Abstract
Background
Canine mammary tumours (CMT) are among the most common types of tumours in female dogs. Diagnosis currently requires invasive tissue biopsies and histological analysis. Tumour cells shed extracellular vesicles (EVs) containing RNAs and proteins with potential for liquid biopsy diagnostics. We aimed to identify CMT subtype-specific proteome profiles by comparing the proteomes of EVs isolated from epithelial cell lines derived from morphologically normal canine mammary tissue, adenomas, and carcinomas.
Methods
Whole-cell protein lysates (WCLs) and EV-lysates were obtained from five canine mammary cell lines: MTH53A (non-neoplastic); ZMTH3 (adenoma); MTH52C (simple carcinoma); 1305, DT1406TB (complex carcinoma); and their proteins identified by LC-MS/MS analyses. Gene Ontology analysis was performed on differentially abundant proteins from each group to identify up- and down-regulated biological processes. To establish CMT subtype-specific proteomic profiles, weighted gene correlation network analysis (WGCNA) was carried out.
Results
WCL and EVs displayed distinct protein abundance signatures while still showing the same increase in adhesion, migration, and motility-related proteins in carcinoma-derived cell lines, and of RNA processing and RNA splicing factors in the adenoma cell line. WGCNA identified CMT stage-specific co-abundant EV proteins, allowing the identification of adenoma and carcinoma EV signatures not seen in WCLs.
Conclusions
EVs from CMT cell lines exhibit distinct protein profiles reflecting malignancy state, allowing us to identify potential biomarkers for canine mammary carcinomas, such as biglycan. Our dataset could therefore potentially serve as a basis for the development of a less invasive clinical diagnostic tool for the characterisation of CMT.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12917-024-04331-1.
Keywords: Canine mammary tumour, Cell culture, Extracellular vesicles, Proteomics, Size exclusion chromatography, WGCNA, Biglycan
Background
Canine mammary tumours (CMT) are the most common tumours in intact female dogs. Approximately 50% of canine mammary neoplasia cases are malignant and the mortality rate is high if left untreated [1]. CMTs have many similar biological features to human breast cancer, including presentation [2], histopathological features [3], biological behaviour [4] and metastatic patterns [5]. Accordingly, dogs are considered good models for human disease. However, there are also distinct differences between both species mostly associated with morphology and clinical presentation. In dogs, carcinomas are classified as simple, when consisting of luminal epithelial cells or myoepithelial components; complex, when made up of both luminal epithelial and myoepithelial components; or mixed, if they contain both epithelial and/or myoepithelial components along with mesenchymal cells [6].
For the diagnosis and classification of CMT, conventional biopsy with histopathology remains the gold standard [7]. However, the morphological heterogeneity of the tumours and the presence of different cell types make an accurate classification difficult [7]. Moreover, the poor prognosis in dogs with large, visible tumours reflects the advanced stage of the pathology, further emphasizing the importance of early detection due to the high malignancy rate [8]. An accurate biomarker for CMT diagnosis with malignant prediction could improve outcomes through intervention at an earlier stage of disease, as well as assess response to treatment, presence of tumour progression, or future prognosis, especially since CMTs represent a very diverse group of tumours and therefore different approaches of treatment will be necessary [9].
In recent years, the study of extracellular vesicles (EVs) has caught the attention of researchers as an emerging and promising tool for disease diagnosis and prognosis [10]. EVs are defined as a heterogeneous group of small lipid-bilayered particles which are constantly released from cells into the extracellular environment. These vesicles transport biological molecules such as lipids, proteins and nucleic acids, playing a key role in cellular communication in multiple physiological and pathological processes [11]. Several studies have shown that EVs play a direct role in the crosstalk between tumour cells and stromal cells, contributing to tumour growth, enhancement of tumour cell invasion, and potential microenvironmental remodelling, leading to tumour cell metastasis [12, 13]. Moreover, EV cargo is highly stable over extended periods of time due to the EV’s lipid bilayer, which prevents degradation by extracellular proteases and nucleases, thereby enhancing their potential for biomarker discovery and clinical diagnosis [14].
In this study, we performed a comprehensive proteome analysis of CMT cell line-derived EVs and paired WCLs from a benign adenoma, as well as one simple and two complex carcinoma-derived cell lines compared to a cell line established from normal mammary tissue. Our aim was to identify molecular markers for disease stage and to provide useful insights into the molecular and biological processes that may be induced by EVs released from tumour cells. By using weighted gene correlation network analysis (WGCNA), a powerful systems biology method described by Langfelder and Horvath, (2008) to identify correlation patterns among genes/proteins [15], we identified co-abundant proteins, pathways relevant to our dataset, as well as key proteins in the proteomics data derived from different WCLs and EVs that may serve as potential biomarkers or therapeutic targets.
Methods
Canine mammary cell lines
The following canine mammary cell lines were used: one non-neoplastic cell line (MTH53A, used as a healthy control), one simple adenoma cell line (ZMTH3), one simple carcinoma cell line (MTH52C) and two complex carcinoma cell lines (1305, DT1406TB). Histological classification of the tissues from which the cell lines have been derived and established was performed by the Department of Pathology of the Stiftung Tierärztliche Hochschule Hannover, Germany [16], according to the proposed classification of canine mammary tumours [17]. Cells were cultured in DMEM/F12 medium (1:1) + GlutaMAX™-l, supplemented with 10% foetal bovine serum (FBS) superior (both from Thermo Fisher Scientific, Paisley, UK), 1% penicillin/streptomycin (P/S) (Life Technologies, Inc., New York, USA) and 1% sodium pyruvate (Sigma-Aldrich, Taufkirchen, Germany). All cells were grown in a humidified incubator at 37 °C and 5% CO2.
Sample preparation for EV isolation
Cells were cultured in T-175 flasks at 90% confluence, rinsed twice with phosphate-buffered saline (PBS) and incubated at 37 °C and 5% CO2 for 48 h in serum-free medium (DMEM/F12) containing 1% pyruvate and 1% P/S. 180 ml of conditioned media was harvested per cell line and centrifuged for 30 min at 2000 x g to remove cells and cell debris. Supernatant was collected and stored at 4 °C until further processing.
EV isolation
The EV isolation protocol was designed and optimised as previously described [18–21], using two replicates per cell line. To initially concentrate EVs, ultrafiltration devices with a cut-off of 50 kDa (Thermo Fisher Scientific) were loaded with conditioned media from each cell line, centrifuged at 3500 x g at 4 °C until the volume was reduced to 300 µl. To isolate EVs, 150 µl of concentrated conditioned culture medium was loaded onto a size-exclusion column (SEC) (Cell Guidance Systems Ltd, Cambridge, UK), and twenty-four 200 µl fractions were eluted by gravity with PBS. This procedure was repeated twice for each cell line and fractions 5–10 were collected and pooled.
Nanoparticle tracking analysis (NTA)
Concentration and size of EVs were analysed using Nanosight NS500 and Nanosight LM14 devices (both from Malvern, Worcestershire, UK). Samples were processed in duplicate and diluted 100-fold in filtered PBS (0.22 μm pore PVDF filter). All analyses were performed at 25 °C and three videos of 30 to 60 s were recorded for each sample. Data were processed and analysed using the NTA 3.2 software (Malvern).
Protein extraction
To prepare WCL from each cell line, cells were cultured in 6-well plates until near-confluency. Cells were rinsed twice with PBS and incubated in serum-free medium for 48 h to mirror EV isolation conditions. Cells were lysed in 200 µl RIPA buffer with protease inhibitors for 20 min on ice, cell lysates were scraped off and passed several times through a 25-gauge needle using a 1 ml syringe. Samples were centrifuged at 13,000 x g at 4 °C for 10 min and supernatant was stored at -20 °C until further use. Protein quantification of all samples was determined by Pierce BCA assay (Thermo Fisher Scientific) according to the manufacturer’s instructions.
For protein isolation from EVs, selected SEC fractions were concentrated with a 2 kDa cut-off centrifugation concentrator device (Thermo Fisher Scientific) to approximately 60 µl at 13,000 x g at 4 °C. Samples were lysed in 60 µl 2x RIPA buffer supplemented with protease inhibitors (#5871 S, Cell Signaling Technology, Leiden, The Netherlands) and centrifuged as described for WCLs.
Immunofluorescence
Sterile 13-mm diameter coverslips (Sarstedt) were placed in a standard 24-well plate, and cell lines were seeded and cultured for 24 h, followed by an additional 48 h in serum-free medium to mimic the conditions used in protein extraction. The cells were washed with cold PBS and fixed with methanol on ice for 15 min. A quenching step with 10 mM glycine was performed for 15 min at room temperature. Coverslips were blocked with 1% BSA/PBST for 30 min at room temperature, followed by incubation in a 50 µL drop of primary antibody dilution in 1% BSA/PBST for 1 h at room temperature (rabbit anti-biglycan (BGN) antibody (16409-1-AP, 1:500, Proteintech, Manchester, UK). Coverslips were washed twice with PBS and incubated with a 50 µL drop of secondary antibody for 1 h at room temperature in the dark (goat-anti rabbit IgG Dylight 488 (1:1000)). The samples were then washed three times with PBS, counterstained and mounted using Prolong Glass with Hoechst (Life Technologies, Willow Creek, Oregon, USA) on a glass slide and cured for 24 h at room temperature in the dark. Images were acquired under identical settings using a Leica DMI6000B inverted microscope and Leica LAS-X software (Leica, Wetzlar, Germany).
Western blotting
10 µg of EV proteins were separated by 12% Bis/Tris SDS-PAGE and transferred to a nitrocellulose membrane via semidry blot. The membrane was blocked for 30 min on 3% milk/TBST. Primary antibodies used were rabbit anti-BGN antibody (1:500, Proteintech), goat anti-CD63 antibody (1:1000, Antibodies-online GmbH, Aachen, Germany) and mouse anti-CD9 antibody (1:1000, Thermo Fisher Scientific). For BGN detection, a pre-treatment of the samples was necessary to deglycosylate the chondroitin sulphate chains of the proteoglycan, using 0.5 µg of chondroitinase ABC (Bio-Techne, Massachusetts, USA) per 10 µg of protein and incubation at 37 °C for 16 h prior to western blotting. Secondary antibodies were as follows: donkey anti-rabbit (NA934), sheep anti-mouse (NA931) horseradish peroxidase-conjugated antibodies (1:10,000, GE Healthcare, Buckinghamshire, UK), and donkey anti-goat (sc-2020) horseradish peroxidase-conjugated antibody (1:10.000, Santa Cruz Biotechnology, Inc., Heidelberg, Germany). A Prime ECL western blotting detection kit (Cytiva, Little Chalfont, Buckinghamshire, UK) was used and imaged using a Fusion imaging system (Vilber Lourmat, Marne-la-Vallée, France).
Proteomics using liquid chromatography with tandem mass spectrometry (LC-MS/MS)
Mass spectrometry was performed as previously described [22, 23] using Sera-Mag SP3 beads (GE Healthcare, Solingen, Germany) and label-free protein quantification. Samples were analysed from two independent isolates, each in duplicate per cell line. 2 µL (20 µg) SpeedBeads magnetic carboxylate-modified particles per sample were prepared by washing twice with 200 µl water. Each 15 µl protein sample was brought to a volume of 50 µl with 100 mM triethylammonium bicarbonate (TEAB). Protein sidechains were reduced by addition of 5 µl 200 mM tris(2-carboxyethyl) phosphine in 100 mM TEAB and incubation for 1 h at 55 °C. The alkylation was performed by adding 5 µl 375 mM iodoacetamide in 100 mM TEAB and incubation at room temperature for 30 min in darkness. 70 µl acetonitrile (ACN) was added and incubated with the prepared beads for 8 min at room temperature. Tubes were placed on a magnetic rack for 2 min, supernatant discarded, and beads washed twice with 200 µl ethanol (70% in water, v/v) and once with ACN. Trypsin (1:50 enzyme to protein ratio, diluted in 100 mM TEAB) (Promega Corporation, Madison, USA) was added to each sample for overnight enzymatic cleavage (16 h) at 37 °C. Thereafter, 200 µl ACN was added to the peptides. After 8 min incubation, samples were incubated for 2 min on a magnetic rack, supernatant was discarded, and beads were washed with 200 µl ACN. Peptides were eluted by addition of water with dimethylsulfoxide (2%, v/v) and sonication for 1 min. After 2 min incubation on a magnetic rack, supernatants were collected, vacuum-dried and reconstituted in water with formic acid (0.1%, v/v).
