Abstract
Background
Long-term management of patients with hypothyroidism on thyroxine replacement requires thyroid function test (TFT) monitoring once every six–12 months as recommended by clinical practice guidelines. This study determined their thyroid function status during two-year follow-up visits in primary care, and the factors influencing their thyroid status, and assessed the optimal interval for TFTs.
Methods
A retrospective cohort study was conducted on adults with a clinical diagnosis code for hypothyroidism in their electronic health records taken from a group of polyclinics in Singapore between July 2017 and June 2020. The follow-up thyroid status was categorized as under-replacement (TSH ≥ 3.70mIU/L), over-replacement (TSH ≤ 0.65mIU/L) or euthyroid (TSH 0.65–3.70mIU/L). The patients’ demographic, clinical and TFT data were analyzed using appropriate statistical tests during the two-year follow-up. Stepwise logistic regression analysis identified the factors associated with suboptimal thyroid control. Kaplan–Meier analysis was used to compare their thyroid function status in association with the interval between TFT monitoring.
Results
Data from 5,749 eligible subjects (mean age 62.1 ± 13.29 years; 79% female; 79.7% Chinese) were analyzed. After a two-year follow-up, 61.9% (n = 3558) of all subjects were euthyroid, with 29.5% (n = 1694) being under-replaced and 8.6% (n = 497) over-replaced. However, thyroid status did not differ significantly with the various dose regimen (daily, segmented, or alternate days) (p = 0.193). Stepwise logistic regression showed that thyroxine under-replacement was significantly associated with the male gender (AOR = 1.25,95%CI = 1.03–1.51,p = 0.02) and obesity (AOR = 1.34,95%CI = 1.08–1.66,p = 0.008). Every unit (μg/kg body weight) increase in the mean daily thyroxine dose was associated with 2.72 times greater odds of over-replacement. When comparing thyroid function monitoring at intervals of 13-24 months, monitoring at shorter intervals (≤ 12 months) was less likely to detect thyroxine under-replacement (AOR = 0.57,95%CI = 0.44–0.74,p < 0.001) and over-replacement (AOR = 0.62,95%CI = 0.41–0.97,p = 0.033). Among the 3,312 adults who were euthyroid at baseline, 22.2%, 41.7% and 59.6% had suboptimal thyroid control at 6, 12 and 24 months respectively (Kaplan–Meier analysis).
Conclusion
Around six in ten patients were euthyroid with thyroxine replacement for hypothyroidism in primary care over two years. Thyroxine under-replacement was associated with male gender and obesity. The proportion of euthyroid patients developing abnormal thyroid function doubled with TFTs at six, 12 and 24-month intervals.
Keywords: Hypothyroidism, Outpatient monitoring, Primary care, Thyroxine, Thyroid function tests
Introduction
Levothyroxine (henceforth referred to as thyroxine), a synthetic form of the thyroid hormone thyroxine, is the most frequently prescribed and clinically effective treatment for hypothyroidism [1]. Hypothyroidism is often managed by primary care physicians. Most patients with hypothyroidism will require lifelong thyroxine replacement therapy (TRT) to alleviate symptoms and prevent long-term consequences [2]. Symptoms range from those at the milder end of the spectrum, such as weight gain and exhaustion, to life-threatening coma. Evidence suggests that an observed mean replacement dose of 1.1 μg per kg per day is required to establish an euthyroid state for Asian adults [3].
Nonetheless, a significant proportion (40%) of adults with hypothyroidism receiving thyroxine replacement failed to attain euthyroid status on follow-up in a cross-sectional study [4]. The risk of being over- and under-replaced ranges from 1.3–19.8% and 3.0–27.5% respectively [3, 5–8]. Poor medication adherence is the most common cause of suboptimal control [2, 8]. Hence, regular thyroid function monitoring is scheduled to ensure medication adherence and prevent complications in sub-optimal thyroxine-treated patients.
A thyroid function test (TFT), measuring serum thyroxine and thyroid stimulating hormone (TSH), is commonly used to assess the thyroid status of patients with thyroid disorders, including those with hypothyroidism. Clinical practice guidelines (CPG) recommend that most adults with hypothyroidism undergo annual thyroid function testing [2]. However, there is limited evidence to support this frequency of thyroid function monitoring. Viswanath et al. have suggested that monitoring at 18-month intervals may be sufficient for hypothyroid adults aged under 60 years of age receiving a stable dose of 100–150 μg of thyroxine [9]. A retrospective population-based cohort study has also reported that patients on a medium dose of thyroxine (75 μg–125 μg) had higher TSH stability to support a longer interval between thyroid function tests [10].
Within a fee-for-service healthcare system, such as those in Singapore, patients pay for their TRT and TFT [3]. The charges for TFT can be substantial, which can lead to missed testing, particularly among patients with lower socioeconomic status. Therefore, a cautious approach should be taken to assess the interval between TFTs, as increasing the frequency of testing will result in additional financial burden on patients and the healthcare system, while reducing frequency may result in sub-optimal thyroxine replacement among patients, leading to subsequent complications.
