Abstract
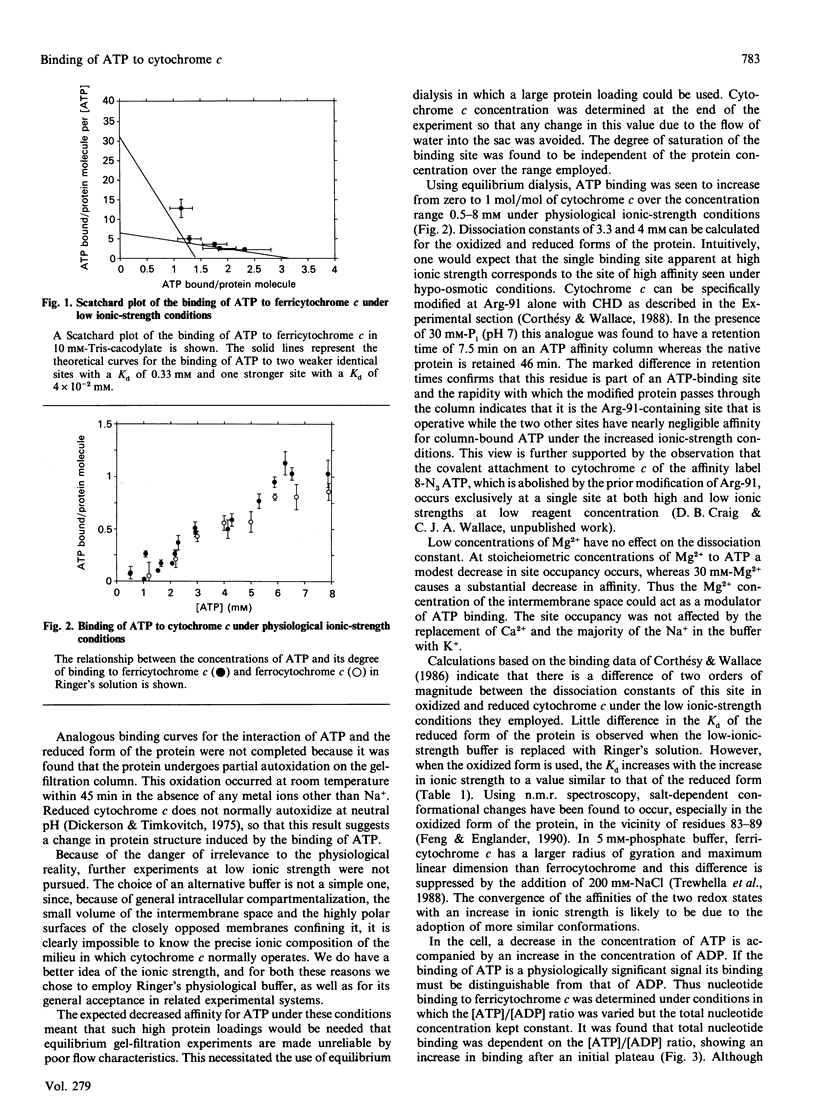
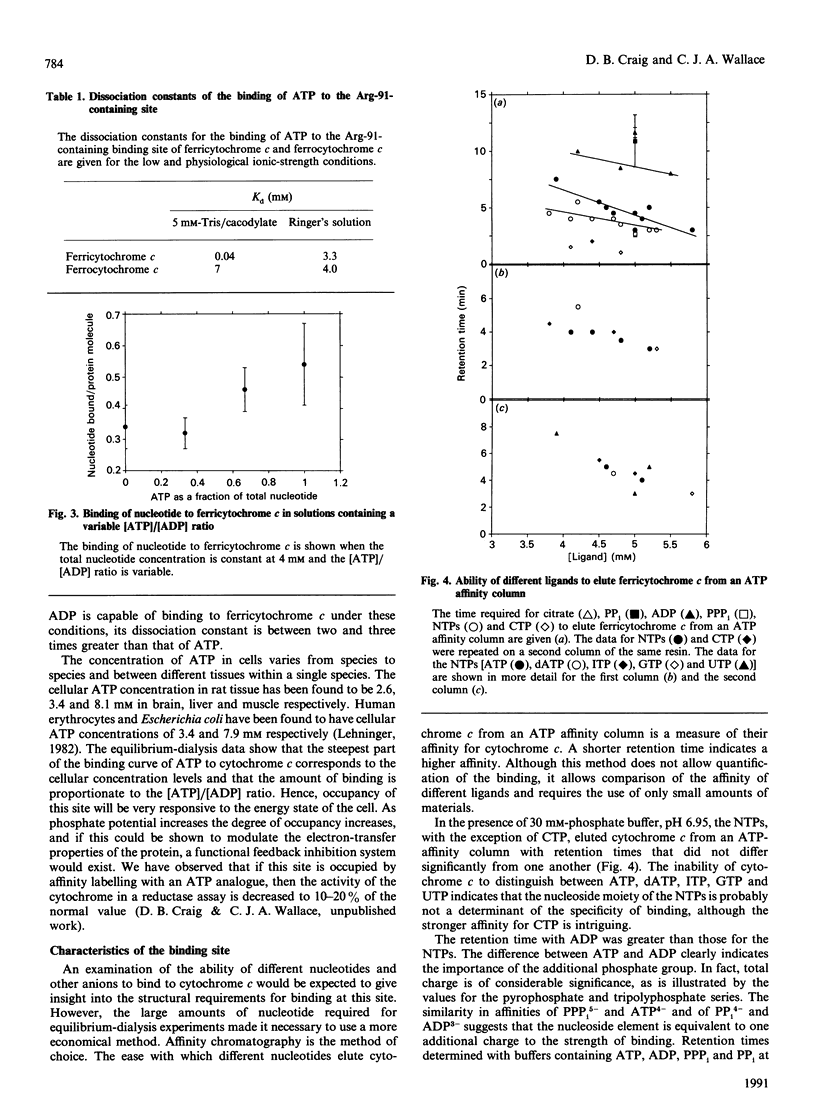
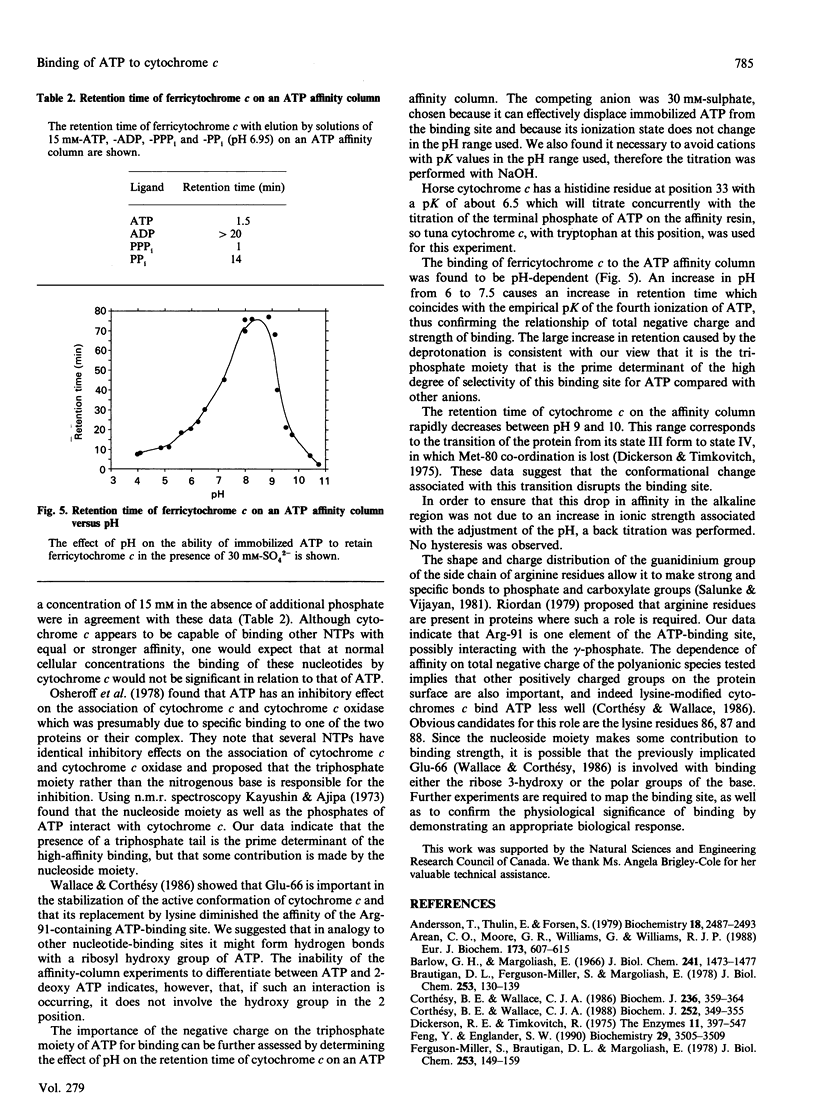
Cytochrome c binds ATP with marked specificity at a site that contains the evolutionarily invariant residue Arg-91. The binding of ATP to this site was studied using equilibrium gel filtration, equilibrium dialysis and affinity chromatography. At physiological ionic strength the affinity is such that the major change in occupancy coincides with the normal cellular ATP concentration range, and the degree of saturation is proportional to the ratio of [ATP]/[ADP]. The specificity of binding at this site is more a function of the degree of phosphorylation of the nucleotide, than of the nature of the nucleoside moiety. Thus under physiological conditions the degree of occupancy of this site is proportional to the energy state of the cell, providing a means for the regulation of the respiratory chain which is sensitive to cytoplasmic ATP levels.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson T., Thulin E., Forsén S. Ion binding to cytochrome c studied by nuclear magnetic quadrupole relaxation. Biochemistry. 1979 Jun 12;18(12):2487–2493. doi: 10.1021/bi00579a008. [DOI] [PubMed] [Google Scholar]
- Arean C. O., Moore G. R., Williams G., Williams R. J. Ion binding to cytochrome c. Eur J Biochem. 1988 May 2;173(3):607–615. doi: 10.1111/j.1432-1033.1988.tb14042.x. [DOI] [PubMed] [Google Scholar]
- Barlow G. H., Margoliash E. Electrophoretic behavior of mammalian-type cytochromes c. J Biol Chem. 1966 Apr 10;241(7):1473–1477. [PubMed] [Google Scholar]
- Brautigan D. L., Ferguson-Miller S., Margoliash E. Definition of cytochrome c binding domains by chemical modification. I. Reaction with 4-chloro-3,5-dinitrobenzoate and chromatographic separation of singly substituted derivatives. J Biol Chem. 1978 Jan 10;253(1):130–139. [PubMed] [Google Scholar]
