panax quinquefolius L. extract and Ginkgo biloba extract (P + G) also significantly decreased endogenous nitrite content in axotomized retinas at 7 dpa, but other mixtures (P + H and G + H) had no effect on endogenous nitrite content compared to the PBS-treated group at 7 dpa. *P < 0.05 vs. PBS treated group at the same time-point; one way ANOVA. n = 3–6 in each group
Menta-FX Did Not Affect NOS Activity
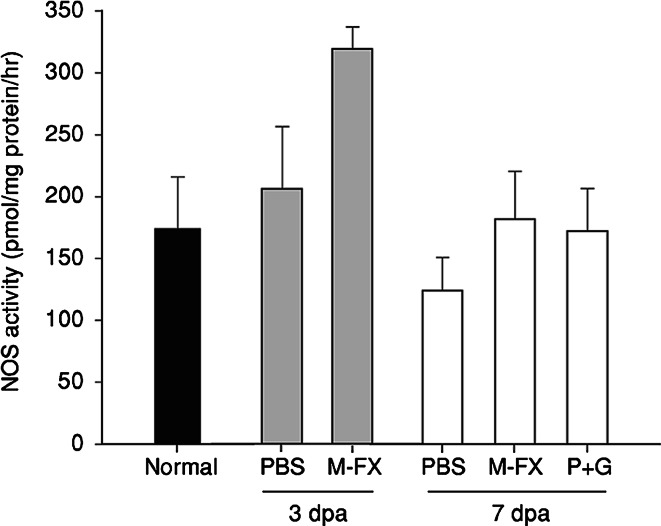
In order to further elucidate if the lowered nitrite content observed in Menta-FX and combination P + G treated retinas was associated with changes in NOS activity, net NOS activity in axotomized retinas with or without herbal extracts treatment was examined. In accordance with the lack of change in nitrite content at 3 dpa in the PBS-treated group, NOS activity was not altered by axotomy at 3 dpa. Menta-FX treatment produced a slight increase in NOS activity at 3 dpa, although the difference was not statistically significant. NOS activity remained comparable to control level in the PBS-treated animals at 7 dpa. Interestingly, 30 mg of Menta-FX and a mixture of PQE and GBE, which significantly decreased nitrite content at 7 dpa, had no effect on NOS activity when compared to the PBS-treated group on day 7 post axotomy (Fig. 2). This suggests that the lowered nitrite content observed was possibly related to scavenging of the NO radical, and not by inhibition of the NOS activity.
Fig. 2.
The effect of Menta-FX (M-FX) and a mixture of Panax quinquefolius L. extract and Ginkgo biloba extract (P + G) on the nitric oxide synthase (NOS) activity in axotomized retinas. NOS activity was not significantly affected by axotomy in the PBS-treated group at 3 and 7 days post-axotomy (dpa). Treatment with Menta-FX and a mixture of Panax quinquefolius L. extract and Ginkgo biloba extract (P + G) did not significantly modulate NOS activity at 3 and 7 dpa, although Menta-FX treatment did slightly increase NOS activity in axotomized retinas at 3 dpa. P > 0.05 for all comparisons; one way ANOVA. n = 3–6 in each group
Menta-FX Had No Effect on SOD Activity
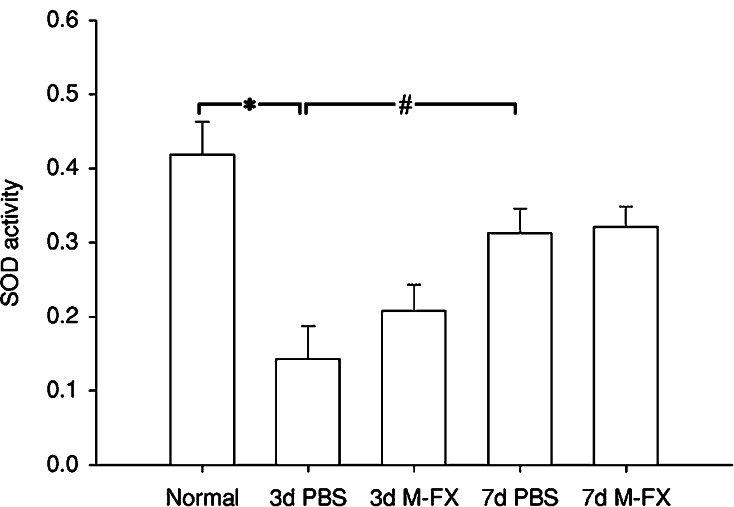
Ginseng saponins have been demonstrated to preserve SOD activity following glutamate toxicity (Kim et al. 1998). In addition, most of the deleterious effects of NO have been associated with the production of peroxynitrite in reaction with superoxide (O−2). It is, therefore, possible that Menta-FX may exert its neuroprotective effect by increasing the activity of SOD, the enzyme responsible for decreasing the superoxide content in cells. Therefore, the effect of Menta-FX on SOD activity was examined. Transection of the optic nerve in the PBS-treated animals resulted in a drop in SOD activity at 3 dpa (P < 0.05 vs. normal). SOD activity was significantly enhanced at 7 dpa compared to that determined at 3 dpa (P < 0.05, 3 dpa PBS vs. 7 dpa PBS), although only partially restored when compared to normal. Daily treatment with 30 mg of Menta-FX had no effect on SOD activity in axotomized retinas at 3 and 7 dpa (Fig. 3). These observations indicate that enhancement of SOD activity may not contribute to the neuroprotective effect of Menta-FX. Since Menta-FX had no effect on SOD activity, the effects of other combinations of PQE, GBE, and HPE on SOD activity were not investigated.
Fig. 3.