Hence, the aim of this study is to determine the thyroid status of hypothyroid subjects treated with TRT based on their TFTs over a two-year observation period within primary care. It also aims to identify the time interval and factors associated with their thyroid status during their follow-up review by primary care physicians. The results will provide insight into management of these patients within primary care services.
Method
Study design, setting and population
A retrospective cohort study was conducted using clinical data extracted from the electronic medical records (EMR) of SingHealth Polyclinics (SHP). It comprised a network of eight public primary care clinics (polyclinics) located across the eastern region of Singapore. These polyclinics, which manage about 1.6 million patient appointments each year, are the study sites [11].
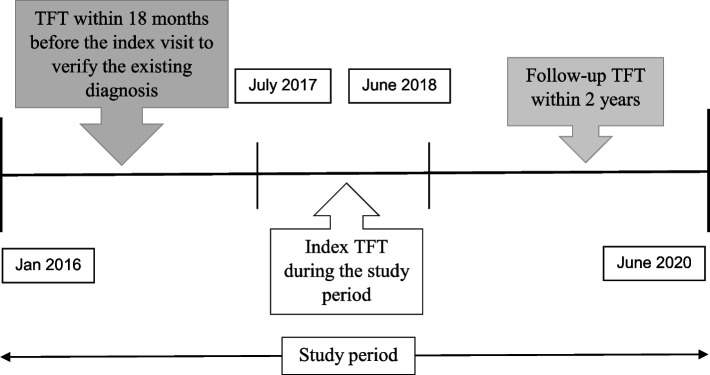
The study population comprised multi-ethnic adults aged 21 years or older, with a clinical diagnosis of hypothyroidism based on having International Classification of Disease 10th edition (ICD-10) code in their EMR. The inclusion criteria for our subjects were as follows: (1) attended a consultation for hypothyroidism at any of the study sites with an index TFT between July 2017 and June 2018; (2) had at least one subsequent TFT within two years of their index test; (3) had at least two prescribed thyroxine therapies between July 2017 and June 2019 (Fig. 1) Subjects without a TFT from 18 months prior to their index TFT and/or thyroxine replacement before the index TFT were excluded from the study. Patients with secondary hypothyroidism, typically followed up in specialty clinics, were not included in the analysis.
Fig. 1.
Illustration to identify Thyroid Function Test (TFT) pairs for subjects with hypothyroidism
The thyroxine replacement regimens could be either daily, segmented (for example: six days of the same treatment and one day at a different dose [1/6], or alternatively [2/5]) or an alternate-day dosing regimen (for example: one day of dose X and the next day of dose Y, then repeat). The weekly total dose was calculated from these regimens and the average daily dose was used in the analysis. The mean daily thyroxine dosage was categorized as low dose replacement (≤ 75 μg/day), medium dose (75–125 μg/day) and high dose (≥ 125 μg/day) [10]. The first thyroxine dose recorded in the study period was considered as the index dose. Body mass index (BMI) was categorized according to the WHO Asian guidelines; underweight (< 18.5 kg/m2), normal (18.5–22.9 kg/m2), overweight (23–27.5 kg/m2) and obese (> 27.5 kg/m2).
Data extraction
The extracted data included demographic variables (age at index visit, gender and ethnicity), clinical parameters (weight and height), thyroxine replacement regimens and TFT results (serum TSH and free T4 levels). The total weekly and mean daily thyroxine doses per kilogram of body weight (BW) of these subjects were calculated as independent variables. The data was de-identified by a data management officer before statistical analysis was performed by a biostatistician.
Outcome definition
The thyroid status of the study subjects is the primary clinical outcome. It is based solely on their TSH level from the first TFT conducted at between 12 and 24 months, with or without an increased or decreased serum T4 level: (1) “euthyroid” (reflecting adequate thyroxine replacement), (2) “hypothyroid” (under-replacement) and (3) “hyperthyroid” (over-replacement). “Hypothyroidism” was defined as TSH ≥ 3.70 mIU/L; “hyperthyroidism” was defined as TSH ≤ 0.65 mIU/L. “Euthyroidism” refers to those subjects with TSH 0.65–3.70 mIU/L [3].
Patient and public involvement statement
In the context of the EMR-based retrospective cohort study, there was no involvement of patients or the public at any stage of the research design, implementation, reporting or dissemination phases.
Statistical analysis
The prevalence of thyroid status based on TSH were presented as frequencies and percentages. Data were presented as frequencies and percentages for categorical variables and tested for association using the chi-square test. Descriptive statistics were calculated for continuous variables and compared against thyroid control status at follow-up using ANOVA. Parametric data were presented in mean values with standard deviation, while non-parametric data were presented in median values with interquartile range. Factors with a significance of p < 0.2 in the bivariate analysis were further analyzed in a stepwise logistic regression model to find the association with sub-optimal thyroid control status. Index TSH was also included as a covariate in the regression model to adjust for potential confounding effects. Adjusted odds ratio, 95% confidence interval and p-value were presented. Statistical significance was set at p < 0.05. Kaplan–Meier analysis was used to study the time to thyroid function status of interest at follow-up for all thyroid statuses at baseline. All TFT results following the index were considered until the occurrence of the event of interest. The subjects were then right censored after the event. To correct for multiple comparisons across different frequencies of TFT monitoring, the Bonferroni correction was utilized. The corrected p-value threshold was set at p < 0.01. All analysis was carried out using R 3.5.2 on RStudio with the tidyverse library.
