Abstract
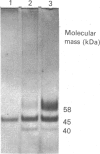
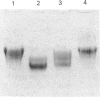
The action of purified rabbit bone stromelysin was investigated on proteoglycan aggregates from pig laryngeal cartilage. The enzyme caused a rapid fall in viscosity of proteoglycan aggregate solution (6 mg/ml), and the products of a partial digest (60% loss of relative viscosity) and a complete digest (95% loss of relative viscosity) were characterized. Analysis by gel chromatography on Sepharose 2B under associative conditions showed that 95% of the glycosaminoglycans in the complete digest were in small-sized fragments, whereas most of the hyaluronan-binding G1 domain and link protein remained intact and bound to hyaluronan. In contrast, there was extensive digestion of the G2 domain which resulted in 76% loss in its detection by immunoassay. Analysis of the partial digest also showed considerable loss (40%) of detection of the G2 domain, but the glycosaminoglycan-rich fragments were much larger than in the complete digest. There was also much less cleavage to create small fragments containing the G1 domain. This was evident on SDS/PAGE analysis where a 58 kDa G1 domain fragment was abundant in the complete digest, but was only present in small amounts in the partial digest. There was also only very limited conversion of link protein from a 44 kDa form to a 40 kDa form. The digestion of proteoglycan aggregate (6 mg/ml) by stromelysin was unaffected by the addition of a high concentration of extra chondroitin sulphate chains (14 mg/ml), and the digestion of proteoglycan monomer showed that the G1 domain was resistant to stromelysin digestion even when not bound to hyaluronan and link protein. The results show that stromelysin degrades the proteoglycan protein core with major cleavages close to, but not within, the G1 domain, and extensive cleavage in other regions. Experiments with purified collagenase, a metalloproteinase structurally related to stromelysin, showed that it too cleaved proteoglycan at several sites within the glycosaminoglycan-rich region of the core protein. Metalloproteinase attack on proteoglycan thus not only occurs with stromelysin but also with collagenase.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Bonnet F., Dunham D. G., Hardingham T. E. Structure and interactions of cartilage proteoglycan binding region and link protein. Biochem J. 1985 May 15;228(1):77–85. doi: 10.1042/bj2280077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell I. K., Roughley P. J., Mort J. S. The action of human articular-cartilage metalloproteinase on proteoglycan and link protein. Similarities between products of degradation in situ and in vitro. Biochem J. 1986 Jul 1;237(1):117–122. doi: 10.1042/bj2370117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney S. L., Bayliss M. T., Collier J. M., Muir H. Electrophoresis of 35S-labeled proteoglycans on polyacrylamide-agarose composite gels and their visualization by fluorography. Anal Biochem. 1986 Jul;156(1):38–44. doi: 10.1016/0003-2697(86)90150-8. [DOI] [PubMed] [Google Scholar]
- Caterson B., Baker J. R., Levitt D., Paslay J. W. Radioimmunoassay of the link proteins associated with bovine nasal cartilage proteoglycan. J Biol Chem. 1979 Oct 10;254(19):9369–9372. [PubMed] [Google Scholar]
- Cooksley S., Hipkiss J. B., Tickle S. P., Holmes-Ievers E., Docherty A. J., Murphy G., Lawson A. D. Immunoassays for the detection of human collagenase, stromelysin, tissue inhibitor of metalloproteinases (TIMP) and enzyme-inhibitor complexes. Matrix. 1990 Oct;10(5):285–291. doi: 10.1016/s0934-8832(11)80183-6. [DOI] [PubMed] [Google Scholar]
- De Blas A. L., Cherwinski H. M. Detection of antigens on nitrocellulose paper immunoblots with monoclonal antibodies. Anal Biochem. 1983 Aug;133(1):214–219. doi: 10.1016/0003-2697(83)90245-2. [DOI] [PubMed] [Google Scholar]
- Doege K., Fernandez P., Hassell J. R., Sasaki M., Yamada Y. Partial cDNA sequence encoding a globular domain at the C terminus of the rat cartilage proteoglycan. J Biol Chem. 1986 Jun 25;261(18):8108–8111. [PubMed] [Google Scholar]
- Dudhia J., Hardingham T. E. The primary structure of human cartilage link protein. Nucleic Acids Res. 1990 Mar 11;18(5):1292–1292. doi: 10.1093/nar/18.5.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Farndale R. W., Sayers C. A., Barrett A. J. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9(4):247–248. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- Fosang A. J., Hardingham T. E. Isolation of the N-terminal globular protein domains from cartilage proteoglycans. Identification of G2 domain and its lack of interaction with hyaluronate and link protein. Biochem J. 1989 Aug 1;261(3):801–809. doi: 10.1042/bj2610801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fosang A. J., Neame P. J., Hardingham T. E., Murphy G., Hamilton J. A. Cleavage of cartilage proteoglycan between G1 and G2 domains by stromelysins. J Biol Chem. 1991 Aug 25;266(24):15579–15582. [PubMed] [Google Scholar]
