Abstract
Non-healing wounds are long-term complications of diabetes mellitus (DM) that increase mortality risk and amputation-related disability and decrease the quality of life. Nitric oxide (NO·)-based treatments (i.e., use of both systemic and topical NO· donors, NO· precursors, and NO· inducers) have received more attention as complementary approaches in treatments of DM wounds. Here, we aimed to highlight the potential benefits of NO·-based treatments on DM wounds through a literature review of experimental and clinical evidence. Various topical NO·-based treatments have been used. In rodents, topical NO·-based therapy facilitates wound healing, manifested as an increased healing rate and a decreased half-closure time. The wound healing effect of NO·-based treatments is attributed to increasing local blood flow, angiogenesis induction, collagen synthesis and deposition, re-epithelization, anti-inflammatory and anti-oxidative properties, and potent broad-spectrum antibacterial effects. The existing literature lacks human clinical evidence on the safety and efficacy of NO·-based treatments for DM wounds. Translating experimental favors of NO·-based treatments of DM wounds into human clinical practice needs conducting clinical trials with well-predefined effect sizes, i.e., wound reduction area, rate of wound healing, and hospital length of stay.
Keywords: angiogenesis, diabetes mellitus, diabetic foot ulcer, inflammation, L-arginine, nitric oxide, nitrite, non-healing wounds, re-epithelization, wound healing
Introduction
Diabetes mellitus (DM), a leading cause of death and disability worldwide, affected 10.5% (536.6 million) of the adult population (20–79 years) in 2021, projected to reach 12.2% (783.2 million) in 2045.1 DM wounds, especially diabetic foot ulcers (DFUs), are life-threatening complications with a prevalence of 4–10% and an annual population-based incidence rate of 1–4.1%.2 The lifetime risk of developing DFU in DM patients is estimated as high as 25%.2 Around 60–80% of these wounds are capable of healing, 10–15% may remain active, and 5–24% lead to amputation of the limb after the first evaluation within 6–18 months.3,4 40–70% of all non-traumatic amputations of the lower limbs occur in patients with DM.4 DFU precedes ~85% of all amputations and 20% of hospital admissions amongst patients with DM.4,5 DFU is mainly caused by lack of foot sensation and high plantar pressure secondary to peripheral neuropathy, ischemia secondary to peripheral artery disease (PAD), and impaired wound healing in patients with DM.6 The updated guidelines on the prevention of DFU considered loss of protective sensation and PAD as the most potent predictors of DM wounds.7
Comprehensive management of DM wounds necessitates a multifaceted approach, encompassing meticulous wound care, optimized glycemic control, pressure offloading, rigorous infection control, debridement of devitalized tissue, and techniques promoting wound closure. Various complementary therapeutic strategies (e.g., growth factors, synthetic drugs, stem cells, and natural products) targeting critical molecules involved in the healing process have been developed to manage DM wounds.8 Nitric oxide (NO·, a multifunctional gasotransmitter) is a critical component of a normal wound healing process.9 Research evidence supports NO·-based therapeutics for DM-wound healing.10,11,12,13,14,15
Here, we focus on the potential benefits of NO·-based treatments on DM wound healing through a literature review of current evidence. Two primary databases, including PubMed and Scopus, were searched for published papers using the search strategy incorporated search terms for core concepts, including “nitric oxide” and “wound healing” alongside terms specific to diabetes (“diabetes mellitus,” “diabetic foot ulcer,” and “diabetic wound”) and mechanisms relevant to wound healing (“angiogenesis,” “inflammation,” and “re-epithelialization”). Additionally, terms like “L-arginine” and “nitrite” were included to capture studies investigating the NO· synthesis pathway, its precursors, and metabolites. The search strings were designed using Boolean operators (AND, OR, NOT) to refine the results and ensure they aligned with the specific focus of the manuscript. Following the outlined search strategy, studies were selected based on the following criteria to ensure their relevance to the review aim. For inclusion, experimental studies had to investigate the effects of NO· or NO·-releasing agents on wound healing, wound closure, and re-epithelialization in DM wound models (in vivo). Clinical studies were included if they assessed the efficacy and safety of NO·-based therapies for promoting wound healing in patients with DM (type 1 or type 2). The primary outcomes of interest were objective measures of wound healing progress (closure rate and closure time) and relevant biological markers (angiogenesis, re-epithelization, inflammation, and infection). As a narrative review, a pre-defined timeframe was not considered when searching databases. However, studies published within the last years were prioritized to capture recent advancements in the field.
Definition of Wound
Traditionally, wounds were defined as a break in tissue continuity caused by external violence (wound) and a lesion with inflammation, a gradual occurrence, and/or a chronic nature caused by an internal factor (ulcer).16 Regardless of origin or internal/external cause, most skin lesions that impair the structural and functional integrity of the skin in the affected site are now called wounds and are primarily classified into acute and chronic.16 Acute wounds heal quickly17 in 5–10 days18 or within 30 days from injury,18,19 whereas chronic wounds are not repaired after 12 weeks of initial insult.17,19
Considering the diverse etiology/pathophysiology, morbidity and mortality, and required therapeutic approaches, reinstating the traditional nomenclature for different skin lesions seems to be essential.16 The most typical wounds can be classified as DM wounds, including DFUs, surgical wounds, venous ulcers, and pressure ulcers.20
Etiology and Classifications of Diabetic Wounds
DM wounds are complex lesions caused by multiple factors.21,22 Both internal (e.g., neuropathy, ischemia, prior deformities, and edema) and external (e.g., mechanical, thermal, and chemical) factors may be associated with the onset of DM wounds.21,23
As indicated in Figure 1, chronic hyperglycemia in DM culminates in the development of wounds through its effects on the peripheral nervous system, vasculature, immune system, and normal healing process. The interplay between hyperglycemia-induced neuropathy, angiopathy, and immunopathy increases sensitivity to the external forces, enhances the onset of lesions, and hinders the normal healing process, altogether leading to developing non-healing DM wounds, including DFUs.23 Neuropathy, encompassing sensory, motor, and autonomic dysfunction causes loss of protective sensation, bone deformity and increased plantar pressures, callus formation, and dry and fissured skin (a favorable environment for fungal infections) rendering the foot highly vulnerable to ulceration, where even minor trauma can trigger a cascade of events leading to DFUs.24 Furthermore, angiopathy manifests as a spectrum of vascular complications, including both microangiopathy and macroangiopathy, leading to a state of reduced oxygen (O2) and nutrient supply known as ischemia. In addition, microvascular complications associated with infections can cause edema.23 Hyperglycemia-induced immunopathy, which makes the environments prone to infections, also contributes to developing DM wounds.23
Figure 1.
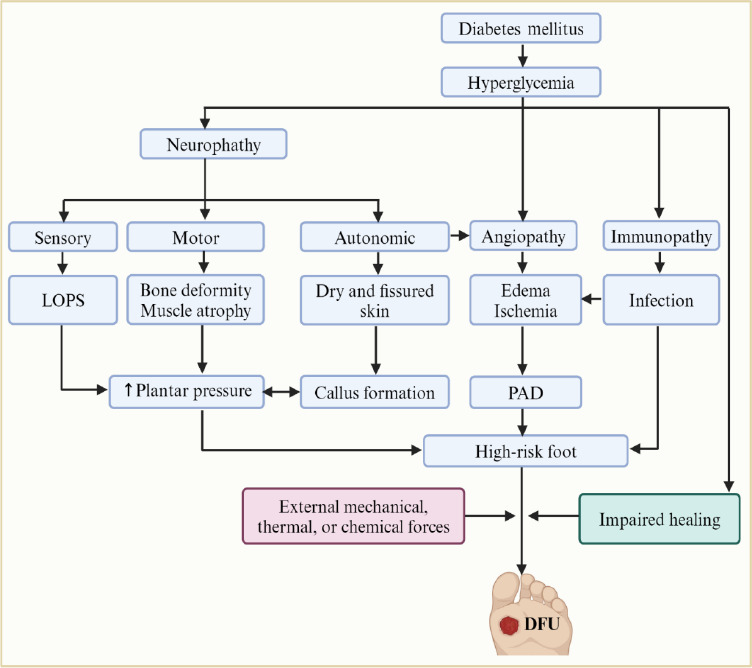
Pathophysiology of diabetic foot ulcer (DFU).
Hyperglycemia, a hallmark of diabetes mellitus (DM), initiates a cascade of pathologic conditions, including neuropathy, angiopathy, and immunopathy, leading to DFU. The hyperglycemia-induced neuropathy encompasses sensory, motor, and autonomic dysfunction. Sensory neuropathy results in loss of protective sensation (LOPS), leading to unnoticed injuries that can progress. Motor dysfunction can contribute to bone deformities and increased plantar pressures, promoting callus formation. Autonomic neuropathy manifests as dry and fissured skin, creating a favorable environment for fungal infections, further increasing vulnerability to ulceration. The hyperglycemia-induced angiopathy manifests as microvascular and macrovascular complications. Microvascular dysfunction disrupts blood flow, leading to ischemia and compromised oxygen and nutrient delivery necessary for healing. Additionally, microvascular complications associated with infections can cause edema. Macrovascular complications, such as peripheral artery disease (PAD), further exacerbate ischemia. On the other hand, hyperglycemia may impair the immune system, potentially increasing susceptibility to infections that further complicate wound healing. Consequently, the interplay between hyperglycemia-induced neuropathy, angiopathy, and immunopathy increases sensitivity to external forces, enhances the onset of lesions, and hinders the normal healing process, leading to the development of non-healing DM wounds, including DFUs. Created with BioRender.com.