Results
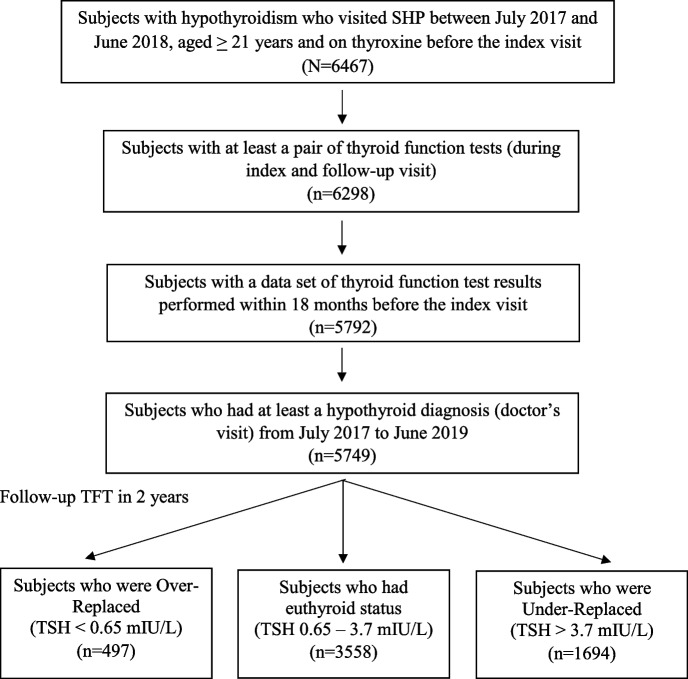
The EMR data included 6,467 study subjects with hypothyroidism, of whom 718 subjects were excluded because they did not have a follow-up TFT and/or did not have a TFT 18 months prior to their index visit. Cohorts of 497 (8.6%), 3,558 (61.9%), and 1,694 (29.5%) subjects in the “Over-replaced,” “Euthyroid,” and “Under-replaced” categories, respectively, were then identified among the 5,749 study subjects based on their follow-up TFT (Fig. 2). The median follow-up duration during the study period was 327 (280–349) days.
Fig. 2.
Flow chart illustrating the derivation of the treatment outcome cohorts. Abbreviation: n number of Study subjects; TFT Thyroid Function Test; TSH Thyroid Stimulating Hormone
Patient demographics
The overall mean age of the study subjects was 62.1 (± 13.29) years. The majority of the study subjects were Chinese (79.7%), followed by Indian (9.9%) and Malay (6.3%) with a preponderance of female subjects (79%). Their median BMI was 24.6 (22–27.7) kg/m2. A large proportion (89.5%) of the subjects had their follow-up TFT within 12 months, while the remainder had their follow-up within between 13 and 24 months. Table 1 shows the study subjects’ descriptive characteristics and the results of the bivariate analysis. Gender, ethnicity, and body mass index were all significantly associated with the follow-up thyroid status (p < 0.001).
Table 1.
Characteristics of the subjects with hypothyroidism on thyroxine medication
| Follow-Up TFTa | |||||
|---|---|---|---|---|---|
| Total | Under-replace | Euthyroid | Over-replace | P-value | |
| Total | 5749 (100) | 1694 (29.5) | 3558 (61.9) | 497 (8.6) | |
| Age | 62.1 (13.29) | 62.25 (13.76) | 62.22 (12.99) | 60.79 (13.69) | 0.07 |
| Gender | < 0.001 | ||||
| Female | 4539 (79) | 1273 (28) | 2865 (63.1) | 401 (8.8) | |
| Male | 1210 (21) | 421 (34.8) | 693 (57.3) | 96 (7.9) | |
| Ethnicity | < 0.001 | ||||
| Chinese | 4583 (79.7) | 1301 (28.4) | 2906 (63.4) | 376 (8.2) | |
| Malay | 365 (6.3) | 130 (35.6) | 190 (52.1) | 45 (12.3) | |
| Indian | 572 (9.9) | 199 (34.8) | 321 (56.1) | 52 (9.1) | |
| Other | 229 (4) | 64 (27.9) | 141 (61.6) | 24 (10.5) | |
| BMIb | < 0.001 | ||||
| Underweight | 156 (4.6) | 58 (37.2) | 90 (57.7) | 8 (5.1) | |
| Normal | 1013 (29.8) | 270 (26.7) | 653 (64.5) | 90 (8.9) | |
| Overweight | 1323 (38.9) | 368 (27.8) | 836 (63.2) | 119 (9) | |
| Obese | 909 (26.7) | 315 (34.7) | 517 (56.9) | 77 (8.5) | |
aThyroid function tests
bBMI Body Mass Index
Thyroxine dosing factors
The mean daily thyroxine dose at the index visit was 1.14 (± 0.43) μg/kg BW. Subjects in both the under-replaced and over-replaced group had a higher mean daily thyroxine dose than those who had euthyroid status (p < 0.001). The most common dosing regimen was daily dose regimen (45.5%), followed by segmented dose regimen (2/5) (27.2%). Compared to subjects on a daily dose and alternate-days dosing regimen, those on a segmented dosing (1/6) regimen had a greater mean daily thyroxine dose (1.23 ± 0.37 μg/kg BW). Nevertheless, there were no significant differences in the follow-up thyroid status between the various dosing regimens (p = 0.193). The median TSH at the index visit was 2.5 (1.3–4.37) mIU/L.
