Abstract
Dysfunction of the proteasome function is known to be a potential mechanism for dopaminergic neuron degeneration. Here, we investigated to determine whether systematic administration of proteasome inhibitor, carbobenzoxy-l-γ-t-butyl-l-glutamyl-l-alanyl-l-leucinal (PSI), causes the increased susceptibility in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated mice. PSI was injected into MPTP-treated mice over a period of 2 weeks. Thereafter, we evaluated the effect of PSI 2, 4, and 8 weeks after the cessation of treatment with PSI. In the present study with HPLC analysis, PSI did not enhance MPTP-induced dopaminergic neurotoxicity in mice. Our present study with Western blot analysis also demonstrated that the reduction of tyrosine hydroxylase (TH) and glial fibrillary acidic protein (GFAP) protein levels in MPTP-treated mice was more pronounced than that in MPTP + PSI-treated animals. These results suggest that proteasome inhibitor did not enhance MPTP neurotoxicity in mice. Our findings suggest that proteasome inhibition is not a reliable model for PD. Thus, our findings provide further valuable information for the pathogenesis of Parkinson’s disease.
Keywords: Proteasome, Parkinson’s disease, Western blot analysis, Dopamine system, Mice
Introduction
Parkinson’s disease (PD) is defined clinically by the presence of bradykinesia, rigidity, resting tremor, and postural instability (Dauer and Przedborski et al. 2003). Emerging reports indicate that PD neurodegeneration is multifactorial (Dauer and Przedborski et al. 2003). The primary pathology of PD is degeneration of dopaminergic neurons in the substantia nigra pars compacta, resulting in loss of the nigrostriatal pathway and a reduction of dopamine levels in the striatum (Braak et al. 2003). Numerous investigations have been reported the mechanisms responsible for dopaminergic neuron degeneration in PD. Oxidative stress, excitotoxicity, depletion of endogenous antioxidants, decreased expression of trophic factors, and dysfunction of protein degradation system are believed to participate in the cascade of events leading to dopaminergic neuronal loss (Jenner 2003; Olanov et al. 2003).
On the other hand, a recent interesting study demonstrates that systemic administration of proteasomal inhibitors causes dopaminergic cell loss in the rat focused attention on the ubiquitin-proteasomal system (UPS) as an important factor in PD pathogenesis (McNaught et al. 2004). The UPS is a major mechanism responsible for the degradation of short-lived, damaged, and misfolded proteins (Sherman and Goldberg 2001). Autosomal recessive juvenile parkinsonism is caused by mutations in the parkin gene (Kitada et al. 1998), a ubiquitin-protein isopeptide ligase (E3) of UPS (Zhang et al. 2000). Furthermore, a previous study suggests that the failure of the UPS to adequately clear unwanted proteins underlies vulnerability and degeneration of the substantia nigra pars compacta in both sporadic and familial PD (McNaught et al. 2003). On the basis of these observations, it is suggested that the impairment of UPS has also been implicated in the pathogenesis of PD.
In both monkeys and mice, the injection of a neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) induces similar neurological and pathological changes that are observed in PD in humans (Burns et al. 1983; Langston et al. 1983; Heikklia et al. 1984). MPTP selectively damages the dopaminergic nigrostriatal system, resulting in the loss of dopaminergic neurons in the substantia nigra and a depletion of dopamine in the striatum. Under strict experimental conditions, however, MPTP does not always damage the nigrostriatal pathway in all injected animals (Bove et al. 2006).
In the present study, therefore, we investigated to determine whether systematic administration of proteasome inhibitor causes the increased susceptibility in MPTP-treated mice.
Materials and Methods
Experimental Animals
Male C57BL/6 mice (Nihon SLC Co., Shizuoka, Japan), 8 weeks of age, were used in this study. The animals were housed in a controlled environment (23 ± 1°C, 50 ± 5% humidity) and were allowed food and tap water ad libitum. The room lights were on between 8:00 and 20:00. In order to examine the synergistic effects of MPTP and PSI (lipophilic proteasome inhibitor, carbobenzoxy-l-γ-t-butyl-l-glutamyl-l-alanyl-l-leucinal, Calbiochem, San Diego, CA, USA), the animals were divided into nine group: (1) vehicle (saline + 70% ethanol)-treated group 2 weeks after the cessation of treatment with 70% ethanol (4 weeks after saline treatment); (2) MPTP-treated group 2 weeks after the cessation of treatment with 70% ethanol (4 weeks after MPTP treatment); (3) MPTP + PSI-treated group 2 weeks after the cessation of treatment with PSI (4 weeks after MPTP treatment); (4) vehicle (saline + 70% ethanol)-treated group 4 weeks after the cessation of treatment with 70% ethanol (6 weeks after saline treatment); (5) MPTP-treated group 4 weeks after the cessation of treatment with 70% ethanol (6 weeks after MPTP treatment); (6) MPTP + PSI-treated group 4 weeks after the cessation of treatment with PSI (6 weeks after MPTP treatment); (7) vehicle (saline + 70% ethanol)-treated group 8 weeks after the cessation of treatment with 70% ethanol (10 weeks after saline treatment); (8) MPTP-treated group 8 weeks after the cessation of treatment with 70% ethanol (10 weeks after MPTP treatment); (9) MPTP + PSI-treated group 8 weeks after the cessation of treatment with PSI (10 weeks after MPTP treatment). In brief, mice were injected intraperitoneally (i.p.) four times with MPTP (20 mg/kg) at 2 h intervals, the total dose per mouse being 80 mg/kg, as described previously (Muramatsu et al. 2003; Kurosaki et al. 2005). Vehicle animals received i.p. four injections of physiological saline. Mice at 5 days after MPTP treatment were with six subcutaneous injections of 3 mg/kg PSI in 70% ethanol, or 70% ethanol alone as vehicle, over a 2-week period (on Mondays, Wednesdays, and Fridays). PSI solution was prepared immediately before each injection by dissolving PSI in 100% ethanol at room temperature, vortexing the solution for 3 min to ensure that no particles remained, then diluting the PSI–100% ethanol solution to 70% with distilled water. All experiments were performed in accordance with the Guidelines for Animal Experiments of the Tokushima University School of Medicine. In addition, a 3 mg/kg dose of the proteasome inhibitor PSI is known to cause the behavioral changes and inhibit proteasome function in the rat brain (Camacho-Arroyo et al. 2002; McNaught et al. 2004). In the present study, therefore, we elected to use a 3 mg/kg dose of PSI.