Obtained peptides were separated using an Ultimate 3000 nano ultra-performance liquid chromatography system (Thermo Fisher Scientific). Peptides were first trapped on an Acclaim PepMap 100 C18 column (Acclaim PepMap 100 C18, nanoViper, 2 μm, 75 μm x 5 cm) (Thermo Fisher Scientific), and subsequently separated on an analytical reverse-phase column Acclaim PepMap 100 C18 (Acclaim PepMap 100 C18, nanoViper, 3 μm, 75 μm x 25 cm) (Thermo Fisher Scientific). The separated peptides were injected into a Q Exactive HF Orbitrap mass spectrometer (Thermo Fisher Scientific) equipped with a TriVersa NanoMate system (Advion, Ithaca, New York, USA). Samples were acquired using parameters described previously [22]. Raw data were processed using Proteome Discoverer 2.5 (Thermo Fisher Scientific). The database search was performed against the UniprotKB reference proteome of Canis lupus familiaris (from 3rd February 2023). As parameters, oxidation of methionine and acetylation of proteins N-termini were set as variable modifications and carbamidomethylation of cysteines as fixed modification. Two missed tryptic cleavages were allowed and proteins with at least two identified peptides and one unique peptide were considered identified. Proteins were quantified by summing the intensities of all unique peptides.
Data processing and statistical analysis
For statistical analysis and visualisation, R v 3.6.1 with the workflow described by the package proteomicsr was used [24], which applies the following packages: mixOmics [25], corrplot [26], limma [27], PerformanceAnalytics [28], dendsort [29], ComplexHeatmap [30], plyr [31], reshape2 [32], xlsx [29], DEP [33], ggsci [34], circlize [35], calibrate [36], ggplot2 [37], readxls [38], qpcR [39], splitstackshape [40], tidyr [41], ggh4x [42], dendextend [43] and Tmisc [44]. Data were log2-transformed, median-normalized, variance-stabilized, and filtered for proteins quantified in at least two of four replicates. Imputation of noise-like values was applied for conditions with no quantification in any of the available replicates. To assess significant changes, a Student’s t-test with Benjamini & Hochberg adjustment for multiple testing was performed, and proteins with adjusted p-values ≤ 0.05 were considered significantly affected.
WGCNA analysis
Normalised intensity data were subjected to Weighted Gene Correlation Analysis (WGCNA) [15, 45] using R v.3.6.1. Modules were created applying the default parameters with the following exceptions: soft power threshold: 21, minimum module size: 50, maximum module size: 200, deep split: 0, merge cut height: 0.4. Modules were correlated to traits using Pearson correlation. For module-trait combinations of particular interest, potential key drivers were determined based on their absolute module membership (MM) and absolute trait significance (TS) ≥ 0.75. Alternatively, the top 20 candidates with highest summed absolute MM and TS were evaluated. Log2(FC)s and adjusted p-values of the identified potential key drivers were used for visualisation.
Gene ontology analysis
Differentially abundant proteins were analysed using gene ontology (GO) functional annotations using ShinyGO 0.80 database [46]. False discovery rate (FDR) cut-off smaller 0.05 was considered significant, and enriched pathways were sorted by fold enrichment (F.E.) values. Venn diagrams were generated for multiple comparisons using the InteractiVenn tool [47].
Results
Protein identification by LC-MS/MS
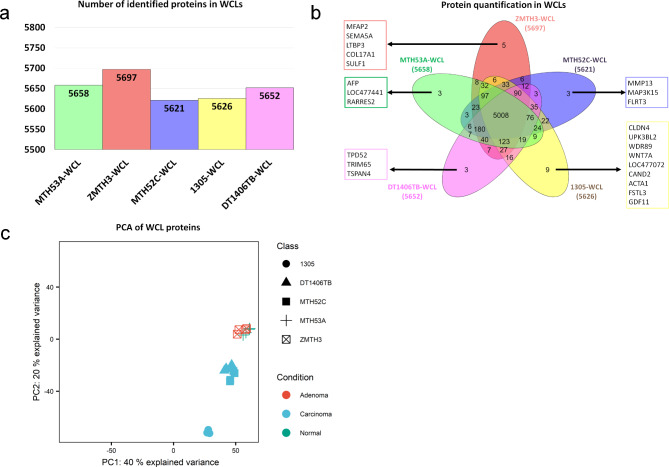
To identify proteins that are specifically associated with normal, adenomatous or cancerous cells, whole-cell lysates (WCLs) from cell lines established from a morphologically normal canine mammary gland (MTH53A), a mammary adenoma (ZMTH3), a simple carcinoma (MTH52C) and two complex carcinomas (1305, DT1406TB) (Additional file 1) were first compared. In total, 5936 proteins were identified by LC-MS/MS, ranging in number between 5621 and 5697 for each WCL sample (Fig. 1a). The vast majority of detected proteins (5006 proteins) was present in all cell lines and only very few cell line-specific proteins (between 3 and 9 per cell line) were detected (Fig. 1b).
Fig. 1.
Mass spectrometry-based profiling of whole-cell proteomes. (a) Protein groups identified in each WCL-derived cell line. (b) Overlap of protein intensities quantified in each WCL-derived cell line and uniquely detected proteins in each cell line. (c) Principal component analysis of WCL proteins of healthy control and CMT cell lines
Principal component analysis (PCA) of these WCLs (Fig. 1c) revealed that the four technical replicates from two independent WCLs per cell line (biological replicates) clustered very closely together, showing good reproducibility. Proteomes of the WCLs of the non-neoplastic cell line MTH53A (healthy control) and the adenoma ZMTH3 clustered together (Fig. 1c), while those from the simple carcinoma MTH52C and complex carcinomas 1305 and DT1406TB formed two separate clusters. Surprisingly, the two complex carcinoma cell lines did not cluster together but instead DT1406TB clustered with to the simple carcinoma MTH52C, while 1305 cells formed a separate cluster.
Cell line specific WCL proteomic profiles allow to distinguish CMT subtypes
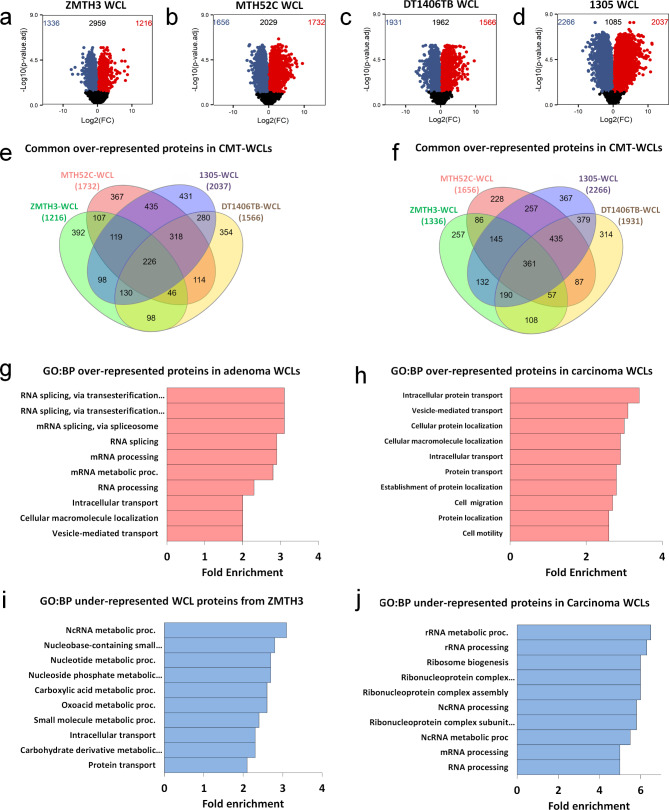
Despite the low variance in the PCA, the WCL proteome of the adenoma ZMTH3 showed significant differences in protein abundance when directly compared against the healthy control MTH53A, with 2552 differentially abundant proteins (adjusted p ≤ 0.05) (Fig. 2a). As expected, WCLs of the carcinoma cell lines showed an even larger difference compared to the healthy control, with between 3388 and 4303 differentially abundant proteins (Fig. 2b-d).
Fig. 2.
Differential analysis in WCL protein abundance levels of CMTs when compared to healthy control MTH53A. Volcano plots of (a) adenoma ZMTH3, (b) Volcano plot of simple carcinoma MTH52C, (c) complex carcinoma DT1406TB, and (d) complex carcinoma 1305, when compared to the healthy control. Red dots in the top right area were over-represented in the CMT cell line relative to MTH53A. Blue dots in the top left area were under-represented in CMT cell line relative to MTH53A. Black dots below the dashed line represent proteins with no statistical difference (p > 0.05). (e) Venn diagram showing the overlap between the WCL proteins over-represented (p < 0.05) in all the CMT cell lines. (f) Gene ontology of over-represented ZMTH3 WCL proteins enriched in biological process pathways (GO: BP) expressed as fold enrichment scores (F.E.), considering a false discovery rate (FDR) < 0.05. (g) Gene ontology of common over-represented WCL proteins of carcinoma cell lines enriched in biological process pathways (GO: BP). (h) Venn diagram showing the overlap between the WCL proteins under-represented (p < 0.05) in all the CMT cell lines. (i) Gene ontology of under-represented ZMTH3 WCL proteins enriched in biological process pathways (GO: BP). (j) Gene ontology of common under-represented WCL proteins of carcinoma cell lines enriched in biological process pathways (GO: BP)
Most over-represented proteins in the adenoma cell line ZMTH3 were associated with RNA-binding and protein expression (EIF1AX, CSRP1, PPIC) (Table 1), suggesting enhanced protein biosynthesis activity. Likewise, top under-represented proteins included those associated with cell adhesion (SERPINB8, DPT), signal transduction (CAPS, DOCK4) and metabolism regulation and homeostasis (CYP39A1, CKB), suggesting a change in adhesiveness together with a dysregulation of transduction pathways to promote cell growth and proliferation.
Table 1.
Top 10 differentially over- and under-represented proteins identified in adenoma WCLs vs. healthy control
| Over-represented proteins | |||
|---|---|---|---|
| Accession number | Protein | Log2FC | Adj. P-value |
| A0A8I3QIQ3 | EIF1AX | 8.865 | 1.72E-04 |
| A0A8I3NCP2 | ACSF2 | 8.733 | 1.04E-03 |
| A0A8I3NAS7 | OCIAD2 | 8.120 | 3.99E-03 |
| A0A8I3NH85 | TNS3 | 8.029 | 4.57E-05 |
| A0A8I3PWS9 | LOC102153069 | 7.829 | 4.56E-04 |
| A0A8P0PEA0 | EBP | 7.563 | 3.15E-06 |
| A0A8I3P342 | ISG15 | 7.047 | 4.40E-05 |
| H6VX52 | FAM83H | 6.540 | 7.28E-04 |
| A0A8I3N5K4 | PPIC | 6.353 | 6.61E-04 |
| A0A8I3P8T5 | CSRP1 | 5.776 | 4.89E-04 |
| Under-represented proteins | |||
| Accession number | Protein | Log2FC | Adj. P-value |
| A0A8I3NQ76 | CYP39A1 | -7.634 | 3.16E-04 |
| P10463 | CAPS | -7.247 | 9.38E-04 |
| A0A8P0PP63 | CNNM2 | -6.508 | 1.89E-04 |
| A0A8P0S626 | SERPINB8 | -5.666 | 9.38E-04 |
| A0A8I3RS96 | DPT | -4.693 | 5.70E-03 |
| A0A8I3SCL9 | DOCK4 | -4.548 | 3.86E-04 |
| A0A8I3S958 | LOC478277 | -4.450 | 9.68E-04 |
| A0A8P0NPZ9 | PLEKHA2 | -4.354 | 6.81E-05 |
| A0A8I3NKV1 | TXNIP | -4.298 | 4.28E-04 |
| P05124 | CKB | -4.284 | 8.63E-06 |
Among the most over-represented proteins in the WCLs of carcinoma cell lines, proteins associated with actin cytoskeleton-rearrangement (TPM4, MYLK, PTK2B) as well as cell adhesion and migration (MDK, EPCAM) were detected (Additional file 2), thus indicating increased migratory and adhesion activity in the carcinoma cell lines. Surprisingly, several of the most under-represented proteins in the carcinoma WCLs included proteins involved in cell cycle regulation (CDK2, CDC45) and transcription regulation (SMARCA4, CHAF1B) (Additional file 2), indicating a dysregulation of cell growth and gene expression in carcinoma cells (for associations of cancer studies with proteins listed in Table 1 and Additional file 2, refer to Additional file 3).