The average number of filled thyroxine prescriptions during the study period was 7.03 (± 2.57), which was significantly associated with differences in follow-up thyroid status (p < 0.001). (Table 2) Subjects who were under- or over-replaced at follow-up had significantly more TFTs compared to euthyroid subjects (p < 0.001). However, sub-optimal thyroid status (both under- and over-replacement) did not differ significantly between follow-up TFT within one year (≤ 12 months) (37.7%) and after one year (13–24 months) (41.3%, p = 0.178). The results are summarized in Table 2.
Table 2.
Association between thyroxine replacement regimen and thyroid status in subjects with hypothyroidism
| Follow-Up TFTa | |||||
|---|---|---|---|---|---|
| Total | Under-replace | Euthyroid | Over-replace | P-value | |
| Index thyroxine Dose regimen | 0.193 | ||||
| Segmented dose (1/6) | 456 (7.9) | 135 (29.6) | 270 (59.2) | 51 (11.2) | |
| Segmented dose (2/5) | 1565 (27.2) | 461 (29.5) | 956 (61.1) | 148 (9.5) | |
| Alternate dose | 1112 (19.3) | 319 (28.7) | 696 (62.6) | 97 (8.7) | |
| Daily dose | 2616 (45.5) | 779 (29.8) | 1636 (62.5) | 201 (7.7) | |
| Index thyroxine Dose per kg BWb | 1.14 (0.43) | 1.15 (0.46) | 1.11 (0.41) | 1.33 (0.43) | < 0.001 |
| Index thyroxine Daily dose | < 0.001 | ||||
| High (≥ 125 μg) | 160 (2.8) | 61 (38.1) | 68 (42.5) | 31 (19.4) | |
| Low (≤ 75 μg) | 2954 (51.4) | 858 (29) | 1931 (65.4) | 165 (5.6) | |
| Medium (75–125 μg) | 2635 (45.8) | 775 (29.4) | 1559 (59.2) | 301 (11.4) | |
| Follow-up period | 0.178 | ||||
| Between 13 and 24 months | 606 (10.5) | 198 (32.7) | 356 (58.7) | 52 (8.6) | |
| Within one year (≤ 12 months) | 5143 (89.5) | 1496 (29.1) | 3202 (62.3) | 445 (8.7) | |
| Number of thyroxine prescriptionsc | 7.03 (2.57) | 7.45 (2.58) | 6.78 (2.51) | 7.38 (2.75) | < 0.001 |
| Number of thyroid function testsd | 4.58 (1.79) | 5.06 (1.88) | 4.27 (1.67) | 5.13 (1.85) | < 0.001 |
aThyroid function tests
bPer kilogram of body weight
cThyroxine prescription within 24 months from index thyroid test
dNumber of thyroid tests within 24 months from index thyroid test
Factors affecting follow-up thyroid status
The proportion of subjects who achieved euthyroid status increased by 4.7% (57.2% to 61.9%) from their thyroid status at the index visit. Stepwise logistic regression after adjusting for index TSH status was performed for follow-up thyroid status (p < 0.2) and showed that patients with a follow-up TFT within one year (≤ 12 months) had lower odds of being diagnosed as under-replaced (AOR = 0.57, 95%CI = 0.44 to 0.74, p < 0.001) and over-replaced (AOR = 0.62, 95%CI = 0.41 to 0.97, p = 0.033), compared to having a TFT at between 13 and 24 months. Adults with hypothyroidism who were under-replaced on follow-up were more likely to be male (AOR = 1.25, 95%CI = 1.03 to 1.51, p = 0.02) and obese (AOR = 1.34, 95%CI = 1.08 to 1.66, p = 0.008) (Table 3). Every unit increase in mean daily thyroxine per kg BW (μg/kg BW) resulted in 2.72 times increased odds of over-replacement (95%CI = 1.99 to 3.75, p < 0.001) and 1.22 times increased odds of under-replacement (95%CI = 1.01 to 1.48, p = 0.047).
Table 3.
