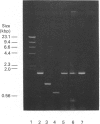
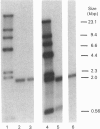
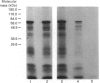
Abstract
cDNA coding for the luciferase in the firefly Photinus pyralis was amplified in vitro to generate cyclic AMP-dependent protein kinase phosphorylation sites. The DNA was transcribed and translated to generate light-emitting protein. A valine at position 217 was mutated to arginine to generate a site RRFS and the heptapeptide kemptide, the phosphorylation site of the porcine pyruvate kinase, was added at the N- or C-terminus of the luciferase. The proteins carrying phosphorylation sites were characterized for their specific activity, pI, effect of pH on the colour of the light emitted and effect of the catalytic subunit of protein kinase A in the presence of ATP. Only one of the recombinant proteins (RRFS) was significantly different from wild-type luciferase. The RRFS mutant had a lower specific activity, lower pH optimum, emitted greener light at low pH and when phosphorylated it decreased its activity by up to 80%. This latter effect was reversed by phosphatase. This recombinant protein is a good candidate to measure for the first time cyclic AMP-dependent phosphorylation in live cells.
Full text
PDF

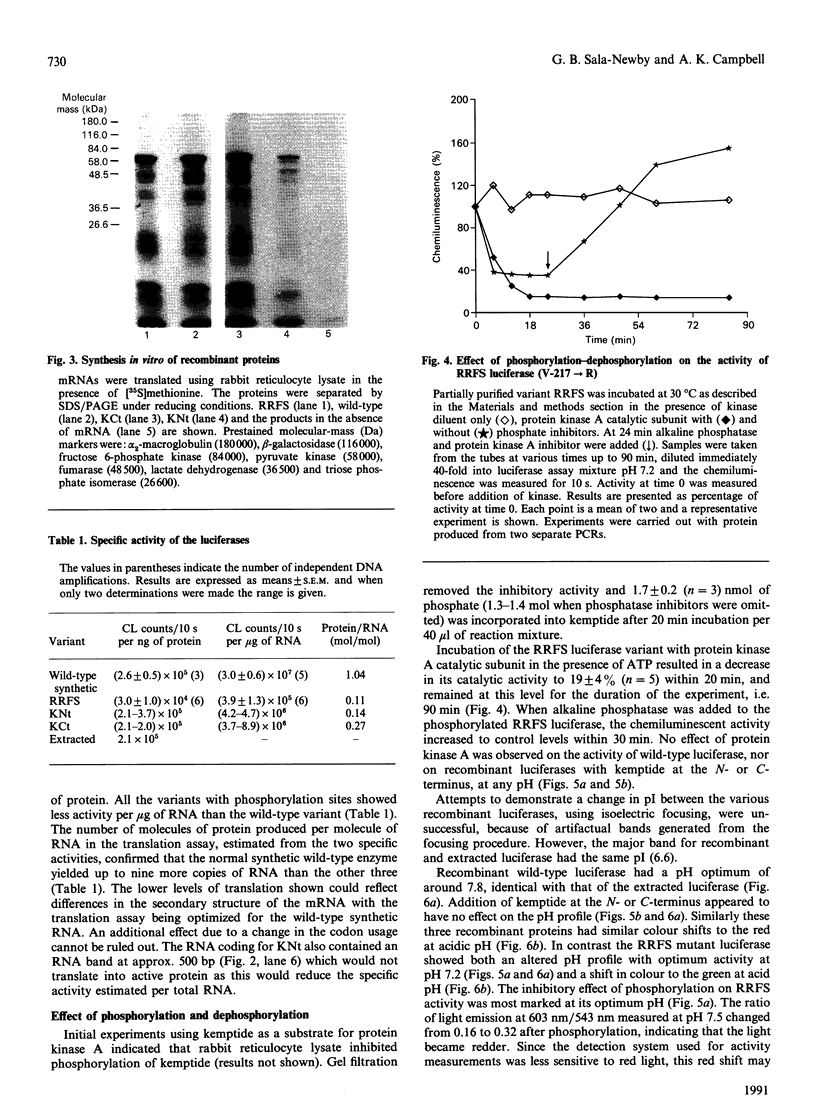
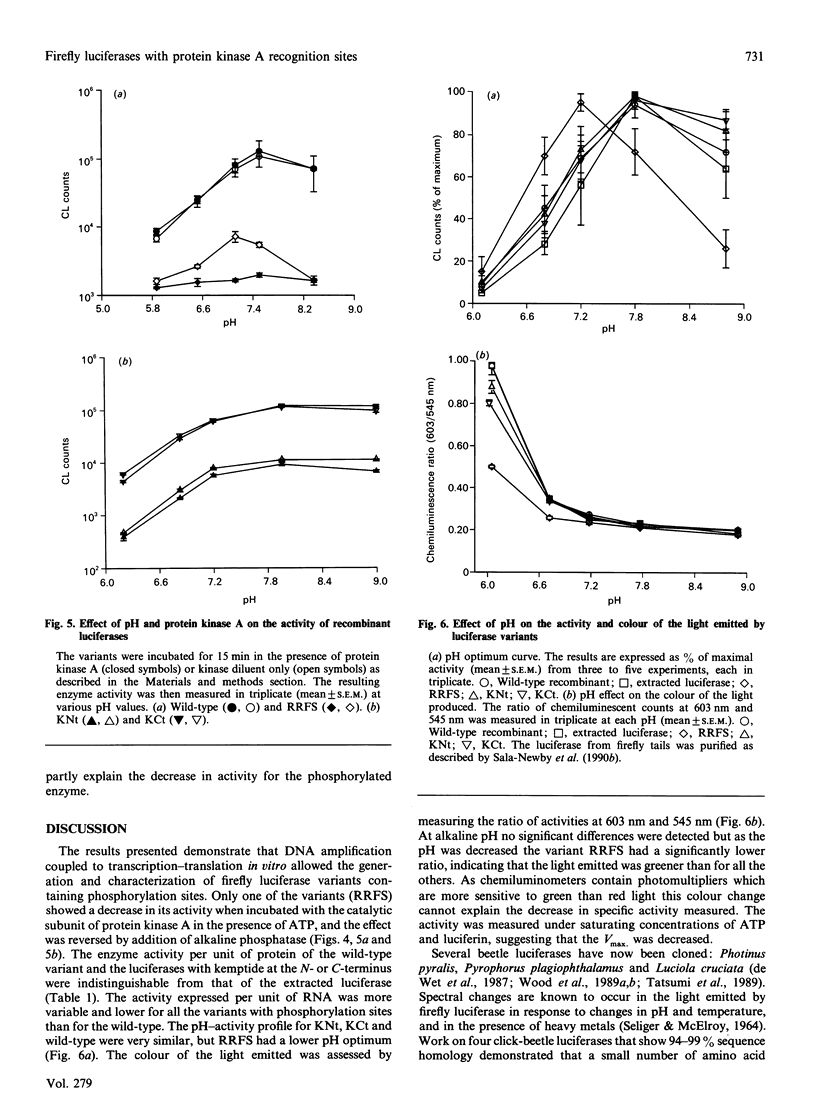

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Cheng H. C., van Patten S. M., Smith A. J., Walsh D. A. An active twenty-amino-acid-residue peptide derived from the inhibitor protein of the cyclic AMP-dependent protein kinase. Biochem J. 1985 Nov 1;231(3):655–661. doi: 10.1042/bj2310655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbold P. H., Rink T. J. Fluorescence and bioluminescence measurement of cytoplasmic free calcium. Biochem J. 1987 Dec 1;248(2):313–328. doi: 10.1042/bj2480313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. Protein phosphorylation and hormone action. Proc R Soc Lond B Biol Sci. 1988 Jul 22;234(1275):115–144. doi: 10.1098/rspb.1988.0040. [DOI] [PubMed] [Google Scholar]
- Davies E. V., Hallett M. B., Campbell A. K. Localized superoxide release by neutrophils can be provoked by a cytosolic calcium 'cloud'. Immunology. 1991 Jun;73(2):228–234. [PMC free article] [PubMed] [Google Scholar]