The effect of Menta-FX (M-FX) on the superoxide dismutase (SOD) activity in axotomized retinas. Axotomy induced a significant reduction in SOD activity in the PBS-treated group at 3 days post-axotomy. However, by 7 days, SOD activity had partially recovered relative to control level. Menta-FX treatment had no effect on SOD activity at 3 or 7 days post-axotomy compared to the PBS-treated group. d = days post-axotomy. *P < 0.05 vs. control; #P < 0.05 vs. 3d PBS; one way ANOVA. n = 5 per group
Menta-FX Significantly Lowered Axotomized RGC Death and Nuclear Fragmentation in Retinal Sections
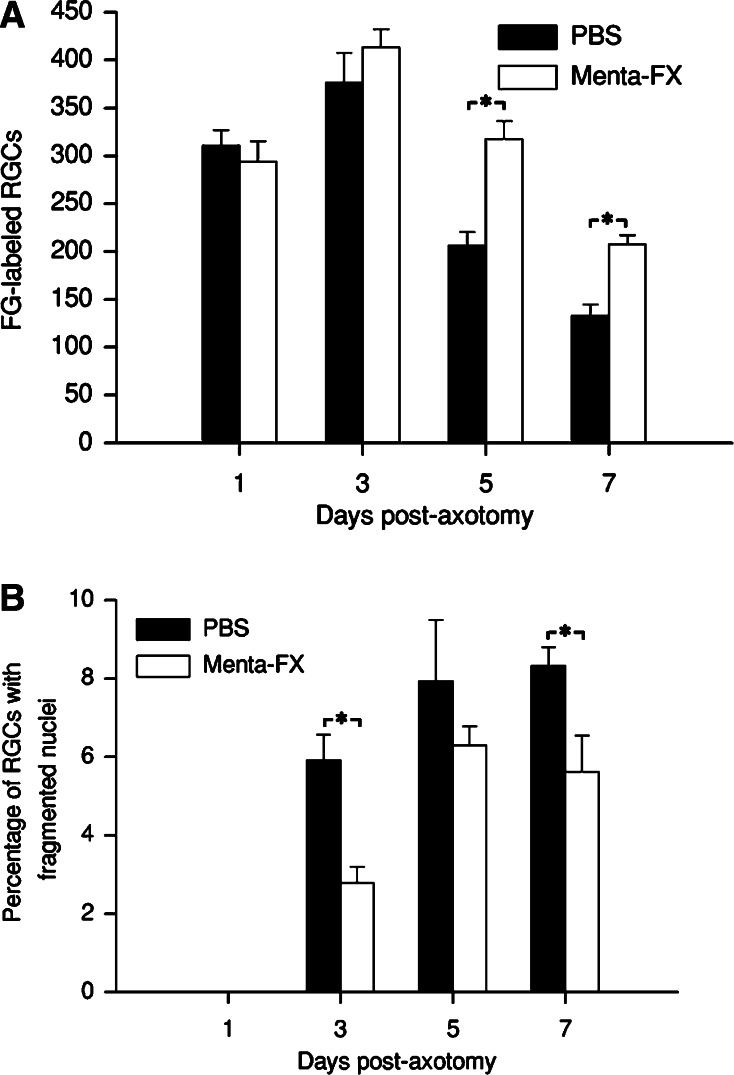
It was previously shown that optic nerve transection results in nuclear fragmentation in the axotomized RGCs (Isenmann et al. 1997). We recently observed that Menta-FX protects against axotomized RGC death, characterized by counting the number of FG-labeled RGCs on retinal flat-mounts (Cheung et al. 2002). In order to elucidate the mechanisms of Menta-FX’s neuroprotective capacity, the effect of Menta-FX on axotomy-induced nuclear fragmentation was examined. In accordance with our previous findings, treatment with 30 mg of Menta-FX significantly increased the number of surviving RGCs at 5 and 7 dpa in retinal sections compared to the PBS-treated group (P < 0.05 vs. PBS-treated group; Fig. 4A). On the other hand, Menta-FX significantly lowered the percentage of nuclear fragmented RGCs at 3 and 7 dpa (P < 0.05 vs. PBS-treated group; Fig. 4B). Menta-FX also slightly decreased the percentage of total RGCs undergoing nuclear fragmentation at 5 dpa, but the difference was not statistically significant (Fig. 4B). Our findings indicate that the Menta-FX enhanced RGCs survival following axotomy, at least in part, by lowering the axotomy-induced nuclear fragmentation in RGCs.
Fig. 4.
(A) The effect of Menta-FX on the survival of axotomized retinal ganglion cells (RGCs) in retinal sections at 1, 3, 5, and 7 days post-axotomy (dpa). Menta-FX significantly increased RGC survival at 5 and 7 dpa compared to the PBS-treated group. *P < 0.05 vs. PBS-treated group at the same time-point; student’s t-test. (B) The effect of Menta-FX on the percentage of retinal ganglion cells (RGCs) displaying fragmented nuclei at 1, 3, 5, and 7 days post-axotomy (dpa). Menta-FX significantly decreased the percentage of RGCs with nuclear fragmentation at 3 and 7 dpa. *P < 0.05 vs. PBS-treated group at the same time-point; student’s t-test. n = 3–4 in each group
Menta-FX Had No Effect on the Percentage of Total RGCs Expressing Activated Caspase-3 Immunoreactivity
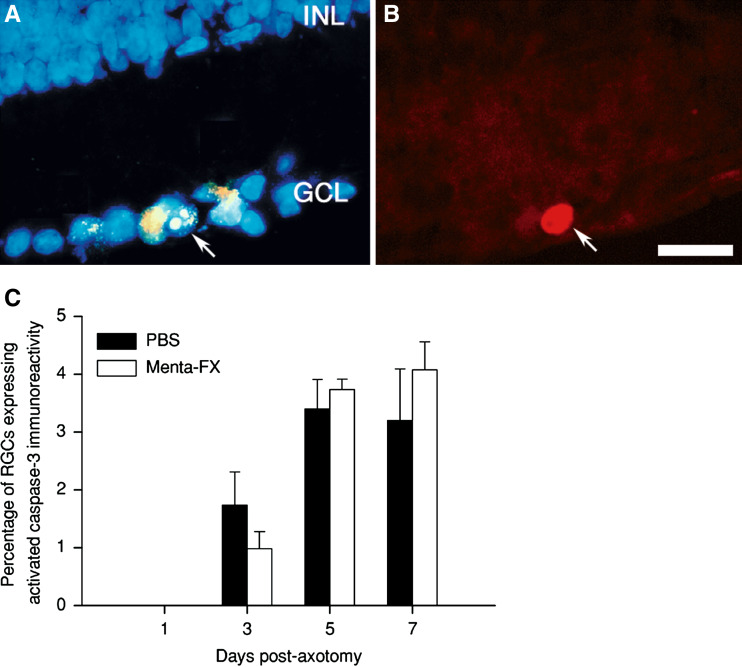
Since it was found that caspase-3 activation co-localized with DNA damage in axotomized RGCs (Kermer et al. 1999), we next examined if the lowered nuclear fragmentation observed after Menta-FX treatment is associated with a decrease in caspase-3 activation. In the PBS-treated groups, activated caspase-3 immunoreactivity was observed solely in the ganglion cell layer (GCL) at all time-points examined. Over 80% of the immunoreactive cells contained FG staining, suggesting that most of the cells examined were RGCs. In addition, all cells displaying activated caspase-3 immunoreactivity exhibited nuclear fragmentation (Fig. 5A, B). Menta-FX treatment did not affect the distribution and morphology of the activated caspase-3 immunoreactivity compared to the PBS-treated group. Surprisingly, Menta-FX did not significantly affect the percentage of RGCs displaying activated caspase-3 immunoreactivity at all time-points examined (Fig. 5C), indicating that Menta-FX had no effect on caspase-3 activation.