Measurement of Dopamine, DOPAC and HVA Levels
The mice were killed by cervical dislocation at 2, 4, and 8 weeks after the cessation of treatment with PSI or 70% ethanol (4, 6, and 10 weeks after MPTP or saline treatment). After cervical dislocation, the striatum were rapidly dissected out and sonicated in ice-cold 0.2 M perchloric acid containing 100 ng/ml isoproterenol as an internal standard. Dopamine, DOPAC (3,4-dihydroxyphenylacetic acid) and HVA (homovanillic acid) were quantified by HPLC with an electrochemical detector (ECD) (Eicom, Kyoto, Japan). Concentrations of dopamine and its metabolites were expressed as μg/g tissue weight, as described previously (Araki et al. 2001; Kurosaki et al. 2005). Each group consisted of 4–5 mice.
Western Blot Analysis
The mice were killed by cervical dislocation 2, 4, and 8 weeks after the cessation of treatment with PSI or 70% ethanol (4, 6, and 10 weeks after MPTP or vehicle treatment). The striatal tissues were homogenized in HEPES-buffered sucrose (0.32 M sucrose containing 4 μg/ml pepstatin, 5 μg/ml aprotinin, 20 μg/ml trypsin inhibitor, 4 μg/ml leupeptin, 0.2 mM phenylmethanesulfonyl fluroride, 2 mM EDTA, 2 mM EGTA, and 20 mM HEPES, pH 7.2) using a microtube homogenizer. Protein concentrations were determined using a BCA kit (PIERCE, IL, USA). The homogenates were solubilized in Laemmli’s sample buffer. Ten micrograms of protein from each sample were separated on 5–20% SDS-PAGE gel using constant current. Separated proteins were electrophoretically transferred to polyvinylidene difluoride (PVDF) membranes (ATTO, Tokyo, Japan) for 1 h with semi-dry blotting system. The PVDF membranes were incubated for 1 h at room temperature with Phosphate-buffered saline containing 0.1% Tween 20 (PBST) and 0.5% skim milk, followed by overnight incubation at room temperature with desired antibodies. The anti-tyrosine hydroxylase (TH) antibody (1:5000, Chemicon International, Inc., Temecula, CA, USA) as a marker of dopaminergic neurons and anti-glial fibrillary acidic protein (GFAP) antibody (1:2000, Sigma, Saint Louis, MO, USA) as a marker of reactive astrocytes were diluted in PBST containing 0.5% skim milk. Membranes were washed three times for 10 min at room temperature and incubated with horseradish peroxidase-conjugated secondary antibody in PBST containing 0.5% Skim milk for 1 h. Immunoreactive bands were visualized by enhanced chemiluminescent autoradiography (ECL Kit, Amersham, IL, USA), according to manufacturer’s instructions. Actin antibody (Sigma, Saint Louis, MO, USA) and α1 subunit of Na+/K+-ATPase protein (Upstate Biotechnology, NY, USA) were used as a house keeping protein to confirm that equal amounts of protein were loaded in each line. Optical densities were determined using a computerized image analysis system (Dolphin-DOC, Kurabo, Osaka, Japan). TH protein levels were expressed as % of vehicle using ratios to actin protein levels. GFAP protein levels were expressed as % of vehicle using ratios to α1 subunit of Na+/K+-ATPase protein levels. Each group consisted of 4–5 mice.
Statistical Analysis
All values were expressed as the means ± SD and statistical significance was evaluated by one-way analysis of variance (ANOVA) followed by Fisher’s PLSD multiple comparison test or Student’s t-test (Stat View version 5.0, SAS Institute Inc., USA).