The etiology, complexity, and severity of DM wounds exhibit significant heterogeneity. This variation encompasses the extent and depth of tissue destruction, the specific anatomical areas affected, and the presence of co-morbidities such as ischemia, infection, edema, and neuropathy.25,26 DFUs, the most prevalent form of DM wounds, can be classified based on the underlying pathophysiology into three main categories, i.e., neuropathic, ischemic, and neuroischemic.27,28 Neuropathic ulcers develop in patients with peripheral neuropathy, whereas ischemic ulcers are associated with PAD in the absence of neuropathy; neuroischemic ulcers represent a combined etiology occurring in patients with both peripheral neuropathy and PAD.26,27,28 Neuropathy can further contribute to developing various lesions beyond ulcers, including arterial, venous, or mixed ulcers.27 This highlights the complex interplay between neuropathy and vascular insufficiency in the pathogenesis of DFUs. While established classifications for DFUs typically focus on underlying pathologies like neuropathy and ischemia, external forces, e.g., accidents, surgical procedures, burns, radiation therapy, thermal injuries, and mechanical trauma, can develop another class of wounds, i.e., “traumatic DM wounds.”23,26 Notably, trauma, as the primary cause of DM wounds,23,29 can occur in the context of any DFU classification; so, considering “traumatic DM wounds” alongside traditional classifications for a more comprehensive understanding of DFU etiology seems to be crucial.23 The primary external causes of “traumatic DM wounds” are likely to be puncture wounds, ill-fitting footwear, and self-care practices.23
Traumatic DM wounds exhibit distinct characteristics and prognoses compared to other classifications.23,28 An ischemic DFU displays features of PAD, i.e., an ankle-brachial index (ABI) < 0.9.28 Ischemic DM wounds present with a pale, yellow, and cool appearance, often accompanied by weak or absent pulses, whereas neuropathic DM wounds are preceded by callus formations and contain fibrotic and hyperkeratotic tissues.23,28 Plantar callus, tinea pedis, onychomycosis, and foot deformity are prevalent in neuropathic and neuroischemic DM wounds.28 Arterial DM wounds manifest with intense pain, a punched-out appearance, a shiny surface, reduced hair growth, pallor upon leg elevation, weak or absent pulses, and delayed capillary refill.23 The average healing time of different types of DM wounds, including neuropathic, neuroischemic, and ischemic ulcers, is reported to be 70, 113, and 233 days, respectively.28 Although traumatic DM wounds often exhibit a poorer prognosis, studies suggest potentially faster healing for those with a normal ABI ≥ 0.8 and in the absence of co-morbidities such as neuropathy, PAD, or infection.26 Several established classification systems have been developed and validated, i.e., Meggitt-Wagner, University of Texas, IDSA (i.e., Infectious Disease Society of America), SINBAD (i.e., acronym for site, ischemia, neuropathy, bacterial infection, area, and depth), WIfI (i.e., acronym for wound, ischemia and foot infection), and PEDIS (i.e., acronym for perfusion, extent, depth, ischemia, sensation).27,30,31 The PEDIS system, developed by the IWGDF group, is a research-based classification system categorizing DFUs based on the five key independent factors.32
Wound Healing in Normal Condition and Diabetes Mellitus
Wound healing is one of the most complex processes in the human body, involving the spatial and temporal synchronization of various cell types with distinct roles in the four phases.33,34,35 The normal wound healing process involves the hemostasis phase (i.e., vasoconstriction, formation of a platelet plug, coagulation, and reinforcement of the platelet plug), the inflammation phase (i.e., immune cell infiltration, cytokine secretion), the proliferation phase [i.e., extracellular matrix (ECM) generation, angiogenesis, and epithelialization], and the remodeling phase (i.e., collagen crosslinking and reorganization).34
Wound formation triggers an immediate response to stop bleeding, known as hemostasis. Damaged arteries constrict rapidly, within minutes, to restrict blood flow and achieve initial hemostasis.17 However, this decrease in blood flow can lead to tissue hypoxia and acidosis within a few minutes,17 triggering the production of vasodilators like NO· and adenosine.18 Simultaneously, clot formation occurs at the injury site as a primary mechanism to prevent further bleeding.17,19 Trapped platelets within the clot are activated, resulting in the degranulation of α-granules and dense granules and releasing cytokines and growth factors, including platelet-derived growth factor, transforming growth factor-beta (TGF-β), epidermal growth factor, and insulin-like growth factors.17,18
The inflammatory phase of wound healing (days 1–5 after wounding) is a critical defense mechanism, preventing infection and initiating tissue repair.17,18 Neutrophils infiltrate the wound by chemotaxis immediately upon wounding and migrate in sustained levels for the first 48 hours, with a peak at 24 hours; neutrophils release reactive oxygen species (ROS) to destroy bacteria and dead host tissue.17 Monocytes are recruited within 48–96 hours post-injury and transform into tissue-activated macrophages at the wound site.36 Macrophages, peaking at 48–72 hours, regulate inflammatory responses, stimulate new blood vessel formation, and promote granulation tissue (GT) growth.15,16 The pro-inflammatory M1-like macrophages, induced by necrotic cells and/or infection, produce pro-inflammatory cytokines, proteases, and ROS to support host defense.37 Finally, lymphocytes arrive later and contribute to the ECM and collagen remodeling, which are essential for successful wound healing. A prolonged inflammatory phase, however, can hinder the healing process.17,18 Later, at 72–120 hours post-injury, lymphocytes initiate wound repair by generating components of the ECM and promoting collagen remodeling, a vital process for restoring tissue integrity.17
The proliferation phase (also called the growth phase) includes angiogenesis, granulation, collagen deposition, re-epithelialization, and wound retraction.17 Following the inflammatory phase, wound healing progresses with angiogenesis, i.e., involved endothelial cell proliferation, migration, and branching, ultimately forming new blood vessels.34 Alongside endothelial cell proliferation, pericytes within the basal lamina become activated, providing scaffolding and structural support for the newly formed blood vessels.38 Additionally, circulating progenitor cells from the bone marrow are also recruited to contribute to new blood vessel formation during wound healing.39 Dominated by activated fibroblasts, GT plays a vital role in wound healing. Fibroblasts provide structural support and contribute to wound contraction by synthesizing new ECM. Additionally, GT serves as a temporary platform for other essential components, including newly formed blood vessels, inflammatory cells, and further ECM deposition.34 Ultimately, during wound remodeling, normal connective tissue gradually replaces this specialized tissue.33,34
Building upon the formation of GT, wound contraction is another crucial aspect of the proliferation phase. This process minimizes the surface area requiring re-epithelialization; collagen fibers realign perpendicularly to the wound edges for increased strength.34 This change in stiffness triggers the transformation of specific fibroblast subpopulations into contractile myofibroblasts, a transient cell type that synthesizes collagen types I and III and exhibits characteristics of contractile smooth muscle.40,41 M2-like macrophages, exhibiting pro-fibrotic capacities, are key players that contribute to both GT formation and wound contraction; they actively produce growth factors, specifically TGF-β1 and platelet-derived growth factor, and influence the persistence of ECM components within the wound environment, lasting up to 10 days after the initial injury.37
Following successful re-epithelialization, the wound proceeds into the remodeling stage with the emergence of a new fibrinolytic profile of resident macrophages within the wound.35 These reprogrammed macrophages, called M2c or Mreg-like macrophages, release proteases and phagocytize unnecessary cells and excess ECM no longer needed for wound closure.37 This activity ensures proper remodeling and prevents the accumulation of ECM and cells, which can lead to scar formation if dysregulated.37
DM wounds exhibit a dysregulated healing cascade due to a confluence of pathological factors. Restricted microvascular perfusion limits O2 and nutrient delivery to the wound bed, compromising tissue regeneration.42 Additionally, deficiencies in key growth factors like insulin-like growth factor-1 and TGF-β impair cellular proliferation and differentiation, essential for timely vasculoneogenesis and wound closure.43 Furthermore, matrix metalloproteinase dysregulation can occur, potentially driven by oxidative stress and advanced glycation end products, leading to excessive degradation of the ECM, which provides structural support and facilitates cell migration during wound healing.44,45,46 Finally, delayed recruitment of inflammatory cells and increased pro- to anti-inflammatory cytokine ratio,47 and excessive ROS production,48,49,50 results in further inhibition of cell proliferation, vasculoneogenesis, M1-to-M2 macrophage polarization, and inflammation-to-proliferation transition, i.e., a critical step during wound healing.51
Nitric Oxide and Wound Healing in Normal State and Diabetes Mellitus
Historically, the involvement of NO· in normal wound healing was first documented indirectly using its precursor L-arginine (L-Arg) in 1978,52 and then directly in 1996–2000 through a series of investigations, including time-course assessment of NO· synthase (NOS) expression and synthesis of NO· metabolites [i.e., nitrate (NO3), nitrite (NO2)] and citrulline during wound healing,53 and pharmacologic-54 and genetic-manipulations55 of NOS enzymes. As reviewed elsewhere,13,19,56 normal wound healing requires NO· (1) in the inflammation phase (for cytokine modulation), (2) in the proliferative phase (for re-epithelialization, angiogenesis, and neo-vascularization), and (3) in the remodeling phase (for collagen deposition).
Building on the established role of NO in wound healing, research conducted in 1997 provided the first evidence linking impaired NO synthesis to the pathophysiology of DM wounds.57 This impairment was in line with lower concentrations (~20–68%) of NO metabolites observed in DM wounds compared to non-DM ones.56 Several contributing factors may explain this deficiency, including downregulated NOS enzymes, including endothelial NOS and inducible NOS (iNOS),58,59,60 defective migration of NO·-producing cells (e.g., macrophages, keratinocytes, and fibroblasts) into the wound, and diminished capacity of these cells to produce NO·.57 Insufficient NO· in DM wounds disrupts natural healing, leading to an impaired inflammatory response,59,61 decreased collagen synthesis,57,61,62,63 inadequate re-epithelization and angiogenesis,60,63 and diminished wound breaking strength (i.e., a good index of functional recovery of healing wounds, measured as the minimum force required to break a wound).60,64 An impaired inflammatory response refers to either a deficient initial inflammatory response (manifests as insufficient initial cytokine production and recruitment of immune cells, leading to delayed debris clearance and impaired wound healing) or excessive/prolonged inflammation (i.e., uncontrolled production of pro-inflammatory cytokines and infiltration of immune cells, preventing the deposition of matrix components, remodeling, and wound closure).65,66
Considering the essential roles of NO· in wound healing, NO·-based treatments have emerged as new approaches for wound healing.10,11,12,13 Several NO·-based therapeutic strategies have been proposed, ranging from systemic administration of NO· donors [e.g., L-Arg, molsidomine (SIN-10), dinitrosyl iron complexes (DNIC)] to topical applications of engineered NO·-based biomaterials.13 Topical NO·-based therapies broadly include gaseous NO·, acidified NO2 creams, NO·-probiotic patches, nanoparticle platforms, and NO·-releasing hydrogels.13 Commonly used NO· donors/NO· releasing substances for topical treatments of DM wounds are inorganic NO2, L-Arg, nitroglycerine (also known as trinitroglycerin, TNG), metal-NO· complexes, N-diazeniumdiolates, and S-nitrosothiols (e.g., S-nitrosoglutathione and S-nitroso-N-acetyl-DL-penicillamine).67,68,69,70 TNG, a common clinically used NO·-releasing drug for management of hypertension and angina pain, produces NO· upon activation by mitochondrial aldehyde dehydrogenase, cytochrome p450 enzymes, and xanthine oxidoreductase.71,72 N-diazeniumdiolates are the most widely studied NO· donors hydrolyzed under physiologic conditions or upon thermal, photochemical, or enzymatic stimuli and release 2-mole equivalents of NO·.73 S-nitrosothiols are naturally occurring NO·-releasing substances (e.g., S-nitrosohemoglobin, S-nitrosoglutathione, S-nitrosocysteine, and S-nitrosoalbumin) biologically produced through thiol nitrosation reaction.74
Gaseous NO· (200–500 ppm) is a straightforward way of NO· delivery to wounds; however, its application has some limitations (e.g., requiring industrial NO· gas cylinders and hospital settings) and concerns (e.g., NO·’s high-reactivity, especially with O2 and producing harmful byproducts like nitrogen dioxide).19,75 NO·-generating acidified-NO2 cream is another simple mode of NO· delivery to the wound tissue. Hydrogels, i.e., highly-hydrated crosslinked polymers (made up of collagen, alginate, hyaluronic acid, gelatin, cellulose, and chitosan), are popular materials for wound dressings because of their flexibility, adhesion, stability, and mimicking native ECM,76 and a platform for NO· storage and delivery that provide a controlled NO· release for sustained exposure to wounds.12
Effects of Nitric Oxide-Based Treatments in Animal Models of Diabetic Wound
An overview of animal models of diabetic wound
Ethical and practical concerns limit the direct investigation of therapeutic interventions, like NO·-based treatments, for DM wounds in humans. Thus, various models in different animal species (e.g., mouse, rat, rabbit, dog, guinea pig, pig, and zebrafish) have been developed to mimic the healing process of DM wounds.77,78 However, these models often only capture a single aspect of the multifaceted nature of human DM wounds. The ongoing challenge is developing a model that resembles the human DM wound environment with acceptable reproducibility, quantifiable interpretation, therapeutic significance, and effective translation into clinical applications.77,78
Rodents, particularly mice and rats, are commonly used animals for studying DM wounds because of their genetic and biological similarities to humans, cost-effectiveness, and ease of handling.77,78,79 Mice have been preferred over rats because of the much larger genetic toolbox available for mice.80 However, having higher body weight, demonstrating lower stress response to human interaction, and increasing availability of genetic tools in rats paved the way for the rising use of rats in wound research.80 The induction of DM wounds in animals includes a two-stage process: (1) Induction of DM, including type 1 (T1DM), i.e., spontaneously developed autoimmune models or chemically-disrupted pancreatic β-cells models [using streptozotocin (STZ), or alloxan81] and type 2 (T2DM), i.e., genetically-manipulated models (e.g., db/db mice, KK-Ay mice, ob/ob mice, Goto-Kakizaki rats) or dietary models (e.g., monosodium glutamate, high-fat diet82,83); (2) Induction of wound.77,78 A network meta-analysis of 267 studies indicates that among all models, only db/db, ob/ob, STZ, and STZ + HFT models display significantly delayed wound healing.84 Upon establishing DM, the wound will be created by cutting, radiation, burning, or other methods, resulting in heterogenic lesions in size and depth. The commonly used wound models are77,85: (1) excision; (2) incision; (3) burn; (4) ischemic; (5) dead space; (6) tape stripping; (7) pressure ulcer; (8) para-biosis (by the surgical joining of two animals at their flank skin, used for study of circulating factors that play a vital role in a different phase of tissue repair and regeneration); (9) denervated (i.e., performed by hemisection of the spinal cord, followed by a 15 mm diameter skin wound, resembling neuropathic DM wound); (10) skinfold chamber (performed using two complementary plates sandwiching a laterally positioned fold of dorsal skin of animal, i.e., a good model for microvascular research); (11) xenograft (performed using putting a xenograft from human skin on the full-thickness wound created earlier in animal, i.e., used for resembles wound healing by re-epithelization); (12) infected wound model.