Fig. 5.
Immunohistochemical detection of caspase-3 activation in axotomized retinas following Menta-FX treatment (A & B). Photomicrographs of the same retinal section stained with DAPI (A) and activated caspase-3 polyclonal antibody (B) taken at 7 days post-axotomy (dpa) following Menta-FX treatment. Note that the activated caspase-3 immunoreactive cell (arrow, B) was found in the GCL, exhibited nuclear fragmentation and contained FG staining (arrow, A). This suggests that the stained cell observed was an apoptotic retinal ganglion cell (RGC). (C) The effect of Menta-FX on the percentage of RGCs displaying activated caspase-3 immunoreactivity at 1, 3, 5, and 7 dpa. Menta-FX failed to significantly modulate the percentage of RGCs with activated caspase-3 immunoreactivity at all time-points examined. Bar = 25 μm. GCL: ganglion cell layer; INL: inner nuclear layer. n = 3–4 per group
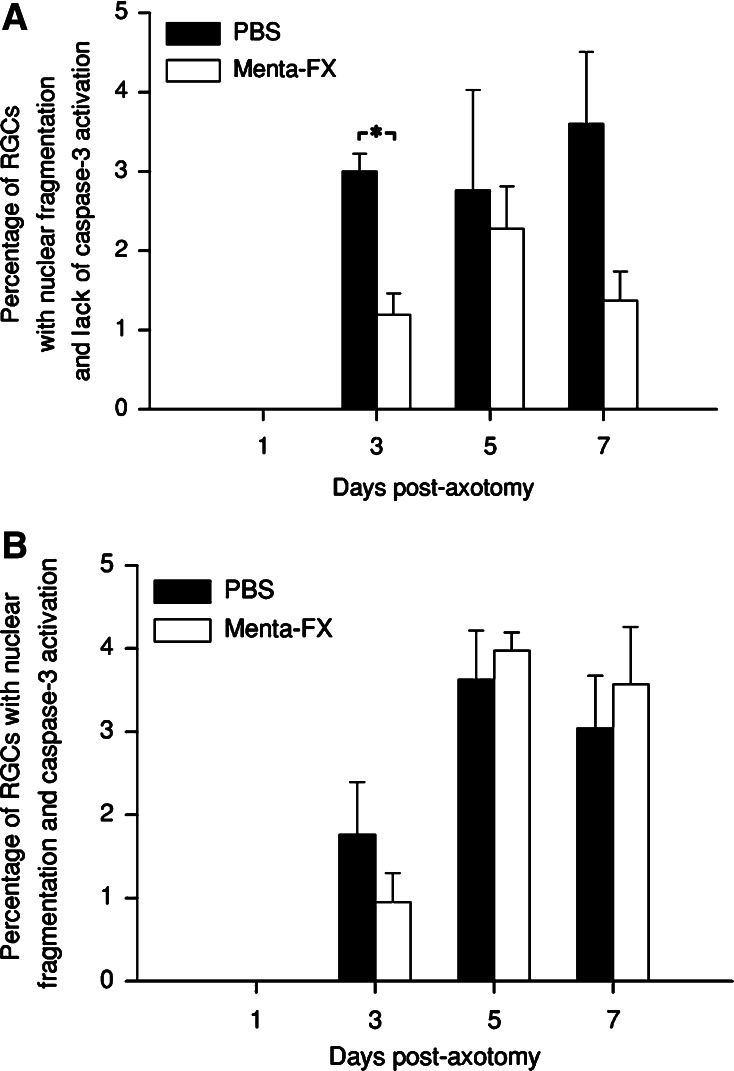
Menta-FX Selectively Affected the Caspase-3-negative Population of Cells Exhibiting Fragmented Nuclei
In the current study, it was observed that although Menta-FX treatment significantly reduced nuclear fragmentation, the percentage of total RGCs exhibiting caspase-3 activation was unchanged. We, therefore, speculate that Menta-FX may selectively lower the proportion of apoptotic cells not expressing activated caspase-3. In order to answer this question, cells with fragmented nuclei quantified at several time-points following vehicle or Menta-FX treatment were separated into two groups according to the presence/absence of caspase-3 activation. We found that among the population of apoptotic cells (characterized by the presence of fragmented nuclei), the caspase-3-positive population consistently represented about 30–50% of the apoptotic cells in the PBS-treated group. This can be observed by comparing the PBS-treated group in Fig. 4B to the PBS-treated group in Fig. 6B. While about 6–8% of all RGCs exhibited fragmented nuclei from 3–7 dpa (Fig. 4B), only about 2–4% of RGCs displayed activated caspase-3-immunoreactivity and nuclear fragmentation in the PBS-treated groups (Fig. 6B).
Fig. 6.