- Gould S. G., Keller G. A., Subramani S. Identification of a peroxisomal targeting signal at the carboxy terminus of firefly luciferase. J Cell Biol. 1987 Dec;105(6 Pt 2):2923–2931. doi: 10.1083/jcb.105.6.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M. B., Davies E. V., Campbell A. K. Oxidase activation in individual neutrophils is dependent on the onset and magnitude of the Ca2+ signal. Cell Calcium. 1990 Nov-Dec;11(10):655–663. doi: 10.1016/0143-4160(90)90020-u. [DOI] [PubMed] [Google Scholar]
- Hooper C. E., Ansorge R. E., Browne H. M., Tomkins P. CCD imaging of luciferase gene expression in single mammalian cells. J Biolumin Chemilumin. 1990 Apr-Jun;5(2):123–130. doi: 10.1002/bio.1170050208. [DOI] [PubMed] [Google Scholar]
- Jenkins T. M., Sala-Newby G., Campbell A. K. Measurement of protein phosphorylation by covalent modification of firefly luciferase. Biochem Soc Trans. 1990 Jun;18(3):463–464. doi: 10.1042/bst0180463. [DOI] [PubMed] [Google Scholar]
- Keller G. A., Gould S., Deluca M., Subramani S. Firefly luciferase is targeted to peroxisomes in mammalian cells. Proc Natl Acad Sci U S A. 1987 May;84(10):3264–3268. doi: 10.1073/pnas.84.10.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp B. E., Graves D. J., Benjamini E., Krebs E. G. Role of multiple basic residues in determining the substrate specificity of cyclic AMP-dependent protein kinase. J Biol Chem. 1977 Jul 25;252(14):4888–4894. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li B. L., Langer J. A., Schwartz B., Pestka S. Creation of phosphorylation sites in proteins: construction of a phosphorylatable human interferon alpha. Proc Natl Acad Sci U S A. 1989 Jan;86(2):558–562. doi: 10.1073/pnas.86.2.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livesey S. A., Martin T. J. Selective activation of the cAMP-dependent protein kinase isoenzymes. Methods Enzymol. 1988;159:105–118. doi: 10.1016/0076-6879(88)59012-2. [DOI] [PubMed] [Google Scholar]