Mammary tumour cell lines from dogs share proteins associated with cancer progression
Evaluating the similarities of significantly over-represented proteins in the adenoma and carcinoma cell lines (Fig. 2e), 226 proteins were identified, of which the most over-represented included proteins involved in signal transduction (NUCB1, ZYK) and apoptosis (RIPK3, CASP8) (Table 2). Interestingly, the RNA-binding protein CSRP1 was one of the most abundant proteins for all CMTs, which has been linked to human breast cancer for its role in alteration of gene regulation, cell growth and differentiation [48]. Moreover, 318 over-represented proteins were common in the three carcinoma cell lines, including SERPINB5 among the most abundant proteins (Table 3), which is expressed in highly aggressive human basal-like breast cancers [49].
Table 2.
Top 20 differentially common over-represented WCL proteins vs. healthy control in CMT cell lines
| Common over-represented proteins | ||||||
|---|---|---|---|---|---|---|
| Accession number | Protein name | Protein abbreviation | ZMTH3 Log2FC |
MTH52C Log2FC |
1305 Log2FC |
DT1406TB Log2FC |
| A0A8I3PIK8 | Alpha-2-HS-glycoprotein | AHSG | 3.875 | 7.537 | 7.181 | 5.387 |
| A0A8I3NGU2 | Folate_rec domain-containing protein | LOC476816 | 5.108 | 6.698 | 5.571 | 6.387 |
| A0A8I3P8T5 | Cysteine and glycine rich protein 1 | CSRP1 | 5.776 | 2.430 | 8.488 | 6.356 |
| A0A8I3N5K4 | Peptidyl-prolyl cis-trans isomerase | PPIC | 6.353 | 2.194 | 6.291 | 5.153 |
| A0A8I3P375 | Hook microtubule tethering protein 3 | HOOK3 | 3.798 | 5.981 | 5.837 | 4.102 |
| A0A8I3Q2T3 | Reticulocalbin 1 | RCN1 | 2.783 | 4.697 | 7.752 | 3.734 |
| A0A8I3PW67 | DLG associated protein 4 | DLGAP4 | 1.708 | 4.136 | 6.593 | 4.383 |
| Q38JA9 | Caspase 8 | CASP8 | 5.210 | 4.122 | 4.978 | 2.240 |
| A0A8I3RP80 | Nucleobindin-1 | NUCB1 | 1.601 | 6.004 | 5.651 | 3.035 |
| A0A8I3N5W9 | Protein kinase domain-containing protein | RIPK3 | 3.977 | 3.259 | 4.929 | 3.830 |
| A0A8I3MQM8 | Prosaposin | PSAP | 1.604 | 4.820 | 6.394 | 2.912 |
| A0A8I3NTX2 | Mitochondrial antiviral signaling protein | MAVS | 2.310 | 4.379 | 6.169 | 2.631 |
| A0A8I3P225 | GLOBIN domain-containing protein | LOC609402 | 1.814 | 5.072 | 6.257 | 1.981 |
| A0A8P0N4U0 | Heme oxygenase | HMOX1 | 2.849 | 4.484 | 4.044 | 3.503 |
| A0A8P0TML4 | Ring finger protein 214 | RNF214 | 2.850 | 5.212 | 3.562 | 3.099 |
| A0A8I3MYL2 | Mitochondrial trans-2-enoyl-CoA reductase | MECR | 1.343 | 2.349 | 7.828 | 3.124 |
| A0A8I3S1U2 | Methyltransferase like 7 A | METTL7A | 3.382 | 1.674 | 4.967 | 4.326 |
| A0A8I3NUI7 | Coatomer subunit zeta | COPZ2 | 2.575 | 2.756 | 4.486 | 4.503 |
| A0A8I3MCD6 | GLOBIN domain-containing protein | HBQ1 | 2.048 | 4.828 | 5.210 | 1.888 |
| A0A8I3NVB0 | Zyxin | ZYX | 1.263 | 3.905 | 5.032 | 3.443 |
| Common under-represented proteins | ||||||
| Accession number | Protein name | Protein abbreviation |
ZMTH3 Log2FC |
MTH52C Log2FC |
1305 Log2FC |
DT1406TB Log2FC |
| A0A8I3NQ76 | Cytochrome P450 family 39 subfamily A member 1 | CYP39A1 | -7.634 | -5.342 | -7.234 | -4.981 |
| A0A8I3NB48 | Stonin-2 | STON2 | -3.664 | -4.148 | -3.505 | -2.718 |
| A0A8I3RWC5 | Pleckstrin homology and RhoGEF domain containing G4 | PLEKHG4 | -3.007 | -5.073 | -5.241 | -0.623 |
| A0A8I3RS96 | Dermatopontin | DPT | -4.693 | -3.372 | -4.739 | -1.009 |
| A0A8I3PI43 | Nucleolar complex protein 3 homolog | NOC3L | -0.736 | -3.834 | -5.105 | -4.048 |
| A0A8I3Q982 | Interferon related developmental regulator 1 | IFRD1 | -2.863 | -1.472 | -5.130 | -4.098 |
| A0A8I3NKM0 | tRNA dimethylallyltransferase | TRIT1 | -1.911 | -2.817 | -4.625 | -2.501 |
| A0A8I3Q4 × 2 | Ubiquitin specific peptidase 19 | USP19 | -1.888 | -2.480 | -4.484 | -2.824 |
| A0A8I3PIR4 | Fibroblast growth factor 7 | GALK2 | -3.957 | -2.692 | -2.801 | -2.029 |
| A0A8I3N128 | Cyclin dependent kinase 2 | CDK2 | -0.388 | -0.379 | -7.813 | -2.540 |
| A0A8I3RUT0 | rRNA adenine N(6)-methyltransferase | DIMT1 | -1.667 | -2.422 | -5.646 | -1.383 |
| A0A8I3NJG0 | TatD DNase domain containing 1 | TATDN1 | -2.207 | -1.452 | -4.510 | -2.850 |
| A0A8I3P8U7 | Cms1 ribosomal small subunit homolog | CMSS1 | -0.995 | -2.899 | -4.866 | -2.214 |
| A0A8I3NFP5 | Neurobeachin like 2 | NBEAL2 | -2.744 | -3.091 | -3.938 | -1.134 |
| A0A8I3PB80 | Hyaluronidase 3 | HYAL3 | -2.310 | -2.229 | -4.172 | -2.080 |
| A0A8I3PRT9 | Inosine-5’-monophosphate dehydrogenase | IMPDH1 | -3.233 | -1.501 | -4.005 | -1.938 |
| A0A8I3Q7R1 | Inosine-5’-monophosphate dehydrogenase | IMPDH2 | -2.070 | -0.621 | -4.916 | -2.830 |
| A0A8P0NPZ9 | Pleckstrin homology domain containing A2 | PLEKHA2 | -4.354 | -1.288 | -2.812 | -1.972 |
| A0A8I3S0T5 | CCAAT enhancer binding protein zeta | CEBPZ | -1.140 | -4.729 | -3.102 | -1.406 |
| A0A8P0NM37 | ACD shelterin complex subunit and telomerase recruitment factor | ACD | -1.818 | -1.722 | -4.163 | -2.623 |
Table 3.
Top 20 differentially common over-represented WCL proteins vs. healthy control in the carcinoma cell lines
| Common over-represented proteins | |||||
|---|---|---|---|---|---|
| Accession number | Protein name | Protein abbreviation |
MTH52C Log2FC |
1305 Log2FC |
DT1406TB Log2FC |
| A0A8I3MKP5 | Serpin B5 | SERPINB5 | 5.987 | 11.184 | 4.273 |
| A0A8I3RRG1 | Heat shock protein family B (small) member 6 | HSPB6 | 6.269 | 9.503 | 4.919 |
| A0A8I3Q497 | RAB8A, member RAS onco family | TPM4 | 7.749 | 8.158 | 1.989 |
| A0A8P0P7T7 | Transforming acidic coiled-coil containing protein 1 | TACC1 | 6.187 | 6.939 | 4.416 |
| A0A8I3N4I3 | Trophoblast glycoprotein | TPBG | 4.280 | 5.255 | 7.631 |
| A0A8I3Q6P3 | RAB8A, member RAS onco family | TPM4 | 7.882 | 6.517 | 2.211 |
| A0A8I3P542 | fatty acid amide hydrolase | FAAH | 3.651 | 7.188 | 5.457 |
| A0A8I3S7B1 | Bridging integrator 1 | BIN1 | 5.290 | 6.640 | 3.024 |
| A0A8I3PMV7 | CutA divalent cation tolerance homolog | CUTA | 5.372 | 7.128 | 2.272 |
| A0A8P0NGG9 | Galectin | LGALS3 | 1.521 | 7.307 | 5.419 |
| A0A8I3NMV9 | PBX homeobox interacting protein 1 | PBXIP1 | 4.881 | 5.810 | 3.389 |
| A0A8I3MUR7 | DAB adaptor protein 2 | DAB2 | 4.991 | 5.599 | 3.370 |
| A0A8I3RR31 | Myosin regulatory light chain 12B | MYL12B | 2.191 | 6.458 | 5.301 |
| A0A8I3NML2 | Clathrin light chain | CLTB | 3.828 | 7.208 | 2.910 |
| A0A8I3MJ17 | Abl interactor 1 | ABI1 | 5.757 | 6.161 | 1.656 |
| A0A8I3NZF1 | Acylphosphatase | ACYP1 | 3.241 | 6.659 | 3.557 |
| A0A8P0SCK9 | IF rod domain-containing protein | KRT86 | 3.257 | 4.057 | 5.955 |
| A0A8P0N5R9 | Glycoprotein nmb | GPNMB | 1.419 | 7.333 | 4.212 |
| A0A8P0P6 × 6 | Cadherin-2 | CDH2 | 1.605 | 5.693 | 5.647 |
| A0A8I3P9R4 | Ectonucleoside triphosphate diphosphohydrolase 3 | ENTPD3 | 3.408 | 5.338 | 4.067 |
| Common under-represented proteins | |||||
| Accession number | Protein name | Protein abbreviation |
MTH52C Log2FC |
1305 Log2FC |
DT1406TB Log2FC |
| A0A8I3PDZ7 | RNA-splicing ligase RtcB homolog | RTCB | -6.061 | -5.268 | -6.013 |
| A0A8I3P3E3 | Transglutaminase 3 | TGM3 | -4.386 | -3.891 | -6.058 |
| A0A8I3PJ19 | SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily a, member 4 | SMARCA4 | -4.700 | -5.013 | -4.087 |
| A0A8I3MM38 | Fibrillarin | FBL | -4.018 | -7.114 | -1.962 |
| A0A8I3RZK8 | Zinc finger CCHC-type containing 3 | ZCCHC3 | -3.665 | -5.718 | -3.381 |
| A0A8P0STD7 | 40 S ribosomal protein S2 | 3 SV | -5.069 | -5.791 | -0.657 |
| A0A8I3PKM8 | Nuclear transcription factor, X-box binding 1 | NFX1 | -3.588 | -4.875 | -3.002 |
| A0A8I3Q3M7 | Cell division cycle 45 | CDC45 | -1.025 | -7.065 | -2.788 |
| A0A8I3PMM3 | Cellular tumor antigen p53 | TP53 | -2.145 | -3.902 | -4.705 |
| A0A8I3N527 | Nuclear RNA export factor 1 | NXF1 | -3.800 | -5.267 | -1.536 |
| A0A8I3PYF0 | FACT complex subunit | SUPT16H | -3.071 | -5.592 | -1.828 |
| A0A8P0SQL8 | Ribosomal protein L37a | 3 SV | -3.965 | -5.827 | -0.663 |
| A0A8I3MSY1 | Nucleosome assembly protein 1 like 1 | NAP1L1 | -1.885 | -4.066 | -4.485 |
| A0A8P0T777 | WD repeat domain 70 | WDR70 | -2.919 | -6.089 | -1.424 |
| A0A8I3NZ09 | Transducin beta like 3 | TBL3 | -1.844 | -3.417 | -4.811 |
| A0A8P0N906 | DNA polymerase epsilon catalytic subunit | POLE | -2.923 | -3.493 | -3.655 |
| A0A8I3P419 | Serine/threonine-protein kinase PRP4 homolog | PRPF4B | -4.051 | -3.781 | -2.207 |
| A0A8I3S988 | Chromosome transmission fidelity factor 18 | CHTF18 | -1.533 | -6.296 | -2.166 |
| A0A8I3MIZ2 | Pumilio RNA binding family member 3 | PUM3 | -3.700 | -3.651 | -2.437 |
| A0A8I3PVA9 | CWC22 spliceosome associated protein homolog | CWC22 | -3.135 | -4.297 | -2.187 |
Additionally, 361 proteins were commonly under-represented in both adenoma and carcinoma cell lines (Fig. 2f), which were mainly associated with DNA replication, transcriptional activation and cell cycle regulation (NOC3L, TRIT1, CDK2, USP19) (Table 2); whereas the carcinoma cell lines shared 435 under-represented proteins, including splicing and chromatin regulator proteins (RTCB, SMARCA4) (Table 3, Additional file 2).