The effect of Menta-FX on the percentage of apoptotic retinal ganglion cells (RGCs) without activated caspase-3 immunoreactivity (A) or with activated caspase-3 immunoreactivity (B) in axotomized retinas. Menta-FX significantly decreased the percentage of apoptotic RGCs dying via a casapse-3-independent pathway at 3 days post-axotomy (dpa). Menta-FX slightly decreased the percentage of non-caspase-3-activated apoptotic RGCs at 5 and 7 dpa, but the difference fell short of statistical significance. On the other hand, Menta-FX did not affect the percentage of apoptotic RGCs that stained positive for activated caspase-3 at all time-points examined. These observations suggest that Menta-FX selectively lowered the population of apoptotic RGCs dying via a caspase-3-independent pathway. *P < 0.05 vs. PBS-treated group at the same time-point; student’s t-test. n = 3–4 per group
Interestingly, Menta-FX treatment significantly reduced the percentage of apoptotic RGCs that were activated casapse-3-negative (P < 0.05 vs. PBS-treated group; Fig. 6A). Menta-FX also reduced the percentage of activated caspase-3-negative apoptotic cells at 5 and 7 dpa, but the difference failed to reach statistical significance. On the contrary, the percentage of apoptotic RGCs expressing activated caspase-3 was slightly increased by Menta-FX treatment at all time-points examined, but the difference was statistically insignificant (Fig. 6B). These findings thus suggest that Menta-FX exerted its anti-apoptotic effect mostly by lowering nuclear fragmentation in a population of RGCs without detectable caspase-3 activation.
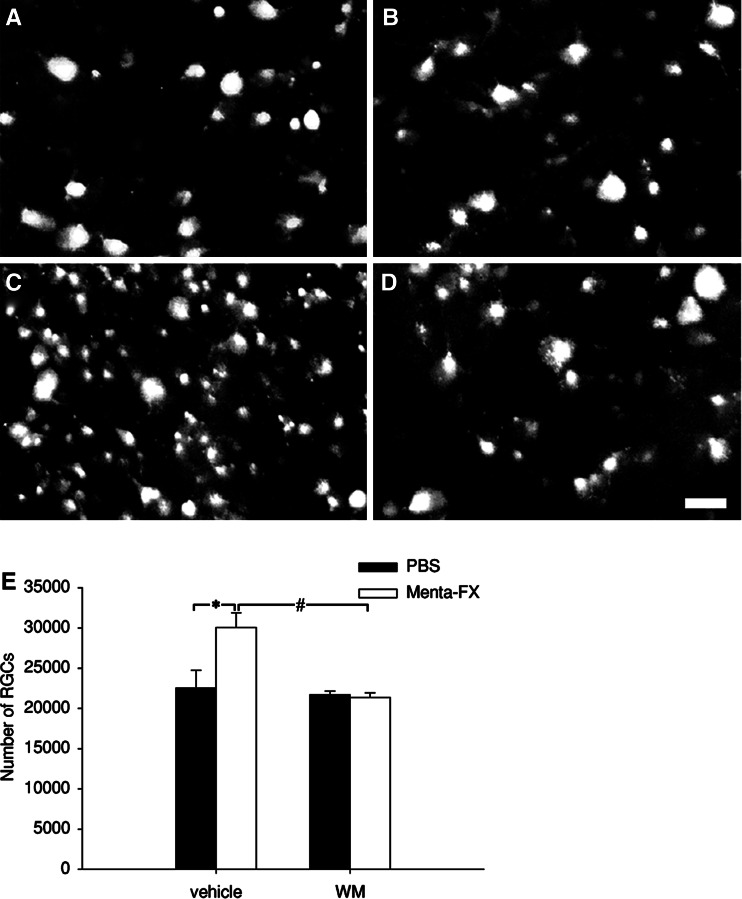
Wortmannin Abolished the Neuroprotective Effect of Menta-FX on Axotomized RGCs
The PI3K/Akt pathway has been observed to serve as important survival signals in neurons (Politi et al. 2001; Kermer et al. 2000b; Klocker et al. 2000; Dudek et al. 1997; Barber et al. 2001). In order to examine if the PI3K/Akt pathway contributes to the neuroprotective effect of Menta-FX, the effects on wortmannin (WM), a PI3K inhibitor, on Menta-FX’s protective effect was examined. In the PBS-treated, vehicle-injected animals, 22529 ± 2201 surviving RGCs remained at 7 dpa. Injection of 0.1 mM WM at 0, 3, and 6 dpa did not affect axotomized RGC survival in the PBS-treated animals (21705 ± 461). Menta-FX, as previously shown, significantly increased RGC survival despite intravitreal injections of 10% DMSO (30044 ± 1824; P < 0.05 vs. PBS-treated, vehicle-injected group). Interestingly, injection of WM completely abolished the neuroprotective effect of Menta-FX on axotomized RGC survival at 7 dpa (21344 ± 603; P < 0.01 vs. Menta-FX-treated, vehicle-injected group; Fig. 7). This indicates that PI3K was an important mediator of the neuroprotective effect of Menta-FX.
Fig. 7.
The effect of wortmannin (WM) on the Menta-FX-induced enhancement in axotomized retinal ganglion cell (RGC) survival. (A–D) Photomicrographs of FG-labeled RGCs taken from retinal flat-mounts treated with (A) oral administration of PBS and 10% DMSO injection; (B) oral administration of PBS and 0.1 mM WM injections; (C) oral administration of Menta-FX and 10% DMSO injections; and (D) oral administration of Menta-FX and 0.1 mM WM injections at 7 days post-axotomy (dpa). (E) A histogram summarizing the effects of wortmannin on the neuroprotective effect of Menta-FX on axotomized RGCs at 7 dpa. Injections of 0.1 mM WM at 0, 3, and 6 dpa did not induce further axotomized RGC death at 7 dpa. Interestingly, wortmannin injections completely abolished the protective effect of Menta-FX on axotomized RGCs. This suggests that PI3K plays a crucial role in the neuroprotective effect of Menta-FX. Bar = 25 μm. *P < 0.05 vs. PBS-treated, vehicle-injected group; #P < 0.05 vs. Menta-FX-treated, WM-injected group; one way ANOVA. n = 4 per group
Menta-FX Had No Effect on Akt Phosphorylation Following Axotomy
Akt has been demonstrated to be an important mediator of PI3K’s anti-apoptotic effect on neurons (Dudek et al. 1997; Franke et al. 1997; Wang et al. 2000; Namikawa et al. 2000; Barber et al. 2001; Sarmiere and Freeman 2001). Since PI3K appeared to play a crucial role in Menta-FX’s neuroprotective effect, it is of interest to examine if Akt is involved in the PI3K-dependent neuroprotective property of Menta-FX. The ability of Menta-FX to affect Akt phosphorylation was investigated. Akt phosphorylation at Ser473 has generally been associated with its activation. We, thus, examined the effect of Menta-FX treatment on Akt phosphorylation at Ser473 at 3 and 7 dpa, two time-points when Menta-FX was observed to significantly lower the percentage of RGCs undergoing nuclear fragmentation. Results from this study indicate that Menta-FX did not affect Akt phosphorylation at 3 or 7 dpa (Fig. 8). Densitometry analysis revealed that pAkt levels at 3 dpa for PBS treated group (111.35 ± 1.5 arbituary value) and Menta-FX treated group (102.23 ± 8.9 arbituary value) were comparable. Similarly, the pAkt levels at 7 dpa for PBS treated group (121.48 ± 7.04 arbituary value) and Menta-FX treated group (118.02 ± 21.9 arbituary value) also fell short of statistical significance. These observations suggest that Akt did not mediate PI3K activity in the neuroprotective effect of Menta-FX.