- Fosang A. J., Tyler J. A., Hardingham T. E. Effect of interleukin-1 and insulin like growth factor-1 on the release of proteoglycan components and hyaluronan from pig articular cartilage in explant culture. Matrix. 1991 Feb;11(1):17–24. doi: 10.1016/s0934-8832(11)80223-4. [DOI] [PubMed] [Google Scholar]
- Frisch S. M., Ruley H. E. Transcription from the stromelysin promoter is induced by interleukin-1 and repressed by dexamethasone. J Biol Chem. 1987 Dec 5;262(34):16300–16304. [PubMed] [Google Scholar]
- Galloway W. A., Murphy G., Sandy J. D., Gavrilovic J., Cawston T. E., Reynolds J. J. Purification and characterization of a rabbit bone metalloproteinase that degrades proteoglycan and other connective-tissue components. Biochem J. 1983 Mar 1;209(3):741–752. doi: 10.1042/bj2090741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. E., Ewins R. J., Muir H. Cartilage proteoglycans. Structure and heterogeneity of the protein core and the effects of specific protein modifications on the binding to hyaluronate. Biochem J. 1976 Jul 1;157(1):127–143. doi: 10.1042/bj1570127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. E., Muir H. The specific interaction of hyaluronic acid with cartillage proteoglycans. Biochim Biophys Acta. 1972 Sep 15;279(2):401–405. doi: 10.1016/0304-4165(72)90160-2. [DOI] [PubMed] [Google Scholar]
- Hardingham T. E. The role of link-protein in the structure of cartilage proteoglycan aggregates. Biochem J. 1979 Jan 1;177(1):237–247. doi: 10.1042/bj1770237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. Proteoglycans: their structure, interactions and molecular organization in cartilage. Biochem Soc Trans. 1981 Dec;9(6):489–497. doi: 10.1042/bst0090489. [DOI] [PubMed] [Google Scholar]
- Heinegård D., Hascall V. C. Aggregation of cartilage proteoglycans. 3. Characteristics of the proteins isolated from trypsin digests of aggregates. J Biol Chem. 1974 Jul 10;249(13):4250–4256. [PubMed] [Google Scholar]
- Kempson G. E., Muir H., Swanson S. A., Freeman M. A. Correlations between stiffness and the chemical constituents of cartilage on the human femoral head. Biochim Biophys Acta. 1970 Jul 21;215(1):70–77. doi: 10.1016/0304-4165(70)90388-0. [DOI] [PubMed] [Google Scholar]
- Kempson G. E., Tuke M. A., Dingle J. T., Barrett A. J., Horsfield P. H. The effects of proteolytic enzymes on the mechanical properties of adult human articular cartilage. Biochim Biophys Acta. 1976 May 28;428(3):741–760. doi: 10.1016/0304-4165(76)90205-1. [DOI] [PubMed] [Google Scholar]
- Martel-Pelletier J., Pelletier J. P., Malemud C. J. Activation of neutral metalloprotease in human osteoarthritic knee cartilage: evidence for degradation in the core protein of sulphated proteoglycan. Ann Rheum Dis. 1988 Oct;47(10):801–808. doi: 10.1136/ard.47.10.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt C. A., Muir H. Gel electrophoresis of proteoglycans and glycosaminoglycans on large-pore composite polyacrylamide-agarose gels. Anal Biochem. 1971 Dec;44(2):612–622. doi: 10.1016/0003-2697(71)90250-8. [DOI] [PubMed] [Google Scholar]
- Morales T. I., Kuettner K. E. The properties of the neutral proteinase released by primary chondrocyte cultures and its action on proteoglycan aggregate. Biochim Biophys Acta. 1982 Jul 12;705(1):92–101. doi: 10.1016/0167-4838(82)90340-5. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Mort J. S., Poole A. R., Roughley P. J. Age-related changes in the structure of proteoglycan link proteins present in normal human articular cartilage. Biochem J. 1983 Jul 15;214(1):269–272. doi: 10.1042/bj2140269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G., Cockett M. I., Stephens P. E., Smith B. J., Docherty A. J. Stromelysin is an activator of procollagenase. A study with natural and recombinant enzymes. Biochem J. 1987 Nov 15;248(1):265–268. doi: 10.1042/bj2480265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G., Hembry R. M., McGarrity A. M., Reynolds J. J., Henderson B. Gelatinase (type IV collagenase) immunolocalization in cells and tissues: use of an antiserum to rabbit bone gelatinase that identifies high and low Mr forms. J Cell Sci. 1989 Mar;92(Pt 3):487–495. doi: 10.1242/jcs.92.3.487. [DOI] [PubMed] [Google Scholar]