In experimental studies, the excision wound model is the most commonly used and clinically relevant model for DM ulcers.77,78,85 The model involves a surgical excision (a circular full-thickness wound ~2 cm²) on the dorsal thoracic region of rodents, which is appropriate for the pharmacological evaluation of new entities and formulations.77,78 The second most common model is the incision wound model (controlled cut), i.e., induced using two para-vertebral long cuts ~4-6cm length made through the skin and cutaneous muscles that are separated ~1.5cm from the midline on each side of the depilated back of the rodents. This model is appropriate for investigating the quality of healed tissue and scar formation.77,78 The pressure wound models, which are more superficial, resulting in partial thickness loss of the dermis, are less likely appropriate as a DM wound model.84
Systemic nitric oxide-based treatments
Table 1 summarizes the effects of systemic NO·-based treatments, including L-Arg and NO· donors, i.e., SIN-10, DNICs on DM wounds in animal studies.62,86,87,88,89
Table 1.
Effects of systemic NO·-based treatment on wound healing in animals with DM
| Study | Year | Species | DM model | Body weight (g) | Wound | Donor | Dose | Route | Endpoint | Findings |
|---|---|---|---|---|---|---|---|---|---|---|
| Witte et al.86 | 2002 | Male rats | STZ | 225–250 | Incision (7 cm) | L-Arg | 2× 1 g/kg/d | G | Wound healing | ↑ Wound fluid NO· metabolite concentrations ↑ Wound breaking strength ↑ Hydroxyproline concentration ↔ Wound fluid arginase activity ↔ Wound fluid ornithine concentration |
| Shi et al.62 | 2003 | Male rats | STZ | 225–266 | Incision (7 cm) | L-Arg | 1 g/kg/d | IP | Wound healing | ↑ Wound fluid NO· concentration ↑ Wound fluid hydroxyproline ↑ Wound breaking strength ↑ Expression of procollagen I, and III |
| Jerônimo et al.87 | 2016 | Male mice | STZ | 20–25 | Incision (3 cm) | L-Arg | 2 g/kg/d | G | Wound healing | ↑ Collagen synthesis and deposition ↑ Vasculoneogenesis ↑ TGF-β expression ↓ Expression of IL-8 ↓ Polymorphonuclear cell infiltration ↓ Fibrosis |
| Witte et al.86 | 2002 | Male rats | STZ | 225–250 | Incision (7 cm) | SIN-10 | 2× 1 mg/kg/d | G | Wound healing | ↑ Wound breaking strength ↑ Hydroxyproline concentration ↑ MMP-2 ↑ Wound fluid arginase activity |
| Schäffer et al.88 | 2007 | Male rats | BBDP | 220–250 | Incision (7 cm) | SIN-10 | 4 mg/kg/d | G | Wound healing | Restoring NO· metabolite concentrations, wound-breaking strength, and collagen deposition in normoglycemic rats ↑ NO· metabolite concentrations, wound-breaking strength, and collagen deposition in hyperglycemic rats |
| Chen et al.89 | 2019 | Female mice | db/db | NR | Ischemic- excision (6 mm) | DNIC-1 | 0.18 mg/kg every other day | IV | Angiogenesis, wound healing | ↑ Healing rate ↓ Wound closure time ↑ Expression of eNOS, MMP-11, CD31, CD34, and VEGF ↑ Angiogenesis ↓ Inflammation |
Control groups received water or normal saline. BBDP: Biobreeding (i.e., an inbred laboratory rat strain that spontaneously develops autoimmune type 1 diabetes mellitus); CD31: endothelial marker; CD34: endothelial marker; DM: diabetes mellitus; DNIC-1: dinitrosyl iron complexe-1; eNOS: endothelial nitric oxide synthase; G: gavage; GSNO: S-nitrosoglutathione; HIF-1: hypoxia-inducible factor-1; IL: interleukin; IP: intraperitoneal; IV: intravenous; L-Arg: L-arginine; MMP: matrix metalloproteinase; NO·: nitric oxide; NR: not reported; SIN-10: molsidomine; STZ: streptozotocin; TGF-β: transforming growth factor-β; VEGF: vascular endothelial growth factor.
L-Arg supplementation restores the impaired NO· synthesis in the DM wound environment and promotes healing factors.62,86,88 The healing effect of systemic supplying L-Arg was associated with increased collagen synthesis and deposition, induction of vasculoneogenesis, and decreased inflammation.62,87 L-Arg supplementation restored NO· metabolites but not ornithine concentration in wound fluid toward normal, an observation indicates that systemic supplying L-Arg is preferentially utilized by NOS enzyme(s), not arginase86; this speculation was supported by evidence obtained in iNOS-knockout mice without DM that failed to improve wound healing in response to L-Arg supplementation.55 L-Arg is catalyzed by M1 macrophages-iNOS to produce NO· at the early phase of wound healing (which promotes vascular repair), and then by M2 macrophages-arginase to produce ornithine at the late phase of wound healing (which promotes tissue repair).68
Supplementation with other NO· donors, e.g., SIN-10 and DNIC-1, also promotes wound healing in animal DM models.61,88,89 Administration of SIN-10 (1 mg/kg twice daily) in STZ-induced DM male rats, compared to saline-treated ones, increased wound-breaking strength, hydroxyproline concentration, matrix metalloproteinase-2 and arginase activity in DM wounds.86 Systemic supplementation with SIN-10 (4 mg/kg/d) improved wound repair in rats with T1DM. However, the healing rate was better in the normoglycemic (insulin-treated) than in hyperglycemic rats. This finding highlights the importance of concurrent glycemic control and systemic NO· boosting to facilitate wound healing among patients with DM.88
Intravenous injection of DNIC-1 (0.18 mg/kg) increased expression of endothelial NOS, matrix metalloproteinase-11, CD31, CD34, vascular endothelial growth factor (VEGF), restored the impaired angiogenesis, and accelerated the recovery rate of wound closure in mice with T2DM.89
The safety profile of systemic NO·-based therapies for DM wound healing is yet to be fully characterized due to limited in vivo data on potential toxicity. However, it is worth noting that L-Arg supplementation has been well-tolerated, as indicated by normal serum levels of urea, creatinine, aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase.87
To sum up, studies provide preliminary evidence for the efficacy of systemic L-Arg and other NO·-based therapies in promoting DM wound healing in animal models. However, generalizability to humans remains to be established due to potential species-specific physiological differences. Whereas short-term safety is indicated by normal serum markers of liver function with L-Arg supplementation, a comprehensive evaluation of long-term safety profiles for all NO· donors is essential. Furthermore, the optimal dosing and specific mechanisms of action for each NO· donor require further elucidation.
Topical nitric oxide-based treatments
Acidified NO2 (3.0% sodium nitrite (NaNO2) + 4.5% citric acid containing cream) was documented to accelerate wound healing [i.e., indicated as decreased half closure time (CT50%) from 8 to 5 days] in rats with T2DM (low dose of STZ + high-fat diet).59 Acidified-NO2 increased the numerical density of basal cells (1070 vs. 936.6 mm3) and epidermal thickness (58.5 vs. 44.3 μm).90 Daily topical administration of a similar formulation with various doses of sodium nitrate (NaNO3) and citric acid on wound healing in male mice with T2DM indicated a wound closure rate of 98.1%, 100%, and 97.4% (in 0.5%/0.75%, 3.0%/4.5% and 9.0%/13.5%, NaNO3/citric acid, respectively), at day 12 after wounding.91 The healing rate, i.e., indicated by both higher wound closure rate (91.8 vs. 60% at day 18) and lower CT50% (12.9 vs. 17.9 days), was significantly greater in the NO·-treated (3.0%/4.5% NaNO3/citric acid) compared to the untreated group when administered on day 2 after wounding (i.e., after the coagulation phase).91 This dose of acidified-NO2 was effective when applied before day 4 after wounding (days 1, 2, and 4), parallel to the migratory/proliferative phase of healing.91
Although topical TNG was proposed as a promising potent healing agent for clinical management of DFUs,92 this idea remains to be investigated. Only one experimental study in rats assessed the healing effect of TNG ointment and gel with and without aloe vera on DM wound reported a significantly lower wound area at day 8 (12.2 vs. 18.7 mm2), higher wound closure (54.9% vs. 31.2%), and higher hydroxyproline content and NO· concentration in the wound tissue (1.45 vs. 0.8 ng/mg protein) in the TNG ointment group compared to the non-treated group.93
Topical application of a newly-developed dual acting NO·-based agent (TOP-N53, NO· donor S-nitroso-N-acetyl-DL-penicillamine + phosphodiesterase 5 inhibitor sildenafil) on full-thickness DM wound in db/db mice, induced angiogenesis (indicated by higher expression of vascular marker CD31) enhanced keratinocyte migration and proliferation (indicated by the length and the thickness of the wound epidermis, respectively) and increased area of GT.94 The favorable effect of TOP-N53 on DM wound healing was confirmed by semiquantitative wound scoring, demonstrating a significant increase in re-epithelialization and a mild acceleration of GT maturation.94
Table 2 summarizes the investigations that used newly developed NO·-releasing hydrogels for promoting wound healing in DM.67,68,69,70,95,96,97,98,99,100,101,102,103 The effects of NO·-releasing hydrogels on DM wound healing are mainly documented in mice. Treatment of DM wounds with NO·-releasing hydrogels in animal models resulted in an increased expression of angiogenesis factors (e.g., hypoxia-inducible factor-1, VEGF, TGF-β), promotion of angiogenesis and neovascularization, inhibition of inflammation by concomitant upregulating of pro-inflammatory cytokines and downregulating anti-inflammatory cytokines, polarization of M1 to M2 macrophages, promoted fibroblast migration and differentiation, and collagen synthesis and deposition.67,68,69,70,96,98,100 NO·-releasing hydrogels also suppressed bacterial growth (i.e., Staphylococcus aureus and Escherichia coli) and facilitated ROS clearance.67,97,102 These effects resulted in accelerated wound healing.67,68,69,70,96,98
Table 2.