Fig. 8.
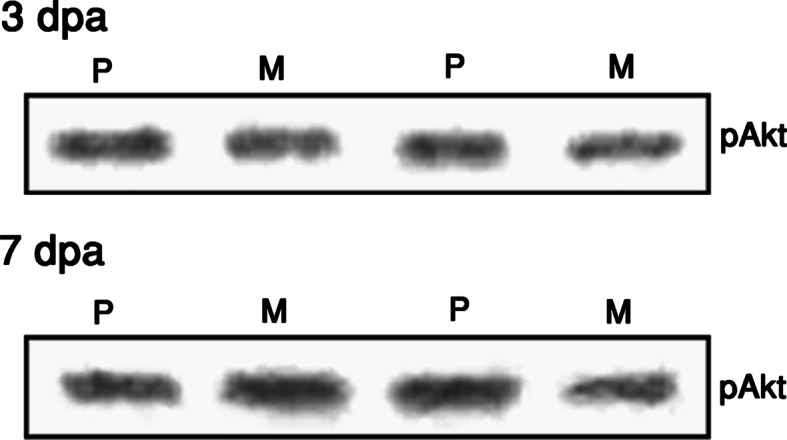
The effect of Menta-FX on Akt phosphorylation in axotomized retinas. Menta-FX treatment did not affect the levels of phospho-Akt (pAkt) at 3 or 7 days post-axotomy (dpa), indicating that Akt may not mediate the role of PI3K in the neuroprotective effect of Menta-FX. P = PBS-treated axotomized retinas; M = Menta-FX treated axotomized retinas. n = 3 per group
Discussions
In the current study, we found that Menta-FX, a mixture of Panax quinquefolius L., Ginkgo biloba and Hypericum perforatum extracts, exhibited NO scavenging property. Furthermore, we found that Menta-FX protected against axotomized RGC death through inhibiting nuclear fragmentation in a PI3K-dependent manner. Interestingly, we found that Menta-FX selectively ameliorated nuclear fragmentation in a population of RGCs that exhibited no detectable caspase-3 activation. Our study, therefore, suggests that Menta-FX may exert its neuroprotective effect through anti-oxidation and suppression of caspase-3-independent nuclear fragmentation.
In order to elucidate the neuroprotective mechanisms of Menta-FX, the involvement of anti-oxidation in Menta-FX’s neuroprotective effect was examined. First, the exhibition of anti-oxidative property by Menta-FX was elucidated in axotomized retinas. Extracts of Panax quinquefolius L., Ginkgo biloba and Hypericum perforatum have been demonstrated to exhibit free radical scavenging capacity (Kitts et al. 2000; Baudouin et al. 1999; Wei et al. 2000; Bastianetto et al. 2000a; Tripathi and Pandey 1999), and limit NO production (Kim et al. 1998; Marcocci et al. 1994). Menta-FX, a mixture of the three, is, therefore, likely to exhibit some anti-oxidative property. Indeed, results from the current study showed that Menta-FX treatment lowered NO content in the retinas without affecting the activity of NOS, suggesting that Menta-FX exhibited a NO scavenging capacity. The demonstration of NO scavenging ability in isolated retinas following Menta-FX treatment indicates indirectly that components of the extracts that reached the retina and exerted local NO scavenging activity in the retina following oral administration of Menta-FX. Furthermore, PQE and GBE, which have been shown to limit NO production in vitro (Kim et al. 1998), also reduced NO content in axotomized retinas when used as a combination. This indicates that despite oral administration, the biological activity of the extracts is somewhat conserved, and provides further support that the extracts component may reach the retina. This observation is in accordance with previous demonstration that components of Ginkgo biloba and Hypericum perforatum are absorbed after oral administration (Franklin et al. 1999; Ranchon et al. 1999).
Secondly, the importance of Menta-FX’s NO scavenging capacity in the neuroprotective effects of Menta-FX was examined. We have previously reported that despite the demonstrated free radical scavenging activity of PQE, GBE, and HPE, higher doses of the three extracts all fail to significantly increase axotomized RGC survival when each is used in separation (Cheung et al. 2002). We thus speculated that the neuroprotective effect of Menta-FX is possibly not a mere summation of the anti-oxidative capacity of the three extracts. Nonetheless, whether anti-oxidation makes partial contribution to Menta-FX’s neuroprotective effect remains unknown. In the current study, neuroprotection by Menta-FX at 7 dpa correlated with its ability to significantly decrease NO content in axotomized retinas. This indicates that NO scavenging may contribute to Menta-FX’s neuroprotective effect. In accordance with this finding, NO by itself has been shown to be toxic to RGCs (Kawasaki et al. 2000), and inhibition of NOS significantly enhances axotomized RGC survival (You et al. 1998; Ball and Keoberle 1999).