- Murphy G., Hembry R. M., Reynolds J. J. Characterization of a specific antiserum to rabbit stromelysin and demonstration of the synthesis of collagenase and stromelysin by stimulated rabbit articular chondrocytes. Coll Relat Res. 1986 Oct;6(4):351–363. doi: 10.1016/s0174-173x(86)80005-x. [DOI] [PubMed] [Google Scholar]
- Mörgelin M., Paulsson M., Hardingham T. E., Heinegård D., Engel J. Cartilage proteoglycans. Assembly with hyaluronate and link protein as studied by electron microscopy. Biochem J. 1988 Jul 1;253(1):175–185. doi: 10.1042/bj2530175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen Q., Murphy G., Roughley P. J., Mort J. S. Degradation of proteoglycan aggregate by a cartilage metalloproteinase. Evidence for the involvement of stromelysin in the generation of link protein heterogeneity in situ. Biochem J. 1989 Apr 1;259(1):61–67. doi: 10.1042/bj2590061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno H., Blackwell J., Jamieson A. M., Carrino D. A., Caplan A. I. Calibration of the relative molecular mass of proteoglycan subunit by column chromatography on Sepharose CL-2B. Biochem J. 1986 Apr 15;235(2):553–557. doi: 10.1042/bj2350553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y., Nagase H., Harris E. D., Jr A metalloproteinase from human rheumatoid synovial fibroblasts that digests connective tissue matrix components. Purification and characterization. J Biol Chem. 1986 Oct 25;261(30):14245–14255. [PubMed] [Google Scholar]
- Paulsson M., Mörgelin M., Wiedemann H., Beardmore-Gray M., Dunham D., Hardingham T., Heinegård D., Timpl R., Engel J. Extended and globular protein domains in cartilage proteoglycans. Biochem J. 1987 Aug 1;245(3):763–772. doi: 10.1042/bj2450763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J. P., Martel-Pelletier J., Malemud C. J. Canine osteoarthritis: effects of endogenous neutral metalloproteoglycanases on articular cartilage proteoglycans. J Orthop Res. 1988;6(3):379–388. doi: 10.1002/jor.1100060309. [DOI] [PubMed] [Google Scholar]
- Perkins S. J., Nealis A. S., Dudhia J., Hardingham T. E. Immunoglobulin fold and tandem repeat structures in proteoglycan N-terminal domains and link protein. J Mol Biol. 1989 Apr 20;206(4):737–753. doi: 10.1016/0022-2836(89)90580-9. [DOI] [PubMed] [Google Scholar]
- Ratcliffe A., Hardingham T. Cartilage proteoglycan binding region and link protein. Radioimmunoassays and the detection of masked determinants in aggregates. Biochem J. 1983 Aug 1;213(2):371–378. doi: 10.1042/bj2130371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe A., Tyler J. A., Hardingham T. E. Articular cartilage cultured with interleukin 1. Increased release of link protein, hyaluronate-binding region and other proteoglycan fragments. Biochem J. 1986 Sep 1;238(2):571–580. doi: 10.1042/bj2380571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandy J. D., Neame P. J., Boynton R. E., Flannery C. R. Catabolism of aggrecan in cartilage explants. Identification of a major cleavage site within the interglobular domain. J Biol Chem. 1991 May 15;266(14):8683–8685. [PubMed] [Google Scholar]
- Sapolsky A. I., Keiser H., Howell D. S., Woessner J. F., Jr Metalloproteases of human articular cartilage that digest cartilage proteoglycan at neutral and acid pH. J Clin Invest. 1976 Oct;58(4):1030–1041. doi: 10.1172/JCI108526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler J. A. Articular cartilage cultured with catabolin (pig interleukin 1) synthesizes a decreased number of normal proteoglycan molecules. Biochem J. 1985 May 1;227(3):869–878. doi: 10.1042/bj2270869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler J. A. Chondrocyte-mediated depletion of articular cartilage proteoglycans in vitro. Biochem J. 1985 Jan 15;225(2):493–507. doi: 10.1042/bj2250493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitham S. E., Murphy G., Angel P., Rahmsdorf H. J., Smith B. J., Lyons A., Harris T. J., Reynolds J. J., Herrlich P., Docherty A. J. Comparison of human stromelysin and collagenase by cloning and sequence analysis. Biochem J. 1986 Dec 15;240(3):913–916. doi: 10.1042/bj2400913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedemann H., Paulsson M., Timpl R., Engel J., Heinegård D. Domain structure of cartilage proteoglycans revealed by rotary shadowing of intact and fragmented molecules. Biochem J. 1984 Nov 15;224(1):331–333. doi: 10.1042/bj2240331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanetti M., Ratcliffe A., Watt F. M. Two subpopulations of differentiated chondrocytes identified with a monoclonal antibody to keratan sulfate. J Cell Biol. 1985 Jul;101(1):53–59. doi: 10.1083/jcb.101.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]