Results
Striatal Dopamine and its Metabolites Content Analysis
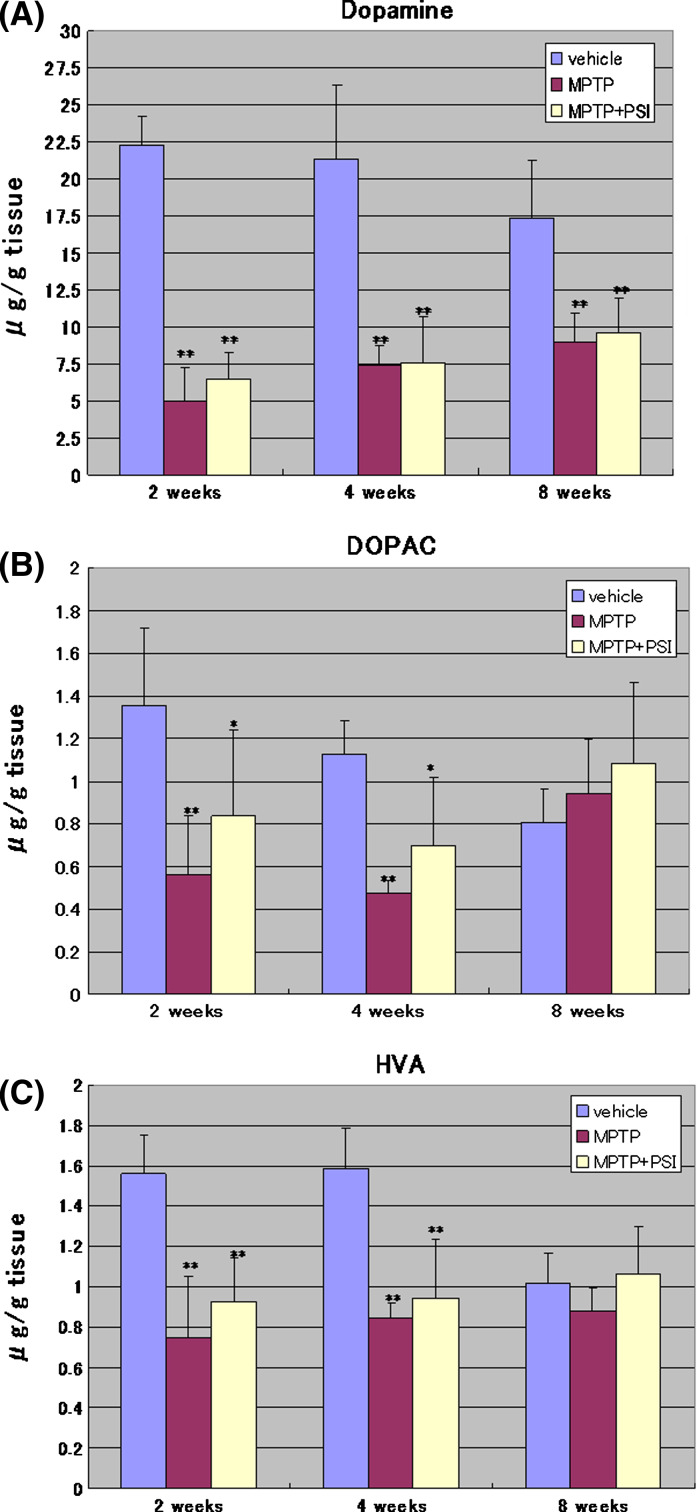
As shown in Fig. 1, the four administrations of MPTP at 2-h intervals to mice decreased significantly the concentration of dopamine, DOPAC and HVA in the striatum 2 weeks after the cessation of treatment with 70% ethanol to 23, 41, and 48% of the vehicle group, respectively. Four weeks after the cessation of treatment with 70% ethanol, the concentration of dopamine, DOPAC and HVA in the striatum was 34, 42, and 53% of the vehicle group, respectively. Eight weeks after the cessation of treatment with 70% ethanol, the concentration of dopamine, DOPAC and HVA in the striatum was 52, 116, and 86% of the vehicle group, respectively. Thus, the concentration of dopamine in the striatum of the MPTP-treated mice showed a tendency to recover the level of the vehicle groups 8 weeks after the cessation of treatment with 70% ethanol. In contrast, no significant changes in the concentration of DOPAC and HVA of the MPTP-treated animals 8 weeks after the cessation of treatment with 70% ethanol were observed in the striatum compared with the vehicle group.
Fig. 1.
Striatal levels of dopamine and its metabolites. Striatal levels of dopamine (A), DOPAC (B), and HVA (C) were determined by HPLC at 2, 4, and 8 weeks after the cessation of treatment with 70% ethanol or PSI. Data were expressed as mean ± SD. Vehicle: vehicle-treated group. MPTP: MPTP-treated group. MPTP + PSI: MPTP + PSI-treated group. * P < 0.05, ** P < 0.01, compared with vehicle-treated group (Fisher’s PLSD multiple comparison test); n = 4–5
In MPTP + PSI-treated mice, on the other hand, the concentration of dopamine, DOPAC and HVA in the striatum 2 weeks after the cessation of treatment with PSI was 30, 62, and 59% of the vehicle group, respectively. Four weeks after the cessation of treatment with PSI, the concentration of dopamine, DOPAC and HVA in the striatum was 35, 62, and 60% of the vehicle group, respectively. Eight weeks after the cessation of treatment with PSI, the concentration of dopamine, DOPAC and HVA in the striatum was 56, 133, and 104% of the vehicle group, respectively. Thus, the concentration of dopamine in the striatum of the MPTP + PSI-treated mice also showed a tendency to recover the level of the vehicle group 8 weeks after the cessation of treatment with PSI. In contrast, no significant changes in the concentration of DOPAC and HVA of the MPTP + PSI-treated animals 8 weeks after the cessation of treatment with PSI were observed in the striatum compared with the vehicle group. Furthermore, no significant changes in the concentration of DOPAC and HVA of the MPTP + PSI-treated animals were found in the striatum compared with MPTP-treated mice 2, 4, and 8 weeks after the cessation of treatment with PSI or 70% ethanol.