- Corthésy B. E., Wallace C. J. The oxidation-state-dependent ATP-binding site of cytochrome c. A possible physiological significance. Biochem J. 1986 Jun 1;236(2):359–364. doi: 10.1042/bj2360359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corthésy B. E., Wallace C. J. The oxidation-state-dependent ATP-binding site of cytochrome c. Implication of an essential arginine residue and the effect of occupancy on the oxidation-reduction potential. Biochem J. 1988 Jun 1;252(2):349–355. doi: 10.1042/bj2520349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y., Englander S. W. Salt-dependent structure change and ion binding in cytochrome c studied by two-dimensional proton NMR. Biochemistry. 1990 Apr 10;29(14):3505–3509. doi: 10.1021/bi00466a012. [DOI] [PubMed] [Google Scholar]
- Ferguson-Miller S., Brautigan D. L., Margoliash E. Definition of cytochrome c binding domains by chemical modification. III. Kinetics of reaction of carboxydinitrophenyl cytochromes c with cytochrome c oxidase. J Biol Chem. 1978 Jan 10;253(1):149–159. [PubMed] [Google Scholar]
- HUMMEL J. P., DREYER W. J. Measurement of protein-binding phenomena by gel filtration. Biochim Biophys Acta. 1962 Oct 8;63:530–532. doi: 10.1016/0006-3002(62)90124-5. [DOI] [PubMed] [Google Scholar]
- Kayushin L. P., Ajipa Y. I. Cytochrome c-nucleotide complexes and the role of unpaired electrons in coupling processes. Ann N Y Acad Sci. 1973 Dec 31;222:255–265. doi: 10.1111/j.1749-6632.1973.tb15267.x. [DOI] [PubMed] [Google Scholar]
- Margalit R., Schejter A. Cytochrome c: a thermodynamic study of relationships among oxidation state, ion-binding and structural parameters. 2. Ion-binding linked to oxidation state. Eur J Biochem. 1973 Feb 1;32(3):500–505. doi: 10.1111/j.1432-1033.1973.tb02634.x. [DOI] [PubMed] [Google Scholar]