Factors associated with under-replaced and over-replaced thyroid status using stepwise logistic regression
| Adjusted Odds Ratio (95%CI) | P-value | |
|---|---|---|
| Under-Replacement (TSH > 3.7 mIU/L)a | ||
| Gender | ||
| Female | Ref | - |
| Male | 1.25 (1.03 to 1.51) | 0.02 |
| BMI | ||
| Underweight | 1.42 (0.96 to 2.08) | 0.077 |
| Normal | Ref | - |
| Overweight | 1.01 (0.83 to 1.24) | 0.885 |
| Obese | 1.34 (1.08 to 1.66) | 0.008 |
| Follow-up period | ||
| Within one year (≤ 12 months) | 0.57 (0.44 to 0.74) | < 0.001 |
| Between 13 and 24 Months | Ref | - |
| Index daily thyroxine dose per kg BW | 1.22 (1.01 to 1.48) | 0.047 |
| Number of thyroid function tests | 1.22 (1.16 to 1.28) | < 0.001 |
| Over-Replacement (TSH < 0.65 mIU/L)a | ||
| BMI | ||
| Underweight | 0.48 (0.20 to 1.02) | 0.074 |
| Normal | Ref | - |
| Overweight | 1.16 (0.85 to 1.58) | 0.343 |
| Obese | 1.29 (0.91 to 1.83) | 0.149 |
| Follow-up period | ||
| Within one year (≤ 12 months) | 0.62 (0.41 to 0.97) | 0.033 |
| Between 13 and 24 months | Ref | - |
| Index daily thyroxine dose per kg BW | 2.72 (1.99 to 3.75) | < 0.001 |
| Number of thyroid function tests | 1.21 (1.12 to 1.31) | < 0.001 |
aAdjusted for index TSH status
Patients who were under-replaced and over-replaced had an increased (1.22X and 1.21X respectively) likelihood of undergoing more TFTs during their review (under-replaced: 95%CI = 1.16 to 1.28, p < 0.001; over-replaced: 95%CI = 1.12 to 1.31, p < 0.001). However, an increased number of TFTs (≥ 6 TFTs) in the two years following the abnormal index TFT was not associated with normalized thyroid function. After adjustment using the Bonferroni correction, subjects who had four (53.5%) or five TFTs (53.7%) within a two-year follow-up significantly achieved euthyroid status compared to six or more TFTs (41.1%) (p < 0.001). (Table 4).
Table 4.
Frequency of follow-up TFTs among subjects with hypothyroidism with sub-optimal control
| Follow Up TFTa | |||||
|---|---|---|---|---|---|
| Total | Under-Replace | Euthyroid | Over-Replace | P-value | |
| Number of TFTs within 24 Monthsa | < 0.001 | ||||
| 1–2 | 139 (5.7) | 58 (5.8) | 68 (5.8) | 13 (4.6) | |
| 3 | 280 (11.4) | 117 (11.6) | 139 (11.9) | 24 (8.5) | |
| 4 | 456 (18.5) | 160 (15.9) | 244 (20.8) | 52 (18.4) | |
| 5 | 553 (22.5) | 204 (20.3) | 297 (25.3) | 52 (18.4) | |
| 6 or more | 1031 (41.9) | 466 (46.4) | 424 (36.2) | 141 (50.1) | |
aThyroid function tests
Optimal time for thyroid function monitoring
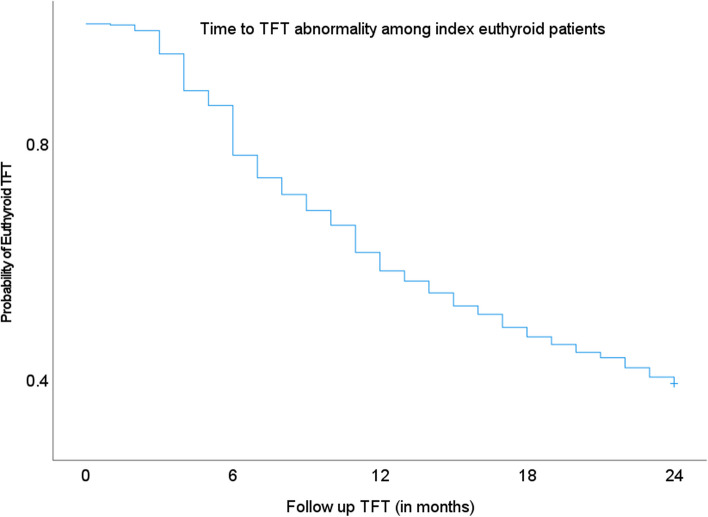
Kaplan–Meier analysis of TFT abnormality after initial euthyroid status demonstrated the largest increase in the proportion of abnormal TFT results at the sixth month (8.4%). (Fig. 3) However, only 22.2% of euthyroid subjects required a change in their thyroxine dose within six months from their index TFT. If thyroid testing were to be conducted annually, it would be able to detect 41.7% of euthyroid subjects that became abnormal. 40.4% of subjects maintained their euthyroid status at the end of two years from the index TFT.
Fig. 3.