Western Blot Analysis of Striatal TH and GFAP Protein
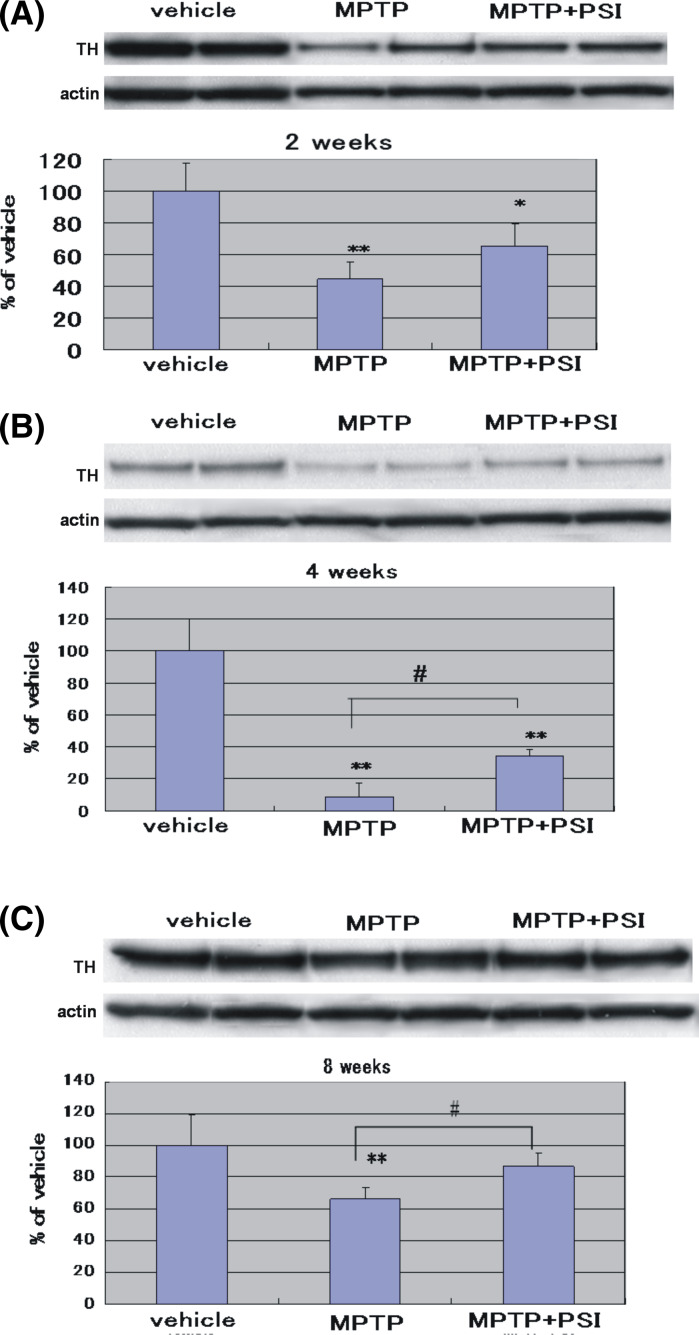
As shown in Fig. 2, the four administrations of MPTP at 2-h intervals to mice produced a significant reduction (45% of the vehicle groups) of TH protein levels in the striatum 2 weeks after the cessation of treatment with 70% ethanol. Four weeks after the cessation of treatment with 70% ethanol, a marked decrease (9% of the vehicle group) of TH protein levels was observed in the striatum of MPTP-treated mice. Eight weeks after the cessation of treatment with 70% ethanol, a mild reduction (67% of the vehicle group) of TH protein levels was found in the striatum of MPTP-treated animals. Thus, TH protein levels in the striatum of the MPTP-treated mice exhibited a tendency to recover the level of the vehicle group 8 weeks after the cessation of treatment with 70% ethanol.
Fig. 2.
Immunoblotting analysis of TH protein levels in the mouse striatum at 2 weeks (A), 4 weeks (B), and 8 weeks (C) after the cessation of treatment with 70% ethanol or PSI. For Western blot analysis, actin protein was detected as a house keeping protein to confirm that equal amounts of protein were loaded in each line. TH protein levels were expressed as % of vehicle (mean ± SD) using ratios to actin protein levels. Vehicle: Vehicle-treated group. MPTP: MPTP-treated group. MPTP + PSI: MPTP + PSI-treated group. * P < 0.05, ** P < 0.01, compared with vehicle-treated group (Fisher’s PLSD multiple comparison test). # P < 0.05, Student’s t-test; n = 4–5
In MPTP + PSI-treated mice, on the other hand, TH protein levels in the striatum 2 weeks after the cessation of treatment with PSI were 65% of the vehicle group. Four weeks after the cessation of treatment with PSI, TH protein levels in the striatum was 34% of the vehicle group. Eight weeks after the cessation of treatment with PSI, the TH protein levels were 87% of the vehicle group. Four and eight weeks after the cessation of treatment with PSI, a significant increase of TH protein levels was observed in the striatum of MPTP + PSI-treated mice compared with MPTP-treated animals. In addition, actin protein was detected as a house keeping protein to confirm that equal amounts of protein were loaded in each line.