Gene ontology assignment to WCL proteins indicates different biological patterns in adenoma and carcinoma cell lines
To further identify biological processes implicated in the set of differentially abundant proteins from each subtype (adenoma, carcinoma) compared to the healthy control, Gene Ontology (GO) analysis was performed. Over-represented proteins from the adenoma ZMTH3 were primarily associated with RNA splicing via transesterification reactions (fold enrichment (F.E.) 3.0), mRNA splicing via spliceosome (F.E. 3.0), mRNA processing (F.E. 2.9), intracellular transport (F.E. 2.0), and vesicle-mediated transport (F.E. 2.0) (Fig. 2g). Likewise, GO analysis on the shared proteins from the three carcinoma cell lines showed that they were significantly involved in biological processes such as intracellular protein transport (F.E. 3.4), vesicle-mediated transport (F.E. 3.1), cellular protein localization (F.E. 3), cell migration (F.E. 2.7) and cell motility (F.E. 2.6) (Fig. 2h).
GO analysis on the differentially under-represented proteins from ZMTH3 showed significant enrichment in proteins associated with ncRNA metabolic process (F.E. 3.1), nucleotide metabolic process (F.E. 2.7), carboxylic acid metabolic process (F.E. 2.6), oxoacid metabolic process (F.E. 2.6), and again intracellular transport (F.E. 2.3) (Fig. 2i). In contrast, common under-represented proteins from the carcinoma cell lines identified pathways associated with rRNA metabolic process (F.E. 6.5); ribosome biogenesis (F.E. 6); ncRNA processing (F.E. 5.8); mRNA processing (F.E. 5) and RNA processing (F.E. 5) (Fig. 2j).
These results demonstrated that WCLs show a large variance in protein abundance patterns among CMT samples when compared to the healthy control. The observed changes were characterised in the adenoma subtype by alterations in abundance of proteins enriched for RNA processing pathways, which indicates a higher protein biosynthesis activity for subsequent cell growth; whereas carcinoma WCLs showed a high abundance of proteins enriched for migratory, motility and adhesion pathways, which may contribute to tumour development and progression. These biological patterns provided a distinctive phenotype for each CMT subtype, representing different pathways of tumorigenic nature.
Extracellular vesicle proteomes show a higher variance than WCLs proteomes
To assess whether the identified differences among WCLs were also reflected in the proteomes of their excreted vesicles, exosomes were isolated from their respective growth media by size exclusion chromatography. As it was not possible with our method to accurately separate exosomes from other secreted extracellular vesicles (EVs) of similar size, these isolated vesicles are simply referred to as “EVs”.
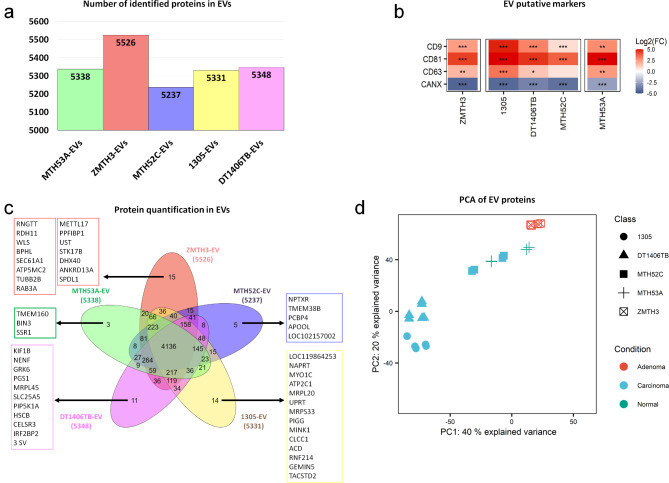
NTA characterisation of EV fractions showed particle concentrations ranging from 2.94 to 10.56 × 1011 particles/ml and the diameter ranged mostly from 120 to 230 nm (Additional file 4). For LC-MS/MS-based proteomics, biological duplicates for healthy control, adenoma, and simple carcinoma EVs (triplicates for both complex carcinoma EVs) and their technical replicates were analysed. Quantified proteins in EVs ranged from 5237 to 5526 (Fig. 3a). To confirm successful EV enrichment, the presence of putative EV markers CD9, CD63 and CD81 in our EV protein extracts was compared to their equivalent WCLs. All three markers were significantly more abundant in the EV protein extracts than in the WCLs (Fig. 3b). In contrast, calnexin (CANX), a protein used as an indicator of intracellular contamination [50], was under-represented in all EV-derived protein extracts. CD9 and CD63 EV markers were also confirmed by western blot (Additional file 5). These results are consistent with a significant enrichment of EVs and very low contamination with non-EV proteins.
Fig. 3.
Mass spectrometry-based profiling of EV proteomes. (a) Protein groups identified in each EV-derived cell line. (b) Heatmap illustrating EV protein markers CD9, CD63, CD81 and calnexin (CANX) in EV-derived protein compared with the WCLs based on their log2-transformed fold-changes. (c) Overlap of protein intensities quantified in each EV-derived cell line. (d) Principal component analysis of EV proteins of healthy control and CMT cell lines
Evaluation of the number of unique and jointly identified EV proteins in each cell line (Fig. 3b) found that similarly to WCLs (Fig. 1b) the majority of proteins (4136) was still identified in all EV samples; however, the percentage of proteins found in all EV fractions was noticeably lower than in the WCLs (69.7% in EVs vs. 84.4% in WCLs). Similarly, the number of unique proteins in EVs, though still very small with only 3–15 proteins, was higher in the complex carcinoma DT1406TB (11 vs. 3), complex carcinoma 1305 (14 vs. 9), and adenoma ZMTH3 (15 vs. 5) EV protein fractions. Hence, the EV proteomes differed more strongly between CMT cell lines than the WCL proteomes.
Despite the very small number of unique proteins in each EV isolate, PCA analysis (Fig. 3d) confirmed a higher variation between the EV proteomes of each cell line compared to the WCLs. Surprisingly, the healthy control MTH53A did not only cluster together with the adenoma cell line ZMTH3 but was also closer to the morphologically similar simple carcinoma cell line MTH52C (Additional file 1). In contrast, EV proteomes of the two complex carcinoma cell lines DT1406TB and 1305 together formed a separate cluster. These results emphasise again the similarities between protein abundance patterns of the healthy control MTH53A and adenoma ZMTH3 cells. It also showed that the EV proteomes did not allow significant separation of normal/benign cell lines from the simple carcinoma MTH52C as was the case for the WCLs.
EVs display larger differences in protein abundance compared to their corresponding WCLs
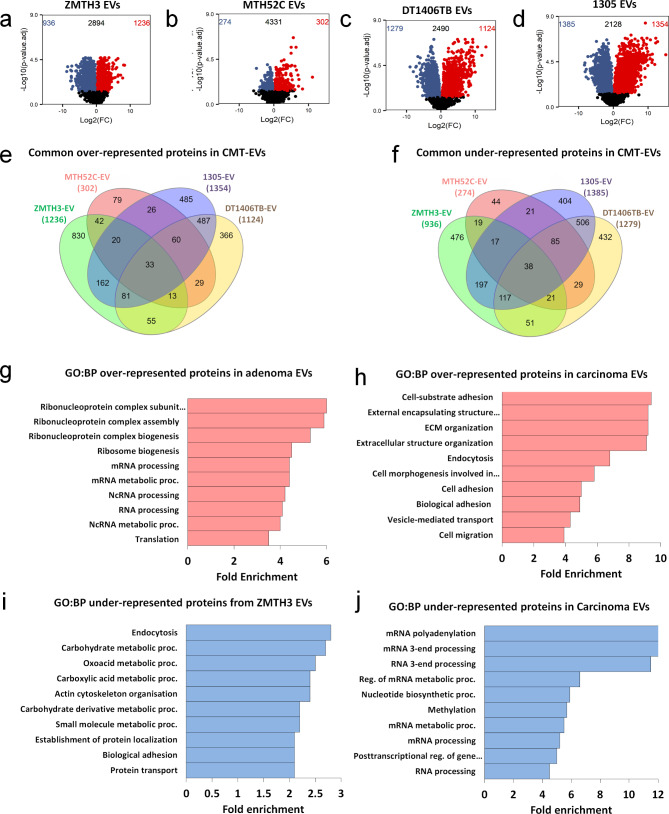
The EV proteome of the adenoma (ZMTH3) showed significant differences from that of the MTH53A cell line (non-neoplastic) when protein abundance levels were directly compared, with 2172 differentially abundant proteins (Fig. 4a). Surprisingly, EV proteomes from the three carcinoma cell lines contained fewer significantly abundant proteins than the WCLs when compared to the healthy control. The simple carcinoma MTH52C EV proteome only had 576 differentially abundant proteins (Fig. 4b), while complex carcinomas 1305 and DT1406TB EVs had 739 and 2403 differentially abundant proteins, respectively (Fig. 4c-d).
Fig. 4.
Differential analysis in EV protein abundance levels of CMTs when compared to healthy control MTH53A. Volcano plots of (a) adenoma ZMTH3, (b) simple carcinoma MTH52C, (c) complex carcinoma DT1406TB, and (d) complex carcinoma 1305, when compared to the healthy control. Red dots in the top right area were over-represented in the CMT cell line relative to MTH53A. Blue dots in the top left area were under-represented in CMT cell line relative to MTH53A. Black dots below the dashed line represent proteins with no statistical difference (p > 0.05). (e) Venn diagram showing the overlap between the EV proteins over-represented (p < 0.05) in all the CMT cell lines. (f) Gene ontology of over-represented ZMTH3 EV proteins enriched in biological process pathways (GO: BP) expressed as fold enrichment scores (F.E.), considering a false discovery rate (FDR) < 0.05. (g) Gene ontology of common over-represented EV proteins of carcinoma cell lines enriched in biological process pathways (GO: BP). (h) Venn diagram showing the overlap between the EV proteins under-represented (p < 0.05) in all the CMT cell lines. (i) Gene ontology of under-represented ZMTH3 EV proteins enriched in biological process pathways (GO: BP). (j) Gene ontology of common under-represented EV proteins of carcinoma cell lines enriched in biological process pathways (GO: BP)
Top over-represented EV proteins from the adenoma ZMTH3 (Table 4) were mainly related to immune response (ISG15, BST2, IFI44), while top under-represented proteins identified in the adenoma ZMTH3 EVs were associated with migratory and adhesion activity (MXRA8, TNN, SERPINB8) (Table 4), similar to the WCLs.
Table 4.