Animal studies investigated effect of NO·-releasing hydrogels on DM wounds
| Study | Year | Species | DM model | BW (g) | Wound model | NO- donor | Concentration in hydrogel | Endpoints | Findings | |
|---|---|---|---|---|---|---|---|---|---|---|
| Liu et al.95 | 2024 | Male mice | STZ | NR | Excision (8 mm)- MRSA- infected | BNN6 | 0.2 mg/mL | Antibacterial, antioxidant, and angiogenic properties | ↑ Re-epithelialization ↑ Collagen deposition ↓ Expression of IL-6 ↑ Expression of IL-10 |
↑ VEGF and CD31 ↓ ROS generation ↑ Wound healing rate Eradication of S.A (97.3%) |
| Huang et al.95 | 2024 | Male mice | STZ | 20-22 | Excision (8 mm) M RSA- infected | GSNO | 50 μmol/mL | Antibacterial, angiogenic, anti-inflammatory, and wound-healing properties | ↑ Ml to M2 polarization ↓ Inflammatory cells ↓ Expression of TNF-α ↑ Fibroblasts ↑ Collagen concentration and deposition |
↑ Expression of VEGF ↑ Re-epithelialization and dermal regeneration ↓ Remaining scar Eradication of S.A and E.C (100%) |
| Yang et al.68 | 2024 | Male mice | STZ+ HFD | 20±5 | Excision (6 mm) | L-Arg | 200 μg/mL | Antioxidant, angiogenic, anti-inf lammatory, and wound-healing properties | ↑ NO· production ↓ ROS generation ↑ O2 in wound tissues ↓ Expression of IL-lβ and IL-6 |
↑ Polarization of M1 to M2 macrophages ↑ Expression of HIF-1α, VEGF, and PCNA |
| Sivaraj et al.59 | 2023 | Female mice | db/db | NR | Excision (6 mm) | NaNO2 | 2 g/100 mL, 10 nM NO•/h | Wound healing | ↑ Tissue density ↑ Collagen networks ↑ Expression of fibronectin, and TGF-β1 |
↑ Wound healing rate ↓ Wound closure time |
| He et al.97 | 2023 | Male mice | STZ | NR | Excision | BNN6 | 2 mg/mL | Antioxidant, angiogenic, anti-inf lammatory, and wound-healing properties | ↑ Wound contraction ↑ Collagen content ↑ Thickness of newly formed epidermis ↑ Angiogenesis |
↓ ROS generation ↑ Expression of VEGF ↓ Expression of TNF-α, Eradication of S.A and E.C (100%) |
| Zhang et al.98 | 2023 | Male mice | STZ | 20–25 | Excision (10 mm) | L-Arg | 1 g | Epithelization, angiogenic, anti-inflammatory, and wound-healing properties | ↑ Epithelial formation ↑ Collagen deposition ↓ Inflammation |
↑ Expression of HIF-1α ↑ Angiogenesis |
| He et al.99 | 2024 | Male rats | STZ | 200–250 | Excision (15 mm) S.A-infected | BNN6 | 2 mg/mL | Antibacterial, angiogenic, anti-inflammatory, and wound healing properties | ↓ ROS generation ↓ Expression of IL-6, TNF-α, CD86 ↑ Expression of IL-10 |
↑ Expression of CD31 and CD206 ↑ Wound healing rate |
| Zhao et al.100 | 2022 | Male rats | STZ | 180–220 | Ischemic-excision (15 mm) | NO· microbubble | 6.0 × 108 NO• microbubbles | Angiogenic, anti-inflammatory, and wound healing properties | ↓ Expression of IL-1β, IL-6, and TNF-α ↑ Expression of IL-10, IL-22, and IL-13 |
↑ Angiogenesis ↑ Blood perfusion at the wounds ↑ Wound healing rate |
| Liu et al.101 | 2022 | Male mice | db/db | NR | Excision (10 mm) | NONOate | 100 μmο1 | Angiogenic and wound healing properties | ↑ Granulation tissue ratio ↑ Vascularization |
↑Expression of CD31 and α-SMA |
| Yang et al.102 | 2021 | Mice† | STZ | NR | Excision S.A- infected | SNP | 200 μg/mL | Antibacterial, angiogenic, anti-inflammatory, and wound healing properties | ↑ HIF-1α protein stability ↑ VEGF secretion from endothelial cells ↑ Wound closure rate |
↓ S.A and E.C (86% and 94%) ↓ Inflammation score |
| Zheng et al.67 | 2024 | Male rats | STZ | 180–220 | Excision (10 mm) | L-Arg + GSNO | 0.5 mg + 3.66 mg/mL | Antibacterial, angiogenic, anti-inf lam matory, antioxidant, and wound healing properties | ↑ Expression of HIF-1α and VEGF ↑ Cell migration rate at the 36-h ↑ Bloodvessel regeneration |
↑ Collagen deposition ↓ S.A and E.C ↑ ROS clearance ↑ Wound healing rate |
| Xie et al.103 | 2024 | Male rats | STZ | NR | Excision (10 mm) S.A-infected | GSNO | 15 g, 25 μmol NO• over 6 h | Antibacterial, angiogenic, and wound healing properties | ↑ Angiogenesis ↑ Wound healing rate ↑ Epidermal thickness |
↓ Scar widths Eradication of S.A and E.C |
| Ahmed et al.70 | 2021 | Rabbits | ALX | 1000±200 | Excision (20 mm) | SNAP | 5% | Angiogenic and wound healing properties | ↑ Wound healing rate ↑Bloodvessels density ↑ Expression of VEGF, PCNA, SDF-1α, Bcl-2, and TGF-β1 |
↑ Hydroxyproline and collagen deposition ↑ Epithelial layer formation ↓ Healing time |
Phosphate-buffered saline-releasing hydrogel was used as control group. † Not reported sex. ALX: Alloxan; Bcl-2: B-cell lymphoma 2; BMSCs: bone marrow stem cells; BNN6: N,N'-di-sec-butyl-N,N'-dinitroso-1,4-phenylenediamine; BW: body weight; CMCS: carboxymethyl ch¡tosan; DM: diabetes mellitus; E.C: Escherichia coli; GSNO: S-nitrosoglutathione; HFD: high-fat diet; HIF-1: hypoxia-inducible factor-1; IL: interleukin; L-Arg: L-arginine; MRSA: methiciIIin-resistant Staphylococcus aureus; NaNO2: sodium nitrite; NO·: nitric oxide; NONOate: diazeniumdiolate; NR: not reported; PCNA: proliferating cell nuclear antigen; ROS: reactive oxygen species; S.A: Staphylococcus aureus; SDF-1α: stromal cell-derived factor-1α; SNAP: S-nitroso-N-acetyl-penicillamine; SNP: sodium nitroprusside; STZ: streptozotocin; TGF-β: transforming growth factor-β; TNF-α: tumor necrosis factor-α; VEGF: vascular endothelial growth factor; α-SMA: α-smooth muscle actin.
Current evidence suggests a gap in knowledge regarding the in vivo safety of NO·-based therapies for DM wounds. Experimental studies have evaluated the biocompatibility of hydrogel-containing NO· donors using cell viability (cytocompatibility) and hemolytic activity (hemocompatibility) assays. These studies demonstrated good biocompatibility and no apparent cytotoxicity on various cell lines, including L929 fibroblasts, HaCaT keratinocytes, and human umbilical vein ECs.73,91,92,93,95,96,97 In addition, no apparent cytotoxicity was shown in wound tissue at the investigated doses96 (refer to Table 2 for details).
Future studies should prioritize translating the promising in vitro biocompatibility of NO·-releasing hydrogels to a clinically relevant in vivo setting. Dose-response studies are essential to establish the optimal therapeutic window for NO· delivery, balancing efficacy and potential cytotoxicity. Long-term in vivo studies are warranted to assess the sustained safety and efficacy of NO·-based treatments, as well as potential delayed adverse effects. Furthermore, exploring synergistic wound healing strategies by combining NO·-based treatments with other established or emerging therapeutic modalities (e.g., growth factors, stem cells) holds promise for accelerating wound closure and improving overall healing outcomes in patients with DM. Through systematic investigation of these key areas, a robust experimental foundation can be established to inform the development of NO·-based treatments for clinical translation in DM wound healing.
Clinical Evidence of Nitric Oxide-Based Treatments on Diabetic Wound
Human clinical trials did not support experimentally investigated beneficial effects of systemic L-Arg supplementation on DM wounds. For instance, a multicenter design randomized double-blind clinical trial in patients with T1DM and T2DM (27.9% were women, mean duration of DM was 13 years, mean glycated hemoglobin was 8 ± 1.5%) reported no additional healing properties (wound closure and time to wound healing) for a 16-week supplementation with L-Arg, glutamine, and β-hydroxy-β-methylbutyrate as an adjunct to standard therapy.104 Only in patients with low albumin (≤ 40 g/L) or decreased limb perfusion (ABI < 1.0) was there evidence of a higher healing rate at week 16 (odds ratio = 1.70, 95% confidence interval = 1.04–2.79, and odds ratio = 1.66, 95% confidence interval = 1.15–2.38, respectively).104
Most recently, a portable on-demand NO·-generating device (NO· jet healing device, NJHD, producing NO· through a simple reaction of NaNO2 with citric acid) has been developed for DM wound healing that overcomes the limitations and concerns of using gaseous NO·.105 Clinical application of NJHD was assessed in patients with DM (22–85 years, glycated hemoglobin < 12%, at least one full-thickness wound below the ankle with 1–16 cm2 area, and ABI > 0.7). Use of NJHD (4 sessions per week, each session 12 minutes with 500 ppm of NO·) significantly reduced wound closure time (19 vs. 25 days, compared to standard therapy).105 Upon 12 minutes of use of NJHD, the relative index of transcutaneous O2 pressure significantly increased, indicating an enhanced blood supply around the wounds.105 A multicenter randomized controlled trial, including 135 participants with T1DM and T2DM (13% were women, fasting serum glucose 178 ± 102 mg/dL) with a chronic (at least 6 weeks), full-thickness DFU, assessed the safety and efficacy of EDX110 (novel dressing system comprising two layers generating NO· in situ with adequate moisturizing that facilitates debridement) in the treatment of DFU compared against an optimal standard of care.106 Both groups received standard care (debridement, offloading, and antimicrobial treatment); however, the NO-treated group also received EDX110 dressing. The result indicated improved healing, with an 88.6% reduction in ulcer area at 12 weeks compared with 46.9% in those receiving standard dressings.106 Wound size reduction of the EDX110-treated patients at 4 weeks was similar to that achieved by the standard dressing at 12 weeks.106
Mechanisms Underlying Wound Healing Effects of Nitric Oxide-Based Treatments
The effectiveness of NO·-based treatments on DM wound healing has been attributed to the anti-microbial,67,95,96,97,102,103 anti-inflammatory,68,95,96,98,99,100 and anti-oxidative properties67,68,95,97,99 of NO· and its positive impacts on promoting healing factors (e.g., growth factors and angiogenesis factors67,68,69,70,95,96,97,99,100,102) facilitating angiogenesis and re-epithelization in the wound.
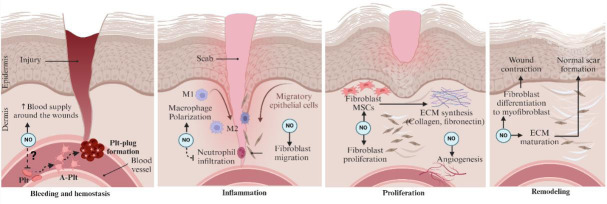
As illustrated in Figure 2, NO·-based therapies are involved in various phases of the wound healing process. The role of NO· in the early wound healing phase is unclear. On the first day of injury (known as hemostasis or coagulation phase), activation of platelets, coagulation, and clot formation are dominant. Theoretically, NO· may be essential for the vasodilation of intact vessels to support enough blood supply around the wound tissue and attenuation of platelet aggregation in the hemostasis phase. However, considering the antiplatelet and anti-thrombotic properties of NO·/NO· donors,107 NO· may prevent platelet activation, attachment, and aggregation, counteracting the hemostasis phase. For instance, treatment of incisional wounds with acidified-NO2 impaired wound healing when applied immediately after wounding (a decreased wound closure rate of 58% vs. 80% and longer CT50% of 9.2 vs. 8.4 days in the treated group compared to control).91 A db/db mouse model of DM that used NO·-releasing hydrogel dressing reported no effect on wound closure when applied in the coagulation phase.108
Figure 2.