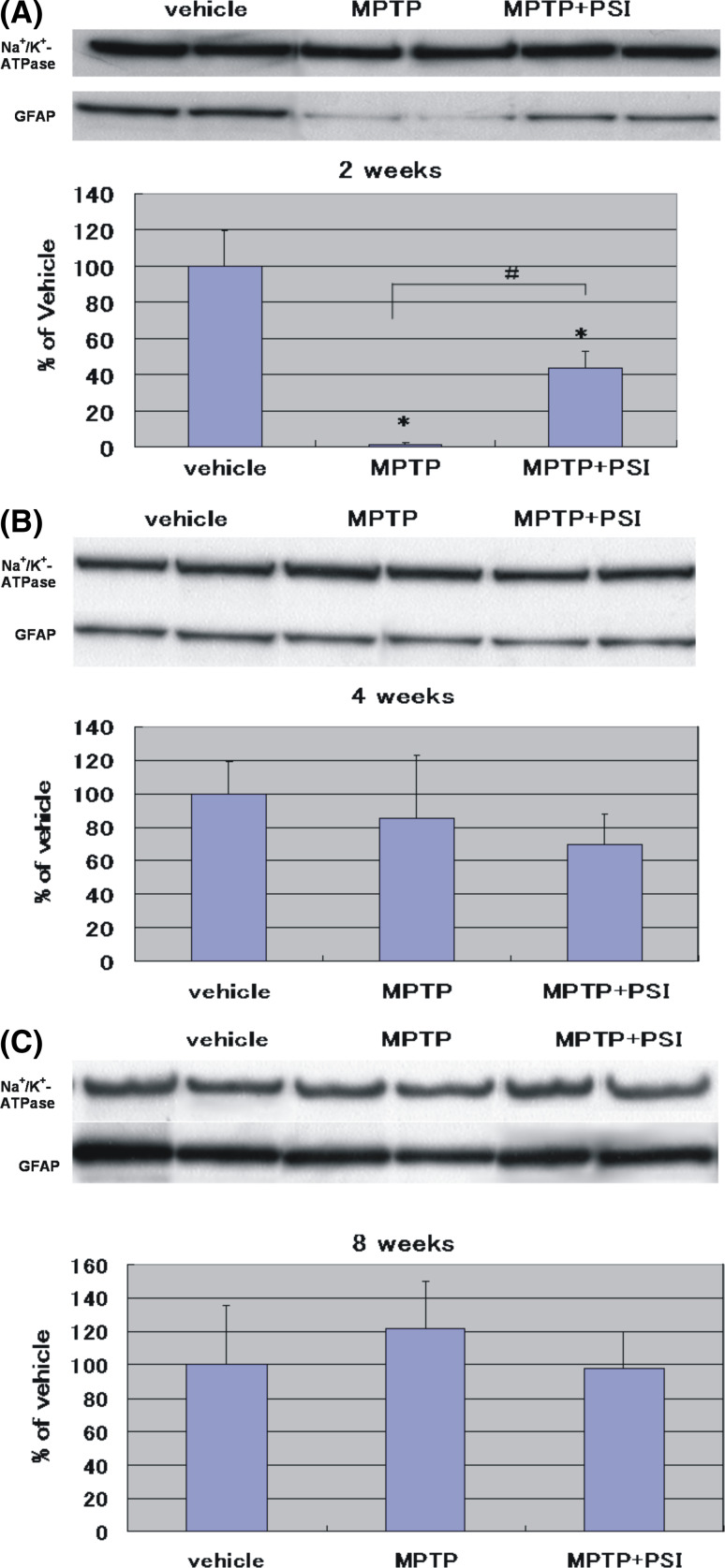
As shown in Fig. 3, the four administrations of MPTP at 2-h intervals to mice produced a marked reduction (2% of the vehicle group) of GFAP protein levels in the striatum 2 weeks after the cessation of treatment with 70% ethanol. Four weeks after the cessation of treatment with 70% ethanol, no significant changes (85% of the vehicle group) of GFAP protein levels were observed in the striatum of MPTP-treated mice. Eight weeks after the cessation of treatment with 70% ethanol, no significant changes of GFAP protein levels were observed in the striatum of MPTP-treated mice. Thus, no significant changes of GFAP protein levels in the striatum of MPTP-treated mice were observed 4 and 8 weeks after the cessation of treatment with 70% ethanol.
Fig. 3.
Immunoblotting analysis of GFAP protein levels in the mouse striatum at 2 weeks (A), 4 weeks (B), and 8 weeks (C) after the cessation of treatment with 70% ethanol or PSI. For Western blot analysis, α1 subunit of Na+/K+-ATPase protein was detected as a house keeping protein to confirm that equal amounts of protein were loaded in each line. GFAP protein levels were expressed as % of vehicle (mean ± SD) using ratios to α1 subunit of Na+/K+-ATPase protein levels. Vehicle: vehicle-treated group. MPTP: MPTP-treated group. MPTP + PSI: MPTP + PSI-treated group. * P < 0.05, ** P < 0.01, compared with vehicle-treated group (Fisher’s PLSD multiple comparison test). # P < 0.05, Student’s t-test; n = 4–5
In MPTP + PSI-treated mice, on the other hand, GFAP protein levels in the striatum 2 weeks after the cessation of treatment with PSI were 44% of the vehicle group. Four weeks after the cessation of treatment with PSI, GFAP protein levels in the striatum was 70% of the vehicle group. Eight weeks after the cessation of treatment with PSI, GFAP protein levels were 98% of the vehicle group. Thus, no significant changes of GFAP protein levels in the striatum of MPTP + PSI-treated mice were observed 4 and 8 weeks after the cessation of treatment with PSI. Two weeks after the cessation of treatment with PSI, furthermore, a significant increase of GFAP protein levels was observed in the striatum of MPTP + PSI-treated mice compared with MPTP-treated animals. In addition, α1 subunit of Na+/K+-ATPase protein was detected as a house keeping protein to confirm that equal amounts of protein were loaded in each line.
Discussion
It is known that proteasome inhibitors are widely distributed in the environment (Kisselev and Goldberg 2001), being produced by bacteria (Fenteany and Schreiber 1998; Sin et al. 1999), fungi (Koguchi et al. 2000), plants (Nam et al. 2001; Kazi et al. 2003; Jana et al. 2004), and possibly the chemical industry (Kisselev and Goldberg 2001). In brief, lactacysin and epoxomicin, which are naturally produced by actinomycetes, are found globally in soil and aquatic habitats of garden and farmland (Cross 1981; Ensign et al. 1993). These microbes can infect root vegetables and potatoes causing scab formation (Cross 1981; Ensign et al. 1993). Thus, it is noteworthy that potent proteasome inhibitors are found in rural areas and in well water, both of which have been shown to be associated with an increased risk of developing PD in epidemiological studies (Priyadarshi et al. 2001). Furthermore, structural analogs and the active pharmacophore of natural and synthetic compounds known to potently inhibit the proteasome, such as PSI, are evident in the environment (Kisselev and Goldberg 2001). The lipophilic proteasome inhibitors including PSI are known to cross the blood-brain barrier in rats (McNaught et al. 2004). Previously, a body of genetic, postmortem and experimental evidence has converged to suggest that a failure of the UPS to degrade unwanted proteins might play a major role in the etiopathogenesis of both familial and sporadic forms of PD (McNaught and Olanow 2003:Petrucelli and Dawson 2004). Several studies also reported that systemic administration of proteasome inhibitors can cause a progressive model of PD in rats (Fornai et al. 2003; McNaught et al. 2004; Miwa et al. 2005; Schapira et al. 2006). The findings suggest that PD is associated with an impaired capacity of the UPS to clear unwanted proteins. From these observations, it is conceivable that the dysfunction of the UPS may play an important role in the pathogenesis of PD. In the present study, therefore, we investigated to determine whether systematic administration of proteasome inhibitor causes the increased susceptibility in MPTP-treated mice.