Top 10 differentially over- and under-represented proteins identified in adenoma EVs vs. healthy control
| Over-represented proteins | |||
|---|---|---|---|
| Accession number | Protein | Log2FC | Adj. P-value |
| A0A8I3QAX6 | CTPS2 | 8.361 | 1.17E-04 |
| A0A8I3NTB5 | CANT1 | 8.065 | 1.12E-04 |
| A0A8I3PAV5 | TCN2 | 7.800 | 2.25E-03 |
| A0A8I3P342 | ISG15 | 7.219 | 1.16E-03 |
| A0A8I3MLA0 | MFAP2 | 6.528 | 1.10E-03 |
| A0A8P0TVC6 | ADAP1 | 6.482 | 1.24E-03 |
| A0A8I3PFH2 | ZPLD1 | 6.409 | 2.46E-05 |
| J9NVI2 | BST2 | 6.396 | 5.88E-03 |
| A0A8I3MKR1 | IFI44 | 6.365 | 1.49E-03 |
| A0A8P0N757 | PRSS23 | 6.058 | 2.00E-04 |
| Under-represented proteins | |||
| Accession number | Protein | Log2FC | Adj. P-value |
| A0A8I3PNQ7 | MXRA8 | -8.773 | 4.98E-04 |
| A0A8I3N7R9 | ANGPTL2 | -8.497 | 1.65E-04 |
| A0A8I3Q6P3 | TPM4 | -7.629 | 4.39E-03 |
| A0A8I3NDQ9 | TNN | -7.596 | 3.69E-04 |
| A0A8I3Q497 | TPM4 | -7.441 | 4.26E-03 |
| A0A8I3PGJ4 | PTX3 | -7.425 | 1.51E-03 |
| A0A8I3MER3 | NN* | -7.400 | 2.04E-03 |
| A0A8I3NUH5 | NDUFB8 | -7.308 | 4.40E-04 |
| A0A8P0T552 | RBP4 | -7.285 | 2.86E-02 |
| A0A8P0S626 | SERPINB8 | -7.266 | 1.40E-04 |
*Rad60-SLD domain-containing protein
Furthermore, top over-represented proteins in carcinoma EVs (Additional file 6) included ECM-related proteins associated with migration and adhesion (LUM, COL14A1, FN1) whereas top under-represented proteins from carcinoma EVs included proteins associated with RNA processing (ELAVL2, SNU13, SARS2) (for associations and references of proteins listed in Table 4 and Additional file 5 with cancer studies, refer to Additional file 7).
CMT-derived EVs contained a set of common proteins with distinct biological behaviour
The number of proteins that were over-represented in all CMT-derived EVs (Fig. 4e) was significantly lower than in the WCLs (33 vs. 226 common proteins; Fig. 2e). Among the most abundant proteins, based on the average of all four cell lines, were ECM-associated glycoproteins OLFML2B and THBS2, as well as the proteoglycan LUM and matrix-metalloproteinase MMP19 (Table 5).
Table 5.
Top 20 differentially common under-represented EV proteins vs. healthy control in CMT cell lines
| Common over-represented proteins | ||||||
|---|---|---|---|---|---|---|
| Accession number | Protein name | Protein abbreviation | ZMTH3 Log2FC |
MTH52C Log2FC |
1305 Log2FC |
DT1406TB Log2FC |
| A0A8I3NTB5 | Galectin-3-binding protein | CANT1 | 8.065 | 3.700 | 12.760 | 9.220 |
| A0A8P0N757 | Serine protease 23 | PRSS23 | 6.058 | 3.657 | 8.085 | 8.682 |
| A0A8I3Q308 | Olfactomedin like 2B | OLFML2B | 1.900 | 5.182 | 7.641 | 9.408 |
| A0A8I3P684 | Lumican | LUM | 2.505 | 11.155 | 1.433 | 8.507 |
| A0A8I3PKT4 | Complement C1s | C1S | 4.888 | 4.440 | 9.560 | 3.265 |
| A0A8I3N2B1 | Beta-1,3-N-acetylglucosaminyltransferase | LFNG | 2.676 | 4.905 | 7.775 | 6.669 |
| A0A8I3QK41 | Pirin | PIR | 1.312 | 3.451 | 4.550 | 8.350 |
| A0A8I3S0C0 | Lysyl oxidase homolog | LOXL3 | 0.979 | 2.957 | 7.004 | 6.529 |
| A0A8I3PRC1 | Semaphorin-3 C | SEMA3C | 3.698 | 1.429 | 5.501 | 4.237 |
| A0A8I3MTX4 | Matrix metallopeptidase 19 | MMP19 | 3.141 | 3.013 | 3.129 | 4.508 |
| A0A8I3MIR7 | Lysophosphatidylcholine acyltransferase 2 | LPCAT2 | 0.925 | 2.086 | 3.281 | 7.370 |
| A0A8I3PN55 | BRO1 domain-containing protein | PDCD6IP | 1.429 | 3.837 | 5.822 | 2.288 |
| A0A8I3S0A8 | Tripartite motif containing 25 | TRIM25 | 3.737 | 2.188 | 4.888 | 1.959 |
| A0A8P0NAE9 | Calpastatin | CAST | 1.081 | 3.637 | 5.163 | 2.655 |
| A0A8I3NB75 | Thrombospondin 2 | THBS2 | 1.132 | 0.915 | 2.530 | 7.351 |
| A0A8I3PVI8 | LRR binding FLII interacting protein 2 | LRRFIP2 | 1.281 | 1.685 | 2.837 | 6.001 |
| A0A8P0TPH1 | Ras-related protein Rab-4 | RAB4A | 2.203 | 2.294 | 3.509 | 2.816 |
| Q38JA9 | Caspase 8 | CASP8 | 1.839 | 1.735 | 2.756 | 3.669 |
| A0A8I3Q6P2 | Progesterone receptor membrane component 2 | PGRMC2 | 1.278 | 0.761 | 4.155 | 3.248 |
| P06625 | Signal recognition particle receptor subunit alpha | SRPRA | 2.452 | 1.162 | 3.140 | 2.625 |
| Common under-represented proteins | ||||||
| Accession number | Protein name | Protein abbreviation |
ZMTH3 Log2FC |
MTH52C Log2FC |
1305 Log2FC |
DT1406TB Log2FC |
| A0A8P0SLL9 | Exportin 6 | XPO6 | -1.488 | -5.010 | -7.770 | -6.686 |
| A0A8I3NJG0 | TatD DNase domain containing 1 | TATDN1 | -3.524 | -1.490 | -5.855 | -3.802 |
| A0A8I3MMH6 | Chromosome 4 C5orf22 homolog | C4H5orf22 | -2.712 | -2.001 | -3.975 | -4.296 |
| A0A8I3PRT9 | Inosine-5’-monophosphate dehydrogenase | IMPDH1 | -3.416 | -2.789 | -4.010 | -1.788 |
| A0A8I3Q4 × 2 | Ubiquitin specific peptidase 19 | USP19 | -0.530 | -1.699 | -3.658 | -5.477 |
| A0A8I3QXV7 | GDP-mannose pyrophosphorylase A | GMPPA | -0.529 | -2.945 | -3.164 | -4.572 |
| A0A8I3MYE7 | Regulation of nuclear pre-mRNA domain-containing protein | RPRD1A | -3.296 | -1.052 | -4.263 | -2.261 |
| A0A8I3S4H8 | Adenosylhomocysteinase like 2 | AHCYL2 | -2.212 | -2.263 | -4.242 | -1.195 |
| A0A8I3PIR4 | Fibroblast growth factor 7 | GALK2 | -1.182 | -1.436 | -1.055 | -5.984 |
| A0A8I3NSZ0 | Argininosuccinate lyase | GUSB | -1.617 | -1.238 | -3.938 | -2.246 |
| A0A8P0NK55 | Dipeptidyl peptidase 9 | DPP9 | -2.086 | -1.861 | -3.587 | -1.368 |
| A0A8I3MP00 | TIP41-like protein | TBX19 | -0.767 | -0.800 | -4.802 | -2.381 |
| A0A8P0TU84 | Core-binding factor subunit beta | CBFB | -1.526 | -0.825 | -3.184 | -3.002 |
| A0A8I3PTA7 | Dihydrolipoyl dehydrogenase, mitochondrial | DLD | -2.055 | -2.534 | -2.086 | -1.690 |
| A0A8I3Q7R1 | Inosine-5’-monophosphate dehydrogenase | IMPDH2 | -1.877 | -1.302 | -3.304 | -1.716 |
| A0A8I3NZF6 | ATPase GET3 | GET3 | -0.356 | -0.947 | -3.553 | -2.850 |
| A0A8I3PVF4 | Methylthioribose-1-phosphate isomerase | MRI1 | -0.989 | -2.024 | -1.683 | -2.792 |
| A0A8I3N997 | Polyadenylate-binding protein | PABPC4 | -2.455 | -1.357 | -1.701 | -1.906 |
| A0A8I3PG05 | Mevalonate kinase | MVK | -0.321 | -1.320 | -3.876 | -1.746 |
| A0A8P0TF63 | Pseudouridine synthase 7 | PUS7 | -1.685 | -0.915 | -2.967 | -1.626 |
Carcinoma EVs shared 60 over-represented proteins, including ECM proteins involved in cell adhesion, motility, wound healing and maintenance of cell shape (FN1, BGN, HAPLN1) (Table 6), which have all been previously identified in human breast cancer and canine mammary cancer studies [51–53]. The EVs of the two complex carcinoma cell lines shared 487 over-represented proteins, mostly associated with angiogenesis, cell migration, adhesion and motility (ECM1, EDIL3, VEGFC) (Additional file 8).
Table 6.
Top 20 differentially common over-represented EV proteins vs. healthy control in the carcinoma cell lines
| Common over-represented proteins | |||||
|---|---|---|---|---|---|
| Accession number | Protein name | Protein abbreviation |
MTH52C Log2FC |
1305 Log2FC |
DT1406TB Log2FC |
| A0A8I3NGU2 | Folate_rec domain-containing protein | LOC476816 | 7.242 | 8.684 | 9.315 |
| A0A8P0TLW8 | Scavenger receptor cysteine rich family member with 5 domains | SSC5D | 8.289 | 10.690 | 5.018 |
| Q28275 | Fibronectin (Fragment) | FN1 | 4.837 | 6.517 | 10.712 |
| A0A8P0SNB9 | Biglycan | BGN | 4.906 | 7.438 | 8.390 |
| A0A8I3PAS0 | Hyaluronan and proteoglycan link protein 1 | HAPLN1 | 6.534 | 2.065 | 11.965 |
| A0A8I3MGS0 | 72 kDa type IV collagenase | MMP2 | 3.103 | 6.260 | 8.838 |
| A0A8I3Q318 | Complement factor I | CFI | 3.497 | 4.469 | 8.752 |
| A0A8I3MZE8 | GM2 ganglioside activator | GM2A | 4.393 | 5.453 | 6.426 |
| A0A8I3PHI4 | Fibronectin | FN1 | 3.907 | 4.087 | 7.910 |
| A0A8I3PMB5 | Secretogranin-3 | SCG3 | 4.982 | 2.825 | 7.256 |
| A0A8I3PPU8 | Thrombospondin 1 | THBS1 | 1.870 | 7.201 | 5.508 |
| A0A8P0P6 × 6 | Cadherin-2 | CDH2 | 2.801 | 4.917 | 5.930 |
| A0A8I3Q5D9 | Procollagen C-endopeptidase enhancer 2 | PCOLCE2 | 2.813 | 3.477 | 7.098 |
| A0A8I3MTJ9 | Laminin subunit gamma 1 | LAMC1 | 3.385 | 5.938 | 3.625 |
| A0A8I3PM12 | L1 cell adhesion molecule | L1CAM | 1.683 | 7.241 | 3.972 |
| A0A8I3PNM0 | G_PROTEIN_RECEP_F3_4 domain-containing protein | GPRC5D | 1.755 | 6.742 | 3.767 |
| A0A8P0TM99 | Pleckstrin homology like domain family B member 2 | PHLDB2 | 2.235 | 6.263 | 3.720 |
| A0A8P0SGF7 | Hyaluronan and proteoglycan link protein 3 | HAPLN3 | 3.031 | 3.779 | 5.017 |
| A0A8I3NXQ5 | Ethylmalonyl-CoA decarboxylase | 3 SV | 1.567 | 4.820 | 5.429 |
| A0A8I3S4E2 | Versican | VCAN | 1.607 | 4.248 | 5.805 |
| Common under-represented proteins | |||||
| Accession number | Protein name | Protein abbreviation |
MTH52C Log2FC |
1305 Log2FC |
DT1406TB Log2FC |
| A0A8I3Q193 | Selenocysteine lyase | SCLY | -1.972 | -5.637 | -5.324 |
| A0A8I3NJ98 | N-sulfoglucosamine sulfohydrolase | SGSH | -2.091 | -4.984 | -4.477 |
| A0A8I3QBZ4 | Taxilin gamma | TXLNG | -0.878 | -4.915 | -5.585 |
| A0A8P0SNX6 | tRNA: m(4)X modification enzyme TRM13 | TRMT13 | -2.591 | -5.158 | -3.569 |
| A0A8P0TLB2 | UBX domain-containing protein 7 | UBXN7 | -2.140 | -4.895 | -3.880 |
| A0A8I3MKV2 | Malignant T-cell-amplified sequence | LOC487150 | -2.309 | -4.124 | -3.935 |
| A0A8I3PUG8 | TATA-box binding protein | TBP | -2.315 | -3.893 | -4.015 |
| A0A8I3RWI4 | Section 1 family domain containing 2 | SCFD2 | -2.539 | -2.898 | -4.163 |
| A0A8I3PMJ4 | DIS3-like exonuclease 2 | DIS3L2 | -3.638 | -2.854 | -2.612 |
| A0A8I3N7E1 | CTP synthase | CTPS1 | -2.172 | -3.350 | -3.558 |
| A0A8I3MKB3 | CYRIA-B_Rac1-bd domain-containing protein | CYFIP2 | -2.791 | -3.719 | -2.455 |
| A0A8I3RYJ8 | Lysine demethylase 2 A | KDM2A | -0.797 | -4.070 | -4.012 |
| A0A8I3RXS7 | 2-(3-amino-3-carboxypropyl)histidine synthase subunit 1 | DPH1 | -2.365 | -2.992 | -3.444 |
| A0A8I3RW28 | ASPSCR1 tether for SLC2A4, UBX domain containing | ASPSCR1 | -1.848 | -4.301 | -2.302 |
| A0A8I3RX66 | thioredoxin-disulfide reductase | TXNRD3 | -1.867 | -4.939 | -1.281 |
| A0A8I3NN15 | Small nuclear ribonucleoprotein Sm D1 | SNRPD1 | -0.857 | -4.393 | -2.586 |
| A0A8P0NJW0 | Metastasis associated 1 | MTA1 | -1.104 | -4.682 | -1.985 |
| A0A8I3S647 | Propionyl-CoA carboxylase alpha chain, mitochondrial | PCCA | -2.235 | -3.662 | -1.763 |
| P10463 | Calcyphosin | CAPS | -1.802 | -2.734 | -2.894 |
| A0A8P0SK07 | Complement component 1 Q subcomponent-binding protein, mitochondrial | C1QBP | -0.944 | -3.812 | -2.622 |
Evaluation of the overlaps of the under-represented proteins in all CMT EVs identified only 38 common proteins mostly related to DNA/RNA processing (TATDN1, IMPDH1, IMPDH2, PAPBC4, PUS7) (Fig. 4f; Table 5). Interestingly the least abundant protein amongst them, XPO6, has previously been reported to be downregulated in human breast cancer (Additional file 7). Moreover, carcinoma EVs shared 85 under-represented proteins involved in regulating the cellular protein metabolism (ASPSCR1, DIS3L2, C1QBP) (Table 6), whereas complex carcinoma EVs shared 506 under-represented proteins, with the least abundant being involved in RNA processing and cell cycle (WDR3, TP53, BRIX1, RPS13) (Additional file 8).