Kaplan–Meier curve showing probability of TFT abnormality among index euthyroid subjects in their subsequent follow-up tests
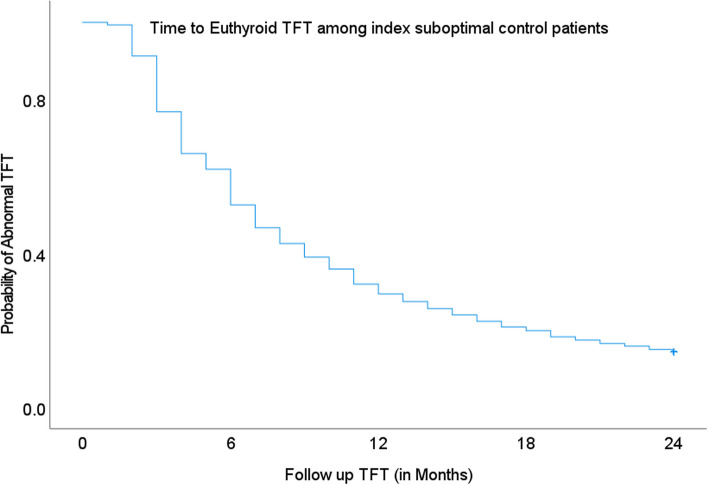
Among the subjects with index sub-optimal thyroid control, TFTs at a three-month follow-up showed that 23.3% achieved euthyroid status. The proportion doubled in the sixth month with 47.5% attaining euthyroid status. Annual TFTs revealed that 31% remained sub-optimal during follow-up at the 12th month. (Fig. 4).
Fig. 4.
Kaplan–Meier curve showing probability of euthyroid TFT among index sub-optimal thyroid control subjects in their subsequent follow-up tests
Discussion
Adults with hypothyroidism on thyroxine replacement undergo periodic clinical and biochemical monitoring every six to 12 months at the polyclinics. The combined prevalence of sub-optimal thyroid control in the study population was 38.1% (29.5% under-replacement and 8.6% over-replacement) over two years. A similar prevalence of under-replacement (27.5%) and over-replacement (12.7%) was described in a local cross-sectional primary care study in 2017 [3]. Studies in western populations reported that the prevalence of under- and over-replacement ranged between 3.0–17.4% and 1.3–19.8% respectively [5–7]. Factors which interfere with thyroxine absorption and bioavailability, such as GI comorbidities or concurrent use of supplements like iron and calcium and poor medication adherence, may result in abnormal thyroid function [12–15].
The association between inadequate thyroxine replacement and male gender and obesity in this study were also reported by Okosieme et al. (2011) in a western population [7]. While males were more likely to be under-replaced in this study population, other studies have reported that females are more susceptible to be under-replaced as they require a higher mean dose [1, 6, 16, 17]. Further exploration using other research methodology is required to understand gender difference in thyroxine replacement. Michalaki et al. have attributed the association between obesity and insufficient thyroxine replacement to disturbances in small bowel motility [18]. This insight highlights the intricate interplay between thyroid function and gastrointestinal physiology, suggesting that suboptimal replacement therapy may exacerbate metabolic imbalances, contributing to weight gain in obese individuals. Building on this, Jonklaas et al. highlighted the importance of personalized dosage adjustments in obese patients undergoing TRT [19]. It was reported that obese individuals may require higher-than-normal doses of thyroxine. This finding underscores the complexity of TRT in obese subjects, emphasizing the need for tailored treatment strategies.
The mean daily thyroxine dose among this cohort of adults with hypothyroidism was 1.14 μg/kg BW, similar to Asian studies which reported a mean daily thyroxine dose of 1.1–1.2 μg/kg BW [3, 8] This is lower than that recommended (1.6 μg/kg BW) by the American Academy of Family Physicians (AAFP) [2]. Okosieme et al. reported a mean daily thyroxine dose of 1.36 μg/kg BW in European patients with hypothyroidism [7]. The thyroxine dose requirement is lower than the AAFP recommendations due to guidelines based on ideal body weight (BMI) or lean body mass instead of actual body weight [20]. Hence, without clarification of these dose requirement guidelines, some patients may receive a higher thyroxine dose. The study subjects who were over-replaced had a higher mean thyroxine dose of 1.33 μg/kg BW compared to the euthyroid (1.11 μg/kg BW) and under-replaced (1.15 μg/kg BW) cohorts. Tan NC et al. reported a similar finding that patients who received a mean daily thyroxine dose of 1.4 μg/kg BW were over-replaced in an Asian population [3].
Subgroup analysis among subjects with index sub-optimal thyroid control (Table 4) showed that the majority with ≤ 5 TFTs achieved euthyroid status compared to those with six or more TFTs within two years. Thus, having more frequent TFTs without addressing the underlying factors leading to sub-optimal thyroid control (such as poor adherence) and titrating the thyroxine dose may not be beneficial in normalizing thyroid function. Hence, primary care physicians should instead investigate patient’s adherence to their prescribed therapy before advocating a TFT, and explore factors which could interfere with their thyroxine replacement, such as concomitant consumption of calcium supplements [12–15].