Since, axotomized RGCs have been recently characterized to die via the activation of caspase-9 and -3 (Kermer et al. 1999, 2000a), we were interested to examine if Menta-FX may exert its neuroprotective effect by attenuating caspase activation. Therefore, the effects of Menta-FX on caspase-3-activation and nuclear fragmentation were examined to further explicate its mechanisms of neuroprotection. We found that Menta-FX exhibited an anti-apoptotic property by inhibiting nuclear fragmentation. Nonetheless, Menta-FX had no effect on caspase-3 activation, suggesting that the reduction of nuclear fragmentation was not achieved by limiting caspase-3 activation. Remarkably, Menta-FX significantly reduced the percentage of caspase-3-negative fragmented nuclei, albeit lacking any effect on caspase-3-dependent nuclear fragmentation. As demonstrated in the current study, caspase-3 activation was observed only in approximately 50% of the apoptotic RGCs. The other 50% of the cells exhibiting fragmented nuclei was referred to the caspase-3-negative population of apoptotic RGCs. The selective reduction in the caspase-3-negative population of apoptotic cells indicates that Menta-FX limited nuclear fragmentation by inhibiting mostly a caspase-3-independent pathway.
The observation that Menta-FX provided neuroprotection without modulating caspase-3 activation is rather surprising because the caspase-9 and -3 pathway is the only known apoptotic pathway taking part in axotomized RGC death. While other apoptotic pathways such as the extrinsic pathway involving death receptors and caspase-8 activation may be implicated in axotomized RGC death, activation of this pathway will eventually result in the activation of caspase-3 (Yuan and Yankner 2000). In addition, various apoptotic stimuli including oxidative stress has been documented to induce neuronal death by activating caspase-3 (Lipton 1999). The ability of Menta-FX to exert neuroprotective effect by modulating selectively a caspase-3-independent pathway, therefore, indicates that a death pathway that is independent of caspase-3 exists and plays an essential role in the demise of the axotomized RGCs.
The identity of the pathways contributing to the caspase-3-independent nuclear fragmentation in axotomized RGCs is unclear. It is not known if one or several pathways are represented by this group of caspase-3-negative dying cells. However, no marked heterogeneity in the morphology of the caspase-3-negative fragmented nuclei was observed in the current study (data not shown). In addition, fragmented nuclear morphology in both the caspase-3-positive or -negative population was comparable, and closely resembled the classical nuclear fragmentation morphology described for apoptotic cells (data not shown) (Darzynkiewics and Traganos 1998). Therefore, this group of caspase-3-negative RGCs was possibly executed via apoptosis. Among the apoptotic proteases, caspase-7 and caspase-3 are similar in their substrates specificity and serve similar function as executioner (Zhivotovsky et al. 1999; Reed 2000; Yuan and Yankner 2000; Blajeski and Kaufmann 1999). Therefore, caspase-7 may take part in nuclear fragmentation when caspase-3 are not involved, as was observed in the axotomized RGCs. Nonetheless, preliminary studies indicate that caspase-7 immunoreactivity failed to colocalize with fragmented nuclei in axotomized retinas at several time-points post-axotomy (unpublished observation), suggesting that caspase-7 is possibly not involved in the caspase-3-independent pathway of nuclear fragmentation.
On the other hand, caspase-independent apoptotic pathways have been documented (Deshmukh and Johnson 2000; Liu et al. 2001). Data from a previous study showed that staurosporin induces apoptosis in a caspase-3-deficient cell line where addition of z-VAD-fmk, a broad-spectrum caspase-inhibitor, has no protective effect (Belmokhtar et al. 2001). This suggests that caspases are not involved in the execution of apoptosis in this caspase-3-deficient cell line, and caspase-independent pathways are potentially present. However, despite the demonstration of caspase-independent apoptotic pathways, the molecules and/or proteases involved remain unidentified. Recent literature reports that apoptosis inducing factor (AIF), a molecule released from the inter-membrane space of mitochondria upon permeabilization of the outer mitochondrial membrane, induces nuclear fragmentation in a caspase-independent manner (Daugas et al. 2000). AIF may, thus, contribute to the caspase-3-independent nuclear fragmentation observed in axotomized RGCs. However, preliminary results indicate that AIF immunoreactivity again did not co-localize with axotomized RGCs (unpublished observation), suggesting that AIF was possibly not involved in the observed caspase-3-independent nuclear fragmentation. Therefore, the identity of the caspase-3-independent apoptotic pathways remains elusive.
Although the identity of the caspase-3-independent pathway remains unknown, results from this study indicate that the neuroprotective effect of Menta-FX was reliant on PI3K activity. Injection of wortmannin, a PI3K inhibitor, abolished the neuroprotective effect of Menta-FX. This suggests that Menta-FX may inhibit nuclear fragmentation by modulating the PI3K/Akt pathway, an important source of survival signals against different types of injury (Politi et al. 2001; Dudek et al. 1997; Barber et al. 2001). Indeed, neuroprotective agents, such as BDNF and IGF have been observed to exert neuroprotective effect on axotomized RGCs via the PI3K pathway (Klocker et al. 2000; Kermer et al. 2000b), thus it is not surprising that Menta-FX also attenuated axotomized RGC death via modulating PI3K activity.
In the majority of the situation where PI3K is implicated as a survival signal, its downstream effector Akt has been indispensable for mediating its neuroprotective effect (Dudek et al. 1997; Roux et al. 2001; Franke et al. 1997; Wang et al. 2000; Namikawa et al. 2000; Barber et al. 2001; Sarmiere and Freeman 2001). Nonetheless, Menta-FX was not observed to modulate the activation of Akt at 3 or 7 dpa, suggesting that the involvement of PI3K in Menta-FX’s neuroprotective effect may be independent from Akt activity. Although Menta-FX may transiently increase Akt activity at time-points other than 3 or 7 dpa, Menta-FX’s inability to modulate Akt phosphorylation is consistent with its observed inability to lower caspase-3 activation since increase in Akt phosphorylation has been associated with a caspase-3 inhibitory effect (Cardone et al. 1998; Barber et al. 2001; Zhou et al. 2000). Therefore, Akt is possibly not involved in the neuroprotective effect of Menta-FX and is unlikely to be the main downstream effector of PI3K in the Menta-FX-treated retina. This implicates other downstream effectors of PI3K such as protein kinase C (PKC) in the PI3K-dependency of Menta-FX’s neuroprotective effect (Nunez and del Peso 1998). In the axotomized RGCs, increase in PKC activity enhances RGC survival in culture (dos and de Araujo 2000). This suggests that PKC may mediate the PI3K-dependency of Menta-FX’s neuroprotective property in axotomized retinas. Alternatively, crosstalk between the PI3K pathway and the Erk pathway, a mitogen-activated protein (MAP) kinase, has been suggested (Fuller et al. 2001). The Erk pathway has been demonstrated to take part in the neuroprotective effect of BDNF in axotomized RGCs (Klocker et al. 2000). Menta-FX may hence mediate its neuroprotective effect by activating the Erk pathway via PI3K. Examining these possibilities will provide essential knowledge on the biological actions of these herbs, and the signaling pathways implicated in axotomized RGC death.