Gene ontology of EV-derived proteins revealed similar biological patterns as WCLs
All over-represented proteins in the adenoma ZMTH3 EVs were again mainly associated with GO biological processes such as ribonucleoprotein complex subunit organisation (F.E. 6.0); ribosome biogenesis (F.E. 4.5); mRNA processing (F.E. 4.4); RNA processing (F.E. 4.0) and translation (F.E. 3.5) (Fig. 4g). Likewise, GO analysis of the common over-represented proteins identified in the carcinoma EVs were enriched for proteins associated with cell-substrate adhesion (F.E. 9.4); ECM organisation (F.E. 9.2); endocytosis (F.E. 6.8); cell adhesion (F.E. 5); vesicle-mediated transport (F.E. 4.3) and cell migration (F.E. 3.9) (Fig. 4h).
Under-represented proteins from the adenoma ZMTH3 EVs were associated with endocytosis (F.E. 2.8); carbohydrate metabolic process (F.E. 2.7); oxoacid metabolic process (F.E. 2.4); actin cytoskeleton organisation (F.E. 2.4); establishment of protein localization (F.E. 2.1) and biological adhesion (F.E. 2.1) pathways (Fig. 4i), whereas shared under-represented proteins in carcinoma EVs were associated with RNA polyadenylation (F.E. 16), RNA 3- end processing (F.E. 11.5), reg. of mRNA metabolic process (F.E. 6.6), RNA catabolic process (F.E. 6), methylation (F.E. 5.7) and mRNA processing (F.E. 5.2) (Fig. 4j).
Overall, CMT cell line-derived EV proteomes presented patterns of protein abundance different to their equivalent WCLs, indicating potential selectivity. Nevertheless, these proteomes still reflected the same biological behaviour of their parent cells (WCLs). While the adenoma subtype was highly abundant in proteins enriched for RNA splicing process, the carcinoma subtype had a high abundance of proteins associated with ECM organisation, a main component of the tumour microenvironment, potentially facilitating cancer progression by promoting cell migration.
EV proteomes allow identification of potential biomarkers for disease state
Given the potential of EV protein cargo to distinguish between different types of CMT, we sought to identify signatures that could serve as biomarkers for each of the tested CMT types based on the abundance of WCL and EV proteins in the CMT cell lines.
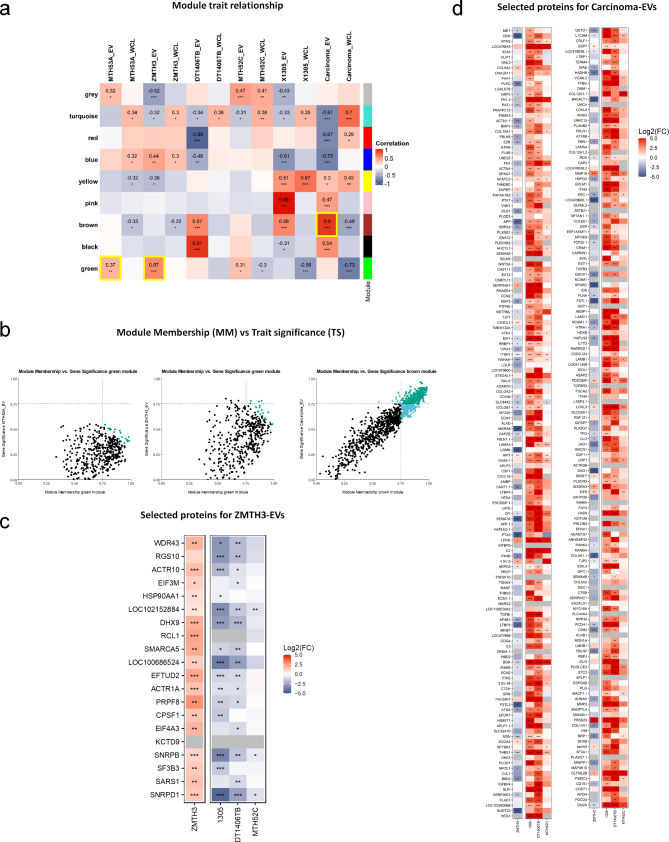
To identify co-abundant proteins that may play an important role in the progression of canine mammary cancer, a weighted gene correlation network analysis (WGCNA) was performed on all available data obtained from the EVs and WCLs of each cell line. Hierarchical cluster analysis identified 9 modules of co-abundant proteins, identified by colours (Additional file 9). Module-trait relationships based on Pearson correlation were assessed to identify modules highly correlating with particular traits (e.g. EV and WCL samples as well as carcinoma and complex carcinoma groups) (Fig. 5a). The most representative modules for each cell line were selected according to their positive correlation (green module for healthy control MTH53A and adenoma ZMTH3 EVs: correlation 0.37 and 0.57, respectively; brown module for carcinoma EVs: correlation 0.9) (Fig. 5a). For these module-trait combinations, absolute module memberships (MM) and trait significances (TS) were determined, and proteins were considered potential key drivers if both values were ≥ 0.75 (Fig. 5b). Using this approach, all proteins in EVs derived from the healthy control MTH53A were below the correlation cut-off (≥ 0.75). Therefore, key drivers could not be identified. Only four key drivers were identified for the adenoma ZMTH3 EVs. Hence, absolute MM and TS values were summed to identify the top 20 key driver candidates for ZMTH3 (Fig. 5c), which were again mostly associated with RNA processing, mRNA processing, and RNA splicing (Table 7). Finally, proteins highly correlating with both simple and complex carcinoma (Fig. 5d) were mainly related to pathways including ECM organisation, cell adhesion, cell motility, and cell migration (Table 7), including ECM-associated proteins previously associated with mammary cancer, such as BGN and FN1 (Additional file 7). As BGN was one of the top most over-represented proteins in the carcinoma signature, western blot was performed on new, independent EV isolates (Additional file 10), which confirmed higher abundance of BGN in the carcinoma EVs. We further performed immunofluorescence to assess the protein abundance in the parent cell lines. Consistent with our proteomic analysis data, no significant differences were found between the cancer cell lines and the normal or adenoma cell lines (Additional file 11). Therefore, BGN may prove useful as a specific EV biomarker for canine mammary carcinomas.
Fig. 5.
Selection of protein “key drivers” for further EV proteomic signatures. a) Heatmap of the EV proteomic profiles selected for each cell line (protein “key drivers”). (a) Module trait relationship. Pearson correlation of co-abundant proteins for each trait (Sample types: WCL and EVs; groups: carcinoma, simple carcinoma, and complex carcinoma) in every module colour. The significance of the correlation is indicated with asterisks (* p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001). Yellow squares highlight the modules of interest for further key driver identification in each subtype (EVs). (b) Module membership (MM) vs trait significance (TS) with a correlation cut-off ≥ 0.75. From left to right: selected module for healthy control MTH53A; selected module (green) for adenoma ZMTH3; selected module (brown) for Carcinoma. Real “key drivers” are shown in green colour. (c) Adenoma ZMTH3 top 20 selected protein key driver. (d) All three carcinoma cell lines-derived protein key drivers. Significance of log2FCs is indicated with asterisks (* p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001)
Table 7.
GO pathway categories from protein profiles (key drivers) in each cell line derived EVs
| Cell type/Cell line | GO pathway categories | Proteins |
|---|---|---|
| Adenoma ZMTH3 | RNA processing | CPSF1 WDR43 EIF4A3 SNRPB DHX9 EFTUD2 SNRPD1 PRPF8 SF3B3 |
| mRNA processing | CPSF1 EIF4A3 SNRPB DHX9 EFTUD2 SNRPD1 PRPF8 SF3B3 | |
| mRNA metabolic process | CPSF1 EIF4A3 SNRPB DHX9 EFTUD2 SNRPD1 PRPF8 SF3B3 | |
| RNA splicing | EIF4A3 SNRPB DHX9 EFTUD2 SNRPD1 PRPF8 SF3B3 | |
| Simple + complex carcinoma | ECM organisation | MMP19 FBLN1 EXT1 TGFBI B4GALT1 ECM2 COL15A1 NTN4 LOXL3 APP PTX3 LOXL4 MMP2 PHLDB2 FBLN5 DAG1 COL6A1 QSOX1 OLFML2B LAMC1 LTBP3 PLOD3 ADAMTS7 COL5A2 VWA1 COL11A1 KAZALD1 |
| Cell adhesion | EZR PLXNB2 FBLN1 EXT1 TGFBI PTPRK PTK7 B4GALT1 ECM2 IGFBP7 NRP1 FAT4 LAMA4 BCAM TENM4 JAG1 ACTN4 P4HB PCDH1 ATRN NTN4 SPINK5 LOXL3 EDIL3 THBS1 CX3CL1 DSP PHLDB2 HAPLN3 PLXNB1 LAMC1 DLG1 NCAM1 RDX FN1 FBN1 ADAM10 MSN THBS3 EFNA1 SRPX2 DSC1 LAMA3 MXRA8 L1CAM FLNA CSF1 FSTL3 LAMB1 WNT5A CCN2 EPDR1 | |
| Cell motility | PLXNB2 PLG FBLN1 EXT1 PTPRK PTK7 B4GALT1 PLA2G7 MET NRP1 LAMA4 PRCP PAK1 JAG1 ATRN NTN4 APP THBS1 CX3CL1 SEMA5A PHLDB2 LGMN FSTL1 DAG1 APOH ENPEP ECM1 GPC1 PLXNB1 NRP2 LAMC1 ARPC5 LRRC15 CTSH GRN RDX FN1 IGFBP5 CXCL16 ADAM10 MSN EFNA1 SRPX2 SPARC SEMA7A LAMA3 MYO18A L1CAM FLNA CSF1 TGFBR3 CD151 LAMB1 WNT5A GNA12 | |
| Cell migration | PLXNB2 PLG EXT1 PTPRK PTK7 B4GALT1 PLA2G7 MET NRP1 LAMA4 PRCP PAK1 JAG1 ATRN NTN4 APP THBS1 CX3CL1 SEMA5A PHLDB2 LGMN FSTL1 DAG1 APOH ENPEP ECM1 GPC1 PLXNB1 NRP2 LAMC1 ARPC5 LRRC15 CTSH GRN RDX FN1 IGFBP5 CXCL16 ADAM10 MSN EFNA1 SRPX2 SPARC SEMA7A LAMA3 MYO18A L1CAM FLNA CSF1 TGFBR3 CD151 LAMB1 WNT5A GNA12 |
Discussion
Over the last decades, EVs have come to the forefront of cancer research due to their role in transferring disease-related signalling molecules, thereby facilitating cell-to-cell communication [1, 54]. Indeed, EV proteins released by highly invasive breast cancer cells can affect the growth and metastatic progression of more benign human breast cancer cells [12, 55]. Previous studies in dogs aimed at characterising EVs in CMT cell lines mainly focused on miRNA content [56]. Here we present the first proteomic analysis of EVs from normal, benign and malignant canine mammary cell lines to identify distinct protein profiles. Such signatures could be used to differentiate between normal mammary gland and CMTs, between CMT types (carcinoma and adenoma), as well as between carcinoma subtypes (simple and complex). Our results form the basis for the development of a potential new serum-EV-based diagnostic procedure for CMT.