The study investigated the optimal frequency of thyroid function testing in individuals with index euthyroid status through Kaplan Meier analysis, revealing an escalating proportion developing abnormal thyroid function over six, 12, and 24 months. The findings offer valuable insights into the prevalence of TFT abnormalities among those receiving TRT over various time intervals. Understanding the temporal patterns of TFT abnormalities among individuals undergoing TRT provides clinicians with valuable information for optimizing treatment regimens and monitoring strategies. The observed spike in TFT abnormality at the sixth month corresponds to the typical thyroid function monitoring interval of between six and 12 months, per local clinical practice guidelines.
The study also highlights the dynamic nature of thyroid function in response to treatment, with a notable increase in the proportion of patients achieving euthyroid status over time. The doubling of euthyroid rates from approximately one in four at three months to nearly half at six months underscores the effectiveness of interventions in restoring thyroid hormones and emphasizes the importance of ongoing monitoring and adjustment of treatment protocols. Similarly, Lindgård Nielsen et al. reported that a significant proportion (67.7%) achieved a clinically acceptable TSH range during the follow-up period, with a median time of 4.3 months to achieve this [21]. These insights underscore the dynamic nature of thyroid function normalization, emphasizing the need for personalized approaches to optimize treatment outcomes and mitigate the risk of complications associated with thyroid dysfunction.
Strengths
Although various studies have investigated the treatment outcome with thyroxine replacement among patients with hypothyroidism, this is likely the first study to focus on the optimal frequency of thyroid function monitoring.
Limitations
The retrospective study has its limitations. While the EMR in our primary care setting use ICD-10-AM coding, certain factors such as pregnancy status, medication usage, and earlier history of hyperthyroidism treatment were not systematically recorded in the EMR. Additionally other variables that reduce thyroxine bioavailability and medication adherence were also not available in the EMR in the primary care setting. Another drawback is that only the first follow-up TFT was arbitrarily used to analyze thyroid status while other subsequent TFTs were excluded from the regression analysis. However consecutive TFTs were utilized for Kaplan–Meier analysis. In addition the thyroid function of the study subjects was classified based solely on TSH levels without reviewing FT3 and FT4 levels.
Conclusion
Around six in ten study subjects were euthyroid with thyroxine replacement for hypothyroidism in primary care over two years. Thyroxine under-replacement was associated with males and obesity. A mean daily thyroxine dose of 1.11 μg/kg BW was required by most of the Asian patients to achieve euthyroid status. Those who were over-replaced had a higher mean daily thyroxine dose than the other two groups of patients. Primary care physicians should take note of the lower daily thyroxine replacement dose for their Asian patients with hypothyroidism, and explore medication adherence and known factors which interfere with thyroxine replacement before considering another TFT.
Acknowledgements
The authors would like to thank P Kin and L Paulpandi for the administrative support and WK Aau from SingHealth Polyclinics Research Department for the data extraction and de-identification.
Abbreviations
- 95%CI
95% Confidence Interval
- AAFP
American Academy of Family Physicians
- ANOVA
Analysis of Variance
- AOR
Adjusted Odds Ratio
- BMI
Body Mass Index
- BW
Body Weight
- CPG
Clinical Practice Guidelines
- EMR
Electronic Medical Records
- FT3
Free Triiodothyronine
- FT4
Free Thyroxine
- GI
Gastro-Intestinal
- ICD
International Classification of Diseases
- SHP
SingHealth Polyclinics
- TFT
Thyroid Function Test
- TSH
Thyroid Stimulating Hormone
- WHO
World Health Organization
Authors’ contributions
TNC conceptualized and designed the study, with input from GK and NDX. NDX performed the data analysis. GK wrote the initial draft of the paper, to which the rest of the authors provided comments. All authors reviewed and approved the final manuscript.
Funding
This research is supported by the SingHealth Duke-NUS Family Medicine Academic Clinical Programme. The publication cost is supported by Research, Singhealth Polyclinics.