Effects of NO·-based treatments at various phases of healing in DM wound.
NO·-based therapies may offer a multifaceted approach to improve DM wound healing. (1) The role of NO· in the hemostatic phase is not clear. (2) At the inflammatory phase, NO· therapy can reduce excessive inflammation while promoting the recruitment of beneficial immune cells like macrophages, which are crucial for debris clearance and healing. (3) NO· may positively influence the proliferative phase by stimulating the growth and migration of endothelial cells, necessary for angiogenesis. This enhanced blood flow can deliver vital oxygen and nutrients to the wound site, promoting epithelialization, i.e., the regeneration of the skin’s surface layer. (4) NO· can regulate collagen deposition during remodeling, potentially influencing scar formation. However, it is important to note that the specific effects of NO·-based therapies on each stage of wound healing require further investigation. Optimizing their dosage and delivery methods will be crucial to stablish their potential for promoting DM wound healing. Created with BioRender.com. A-Plt: Activated platelet; ECM: extracellular matrix; MSCs: mesenchymal stem cells; NO·: nitric oxide; Plt: platelet.
At the inflammatory phase, NO· acts as a pro-inflammatory mediator, facilitating the migration and activation of neutrophils and macrophages, i.e., essential for clearing debris and initiating the healing process. NO· also modulates the production of cytokines, both pro-inflammatory [e.g., interleukin (IL)-1β, tumor necrosis factor-α] and anti-inflammatory (e.g., IL-10); this balanced response is crucial for a controlled inflammatory environment.109 Beyond its initial pro-inflammatory role, NO· appears to play a critical role in downregulating the inflammatory phase through inhibiting RANTES (regulated upon activation, normal T cell expressed and secreted), i.e., a monocyte chemoattractant; NO·-mediated suppression of RANTES might initiate the transition from inflammation to the regenerative phase.110 Additionally, NO· may decrease the expression of monocyte chemoattractant protein-1 by hyperproliferative keratinocytes at the wound edge.111 Finally, NO· may contribute to reduced chemoattraction by activating TGF-β1, which could subsequently suppress the expression of iNOS.109 In contrast to its initial pro-inflammatory role, NO·, in later stages, promotes a more anti-inflammatory environment by inducing apoptosis of neutrophils and macrophages to prevent excessive and prolonged inflammation hindering normal healing.109,112 Overall, this evidence highlights the complex interplay between NO· and various signaling molecules regulating the inflammatory response during wound healing.109
NO·-releasing treatments displayed potent antimicrobial activity in wounds.96,97,113 The antimicrobial properties of NO· donors, e.g., S-nitrosoglutathione, are attributed to their interaction with various proteins, DNA, and bacteria enzymes by forming reactive nitrogen species and induction of nitrosative stress.114 Staphylococcus aureus, i.e., the most frequently isolated bacterium from DFU causing severe necrotic infections and leading to amputations, is eradicated by NO3.113 Under hypoxic conditions (like that observed in DM wounds), Staphylococcus aureus survives by switching metabolic flux between fermentative growth and anaerobic NO3 respiration, depending on the availability of NO2 and NO3 (that serve as terminal electron acceptors); lack of NO2/NO3 impairs anaerobic respiration leading to fermentative growth of Staphylococcus aureus that increases biofilm formation and expression of Staphylococcal toxins.113 NO3 significantly promoted anaerobic respiration and inhibited the expression of Staphylococcus aureus virulence factors and overgrowth.113 Likewise, L-Arg increases NO· synthesis, increasing NO2/NO3 concentration in the wound and promoting NO3 respiration of Staphylococcus aureus and its virulence in hypoxic DM wounds.113
NO·-based treatments can enhance wound healing by inducing fibroblast migration, decreasing neutrophil infiltration, and polarizing M1-to-M2 macrophages at the inflammatory phase.68,96,115 M1-macrophages act as a major source of pro-inflammatory cytokines (i.e., tumor necrosis factor-α, IL-1β, IL-6, IL-12, IL-23) involving in pathogen phagocytosis and removing damaged cells, whereas M2-phenotype plays an anti-inflammatory role by upregulating IL-10, TGF-β1, and IL-12 and has repair and regeneration functions in wound healing process.116,117 Through macrophage polarization, NO· can facilitate the transition of wounds from the inflammatory phase to the proliferative phase, which in turn enhances collagen deposition.67,96
NO· also promotes wound healing in the proliferative phase, i.e., characterized by angiogenesis, GT formation, re-epithelialization, and collagen deposition.118,119 NO· increases expression of stromal cell-derived factor-1α (SDF-1α), a key factor of DM wound healing120 that involves the migration, recruitment, and retention of endothelial progenitor cells and promotion of angiogenesis through activation of heme oxygenase 1.121 SDF-1α enhances epidermal stem cell migration and proliferation, accelerating wound healing.122 SDF-1α acts as a potent chemokine for bone marrow-derived stromal stem cells expressing C-X-C chemokine receptor type 4 (a SDF-1α receptor), which in turn facilitates recruiting bone marrow-derived stromal stem cells to wound tissues, promotes secretion of growth factors by bone marrow-derived stromal stem cells and neovascularization.123 NO· upregulates VEGF,67,69,70 an important pro-angiogenic growth factor that induces vasculogenesis and angiogenesis and acts as a chemoattractant for angioblasts during the wound-healing process.124 VEGF-induced angiogenesis and subsequent perfusion might enhance the nutrient supply of wound tissues.70 Along with VEGF, NO· treatment induces another important angiogenic factor, CD31 (also known as platelet/endothelial cell adhesion molecule-169,125); CD31 promotes EC-cell adhesion, cellular transmigration, and diapedesis, angiogenesis, and vascular integrity maintenance,126 resulting in wound healing at early stages.125 NO· upregulates B-cell lymphoma 2 (Bcl-2, a mitochondrial protein preventing apoptosis) expression in the wound tissue70; upregulated expression of Bcl-2 has been shown to improve cell survival and differentiation of neuroepithelial stem cells.127 The expression of proliferating cell nuclear antigen (a cell-proliferating gene) in the wound tissue increases upon NO· treatment.70 Upregulated proliferating cell nuclear antigen may facilitate cell viability and cell proliferation in mesenchymal stem cells,70,128 the self-renewing multipotent stem cells that coordinate the healing process by recruiting other host cells and secreting growth factors and matrix proteins (e.g., ECM proteins).129
NO·-based treatments promote wound healing in the remodeling phase, i.e., characterized by developing new epithelium and normal scar formation through establishing a balance between synthesis and degradation of ECM (i.e., collagen, fibronectin, and other ECM components),17,130 and wound contraction via myofibroblasts.119 NO· upregulates expression of α-smooth muscle actin, a factor facilitating differentiation of fibroblasts into myofibroblasts (i.e., shared phenotypes of both fibroblasts and smooth muscle cells act essentially in collagen deposition and wound healing101); α-smooth muscle actin‐expressing myofibroblasts promotes contraction and accelerates wound closure and upregulates both ECM components and matrix-degrading proteases.131,132 NO· treatment upregulates expression of fibronectin and TGF-β1 throughout the healing process.69 Fibronectin is a large glycoprotein that crosslinks ECM and integrins and acts as a building block facilitating the maturation of ECM, GT, and re-epithelization.133
Conclusion and Perspective
Existing experimental evidence suggests a promising role for NO·-based therapies in DM wounds. Translating findings from animal studies to clinical practice is challenging. Although animal models are valuable tools, their limitations in simulating the multifaceted nature of DM wounds in humans must be considered when interpreting results. Furthermore, humans and rodents have distinct skin morphophysiology (e.g., skin thickness, epidermis layers, adherence to underlying tissues), immunology, and genetics; their skin differentially expresses several immunologically related genes and has specific stem cell niches.134 The wound healing process also differs between rodents and humans; for instance, wound healing in mice is much faster than in humans and heavily relies upon wound contraction (i.e., mediated by a unique muscle layer, panniculus carnosus) than re-epithelialization.135 Panniculus carnosus is considered vestigial in humans and found only in specific anatomical regions, including the palm, neck, and heel.136 The relevance of animal models to the human pathophysiology of DM wounds remains a critical issue because a specific model cannot capture all underlying causes of healing defects in patients with DM.77 However, animal models help investigate specific pathways underlying DM wounds (e.g., the relationship between hyperglycemia, microvascular dysfunction, neuropathy, and impaired healing).77,78,84 Heterogeneous protocol of DM and wound inductions, use of different NO· donors and diverse NO·-releasing biomaterials and platforms, confounding variables (e.g., age and sex of animals, duration of DM, use of a splint to inhibit early wound contraction) making between-studies comparisons implausible, is another challenge of bench-to-bedside application of NO·. In the splinted wound model, silicone splints are used around the wound to prevent wound contracture by the panniculus carnosus muscle and to promote tissue granulation formation to mimic human the wound healing process.69,137
A critical limitation of the current research field is the paucity of robust clinical trials investigating NO·-based therapies in DM wounds. The lack of human data necessitates well-designed randomized clinical trials to definitively assess the safety and efficacy of NO·-based therapies. These trials should consider well-defined primary endpoints, i.e., wound reduction area, rate of wound healing, and hospital length of stay. Developing a simple, cost-effective, and controlled mode of NO· delivery to wounds with a stable formulation is a promising approach in DM wound treatment.
Some concerns, including the short half-life of NO· and its highly-reactive properties generating excessive amounts of toxic molecules (e.g., peroxynitrite, nitrogen dioxides), uncontrolled release of NO· in the wound tissue, and potential systemic effects (e.g., acute hypotensive effects) remain to be addressed. Some open questions might call for further attention, e.g., the optimum intervention time with NO·-releasing materials and duration.91,138 For instance, NO· improves the healing process (indicated as wound contraction and re-epithelialization) when applied every day at the inflammatory and proliferative phases (corresponding to the day of lesion until the sixth day after wounding) rather than at the inflammatory or the proliferative phase per se.138
Funding Statement
Funding: This work was supported by Shahid Beheshti University of Medical Sciences, Tehran, Iran (No. 43009719).
Footnotes
Conflicts of interest
The authors declare that they have no competing interests.
Data availability statement:
No additional data are available.