In conclusions, results from this study demonstrate that a neuroprotective mixture of herbal extracts, Menta-FX, exhibited anti-oxidative property and an anti-apoptotic property through inhibiting a caspase-3-independent pathway of nuclear fragmentation. PI3K played a crucial role in the neuroprotective effect of Menta-FX, but the survival effect was not mediated by Akt. Findings from the current study indicate that Menta-FX exhibits direct anti-apoptotic property. Furthermore, the selective effect of Menta-FX on the caspase-3-independent apoptotic pathway renders Menta-FX a valuable tool for uncovering the identity of this pathway, thereby expanding our understanding on the mechanisms of apoptosis.
Acknowledgements
The authors would like to thank Miss Reiko Cheung and Mr. K.K. Yip for their technical assistance in the Western blotting. This research is supported by donations from the Hong Kong Charitable Foundation, and Ms. Annie Tsao Wen Wei, and an ASD grant from the Hong Kong Polytechnic University, China. Z.H. Cheung was supported by a scholarship from the Croucher Foundation, Hong Kong.
References
- Ball AK, Keoberle PD (1999) Nitric oxide synthase inhibition delays axonal degeneration and promotes the survival of axotomized retinal ganglion cells. Exp Neurol 158:366–381 [DOI] [PubMed] [Google Scholar]
- Barber AJ, Nakamura M, Wolpert EB, Reiter CE, Seigel GM, Antonetti DA, Gardner TW (2001) Insulin rescues retinal neurons from apoptosis by a phosphatidylinositol 3-kinase/Akt-mediated mechanism that reduces the activation of caspase-3. J Biol Chem 276:32814–32821 [DOI] [PubMed] [Google Scholar]
- Bastianetto S, Ramassamy C, Dore S, Christen Y, Poirier J, Quirion R (2000) The Ginkgo biloba extract (EGb 761) protects hippocampal neurons against cell death induced by beta-amyloid. Eur J Neurosci 12:1882–1890 [DOI] [PubMed] [Google Scholar]
- Baudouin C, Pisella PJ, Ettaiche M, Goldschild M, Becquet F, Gastaud P, Droy-Lefaix MT (1999) Effects of EGb761 and superoxide dismutase in an experimental model of retinopathy generated by intravitreal production of superoxide anion radical. Graefes Arch Clin Exp Ophthalmol 237:58–66 [DOI] [PubMed] [Google Scholar]
- Belmokhtar CA, Hillion J, Segal-Bendirdjian E (2001) Staurosporine induces apoptosis through both caspase-dependent and caspase-independent mechanisms. Oncogene 20:3354–3362 [DOI] [PubMed] [Google Scholar]
- Blajeski AL, Kaufmann SH (1999) Methods for detecting proteolysis during apoptosis in intact cells. In: Studzinski GP (ed) Apoptosis: a practical approach. Oxford University Press, New York, pp 215–238 [Google Scholar]
- Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC (1998) Regulation of cell death protease caspase-9 by phosphorylation. Science 282:1318–1321 [DOI] [PubMed] [Google Scholar]
- Cheung ZH, So KF, Lu Q, Yip HK, Wu W, Shan JJ, Pang PK, Chen CF (2002) Enhanced survival and regeneration of axotomized retinal ganglion cells by a mixture of herbal extracts. J Neurotrauma 19:369–378 [DOI] [PubMed] [Google Scholar]
- Cheung ZH, Chan YM, Siu FK, Yip HK, Wu W, Leung MC, So KF (2004) Regulation of caspase activation in axotomized retinal ganglion cells. Mol Cell Neurosci 25:383–393 [DOI] [PubMed] [Google Scholar]
- Darzynkiewics Z, Traganos F (1998) Measurement of apoptosis. In: Al-Rubeai M (ed) Apoptosis. Springer, Berlin, pp 33–74 [Google Scholar]
- Daugas E, Nochy D, Ravagnan L, Loeffler M, Susin SA, Zamzami N, Kroemer G (2000) Apoptosis-inducing factor (AIF): a ubiquitous mitochondrial oxidoreductase involved in apoptosis. FEBS Lett 476:118–123 [DOI] [PubMed] [Google Scholar]
- Deshmukh M, Johnson EM Jr (2000) Staurosporine-induced neuronal death: multiple mechanisms and methodological implications. Cell Death Differ 7:250–261 [DOI] [PubMed] [Google Scholar]
- Dos SA, De Araujo EG (2000) The effect of PKC activation on the survival of rat retinal ganglion cells in culture. Brain Res 853:338–343 [DOI] [PubMed] [Google Scholar]
- Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM, Segal RA, Kaplan DR, Greenberg ME (1997) Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science 275:661–665 [DOI] [PubMed] [Google Scholar]
- Franke TF, Kaplan DR, Cantley LC (1997) PI3K: downstream AKTion blocks apoptosis. Cell 88:435–437 [DOI] [PubMed] [Google Scholar]
- Franklin M, Chi J, McGavin C, Hockney R, Reed A, Campling G, Whale RW, Cowen PJ (1999) Neuroendocrine evidence for dopaminergic actions of hypericum extract (LI 160) in healthy volunteers. Biol Psychiatry 46:581–584 [DOI] [PubMed] [Google Scholar]
- Fuller G, Veitch K, Ho LK, Cruise L, Morris BJ (2001) Activation of p44/p42 MAP kinase in striatal neurons via kainate receptors and PI3 kinase. Brain Res Mol Brain Res 89:126–132 [DOI] [PubMed] [Google Scholar]
- Isenmann S, Wahl C, Krajewski S, Reed JC, Bahr M (1997) Up-regulation of Bax protein in degenerating retinal ganglion cells precedes apoptotic cell death after optic nerve lesion in the rat. Eur J Neurosci 9:1763–1772 [DOI] [PubMed] [Google Scholar]