In the present study, the four administrations of MPTP at 2-h intervals to mice decreased significantly the concentration of dopamine, DOPAC and HVA in the striatum 2 and 4 weeks after the cessation of treatment with 70% ethanol. Eight weeks after the cessation of treatment with 70% ethanol, however, the concentration of dopamine, DOPAC and HVA in the striatum was 52, 116, and 86% of the vehicle group, respectively. Thus, the concentration of dopamine in the striatum of the MPTP-treated mice showed a tendency to recover the level of the vehicle groups 8 weeks after the cessation of treatment with 70% ethanol. In contrast, no significant changes in the concentration of DOPAC and HVA of the MPTP-treated animals 8 weeks after the cessation of treatment with 70% ethanol were observed in the striatum compared with the vehicle group. On the other hand, no significant changes in the concentration of DOPAC and HVA of the MPTP + PSI-treated animals were found in the striatum compared with MPTP-treated mice 2, 4, and 8 weeks after the cessation of treatment with PSI or 70% ethanol. A recent study demonstrates that HPLC postmortem neurochemistry after systemic PSI (3 mg/kg, s.c., six times over 2 weeks) treatment did not show a significant difference in striatal dopamine levels of C57 BL/6 mice when comparing MPTP with MPTP + PSI treatment (Hirst and Ferger 2008). These findings are consistent with the present results with our HPLC analysis.
In our Western blot analysis, the four administrations of MPTP at 2-h intervals to mice produced a significant and marked reduction (45 and 9% of the vehicle group) of TH protein levels in the striatum 2 and 4 weeks after the cessation of treatment with 70% ethanol. Eight weeks after the cessation of treatment with 70% ethanol, however, a mild reduction (67% of the vehicle group) of TH protein levels was found in the striatum of MPTP-treated animals. Thus, TH protein levels in the striatum of the MPTP-treated mice exhibited a tendency to recover the level of the vehicle group 8 weeks after the cessation of treatment with 70% ethanol. On the other hand, TH protein levels in the striatum 2 weeks after the cessation of treatment with PSI or 70% ethanol was similar between MPTP-treated and MPTP + PSI-treated mice. Four and eight weeks after the cessation of treatment with PSI, however, a significant increase of TH protein levels was observed in the striatum of MPTP + PSI-treated mice compared with MPTP-treated animals. It is suggested that impairment of the UPS has been implicated in the pathogenesis of PD. However, recent interesting studies report that proteasome inhibitors cannot cause loss of nigral tyrosine hydroxylase neurons in rats and monkeys (Bove et al. 2006; Kordower et al. 2006). Several experimental studies also demonstrate that proteasome inhibition is also able to provide neuroprotection (Phillips et al. 2000; van Leyen et al. 2005; Yamamoto et al. 2007). These findings are, at least in part, consistent with our present findings. Therefore, the role of proteasome inhibition in the etiopathogenesis of PD remains to be clearly determined. On the basis of these observations and our findings, the present study suggests that proteasome inhibitor PSI did not enhance MPTP-induced dopaminergic neurotoxicity in mice. Furthermore, our present study demonstrates that the reduction of TH protein levels in MPTP-treated mice was more pronounced than that in MPTP + PSI-treated animals. These results suggest that proteasome inhibitor did not enhance MPTP neurotoxicity in mice.
On the other hand, the four administrations of MPTP at 2-h intervals to mice produced a marked reduction (2% of the vehicle group) of GFAP protein levels in the striatum 2 weeks after the cessation of treatment with 70% ethanol. Thereafter, no significant changes of GFAP protein levels were observed in the striatum of MPTP-treated mice 4 and 8 weeks after the cessation of treatment with 70% ethanol. In MPTP + PSI-treated mice, on the other hand, GFAP protein levels in the striatum 2 weeks after the cessation of treatment with PSI were 44% of the vehicle group. However, no significant changes of GFAP protein levels in the striatum of MPTP + PSI-treated mice were observed 4 and 8 weeks after the cessation of treatment with PSI. Two weeks after the cessation of treatment with PSI, furthermore, a significant increase of GFAP protein levels was observed in the striatum of MPTP + PSI-treated mice compared with MPTP-treated animals. GFAP-positive astrocytes are known to play a central role in the defense of brain and to exert a variety of neuroprotective functions (Morale et al. 2006). Thus, the astrocytes express crucial neurotrophic molecules, regulating growth, differentiation and survival of neurons (Dringen et al. 2000; Gallo et al. 2000). Furthermore, it is known that GFAP-null mice are highly susceptible to brain damage, such as cerebral ischemia (Nawashiro et al. 2000). These observations suggest that GFAP-positive astrocytes may have a beneficial effect against MPTP neurotoxicity in mice. In the present study, the reduction of GFAP protein levels in MPTP-treated mice was more pronounced than that in MPTP + PSI-treated animals 2 weeks after the cessation of treatment with PSI. Therefore, the present study suggests that the injection of MPTP can cause a severe reduction of GFAP protein levels in the mouse striatum compared with the administration of MPTP and PSI. Furthermore, our findings demonstrate that PSI may attenuate a severe reduction of GFAP protein levels in the striatum caused by MPTP. However, further detailed investigation is required to clarify our findings.