- Margoliash E., Barlow G. H., Byers V. Differential binding properties of cytochrome c: possible relevance for mitochondrial ion transport. Nature. 1970 Nov 21;228(5273):723–726. doi: 10.1038/228723a0. [DOI] [PubMed] [Google Scholar]
- Riordan J. F. Arginyl residues and anion binding sites in proteins. Mol Cell Biochem. 1979 Jul 31;26(2):71–92. doi: 10.1007/BF00232886. [DOI] [PubMed] [Google Scholar]
- Salunke D. M., Vijayan M. Specific interactions involving guanidyl group observed in crystal structures. Int J Pept Protein Res. 1981 Oct;18(4):348–351. doi: 10.1111/j.1399-3011.1981.tb02992.x. [DOI] [PubMed] [Google Scholar]
- Stellwagen E., Shulman R. G. Nuclear magnetic resonance study of exchangeable protons in ferrocytochrome c. J Mol Biol. 1973 Apr 25;75(4):683–698. doi: 10.1016/0022-2836(73)90301-x. [DOI] [PubMed] [Google Scholar]
- Taborsky G., McCollum K. Phosphate binding by cytochrome c. Specific binding site involved in the formation and reactivity of a complex of ferricytochrome c, ferrous ion, and phosphate. J Biol Chem. 1979 Aug 10;254(15):7069–7075. [PubMed] [Google Scholar]
- Trewhella J., Carlson V. A., Curtis E. H., Heidorn D. B. Differences in the solution structures of oxidized and reduced cytochrome c measured by small-angle X-ray scattering. Biochemistry. 1988 Feb 23;27(4):1121–1125. doi: 10.1021/bi00404a007. [DOI] [PubMed] [Google Scholar]
- Wallace C. J., Corthésy B. E. Protein engineering of cytochrome c by semisynthesis: substitutions at glutamic acid 66. Protein Eng. 1986 Oct-Nov;1(1):23–27. [PubMed] [Google Scholar]
- Wallace C. J., Rose K. The semisynthesis of analogues of cytochrome c. Modifications of arginine residues 38 and 91. Biochem J. 1983 Dec 1;215(3):651–658. doi: 10.1042/bj2150651. [DOI] [PMC free article] [PubMed] [Google Scholar]