- Murray K. J., England P. J., Lynham J. A., Mills D., Schmitz-Peiffer C., Reeves M. L. Use of a synthetic dodecapeptide (malantide) to measure the cyclic AMP-dependent protein kinase activity ratio in a variety of tissues. Biochem J. 1990 May 1;267(3):703–708. doi: 10.1042/bj2670703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sala-Newby G., Kalsheker N., Campbell A. K. Production of translatable firefly luciferase mRNA in vitro from cloned cDNA. Biochem Soc Trans. 1990 Jun;18(3):459–460. doi: 10.1042/bst0180459. [DOI] [PubMed] [Google Scholar]
- Sala-Newby G., Kalsheker N., Campbell A. K. Removal of twelve C-terminal amino acids from firefly luciferase abolishes activity. Biochem Biophys Res Commun. 1990 Oct 30;172(2):477–482. doi: 10.1016/0006-291x(90)90697-l. [DOI] [PubMed] [Google Scholar]
- Seliger H. H., McElroy W. D. THE COLORS OF FIREFLY BIOLUMINESCENCE: ENZYME CONFIGURATION AND SPECIES SPECIFICITY. Proc Natl Acad Sci U S A. 1964 Jul;52(1):75–81. doi: 10.1073/pnas.52.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoflet E. S., Koeberl D. D., Sarkar G., Sommer S. S. Genomic amplification with transcript sequencing. Science. 1988 Jan 29;239(4839):491–494. doi: 10.1126/science.3340835. [DOI] [PubMed] [Google Scholar]
- Tatsumi H., Masuda T., Kajiyama N., Nakano E. Luciferase cDNA from Japanese firefly, Luciola cruciata: cloning, structure and expression in Escherichia coli. J Biolumin Chemilumin. 1989 Apr-Jun;3(2):75–78. doi: 10.1002/bio.1170030208. [DOI] [PubMed] [Google Scholar]
- Wood K. V., Lam Y. A., McElroy W. D., Seliger H. H. Bioluminescent click beetles revisited. J Biolumin Chemilumin. 1989 Jul;4(1):31–39. doi: 10.1002/bio.1170040110. [DOI] [PubMed] [Google Scholar]
- Wood K. V., Lam Y. A., Seliger H. H., McElroy W. D. Complementary DNA coding click beetle luciferases can elicit bioluminescence of different colors. Science. 1989 May 12;244(4905):700–702. doi: 10.1126/science.2655091. [DOI] [PubMed] [Google Scholar]
- Zetterqvist O., Ragnarsson U., Humble E., Berglund L., Engström L. The minimum substrate of cyclic AMP-stimulated protein kinase, as studied by synthetic peptides representing the phosphorylatable site of pyruvate kinase (type L) of rat liver. Biochem Biophys Res Commun. 1976 Jun 7;70(3):696–703. doi: 10.1016/0006-291x(76)90648-3. [DOI] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]