In conclusion, the present study indicates that the proteasome inhibitor PSI did not enhance MPTP-induced dopaminergic neurotoxicity in mice. Our present study also demonstrates that the reduction of striatal TH and GFAP protein levels in MPTP-treated mice was more pronounced than that in MPTP + PSI-treated animals. Thus, our findings demonstrate that proteasome inhibition is not a reliable model for PD.
Acknowledgments
This study was supported in part by Grant-in-Aid for Scientific Research (136700627 and 13671095) from the Ministry of Science and Education in Japan.
Footnotes
Naoto Kadoguchi and Masahiro Umeda contributed equally to this work.
References
- Araki T, Kumagai T, Tanaka K, Matsubara M, Kato H, Itoyama Y, Imai Y (2001) Neuroprotective effect of riluzole in MPTP-treated mice. Brain Res 918:176–181 [DOI] [PubMed] [Google Scholar]
- Bove J, Zhou C, Jackson-Lewis V, Taylor J, Chu Y, Rideout HJ, Wu DC, Kordower JH, Petrucelli L, Przedbroski S (2006) Proteasome inhibition and Parkinson’s disease modeling. Ann Neurol 60:260–264 [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24:197–211 [DOI] [PubMed] [Google Scholar]
- Burns RS, Chiueh CC, Markey SP, Ebert MH, Jacobowitz DM, Kopin IJ (1983) A primate model of parkinsonism: selective destruction of dopaminergic neurons in the pars compacta of the substantia nigra by N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Proc Natl Acad Sci USA 80:4546–4550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho-Arroyo I, Villamar-Cruz O, Gonzalez-Arenas A, Guerra-Araiza C (2002) Participation of the 26S proteasome in the regulation of progesterone concentrations in the rat brain. Neuroendocrinology 76:267–271 [DOI] [PubMed] [Google Scholar]
- Cross T (1981) Aquatic actinomycetes: a critical survey of the occurrence, growth and role of actinomycetes in aquatic habitats. J Appl Bacteriol 50:397–423 [DOI] [PubMed] [Google Scholar]
- Dauer W, Przedborski S (2003) Parkinson’s disease: mechanisms and models. Neuron 39:889–909 [DOI] [PubMed] [Google Scholar]
- Dringen R, Gutterer JM, Hirrlinger J (2000) Gultathione metabolism in brain. Metabolic interaction between astrocytes and neurons in the defense against reactive oxygen species. Eur J Biochem 267:4912–4916 [DOI] [PubMed] [Google Scholar]
- Ensign JC, Normand P, Burden JP, Yallop CA (1993) Physiology of some actinomycete genera. Res Microbiol 144:657–660 [DOI] [PubMed] [Google Scholar]
- Fenteany G, Schreiber SL (1998) Latacystin, proteasome function, and cell fate. J Biol Chem 273:8545–8548 [DOI] [PubMed] [Google Scholar]
- Fornai F, Lenzi P, Gesi M, Ferrucci M, Lazzeri G, Busceti CL, Ruffoli R, Soldani P, Ruggieri S, Alessandri MG, Paparelli A (2003) Fine structure and biochemical mechanisms underlying nigrostriatal inclusions and cell death after proteasome inhibition. J Neurosci 23:8955–8966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo F, Morale MC, Spaima-Purrello V, Tirolo C, Testa N, Farinella Z, Avola R, Beaudet A, Marchetti B (2000) Basic fibroblast growth factor (bFGF) acts on both neurons and glia to mediate the neurotrophic effects of astrocytes on LHRH neurons in culture. Synapse 36:233–253 [DOI] [PubMed] [Google Scholar]
- Heikkila RE, Hess A, Duvoisin RC (1984) Dopaminergic neurotoxicity of 1-methyl-4-phenyl-1,2,5,6-tetrahydropyidine in mice. Science 224:1451–1453 [DOI] [PubMed] [Google Scholar]
- Hirst SJ, Ferger B (2008) Systemic proteasomal inhibitor exposure enhances dopamine turnover and decreases dopamine levels but does not affect MPTP-induced striatal dopamine depletion in mice. Synapse 62:85–90 [DOI] [PubMed] [Google Scholar]
- Jana NR, Dikshit P, Goswami A, Nukina N (2004) Inhibition of poteasomal function by curcumin induces apoptosis through mitochondrial pathway. J Biol Chem 279:11680–11685 [DOI] [PubMed] [Google Scholar]
- Jenner P (2003) Oxidative stress in Parkinson’s disease. Ann Neurol 53:S26–S38 [DOI] [PubMed] [Google Scholar]
- Kazi A, Urbizu DA, Kuhn DJ, Acebo AL, Jackson ER, Greenfelder GP, Kumar NB, Dou QP (2003) A natural musaceas plant extract inhibits proteasome activity and induces apotosis selectively in human tumor and transformed, but not normal and non-transformed, cells. Int J Mol Med 12:879–887 [PubMed] [Google Scholar]
- Kisselev AF, Goldberg AL (2001) Proteasome inhibitiors: from research tools to drug candidates. Chem Biol 8:739–758 [DOI] [PubMed] [Google Scholar]
- Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, Yokochi M, Mizuno Y, Shimizu N (1998) Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 392:605–608 [DOI] [PubMed] [Google Scholar]