PCA analysis and hierarchical clustering (Figs. 1c and 3d + 5) confirmed that proteins identified in WCLs and EVs depend on CMT subtype. One exception was the simple carcinoma MTH52C EV proteome, which clustered together with the healthy and benign control despite a higher variance in WCL sample types. This was also reflected by a reduced number of differentially abundant proteins in these EVs compared to the two other carcinoma-derived cell lines and may reflect the similar 2D morphology of simple carcinoma MTH52C and healthy control MTH53A cell lines. Nevertheless, simple carcinoma MTH52C cell line EVs contained a number of proteins that were also found in the two other carcinoma cell lines and are therefore likely to reflect their nature as carcinoma-derived cells. Tumours are diverse and therefore phenotypic diversity of canine mammary tumours can be expected to be accompanied by a respective variance in protein abundance patterns, as in human breast cancer [57].
Unlike the carcinoma cell lines, the adenoma ZMTH3 was highly abundant in proteins involved in RNA processing and RNA splicing in WCLs and EVs, respectively, processes that are major mediators of proteome diversity by interacting in more than one cancer hallmark, establishing complex regulatory networks that simultaneously coordinate multiple cancer characteristics [58]. One possible explanation for this biological difference between the adenoma and carcinoma cell types is that RNA-splicing factors may be required for cancer initiation, while being dispensable for tumour maintenance [59]. Interestingly, a recent proteomic study identified an enrichment of mRNA processing-associated proteins among phosphoproteins down-regulated in metastatic human breast cancer patients compared to non-metastatic cancers [60], indicating that a change in mRNA processing may be a specific trait for more aggressive mammary cancers.
The carcinoma subtype showed high abundance of proteins enriched for cell migration, adhesion, and motility in both WCLs and EV proteomes consistent with an increased migratory ability of carcinoma cells [61]. This was highlighted by a high over-representation of proteins associated with cytoskeleton organisation in the carcinoma cell-line WCLs. Moreover, reorganization of intermediate filaments, such as vimentin (average log2-fold change 1.23 in WCLs), in tumour cells is associated with epithelial-mesenchymal transition (EMT), promoting migratory and invasive activity of cancer cells of more aggressive phenotypes [61, 62]. Further, the carcinoma-derived EVs had a high abundance of ECM-related proteins consistent with their role in facilitating cell survival, growth, migration and invasion of cells [63].
It was noticeable that EVs did not purely reflect the parental WCL protein content but showed different protein abundance patterns; yet the same biological behaviour was still described in each CMT subtype. Nevertheless, it remains unclear whether there is a selective mechanism for sorting proteins into EVs in these cells. The higher concentration of specific proteins in EVs compared to WCLs suggests that there may be a targeted mechanism to direct these proteins into EVs, which could be cancer-specific [64]. Further studies should determine whether such an EV protein cargo sorting mechanism responds to a functional purpose, and what the biological effect of this selection in the tumour microenvironment is.
Co-abundant proteins were identified for the adenoma and carcinoma EV subtypes by WGCNA (Fig. 5). With this approach, proteins showing specific regulation in carcinoma EVs were identified, which may serve as potential biomarkers and/or therapeutic targets for CMT in the future. Unfortunately, the signature for the adenoma ZMTH3 was composed of proteins that did not show convincing correlation with the module and the trait, indicating that the selection of protein key drivers is not representative enough to establish a reliable adenoma signature based on these data. Nevertheless, the identified potential candidates show consistent and specific regulation over the different conditions investigated here, suggesting them to be a suitable starting point for the development of a representative and reliable signature. Notably, 9 out of the 20 co-abundant proteins were associated with RNA processing, mRNA processing and RNA splicing, correlating with the gene ontology results in the EV proteome and thus providing functional insights into the adenoma CMT phenotype. Further investigations are needed to establish a specific signature for benign mammary tumours in dogs.
Despite the differences in EV protein abundance between simple carcinoma MTH52C and complex carcinoma cell lines, there were expected similarities in biological processes resulting in a clear carcinoma protein pattern. Thus, the carcinoma signature included many of the most over-represented proteins that were strongly associated with ECM organisation, cell adhesion, cell motility, and cell migration, similar to results from previous proteomic studies of human breast cancer EVs [12, 54]. This is also consistent with observations from a previous cDNA microarray study on spontaneous CMTs, in which the majority of differentially expressed genes coded for proteins involved in cell motility, cytoskeletal organisation and ECM production [65]. In addition, proteomic analysis of cancer-associated stroma from 14 formalin-fixed paraffin-embedded canine mammary carcinomas also identified an over-representation of proteins associated with the ECM and cytoskeleton [66]. These observations show that the proteomes of the isolated EVs not only resemble those of the total proteomes of the CMT subtype but also reflect the biological processes related to carcinogenesis, both in vitro and in vivo. Taken together, our data is consistent with previous evidence that EVs could play a crucial role in cell behaviour changes, which may lead to cancer progression and therefore have strong potential for diagnostic purposes.
Two proteins included in the carcinoma EV signature were the ECM proteins biglycan (BGN) and fibronectin (FN1), which were among the most over-represented ECM-related proteins in EV isolates from all carcinoma cell lines (Table 6; Fig. 5c). BGN plays major roles in cellular processes including migration, adhesion, inflammation, cell growth and apoptosis [67]. In dogs, BGN upregulation has been identified in cancer-associated stroma of malignant CMTs, both in proteomic and transcriptomic approaches, and these results were consistent with human breast cancer studies [52]. FN1 is a major component of the ECM involved in cell adhesion, proliferation, migration, blood coagulation, wound healing and embryogenesis [51]. In human breast cancer tissues, FN1 is upregulated compared with normal tissues, and correlates with poor clinical outcomes [68]. Both FN1 and BGN correlate positively in a transcriptomic study between canine mammary carcinomas and human breast cancer [69]. Therefore, our findings support and strengthen the potential role for these proteins as CMT biomarkers in EVs.
In our isolation procedure we were unable to distinguish between exosomes and microvesicles. However, the EVs of the different cell lines had similar size distributions though varying amounts of CD9 and CD63 protein abundance. This might indicate that the isolated EVs contained different proportions of exosomes and MVs [70]. Nevertheless, the higher biglycan abundance in the carcinoma EVs was clearly not due to these differences as MTH53A EVs expressed similar CD9 levels as the complex carcinoma cell lines, but biglycan was hardly detectable (Additional file 10).
Apart from the obvious limitation that in vitro cell systems cannot represent the whole spectrum of CMTs, another major limitation in our study is that EV isolation from cell culture media only provides relatively low yields; therefore, it cannot be excluded that low-level proteins have not been detected. Moreover, using serum-free medium was exclusively chosen to avoid any possible contamination for proteomics application. We acknowledge this might affect the cellular response and therefore we cannot exclude that some proteins might be detected because of cell starvation. Another important limitation was the scarcity of canine proteomic data in the UniProtKB database as many canine proteins were not mapped. Nevertheless, our data suggests that subtyping disease stages from EVs proteins is feasible and that our proteomic analyses could now form the basis to develop biomarkers suitable for clinical diagnostics of canine mammary cancers. Follow-up studies will now investigate whether these CMT signatures can also be detected in serum samples from clinical patients and even identify CMT subtypes.
Conclusions
Our study is the first to describe the proteomic profiles of WCLs and EVs from canine mammary tumour-derived cell lines. We found that WCLs can be used to distinguish different CMT phenotypes based on protein abundance, and that EVs derived from CMTs resemble the biological behaviour of their (parental) WCLs. Thus, EVs could potentially be used as diagnostic tools for detecting specific biomarkers of disease state in liquid biopsies, enabling the prediction of tumour development and progression in conjunction with conventional techniques. BGN was one of the most over-represented proteins in the carcinoma EV signature and shows potential as an EV-biomarker for canine mammary tumours. However, further potential markers, including FN1, require further verification. Equally, further studies with larger CMT subtype cohorts as well as patient material will be needed to experimentally validate these protein markers. Given the apparent similarities between canine mammary cancer and human breast cancer, it is possible that such data could also be valuable in the diagnosis of the human disease.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank Ms. Ann-Cathrin Schmitt and Prof. Dr. Rainer Haag (Institute of Chemistry and Biochemistry, Department of Biology, Chemistry and Pharmacy, Freie Universität Berlin, as well as Drs. Isabella Tavernaro and Ute Resch-Genger (Division Biophotonics, Department of Analytical Chemistry, Reference Materials, Bundesanstalt für Materialforschung und -prüfung (BAM)) for the access to the NTA systems and their assistance. Furthermore, the authors would like to take this opportunity to thank the UFZ-funded ProMetheus platform for proteomics and metabolomics for the support of this project. In addition, the authors want to thank Maj Schuster for excellent technical assistance.
Abbreviations
- CMT
Canine mammary tumour
- EVs
Extracellular vesicles
- WCL
Whole-cell lysate
- FBS
Foetal bovine serum
- SEC
Size exclusion chromatography
- NTA
Nanoparticle tracking analysis
- LC-MS/MS
Liquid chromatography with tandem mass spectrometry
- TMT
Tandem mass tag
- TEAB
Triethylammonium bicarbonate
- ACN
Acetonitrile
- log2(FC)
Log2-transformed fold change
- WGCNA
Weighted gene correlation network analysis
- GO
Gene ontology
- FDR
False discovery rate
- PCA
Principal component analysis
- F.E
Fold enrichment
- ECM
Extracellular matrix
- BGN
Biglycan
Author contributions
T.G.R. wrote the original manuscript, performed experiments, and analysed data. I.K., K.S., G.L. and M.v.B. performed data acquisition using mass spectrometry-based proteomics and subsequent data processing and analysis. E.M.P., I.N., and H.M.E. provided experimental material. T.S. revised and edited the manuscript, designed the study, analysed data, and supervised planning and execution. M.A.R. revised and edited the manuscript, supervised planning and execution, and provided experimental advice. R.E. revised and edited the manuscript and supervised planning and execution. All authors contributed to the writing of the manuscript, and have read and approved the final version of the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. T. Gutierrez-Riquelme is a scholarship holder of the doctoral bilateral agreement DAAD/Becas Chile program (folio number 62190010) between the German and Chilean governments.
Data availability
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE [71] partner repository with the dataset identifier PXD046033 and 10.6019/PXD046033. Reviewer account details:
Username: reviewer_pxd046033@ebi.ac.uk.
Password: dMG0adD3.