References
- 1.Sun H, Saeedi P, Karuranga S, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. doi: 10.1016/j.diabres.2021.109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293:217–228. doi: 10.1001/jama.293.2.217. [DOI] [PubMed] [Google Scholar]
- 3.Iversen MM, Tell GS, Riise T, et al. History of foot ulcer increases mortality among individuals with diabetes: ten-year follow-up of the Nord-Trøndelag Health Study, Norway. Diabetes Care. 2009;32:2193–2199. doi: 10.2337/dc09-0651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moxey PW, Gogalniceanu P, Hinchliffe RJ, et al. Lower extremity amputations--a review of global variability in incidence. Diabet Med. 2011;28:1144–1153. doi: 10.1111/j.1464-5491.2011.03279.x. [DOI] [PubMed] [Google Scholar]
- 5.Snyder RJ, Hanft JR. Diabetic foot ulcers--effects on QOL, costs, and mortality and the role of standard wound care and advanced-care therapies. Ostomy Wound Manage. 2009;55:28–38. [PubMed] [Google Scholar]
- 6.Rümenapf G, Abilmona N, Morbach S, Sigl M. Peripheral arterial disease and the diabetic foot syndrome: neuropathy makes the difference! A narrative review. J Clin Med. 2024;13:2141. doi: 10.3390/jcm13072141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bus SA, Sacco ICN, Monteiro-Soares M, et al. Guidelines on the prevention of foot ulcers in persons with diabetes (IWGDF 2023 update) Diabetes Metab Res Rev. 2024;40:e3651. doi: 10.1002/dmrr.3651. [DOI] [PubMed] [Google Scholar]
- 8.Patel S, Srivastava S, Singh MR, Singh D. Mechanistic insight into diabetic wounds: Pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed Pharmacother. 2019;112:108615. doi: 10.1016/j.biopha.2019.108615. [DOI] [PubMed] [Google Scholar]
- 9.Luo JD, Chen AF. Nitric oxide: a newly discovered function on wound healing. Acta Pharmacol Sin. 2005;26:259–264. doi: 10.1111/j.1745-7254.2005.00058.x. [DOI] [PubMed] [Google Scholar]
- 10.Wu M, Lu Z, Wu K, Nam C, Zhang L, Guo J. Recent advances in the development of nitric oxide-releasing biomaterials and their application potentials in chronic wound healing. J Mater Chem B. 2021;9:7063–7075. doi: 10.1039/d1tb00847a. [DOI] [PubMed] [Google Scholar]
- 11.Ahmed R, Augustine R, Chaudhry M, et al. Nitric oxide-releasing biomaterials for promoting wound healing in impaired diabetic wounds: State of the art and recent trends. Biomed Pharmacother. 2022;149:112707. doi: 10.1016/j.biopha.2022.112707. [DOI] [PubMed] [Google Scholar]
- 12.Tavares G, Alves P, Simões P. Recent advances in hydrogel-mediated nitric oxide delivery systems targeted for wound healing applications. Pharmaceutics. 2022;14:1377. doi: 10.3390/pharmaceutics14071377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinto RV, Carvalho S, Antunes F, Pires J, Pinto ML. Emerging nitric oxide and hydrogen sulfide releasing carriers for skin wound healing therapy. ChemMedChem. 2022;17:e202100429. doi: 10.1002/cmdc.202100429. [DOI] [PubMed] [Google Scholar]
- 14.Bahadoran Z, Mirmiran P, Hosseinpanah F, Ghasemi A. Clinical applications of nitric oxide in diabetic wound healing. Iran J Endocrinol Metab. 2023;25:56–64. [Google Scholar]
- 15.Afzali H, Norouzirad R, Khaksari M, Ghasemi A. The role of nitric oxide donors in wound healing in diabetes mellitus. Iran J Endocrinol Metab. 2019;21:46–57. [Google Scholar]
- 16.Hermans MH. wounds and ulcers: back to the old nomenclature. Wounds. 2010;22:289–293. [PubMed] [Google Scholar]
- 17.Young A, McNaught C-E. The physiology of wound healing. Surgery (Oxford) 2011;29:475–479. [Google Scholar]
- 18.Velnar T, Bailey T, Smrkolj V. The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res. 2009;37:1528–1542. doi: 10.1177/147323000903700531. [DOI] [PubMed] [Google Scholar]
- 19.Malone-Povolny MJ, Maloney SE, Schoenfisch MH. Nitric oxide therapy for diabetic wound healing. Adv Healthc Mater. 2019;8:e1801210. doi: 10.1002/adhm.201801210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel Y, Shah T, Dhar MK, et al. Integrated image and location analysis for wound classification: a deep learning approach. Sci Rep. 2024;14:7043. doi: 10.1038/s41598-024-56626-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Triana-Ricci R, Martinez-de-Jesús F, Aragón-Carreño MP, et al. Management recommendations for diabetic foot patients. Instructional course. Rev Colomb Ortop Traumatol. 2021;35:330–357. [Google Scholar]
- 22.Li M. Guidelines and standards for comprehensive clinical diagnosis and interventional treatment for diabetic foot in China (Issue 7.0) J Interv Med. 2021;4:117–129. doi: 10.1016/j.jimed.2021.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abu-Qamar MZ, Kemp V, Whitehead L. Foot ulcers associated with external trauma among people with diabetes: An integrative review of the origin of trauma and outcomes. Int J Nurs Stud. 2021;114:103822. doi: 10.1016/j.ijnurstu.2020.103822. [DOI] [PubMed] [Google Scholar]
- 24.Eleftheriadou I, Kokkinos A, Liatis S, et al. Atlas of the diabetic foot. John Wiley & Sons Ltd; 2019. [Google Scholar]
- 25.Alexiadou K, Doupis J. Management of diabetic foot ulcers. Diabetes Ther. 2012;3:4. doi: 10.1007/s13300-012-0004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jais S. Various types of wounds that diabetic patients can develop: a narrative review. Clin Pathol. 2023;16:2632010x231205366. doi: 10.1177/2632010X231205366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Camilleri A, Gatt A, Formosa C. Inter-rater reliability of four validated diabetic foot ulcer classification systems. J Tissue Viability. 2020;29:284–290. doi: 10.1016/j.jtv.2020.09.002. [DOI] [PubMed] [Google Scholar]
- 28.Yotsu RR, Pham NM, Oe M, et al. Comparison of characteristics and healing course of diabetic foot ulcers by etiological classification: neuropathic, ischemic, and neuro-ischemic type. J Diabetes Complications. 2014;28:528–535. doi: 10.1016/j.jdiacomp.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 29.Oe M, Saad SS, Jais S, Sugama J. Differences in characteristics between first-ever foot ulcer and recurrent foot ulcer in patients with diabetes: prospective observational study. Health Sci Rep. 2024;7:e2018. doi: 10.1002/hsr2.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brocklehurst JD. The validity and reliability of the SINBAD classification system for diabetic foot ulcers. Adv Skin Wound Care. 2023;36:1–5. doi: 10.1097/ASW.0000000000000050. [DOI] [PubMed] [Google Scholar]
- 31.Oyibo SO, Jude EB, Tarawneh I, Nguyen HC, Harkless LB, Boulton AJ. A comparison of two diabetic foot ulcer classification systems: the Wagner and the University of Texas wound classification systems. Diabetes Care. 2001;24:84–88. doi: 10.2337/diacare.24.1.84. [DOI] [PubMed] [Google Scholar]
- 32.Schaper NC. Diabetic foot ulcer classification system for research purposes: a progress report on criteria for including patients in research studies. Diabetes Metab Res Rev. 2004;20(Suppl 1):S90–95. doi: 10.1002/dmrr.464. [DOI] [PubMed] [Google Scholar]
- 33.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 34.Rodrigues M, Kosaric N, Bonham CA, Gurtner GC. Wound healing: a cellular perspective. Physiol Rev. 2019;99:665–706. doi: 10.1152/physrev.00067.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cioce A, Cavani A, Cattani C, Scopelliti F. Role of the skin immune system in wound healing. Cells. 2024;13:624. doi: 10.3390/cells13070624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park JE, Barbul A. Understanding the role of immune regulation in wound healing. Am J Surg. 2004;187:11S–16S. doi: 10.1016/S0002-9610(03)00296-4. [DOI] [PubMed] [Google Scholar]
- 37.Sindrilaru A, Scharffetter-Kochanek K. Disclosure of the culprits: macrophages-versatile regulators of wound healing. Adv Wound Care (New Rochelle) 2013;2:357–368. doi: 10.1089/wound.2012.0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ansell DM, Izeta A. Pericytes in wound healing: friend or foe? Exp Dermatol. 2015;24:833–834. doi: 10.1111/exd.12782. [DOI] [PubMed] [Google Scholar]
- 39.Kosaraju R, Rennert RC, Maan ZN, et al. Adipose-derived stem cell-seeded hydrogels increase endogenous progenitor cell recruitment and neovascularization in wounds. Tissue Eng Part A. 2016;22:295–305. doi: 10.1089/ten.tea.2015.0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hinz B, Gabbiani G. Cell-matrix and cell-cell contacts of myofibroblasts: role in connective tissue remodeling. Thromb Haemost. 2003;90:993–1002. doi: 10.1160/TH03-05-0328. [DOI] [PubMed] [Google Scholar]
- 41.Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200:500–503. doi: 10.1002/path.1427. [DOI] [PubMed] [Google Scholar]
- 42.Sharp A, Clark J. Diabetes and its effects on wound healing. Nurs Stand. 2011;25:41–47. doi: 10.7748/ns2011.07.25.45.41.c8626. [DOI] [PubMed] [Google Scholar]
- 43.Bitar MS, Labbad ZN. Transforming growth factor-beta and insulin-like growth factor-I in relation to diabetes-induced impairment of wound healing. J Surg Res. 1996;61:113–119. doi: 10.1006/jsre.1996.0090. [DOI] [PubMed] [Google Scholar]
- 44.Lobmann R, Ambrosch A, Schultz G, Waldmann K, Schiweck S, Lehnert H. Expression of matrix-metalloproteinases and their inhibitors in the wounds of diabetic and non-diabetic patients. Diabetologia. 2002;45:1011–1016. doi: 10.1007/s00125-002-0868-8. [DOI] [PubMed] [Google Scholar]
- 45.Wall SJ, Bevan D, Thomas DW, Harding KG, Edwards DR, Murphy G. Differential expression of matrix metalloproteinases during impaired wound healing of the diabetes mouse. J Invest Dermatol. 2002;119:91–98. doi: 10.1046/j.1523-1747.2002.01779.x. [DOI] [PubMed] [Google Scholar]
- 46.Ayuk SM, Abrahamse H, Houreld NN. The role of matrix metalloproteinases in diabetic wound healing in relation to photobiomodulation. J Diabetes Res. 2016;2016:2897656. doi: 10.1155/2016/2897656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nirenjen S, Narayanan J, Tamilanban T, et al. Exploring the contribution of pro-inflammatory cytokines to impaired wound healing in diabetes. Front Immunol. 2023;14:1216321. doi: 10.3389/fimmu.2023.1216321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang G, Yang F, Zhou W, Xiao N, Luo M, Tang Z. The initiation of oxidative stress and therapeutic strategies in wound healing. Biomed Pharmacother. 2023;157:114004. doi: 10.1016/j.biopha.2022.114004. [DOI] [PubMed] [Google Scholar]
- 49.Rasik AM, Shukla A. Antioxidant status in delayed healing type of wounds. Int J Exp Pathol. 2000;81:257–263. doi: 10.1046/j.1365-2613.2000.00158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mudge BP, Harris C, Gilmont RR, Adamson BS, Rees RS. Role of glutathione redox dysfunction in diabetic wounds. Wound Repair Regen. 2002;10:52–58. doi: 10.1046/j.1524-475x.2002.10803.x. [DOI] [PubMed] [Google Scholar]