- Koguchi Y, Kohno J, Nishio M, Takahashi K, Okuda T, Ohnuki T, Komatsubara S (2000) TMC-95A, B, C, and D, novel proteasome inhibitors produced by Apiospora montagnei Sacc. TC 1093. Taxonomy, production, isolation, and biological activities. J Antibiot 53:105–109 [DOI] [PubMed] [Google Scholar]
- Kordower JH, Kanaan NM, Chu Y, Babu RS, Stansell J, Terpstra BT, Sortwell CE, Strece-Collier K, Collier TJ (2006) Failure of proteasome inhibitor administration to provide a model of Parkinson’s disease in rats and monkeys. Ann Neurol 60: 264–268 [DOI] [PubMed] [Google Scholar]
- Kurosaki R, Muramatsu Y, Kato H, Watanabe Y, Imai Y, Itoyama Y, Araki T (2005) Effect of angiotensin-converting enzyme inhibitor perindopril on interneurons in MPTP-treated mice. Eur Neuropsychopharmacol 15:57–67 [DOI] [PubMed] [Google Scholar]
- Langston JW, Ballard P, Terud JW, Irwin I (1983) Chronic parkinsonism in humans due to a product of meperidine-analog synthesis. Science 219:979–980 [DOI] [PubMed] [Google Scholar]
- McNaught KS, Belizaire R, Isacson O, Jenner P, Olanow CW (2003) Altered proteasomal function in sporadic Parkinson’s disease. Exp Neurol 179:38–46 [DOI] [PubMed] [Google Scholar]
- McNaught KS, Perl DP, Brownell AL, Olanow CW (2004) Systemic exposure to proteasome inhibitors causes a progressive model of Parkinson’s disease. Ann Neurol 56:149–162 [DOI] [PubMed] [Google Scholar]
- Miwa H, Kubo T, Suzuki A, Nishi K, Kondo T (2005) Retrograde dopaminergic neuron degeneration following intrastriatal proteasome inhibition. Neurosci Lett 380:93–98 [DOI] [PubMed] [Google Scholar]
- Morale MC, Serra PA, L’episcopo F, Tirolo C, Caniglia S, Testa N, Gennuso F, Giaquinta G, Rocchitta G, Desole MS, Miele E, Marchetti B (2006) Estrogen, neuroinflammation and neuroprotection in Parkinson’s disease: glia dictates resistance versus vulnerability to neurodegeneration. Neuroscience 138:869–878 [DOI] [PubMed] [Google Scholar]
- Muramatsu Y, Kurosaki R, Watanabe H, Michimata M, Matsubara M, Imai Y, Araki T (2003) Expression of S100 protein is related to neuronal damage in MPTP-treated mice. Glia 42:307–313 [DOI] [PubMed] [Google Scholar]
- Nam S, Smith DM, Dou QP (2001) Ester bond-containing tea polyphenols potently inhibit proteasome activity in vitro and in vivo. J Biol Chem 276:13322–13330 [DOI] [PubMed] [Google Scholar]
- Nawashiro H, Brenner M, Fukui S, Shimada K, Hallenbeck JM (2000) High susceptibility to cerebral ischemia in GFAP-null mice. J Cereb Blood Flow Metab 20:1040–1044 [DOI] [PubMed] [Google Scholar]
- Olanov CW, Schapira AH, Agid Y (2003) Neuroprotection of Parkinson’s disease: Prospects and promises. Ann Neurol 53(Suppl 3):S1–S2 [DOI] [PubMed] [Google Scholar]
- Petrucelli L, Dawson TM (2004) Mechanism of neurodegenerative disease: role of the ubiquitin proteasome system. Ann Med 36:315–320 [DOI] [PubMed] [Google Scholar]
- Phillips JB, Williams AJ, Adams J, Elliott PJ, Tortella FC (2000) Proteasome inhibitor PS519 reduces infarction and attenuates leukocyte infiltration in rat model of focal cerebral ischemia. Stroke 31:1686–1693 [DOI] [PubMed] [Google Scholar]
- Priyadarshi A, Khuder SA, Schaub EA, Priyadarshi SS (2001) Environmental risk factors and Parkinson’s disease: a metaanalysis. Environ Res 86:122–127 [DOI] [PubMed] [Google Scholar]
- Schapira AH, Cletter MW, Muddle JR, Workman JM, Cooper JM, King RH (2006) Proteasomal inhibition causes loss of nigral tyrosine hydroxylase neurons. Ann Neurol 60:253–255 [DOI] [PubMed] [Google Scholar]
- Sherman NY, Goldberg AL (2001) Cellular defenses against unfolded proteins: a cell biologist thinks about neurodegenerative diseases. Neuron 29:15–32 [DOI] [PubMed] [Google Scholar]
- Sin N, Kim KB, Elofsson M, Meng L, Auth H, Kwok BH, Crews CM (1999) Total synthesis of the potent proteasome inhibitor epoxomicin: a useful tool for understanding proteasome biology. Bioorg Med Chem Lett 9:2283–2288 [DOI] [PubMed] [Google Scholar]
- van Leyen K, Siddiq A, Ratan RR, Lo EH (2005) Proteasome inhibition protects HT22 neuronal cells from oxidative glutamate toxicity. J Neurochem 92:824–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N, Sawada H, Izumi Y, Kume T, Katsuki H, Shimohama S, Akaike A (2007) Proteasome inhibition induces glutathione synthesis and protects cells from oxidative stress. J Biol Chem 282:4364–4372 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Gao J, Chung KK, Huang H, Dawson VL, Dawson TM (2000) Parkin functions as an E2-dependent ubiquitin-protein ligase and promotes the degradation of the synaptic vesicle-associated protein, CDCrel-1. Proc Natl Acad Sci USA 97:13354–13359 [DOI] [PMC free article] [PubMed] [Google Scholar]