- Kawasaki A, Otori Y, Barnstable CJ (2000) Muller cell protection of rat retinal ganglion cells from glutamate and nitric oxide neurotoxicity. Invest Ophthalmol Vis Sci 41:3444–3450 [PubMed] [Google Scholar]
- Kermer P, Klocker N, Labes M, Thomsen S, Srinivasan A, Bahr M (1999) Activation of caspase-3 in axotomized rat retinal ganglion cells in vivo. FEBS Lett 453:361–364 [DOI] [PubMed] [Google Scholar]
- Kermer P, Ankerhold R, Klocker N, Krajewski S, Reed JC, Bahr M (2000a) Caspase-9: involvement in secondary death of axotomized rat retinal ganglion cells in vivo. Brain Res Mol Brain Res 85:144–150 [DOI] [PubMed] [Google Scholar]
- Kermer P, Klocker N, Labes M, Bahr M (2000b) Insulin-like growth factor-I protects axotomized rat retinal ganglion cells from secondary death via PI3-K-dependent Akt phosphorylation and inhibition of caspase-3 in vivo. J Neurosci 20:2–8 [PubMed] [Google Scholar]
- Kim YC, Kim SR, Markelonis GJ, Oh TH (1998) Ginsenosides Rb1 and Rg3 protect cultured rat cortical cells from glutamate-induced neurodegeneration [published erratum appears in J Neurosci Res 1998 Oct 1; 54(1): 123]. J Neurosci Res 53:426–432 [DOI] [PubMed] [Google Scholar]
- Kitts DD, Wijewickreme AN, Hu C (2000) Antioxidant properties of a North American ginseng extract. Mol Cell Biochem 203:1–10 [DOI] [PubMed] [Google Scholar]
- Klocker N, Kermer P, Weishaupt JH, Labes M, Ankerhold R, Bahr M (2000) Brain-derived neurotrophic factor-mediated neuroprotection of adult rat retinal ganglion cells in vivo does not exclusively depend on phosphatidyl-inositol-3′-kinase/protein kinase B signaling. J Neurosci 20:6962–6967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao J, Xu X, Wargovich MJ (2000) Direct reprobing with anti-beta-actin antibody as an internal control for western blotting analysis. Biotechniques 28:216–218 [DOI] [PubMed] [Google Scholar]
- Lipton SA (1999) Neuronal protection and destruction by NO. Cell Death Differ 6:943–951 [DOI] [PubMed] [Google Scholar]
- Liu W, Liu R, Chun JT, Bi R, Hoe W, Schreiber SS, Baudry M (2001) Kainate excitotoxicity in organotypic hippocampal slice cultures: evidence for multiple apoptotic pathways. Brain Res 916:239–248 [DOI] [PubMed] [Google Scholar]
- Marcocci L, Maguire JJ, Droy-Lefaix MT, Packer L (1994) The nitric oxide-scavenging properties of Ginkgo biloba extract EGb 761. Biochem Biophys Res Commun 201:748–755 [DOI] [PubMed] [Google Scholar]
- Namikawa K, Honma M, Abe K, Takeda M, Mansur K, Obata T, Miwa A, Okado H, Kiyama H (2000) Akt/protein kinase B prevents injury-induced motoneuron death and accelerates axonal regeneration. J Neurosci 20:2875–2886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez G, Del Peso L (1998) Linking extracellular survival signals and the apoptotic machinery. Curr Opin Neurobiol 8:613–618 [DOI] [PubMed] [Google Scholar]
- Politi LE, Rotstein NP, Salvador G, Giusto NM, Insua MF (2001) Insulin-like growth factor-I is a potential trophic factor for amacrine cells. J Neurochem 76:1199–1211 [DOI] [PubMed] [Google Scholar]
- Ranchon I, Gorrand JM, Cluzel J, Droy-Lefaix MT, Doly M (1999) Functional protection of photoreceptors from light-induced damage by dimethylthiourea and Ginkgo biloba extract. Invest Ophthalmol Vis Sci 40:1191–1199 [PubMed] [Google Scholar]
- Reed JC (2000) Mechanisms of apoptosis. Am J Pathol 157:1415–1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux PP, Bhakar AL, Kennedy TE, Barker PA (2001) The p75 neurotrophin receptor activates Akt (protein kinase B) through a phosphatidylinositol 3-kinase-dependent pathway. J Biol Chem 276:23097–23104 [DOI] [PubMed] [Google Scholar]
- Sarmiere PD, Freeman RS (2001) Analysis of the nf-kappab and pi 3-kinase/akt survival pathways in nerve growth factor-dependent neurons. Mol Cell Neurosci 18:320–331 [DOI] [PubMed] [Google Scholar]
- Tripathi YB, Pandey E (1999) Role of alcoholic extract of shoot of Hypericum perforatum Linn on lipid peroxidation and various species of free radicals in rats. Indian J Exp Biol 37:567–571 [PubMed] [Google Scholar]
- Wang X, Mccullough KD, Franke TF, Holbrook NJ (2000) Epidermal growth factor receptor-dependent Akt activation by oxidative stress enhances cell survival. J Biol Chem 275:14624–14631 [DOI] [PubMed] [Google Scholar]
- Wei T, Ni Y, Hou J, Chen C, Zhao B, Xin W (2000) Hydrogen peroxide-induced oxidative damage and apoptosis in cerebellar granule cells: protection by Ginkgo biloba extract. Pharmacol Res 41:427–433 [DOI] [PubMed] [Google Scholar]
- You SW, Tay D, So KF, Yip HK, Lau KC (1998) Effects of nitric oxide synthase inhibitor on the neuronal survival and axonal regeneration of retinal ganglion cells in hamsters. Proc Aust Neuroscience Soc 9:98 [Google Scholar]
- Yuan J, Yankner BA (2000) Apoptosis in the nervous system. Nature 407:802–809 [DOI] [PubMed] [Google Scholar]
- Zhivotovsky B, Samali A, Gahm A, Orrenius S (1999) Caspases: their intracellular localization and translocation during apoptosis. Cell Death Differ 6:644–651 [DOI] [PubMed] [Google Scholar]
- Zhou H, Li XM, Meinkoth J, Pittman RN (2000) Akt regulates cell survival and apoptosis at a postmitochondrial level. J Cell Biol 151:483–494 [DOI] [PMC free article] [PubMed] [Google Scholar]