Availability of data and materials
The datasets analyzed during the current study are not publicly available as they contain information that are sensitive to the study institution. They may be made available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Approval for exemption from review was obtained from the SingHealth Centralized Institution Review Board in 2021 (SingHealth CIRB Reference Number: 2021/2231) due to the use of de-identified data in this retrospective study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chiovato L, Magri F, Carlé A. Hypothyroidism in context: where we’ve been and where we’re going. Adv Ther. 2019;36:47–58. 10.1007/s12325-019-01080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaitonde DY, Rowley KD, Sweeney LB. Hypothyroidism: an update. Am Fam Physician. 2012;86(3):244–51. [PubMed] [Google Scholar]
- 3.Tan NC, Chew RQ, Koh YLE, Subramanian RC, Sankari U, Meyappan M, et al. Primary hypothyroidism in the community: lower daily dosages of levothyroxine replacement therapy for Asian patients. Medicine (Baltimore). 2017;96(7):e6145. 10.1097/MD.0000000000006145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160(4):526–34. 10.1001/archinte.160.4.526. [DOI] [PubMed] [Google Scholar]
- 5.La Cour JL, Medici BR, Grand MK, Nicolaisdottir DR, Lind B, Faber J, et al. Risk of over-and under-treatment with levothyroxine in primary care in Copenhagen. Denmark Eur J Endocrinol. 2021;185(5):673–9. 10.1530/EJE-21-0485. [DOI] [PubMed] [Google Scholar]
- 6.Kostev K. Frequency of over- and under-treatment with levothyroxine in primary care in Germany. Eur J Endocrinol. 2022;186(3):L5. 10.1530/EJE-21-0916. [DOI] [PubMed] [Google Scholar]
- 7.Okosieme OE, Belludi G, Spittle K, Kadiyala R, Richards J. Adequacy of thyroid hormone replacement in a general population. QJM. 2011;104(5):395–401. 10.1093/qjmed/hcq222. [DOI] [PubMed] [Google Scholar]
- 8.Yavuz DG, Yazıcı D, Keskin L, Atmaca A, Sancak S, Saraç F, et al. Out-of-reference range thyroid-stimulating hormone levels in levothyroxine-treated primary hypothyroid patients: a multicenter observational study. Front Endocrinol (Lausanne). 2017;8:215. 10.3389/fendo.2017.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Viswanath AK, Avenell A, Philip S, Acharya SH, Maclennan G, Dalziel K, et al. Is annual surveillance of all treated hypothyroid patients necessary? BMC Endocr Disord. 2007;7:4. 10.1186/1472-6823-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caty SA, Marta OB, Jordi R, Leonardo G, Inaki G, Elisabeth M. Factors associated with the stability of thyroid-stimulating hormone values in hypothyroidism. Arch Med. 2017;9(2):13. 10.21767/1989-5216.1000214. [Google Scholar]
- 11.SingHealth. SingHealth Duke-NUS Academic Medical Centre Annual Report 2021/2022 Spearheading the Future; 2022. Available at SingHealth Annual Report 21–22_facts_and_figures.pdf. Accessed 15 May 2023.
- 12.Caron P, Grunenwald S, Persani L, Borson-Chazot F, Leroy R, Duntas L. Factors influencing the levothyroxine dose in the hormone replacement therapy of primary hypothyroidism in adults. Rev Endocr Metab Disord. 2021;23(3):463–83. 10.1007/s11154-021-09691-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chakera AJ, Pearce SH, Vaidya B. Treatment for primary hypothyroidism: current approaches and future possibilities. Drug Des Devel Ther. 2012;6:1–11. 10.2147/DDDT.S12894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McMillan M, Rotenberg KS, Vora K, Sterman AB, Thevathasan L, Ryan MF, et al. Comorbidities, concomitant medications, and diet as factors affecting levothyroxine therapy: results of the CONTROL surveillance project. Drugs R D. 2016;16(1):53–68. 10.1007/s40268-015-0116-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hueston WJ. Treatment of hypothyroidism. Am Fam Physician. 2001;64(10):1717–24. Erratum in: Am Fam Physician. 2002;65(12):2438. [PubMed] [Google Scholar]
- 16.Devdhar M, Drooger R, Pehlivanova M, Singh G, Jonklaas J. Levothyroxine replacement doses are affected by gender and weight, but not age. Thyroid. 2011;21(8):821–7. 10.1089/thy.2011.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robertson HM, Narayanaswamy AK, Pereira O, Copland SA, Herriot R, McKinlay AW, et al. Factors contributing to high levothyroxine doses in primary hypothyroidism: an interventional audit of a large community database. Thyroid. 2014;24(12):1765–71. 10.1089/thy.2013.0661. [DOI] [PubMed] [Google Scholar]
- 18.Michalaki MA, Gkotsina MI, Mamali I, Markantes GK, Faltaka A, Kalfarentzos F, et al. Impaired pharmacokinetics of levothyroxine in severely obese volunteers. Thyroid. 2011;21(5):477–81. 10.1089/thy.2010.0149. [DOI] [PubMed] [Google Scholar]
- 19.Jonklaas J, Bianco AC, Bauer AJ, Burman KD, Cappola AR, Celi FS, et al; American Thyroid Association Task Force on Thyroid Hormone Replacement. Guidelines for the treatment of hypothyroidism: prepared by the American thyroid association task force on thyroid hormone replacement. Thyroid. 2014;24(12):1670–751. 10.1089/thy.2014.0028. [DOI] [PMC free article] [PubMed]
- 20.Muncie HL Jr. Weight-based levothyroxine dosage adjustment for hypothyroidism. Am Fam Physician. 2022;105(1):6–7. [PubMed] [Google Scholar]
- 21.Lindgård Nielsen J, Karmisholt J, Bülow Pedersen I, Carlé A. Prevalence and predictors of adequate treatment of overt hypothyroidism - a population-based study. EXCLI J. 2022;6(21):104–16. 10.17179/excli2021-4291. PMID: 35145368; PMCID: PMC8822305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study are not publicly available as they contain information that are sensitive to the study institution. They may be made available from the corresponding author on reasonable request.