- 51.Landén NX, Li D, Ståhle M. Transition from inflammation to proliferation: a critical step during wound healing. Cell Mol Life Sci. 2016;73:3861–3885. doi: 10.1007/s00018-016-2268-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seifter E, Rettura G, Barbul A, Levenson SM. Arginine: an essential amino acid for injured rats. Surgery. 1978;84:224–230. [PubMed] [Google Scholar]
- 53.Schäffer MR, Tantry U, van Wesep RA, Barbul A. Nitric oxide metabolism in wounds. J Surg Res. 1997;71:25–31. doi: 10.1006/jsre.1997.5137. [DOI] [PubMed] [Google Scholar]
- 54.Schaffer MR, Tantry U, Gross SS, Wasserburg HL, Barbul A. Nitric oxide regulates wound healing. J Surg Res. 1996;63:237–240. doi: 10.1006/jsre.1996.0254. [DOI] [PubMed] [Google Scholar]
- 55.Shi HP, Efron DT, Most D, Tantry US, Barbul A. Supplemental dietary arginine enhances wound healing in normal but not inducible nitric oxide synthase knockout mice. Surgery. 2000;128:374–378. doi: 10.1067/msy.2000.107372. [DOI] [PubMed] [Google Scholar]
- 56.Afzali H, Ranjbar T, Kashfi K, Ghasemi A. Role of nitric oxide in diabetic wound healing. In: Ghasemi A, Kashfi K, Bahadoran Z, editors. The role of nitric oxide in type 2 diabetes. Bentham Books; 2022. [Google Scholar]
- 57.Schäffer MR, Tantry U, Efron PA, Ahrendt GM, Thornton FJ, Barbul A. Diabetes-impaired healing and reduced wound nitric oxide synthesis: a possible pathophysiologic correlation. Surgery. 1997;121:513–519. doi: 10.1016/s0039-6060(97)90105-7. [DOI] [PubMed] [Google Scholar]
- 58.Stallmeyer B, Anhold M, Wetzler C, Kahlina K, Pfeilschifter J, Frank S. Regulation of eNOS in normal and diabetes-impaired skin repair: implications for tissue regeneration. Nitric Oxide. 2002;6:168–177. doi: 10.1006/niox.2001.0407. [DOI] [PubMed] [Google Scholar]
- 59.Afzali H, Khaksari M, Norouzirad R, Jeddi S, Kashfi K, Ghasemi A. Acidified nitrite improves wound healing in type 2 diabetic rats: Role of oxidative stress and inflammation. Nitric Oxide. 2020;103:20–28. doi: 10.1016/j.niox.2020.07.001. [DOI] [PubMed] [Google Scholar]
- 60.Bitto A, Irrera N, Pizzino G, et al. Activation of the EPOR-β common receptor complex by cibinetide ameliorates impaired wound healing in mice with genetic diabetes. Biochim Biophys Acta Mol Basis Dis. 2018;1864:632–639. doi: 10.1016/j.bbadis.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 61.Witte MB, Kiyama T, Barbul A. Nitric oxide enhances experimental wound healing in diabetes. Br J Surg. 2002;89:1594–1601. doi: 10.1046/j.1365-2168.2002.02263.x. [DOI] [PubMed] [Google Scholar]
- 62.Shi HP, Most D, Efron DT, Witte MB, Barbul A. Supplemental L-arginine enhances wound healing in diabetic rats. Wound Repair Regen. 2003;11:198–203. doi: 10.1046/j.1524-475x.2003.11308.x. [DOI] [PubMed] [Google Scholar]
- 63.Tatmatsu-Rocha JC, Ferraresi C, Hamblin MR, et al. Low-level laser therapy (904nm) can increase collagen and reduce oxidative and nitrosative stress in diabetic wounded mouse skin. J Photochem Photobiol B. 2016;164:96–102. doi: 10.1016/j.jphotobiol.2016.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Laing T, Hanson R, Chan F, Bouchier-Hayes D. Effect of pravastatin on experimental diabetic wound healing. J Surg Res. 2010;161:336–340. doi: 10.1016/j.jss.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 65.Pierce GF. Inflammation in nonhealing diabetic wounds: the space-time continuum does matter. Am J Pathol. 2001;159:399–403. doi: 10.1016/S0002-9440(10)61709-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saidian M, Lakey JRT, Ponticorvo A, et al. Characterisation of impaired wound healing in a preclinical model of induced diabetes using wide-field imaging and conventional immunohistochemistry assays. Int Wound J. 2019;16:144–152. doi: 10.1111/iwj.13005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zheng Y, Yang D, Gao B, et al. A DNA-inspired injectable adhesive hydrogel with dual nitric oxide donors to promote angiogenesis for enhanced wound healing. Acta Biomater. 2024;176:128–143. doi: 10.1016/j.actbio.2024.01.026. [DOI] [PubMed] [Google Scholar]
- 68.Yang Y, Yang Y, Jiang J, et al. Arginine-nanoenzyme with timely angiogenesis for promoting diabetic wound healing. ACS Appl Mater Interfaces. 2024;16:9640–9655. doi: 10.1021/acsami.3c13072. [DOI] [PubMed] [Google Scholar]
- 69.Sivaraj D, Noishiki C, Kosaric N, et al. Nitric oxide-releasing gel accelerates healing in a diabetic murine splinted excisional wound model. Front Med (Lausanne) 2023;10:1060758. doi: 10.3389/fmed.2023.1060758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ahmed R, Afreen A, Tariq M, et al. Bone marrow mesenchymal stem cells preconditioned with nitric-oxide-releasing chitosan/PVA hydrogel accelerate diabetic wound healing in rabbits. Biomed Mater. 2021;16:035014. doi: 10.1088/1748-605X/abc28b. [DOI] [PubMed] [Google Scholar]
- 71.Opelt M, Eroglu E, Waldeck-Weiermair M, et al. Formation of nitric oxide by aldehyde dehydrogenase-2 is necessary and sufficient for vascular bioactivation of nitroglycerin. J Biol Chem. 2016;291:24076–24084. doi: 10.1074/jbc.M116.752071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Agvald P, Adding LC, Artlich A, Persson MG, Gustafsson LE. Mechanisms of nitric oxide generation from nitroglycerin and endogenous sources during hypoxia in vivo. Br J Pharmacol. 2002;135:373–382. doi: 10.1038/sj.bjp.0704489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li B, Ming Y, Liu Y, et al. Recent developments in pharmacological effect, mechanism and application prospect of diazeniumdiolates. Front Pharmacol. 2020;11:923. doi: 10.3389/fphar.2020.00923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Broniowska KA, Hogg N. The chemical biology of S-nitrosothiols. Antioxid Redox Signal. 2012;17:969–980. doi: 10.1089/ars.2012.4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miller CC, Miller MK, Ghaffari A, Kunimoto B. Treatment of chronic nonhealing leg ulceration with gaseous nitric oxide: a case study. J Cutan Med Surg. 2004;8:233–238. doi: 10.1007/s10227-004-0106-8. [DOI] [PubMed] [Google Scholar]
- 76.Norahan MH, Pedroza-González SC, Sánchez-Salazar MG, Álvarez MM, Trujillo de Santiago G. Structural and biological engineering of 3D hydrogels for wound healing. Bioact Mater. 2023;24:197–235. doi: 10.1016/j.bioactmat.2022.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sanapalli BKR, Yele V, Singh MK, Thaggikuppe Krishnamurthy P, Karri V. Preclinical models of diabetic wound healing: a critical review. Biomed Pharmacother. 2021;142:111946. doi: 10.1016/j.biopha.2021.111946. [DOI] [PubMed] [Google Scholar]
- 78.Rai V, Moellmer R, Agrawal DK. Clinically relevant experimental rodent models of diabetic foot ulcer. Mol Cell Biochem. 2022;477:1239–1247. doi: 10.1007/s11010-022-04372-w. [DOI] [PubMed] [Google Scholar]
- 79.Ghanbari M, Salkovskiy Y, Carlson MA. The rat as an animal model in chronic wound research: An update. Life Sci. 2024;351:122783. doi: 10.1016/j.lfs.2024.122783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ellenbroek B, Youn J. Rodent models in neuroscience research: is it a rat race? Dis Model Mech. 2016;9:1079–1087. doi: 10.1242/dmm.026120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ghasemi A, Jeddi S. Streptozotocin as a tool for induction of rat models of diabetes: a practical guide. EXCLI J. 2023;22:274–294. doi: 10.17179/excli2022-5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gheibi S, Kashfi K, Ghasemi A. A practical guide for induction of type-2 diabetes in rat: Incorporating a high-fat diet and streptozotocin. Biomed Pharmacother. 2017;95:605–613. doi: 10.1016/j.biopha.2017.08.098. [DOI] [PubMed] [Google Scholar]
- 83.Bahadoran Z, Mirmiran P, Ghasemi A. Monosodium glutamate (MSG)-induced animal model of type 2 diabetes. Methods Mol Biol. 2019;1916:49–65. doi: 10.1007/978-1-4939-8994-2_3. [DOI] [PubMed] [Google Scholar]
- 84.Couturier A, Calissi C, Cracowski JL, Sigaudo-Roussel D, Khouri C, Roustit M. Mouse models of diabetes-related ulcers: a systematic review and network meta-analysis. EBioMedicine. 2023;98:104856. doi: 10.1016/j.ebiom.2023.104856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sami DG, Heiba HH, Abdellatif A. Wound healing models: a systematic review of animal and non-animal models. Wound Med. 2019;24:8–17. [Google Scholar]
- 86.Witte MB, Thornton FJ, Tantry U, Barbul A. L-Arginine supplementation enhances diabetic wound healing: involvement of the nitric oxide synthase and arginase pathways. Metabolism. 2002;51:1269–1273. doi: 10.1053/meta.2002.35185. [DOI] [PubMed] [Google Scholar]
- 87.Jerônimo MS, Barros AD, Morita IV, et al. Oral or topical administration of L-arginine changes the expression of TGF and iNOS and results in early wounds healing. Acta Cir Bras. 2016;31:586–596. doi: 10.1590/S0102-865020160090000003. [DOI] [PubMed] [Google Scholar]
- 88.Schäffer M, Bongartz M, Fischer S, Proksch B, Viebahn R. Nitric oxide restores impaired healing in normoglycaemic diabetic rats. J Wound Care. 2007;16:311–316. doi: 10.12968/jowc.2007.16.7.27057. [DOI] [PubMed] [Google Scholar]
- 89.Chen YJ, Wu SC, Wang HC, et al. Activation of angiogenesis and wound healing in diabetic mice using NO-delivery dinitrosyl iron complexes. Mol Pharm. 2019;16:4241–4251. doi: 10.1021/acs.molpharmaceut.9b00586. [DOI] [PubMed] [Google Scholar]
- 90.Afzali H, Khaksari M, Jeddi S, Kashfi K, Abdollahifar MA, Ghasemi A. Acidified nitrite accelerates wound healing in type 2 diabetic male rats: a histological and stereological evaluation. Molecules. 2021;26:1872. doi: 10.3390/molecules26071872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Weller R, Finnen MJ. The effects of topical treatment with acidified nitrite on wound healing in normal and diabetic mice. Nitric Oxide. 2006;15:395–399. doi: 10.1016/j.niox.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 92.Mikaili P, Moloudizargari M, Aghajanshakeri S. Treatment with topical nitroglycerine may promote the healing process of diabetic foot ulcers. Med Hypotheses. 2014;83:172–174. doi: 10.1016/j.mehy.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 93.Hotkar MS, Avachat AM, Bhosale SS, Oswal YM. Preliminary investigation of topical nitroglycerin formulations containing natural wound healing agent in diabetes-induced foot ulcer. Int Wound J. 2015;12:210–217. doi: 10.1111/iwj.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ben-Yehuda Greenwald M, Tacconi C, Jukic M, et al. A dual-acting nitric oxide donor and phosphodiesterase 5 inhibitor promotes wound healing in normal mice and mice with diabetes. J Invest Dermatol. 2021;141:415–426. doi: 10.1016/j.jid.2020.05.111. [DOI] [PubMed] [Google Scholar]
- 95.Liu L, Zheng J, Li S, et al. Nitric oxide-releasing multifunctional catechol-modified chitosan/oxidized dextran hydrogel with antibacterial, antioxidant, and pro-angiogenic properties for MRSA-infected diabetic wound healing. Int J Biol Macromol. 2024;263:130225. doi: 10.1016/j.ijbiomac.2024.130225. [DOI] [PubMed] [Google Scholar]
- 96.Huang Q, Yang Z, Tao X, et al. Sprayable chitosan nanogel with nitric oxide to accelerate diabetic wound healing through bacteria inhibition, biofilm eradication and macrophage polarization. Int J Biol Macromol. 2024;254:127806. doi: 10.1016/j.ijbiomac.2023.127806. [DOI] [PubMed] [Google Scholar]
- 97.He J, Li Z, Wang J, et al. Photothermal antibacterial antioxidant conductive self-healing hydrogel with nitric oxide release accelerates diabetic wound healing. Compos B Eng. 2023;266:110985. [Google Scholar]
- 98.Zhang L, Yang J, Liu W, et al. A phellinus igniarius polysaccharide/chitosan-arginine hydrogel for promoting diabetic wound healing. Int J Biol Macromol. 2023;249:126014. doi: 10.1016/j.ijbiomac.2023.126014. [DOI] [PubMed] [Google Scholar]
- 99.He C, Bi S, Zhang R, et al. A hyaluronic acid hydrogel as a mild photothermal antibacterial, antioxidant, and nitric oxide release platform for diabetic wound healing. J Control Release. 2024;370:543–555. doi: 10.1016/j.jconrel.2024.05.011. [DOI] [PubMed] [Google Scholar]
- 100.Zhao Y, Luo L, Huang L, et al. In situ hydrogel capturing nitric oxide microbubbles accelerates the healing of diabetic foot. J Control Release. 2022;350:93–106. doi: 10.1016/j.jconrel.2022.08.018. [DOI] [PubMed] [Google Scholar]
- 101.Liu Y, Guo S, Wei S, et al. A novel nitric oxide-releasing gel for diabetic wounds. PRRS. 2022;1:24–33. [Google Scholar]
- 102.Yang Y, Huang K, Wang M, et al. Ubiquitination flow repressors: enhancing wound healing of infectious diabetic ulcers through stabilization of polyubiquitinated hypoxia-inducible factor-1α by theranostic nitric oxide nanogenerators. Adv Mater. 2021;33:e2103593. doi: 10.1002/adma.202103593. [DOI] [PubMed] [Google Scholar]
- 103.Xie J, Liu G, Chen R, et al. NIR-activated electrospun nanodetonator dressing enhances infected diabetic wound healing with combined photothermal and nitric oxide-based gas therapy. J Nanobiotechnology. 2024;22:232. doi: 10.1186/s12951-024-02474-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Armstrong DG, Hanft JR, Driver VR, et al. Effect of oral nutritional supplementation on wound healing in diabetic foot ulcers: a prospective randomized controlled trial. Diabet Med. 2014;31:1069–1077. doi: 10.1111/dme.12509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bian H, Wang J, Shan X, et al. A portable on-demand therapeutic nitric oxide generation apparatus: New strategy for diabetic foot ulcers. Chem Eng J. 2024;480:148088. [Google Scholar]
- 106.Edmonds ME, Bodansky HJ, Boulton AJM, et al. Multicenter, randomized controlled, observer-blinded study of a nitric oxide generating treatment in foot ulcers of patients with diabetes-ProNOx1 study. Wound Repair Regen. 2018;26:228–237. doi: 10.1111/wrr.12630. [DOI] [PubMed] [Google Scholar]
- 107.Bassenge E. Antiplatelet effects of endothelium-derived relaxing factor and nitric oxide donors. Eur Heart J. 1991;12Suppl E:12–15. doi: 10.1093/eurheartj/12.suppl_e.12. [DOI] [PubMed] [Google Scholar]
- 108.Masters KS, Leibovich SJ, Belem P, West JL, Poole-Warren LA. Effects of nitric oxide releasing poly(vinyl alcohol) hydrogel dressings on dermal wound healing in diabetic mice. Wound Repair Regen. 2002;10:286–294. doi: 10.1046/j.1524-475x.2002.10503.x. [DOI] [PubMed] [Google Scholar]
- 109.Schwentker A, Vodovotz Y, Weller R, Billiar TR. Nitric oxide and wound repair: role of cytokines? Nitric Oxide. 2002;7:1–10. doi: 10.1016/s1089-8603(02)00002-2. [DOI] [PubMed] [Google Scholar]
- 110.Frank S, Kämpfer H, Wetzler C, Stallmeyer B, Pfeilschifter J. Large induction of the chemotactic cytokine RANTES during cutaneous wound repair: a regulatory role for nitric oxide in keratinocyte-derived RANTES expression. Biochem J. 2000;347(Pt 1):265–273. [PMC free article] [PubMed] [Google Scholar]
- 111.Wetzler C, Kämpfer H, Pfeilschifter J, Frank S. Keratinocyte-derived chemotactic cytokines: expressional modulation by nitric oxide in vitro and during cutaneous wound repair in vivo. Biochem Biophys Res Commun. 2000;274:689–696. doi: 10.1006/bbrc.2000.3170. [DOI] [PubMed] [Google Scholar]
- 112.Pérez S, Rius-Pérez S. Macrophage polarization and reprogramming in acute inflammation: a redox perspective. Antioxidants (Basel) 2022;11:1394. doi: 10.3390/antiox11071394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Baker CL, Seo KS, Park N, et al. L-arginine supplementation abrogates hypoxia-induced virulence of Staphylococcus aureus in a murine diabetic pressure wound model. mSphere. 2024;9:e0077423. doi: 10.1128/msphere.00774-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Qian H, Ye Z, Pi L, Ao J. Roles and current applications of S-nitrosoglutathione in anti-infective biomaterials. Mater Today Bio. 2022;16:100419. doi: 10.1016/j.mtbio.2022.100419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tu C, Lu H, Zhou T, et al. Promoting the healing of infected diabetic wound by an anti-bacterial and nano-enzyme-containing hydrogel with inflammation-suppressing, ROS-scavenging, oxygen and nitric oxide-generating properties. Biomaterials. 2022;286:121597. doi: 10.1016/j.biomaterials.2022.121597. [DOI] [PubMed] [Google Scholar]
- 116.Kotwal GJ, Chien S. Macrophage differentiation in normal and accelerated wound healing. Results Probl Cell Differ. 2017;62:353–364. doi: 10.1007/978-3-319-54090-0_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Krzyszczyk P, Schloss R, Palmer A, Berthiaume F. The role of macrophages in acute and chronic wound healing and interventions to promote pro-wound healing phenotypes. Front Physiol. 2018;9:419. doi: 10.3389/fphys.2018.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rousselle P, Braye F, Dayan G. Re-epithelialization of adult skin wounds: cellular mechanisms and therapeutic strategies. Adv Drug Deliv Rev. 2019;146:344–365. doi: 10.1016/j.addr.2018.06.019. [DOI] [PubMed] [Google Scholar]
- 119.Reinke JM, Sorg H. Wound repair and regeneration. Eur Surg Res. 2012;49:35–43. doi: 10.1159/000339613. [DOI] [PubMed] [Google Scholar]
- 120.Badillo AT, Chung S, Zhang L, Zoltick P, Liechty KW. Lentiviral gene transfer of SDF-1alpha to wounds improves diabetic wound healing. J Surg Res. 2007;143:35–42. doi: 10.1016/j.jss.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 121.Deshane J, Chen S, Caballero S, et al. Stromal cell-derived factor 1 promotes angiogenesis via a heme oxygenase 1-dependent mechanism. J Exp Med. 2007;204:605–618. doi: 10.1084/jem.20061609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Guo R, Chai L, Chen L, et al. Stromal cell-derived factor 1 (SDF-1) accelerated skin wound healing by promoting the migration and proliferation of epidermal stem cells. In Vitro Cell Dev Biol Anim. 2015;51:578–585. doi: 10.1007/s11626-014-9862-y. [DOI] [PubMed] [Google Scholar]
- 123.Xu X, Zhu F, Zhang M, et al. Stromal cell-derived factor-1 enhances wound healing through recruiting bone marrow-derived mesenchymal stem cells to the wound area and promoting neovascularization. Cells Tissues Organs. 2013;197:103–113. doi: 10.1159/000342921. [DOI] [PubMed] [Google Scholar]
- 124.Johnson KE, Wilgus TA. Vascular endothelial growth factor and angiogenesis in the regulation of cutaneous wound repair. Adv Wound Care (New Rochelle) 2014;3:647–661. doi: 10.1089/wound.2013.0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang Y, Tang K, Chen B, et al. A polyethylenimine-based diazeniumdiolate nitric oxide donor accelerates wound healing. Biomater Sci. 2019;7:1607–1616. doi: 10.1039/c8bm01519h. [DOI] [PubMed] [Google Scholar]
- 126.Privratsky JR, Newman PJ. PECAM-1: regulator of endothelial junctional integrity. Cell Tissue Res. 2014;355:607–619. doi: 10.1007/s00441-013-1779-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu W, Yue W, Wu R. Overexpression of Bcl-2 promotes survival and differentiation of neuroepithelial stem cells after transplantation into rat aganglionic colon. Stem Cell Res Ther. 2013;4:7. doi: 10.1186/scrt155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kim S, Yoon YM, Han YS, Lee JH, Hur J, Lee SH. Administration of Cripto in GRP78 overexpressed human MSCs enhances stem cell viability and angiogenesis during human MSC transplantation therapy. Cell Prolif. 2018;51:e12463. doi: 10.1111/cpr.12463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Maxson S, Lopez EA, Yoo D, Danilkovitch-Miagkova A, Leroux MA. Concise review: role of mesenchymal stem cells in wound repair. Stem Cells Transl Med. 2012;1:142–149. doi: 10.5966/sctm.2011-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kunkemoeller B, Kyriakides TR. Redox signaling in diabetic wound healing regulates extracellular matrix deposition. Antioxid Redox Signal. 2017;27:823–838. doi: 10.1089/ars.2017.7263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hinz B, Celetta G, Tomasek JJ, Gabbiani G, Chaponnier C. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell. 2001;12:2730–2741. doi: 10.1091/mbc.12.9.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.El Kahi CG, Atiyeh BS, Abdallah Hajj Hussein I, et al. Modulation of wound contracture alpha-smooth muscle actin and multispecific vitronectin receptor integrin alphavbeta3 in the rabbit’s experimental model. Int Wound J. 2009;6:214–224. doi: 10.1111/j.1742-481X.2009.00597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lenselink EA. Role of fibronectin in normal wound healing. Int Wound J. 2015;12:313–316. doi: 10.1111/iwj.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zomer HD, Trentin AG. Skin wound healing in humans and mice: challenges in translational research. J Dermatol Sci. 2018;90:3–12. doi: 10.1016/j.jdermsci.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 135.Liu A, Long Y, Li J, et al. Accelerated complete human skin architecture restoration after wounding by nanogenerator-driven electrostimulation. J Nanobiotechnology. 2021;19:280. doi: 10.1186/s12951-021-01036-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nasir NJM, Corrias A, Heemskerk H, et al. The panniculus carnosus muscle: a missing link in the chronicity of heel pressure ulcers? J R Soc Interface. 2022;19:20210631. doi: 10.1098/rsif.2021.0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Galiano RD, Michaels Jt, Dobryansky M, Levine JP, Gurtner GC. Quantitative and reproducible murine model of excisional wound healing. Wound Repair Regen. 2004;12:485–492. doi: 10.1111/j.1067-1927.2004.12404.x. [DOI] [PubMed] [Google Scholar]
- 138.Amadeu TP, Seabra AB, de Oliveira MG, Monte-Alto-Costa A. Nitric oxide donor improves healing if applied on inflammatory and proliferative phase. J Surg Res. 2008;149:84–93. doi: 10.1016/j.jss.2007.10.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.