Declarations
Ethics approval and consent to participate
The study does not use live animals or primary cells but only established cell lines as referenced. Therefore, no ethical approval was required.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Petroušková P, Hudáková N, Maloveská M, Humeník F, Cizkova D. Non-exosomal and exosome-derived miRNAs as promising biomarkers in Canine Mammary Cancer. Life. 2022;12(4):524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abadie J, Nguyen F, Loussouarn D, Peña L, Gama A, Rieder N, Belousov A, Bemelmans I, Jaillardon L, Ibisch C. Canine invasive mammary carcinomas as models of human breast cancer. Part 2: immunophenotypes and prognostic significance. Breast Cancer Res Treat. 2018;167(2):459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rasotto R, Berlato D, Goldschmidt MH, Zappulli V. Prognostic significance of canine mammary tumor histologic subtypes: an observational cohort study of 229 cases. Vet Pathol. 2017;54(4):571–8. [DOI] [PubMed] [Google Scholar]
- 4.Uva P, Aurisicchio L, Watters J, Loboda A, Kulkarni A, Castle J, Palombo F, Viti V, Mesiti G, Zappulli V. Comparative expression pathway analysis of human and canine mammary tumors. BMC Genomics. 2009;10(1):1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klopfleisch R, Klose P, Weise C, Bondzio A, Multhaup G, Einspanier R, Gruber AD. Proteome of metastatic canine mammary carcinomas: similarities to and differences from human breast cancer. J Proteome Res. 2010;9(12):6380–91. [DOI] [PubMed] [Google Scholar]
- 6.De Las Mulas JM, Reymundo C, De Los Monteros AE, Millán Y, Ordás J. Calponin expression and myoepithelial cell differentiation in canine, feline and human mammary simple carcinomas. Vet Comp Oncol. 2004;2(1):24–35. [DOI] [PubMed] [Google Scholar]
- 7.Canadas A, França M, Pereira C, Vilaça R, Vilhena H, Tinoco F, Silva MJ, Ribeiro J, Medeiros R, Oliveira P. Canine mammary tumors: comparison of classification and grading methods in a survival study. Vet Pathol. 2019;56(2):208–19. [DOI] [PubMed] [Google Scholar]
- 8.Burrai GP, Gabrieli A, Moccia V, Zappulli V, Porcellato I, Brachelente C, Pirino S, Polinas M, Antuofermo E. A statistical analysis of risk factors and biological behavior in canine mammary tumors: a multicenter study. Animals. 2020;10(9):1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaszak I, Witkowska-Piłaszewicz O, Domrazek K, Jurka P. The novel diagnostic techniques and biomarkers of canine mammary tumors. Veterinary Sci. 2022;9(10):526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu J, Chen Y, Pei F, Zeng C, Yao Y, Liao W, Zhao Z. Extracellular vesicles in liquid biopsies: potential for disease diagnosis. BioMed Research International 2021, 2021. [DOI] [PMC free article] [PubMed]
- 11.Van Niel G, d’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19(4):213–28. [DOI] [PubMed] [Google Scholar]
- 12.Jordan KR, Hall JK, Schedin T, Borakove M, Xian JJ, Dzieciatkowska M, Lyons TR, Schedin P, Hansen KC, Borges VF. Extracellular vesicles from young women’s breast cancer patients drive increased invasion of non-malignant cells via the focal adhesion kinase pathway: a proteomic approach. Breast Cancer Res. 2020;22(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou B, Xu K, Zheng X, Chen T, Wang J, Song Y, Shao Y, Zheng S. Application of exosomes as liquid biopsy in clinical diagnosis. Signal Transduct Target Therapy. 2020;5(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim J-H, Kim E, Lee MY. Exosomes as diagnostic biomarkers in cancer. Mol Cell Toxicol. 2018;14(2):113–22. [Google Scholar]
- 15.Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics. 2008;9(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hammer SC, Becker A, Rateitschak K, Mohr A, Lüder Ripoli F, Hennecke S, Junginger J, Hewicker-Trautwein M, Brenig B, Ngezahayo A. Longitudinal claudin gene expression analyses in canine mammary tissues and thereof derived primary cultures and cell lines. Int J Mol Sci. 2016;17(10):1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldschmidt M, Peña L, Rasotto R, Zappulli V. Classification and grading of canine mammary tumors. Vet Pathol. 2011;48(1):117–31. [DOI] [PubMed] [Google Scholar]
- 18.Abramowicz A, Marczak L, Wojakowska A, Zapotoczny S, Whiteside TL, Widlak P, Pietrowska M. Harmonization of exosome isolation from culture supernatants for optimized proteomics analysis. PLoS ONE. 2018;13(10):e0205496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel GK, Khan MA, Zubair H, Srivastava SK, Khushman M, Singh S, Singh AP. Comparative analysis of exosome isolation methods using culture supernatant for optimum yield, purity and downstream applications. Sci Rep. 2019;9(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benedikter BJ, Bouwman FG, Vajen T, Heinzmann AC, Grauls G, Mariman EC, Wouters EF, Savelkoul PH, Lopez-Iglesias C, Koenen RR. Ultrafiltration combined with size exclusion chromatography efficiently isolates extracellular vesicles from cell culture media for compositional and functional studies. Sci Rep. 2017;7(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monguió-Tortajada M, Morón‐Font M, Gámez‐Valero A, Carreras‐Planella L, Borràs FE, Franquesa M. Extracellular‐vesicle isolation from different biological fluids by size‐exclusion chromatography. Curr Protoc Stem Cell Biol. 2019;49(1):e82. [DOI] [PubMed] [Google Scholar]
- 22.Wang Z, Karkossa I, Großkopf H, Rolle-Kampczyk U, Hackermüller J, von Bergen M, Schubert K. Comparison of quantitation methods in proteomics to define relevant toxicological information on AhR activation of HepG2 cells by BaP. Toxicology. 2021;448:152652. [DOI] [PubMed] [Google Scholar]
- 23.Hughes CS, Moggridge S, Müller T, Sorensen PH, Morin GB, Krijgsveld J. Single-pot, solid-phase-enhanced sample preparation for proteomics experiments. Nat Protoc. 2019;14(1):68–85. [DOI] [PubMed] [Google Scholar]
- 24.Karkossa I. Proteomicsr: An Analysis Pipeline for Label-Based and Label-free Proteomics Data (1.0. 0). Zenodo 2023.
- 25.Rohart F, Gautier B, Singh A, Lê Cao K-A. mixOmics: an R package for ‘omics feature selection and multiple data integration. PLoS Comput Biol. 2017;13(11):e1005752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei T, Simko V, Levy M. package corrplot: Visualization of a Correlation Matrix. 2017. Version 084 2021.
- 27.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43(7):e47–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peterson BG, Carl P, Boudt K, Bennett R, Ulrich J, Zivot E, Cornilly D, Hung E, Lestel M, Balkissoon K. Package ‘performanceanalytics’. R Team Cooperation. 2018;3:13–4. [Google Scholar]
- 29.Sakai R, Winand R, Verbeiren T, Moere AV, Aerts J. dendsort: modular leaf ordering methods for dendrogram representations in R. F1000Research 2014, 3. [DOI] [PMC free article] [PubMed]
- 30.Gu Z, Eils R, Schlesner M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics. 2016;32(18):2847–9. [DOI] [PubMed] [Google Scholar]
- 31.Wickham H. The split-apply-combine strategy for data analysis. J Stat Softw. 2011;40:1–29. [Google Scholar]
- 32.Wickham H. Reshaping data with the reshape package. J Stat Softw. 2007;21:1–20. [Google Scholar]
- 33.Zhang X, Smits AH, van Tilburg G, Ovaa H, Huber W, Vermeulen M. Proteome-wide identification of ubiquitin interactions using UbIA-MS. Nat Protoc. 2018;13(3):530–50. [DOI] [PubMed] [Google Scholar]
- 34.Xiao N. Ggsci: scientific journal and sci-fi themed color palettes for ‘ggplot2’. R Package Version. 2018;2:9. [Google Scholar]
- 35.Gu Z, Gu L, Eils R, Schlesner M, Brors B. Circlize implements and enhances circular visualization in R. Bioinformatics. 2014;30(19):2811–2. [DOI] [PubMed] [Google Scholar]
- 36.Graffelman J, van Eeuwijk F. Calibration of multivariate scatter plots for exploratory analysis of relations within and between sets of variables in genomic research. Biometrical Journal: J Math Methods Biosci. 2005;47(6):863–79. [DOI] [PubMed] [Google Scholar]
- 37.Wickham H. Data analysis. ggplot2. Springer; 2016. pp. 189–201.
- 38.Wickham H, Bryan J, Kalicinski M, Valery K, Leitienne C, Colbert B, Hoerl D, Miller E, Bryan MJ. Package ‘readxl’. Computer Software] https://readxl tidyverse org 2019.
- 39.Spiess A-N, Spiess MA-N. Package ‘qpcR’. Model Anal Real-Time PCRdata Httpscran R-Proj’(OrgwebpackagesqpcRqpcR Pdf) 2018.
- 40.Mahto A. Splitstackshape: Stack and reshape datasets after splitting concatenated values. R Package Version 2019, 1(8).
- 41.Wickham H, Henry L. Tidyr: tidy messy data. R Package Version. 2020;1(2):397. [Google Scholar]
- 42.Van den Brand T. Ggh4x: Hacks for Ggplot2. 2020.
- 43.Galili T. Dendextend: an R package for visualizing, adjusting and comparing trees of hierarchical clustering. Bioinformatics. 2015;31(22):3718–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Turner S. Tmisc: Turner Miscellaneous. R package version 01 2019, 22.
- 45.Langfelder P, Horvath S. Fast R functions for robust correlations and hierarchical clustering. J Stat Softw 2012, 46(11). [PMC free article] [PubMed]
- 46.Ge SX, Jung D, Yao R. ShinyGO: a graphical gene-set enrichment tool for animals and plants. Bioinformatics. 2020;36(8):2628–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heberle H, Meirelles GV, da Silva FR, Telles GP, Minghim R. InteractiVenn: a web-based tool for the analysis of sets through Venn diagrams. BMC Bioinformatics. 2015;16(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.López-Cortés A, Cabrera-Andrade A, Vazquez-Naya JM, Pazos A, Gonzales-Diaz H, Paz-y-Mino C, Guerrero S, Perez-Castillo Y, Tejera E, Munteanu CR. Prediction of breast cancer proteins involved in immunotherapy, metastasis, and RNA-binding using molecular descriptors and artificial neural networks. Sci Rep. 2020;10(1):8515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Umekita Y, Ohi Y, Souda M, Rai Y, Sagara Y, Sagara Y, Tamada S, Tanimoto A. Maspin expression is frequent and correlates with basal markers in triple-negative breast cancer. Diagn Pathol. 2011;6:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.An T, Qin S, Sun D, Huang Y, Hu Y, Li S, Zhang H, Li B, Situ B, Lie L. Unique Protein Profiles of Extracellular Vesicles as diagnostic biomarkers for early and advanced Non-small Cell Lung Cancer. Proteomics. 2019;19(12):1800160. [DOI] [PubMed] [Google Scholar]
- 51.Zhang X-X, Luo J-H, Wu L-Q. FN1 overexpression is correlated with unfavorable prognosis and immune infiltrates in breast cancer. Front Genet. 2022;13:913659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ettlin J, Bauer A, Opitz L, Malbon A, Markkanen E. Deciphering stromal changes between metastatic and non-metastatic canine mammary carcinomas. J Mammary Gland Biol Neoplasia. 2023;28(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chang SC, Yuan SHC, Li CY, Chang HM, Wang HC, Pan YA, Hsueh PC, Wu CC, Yang Y, Liu HP. Significant association of serum autoantibodies to TYMS, HAPLN1 and IGFBP5 with early stage canine malignant mammary tumours. Vet Comp Oncol. 2021;19(1):172–82. [DOI] [PubMed] [Google Scholar]
- 54.Rontogianni S, Synadaki E, Li B, Liefaard MC, Lips EH, Wesseling J, Wu W, Altelaar M. Proteomic profiling of extracellular vesicles allows for human breast cancer subtyping. Commun Biology. 2019;2(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee J-E, Moon P-G, Cho Y-E, Kim Y-B, Kim I-S, Park H, Baek M-C. Identification of EDIL3 on extracellular vesicles involved in breast cancer cell invasion. J Proteom. 2016;131:17–28. [DOI] [PubMed] [Google Scholar]
- 56.Fish EJ, Martinez-Romero EG, DeInnocentes P, Koehler JW, Prasad N, Smith AN, Bird RC. Circulating microRNA as biomarkers of canine mammary carcinoma in dogs. J Vet Intern Med. 2020;34(3):1282–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perou CM, Sørlie T, Eisen MB, Van De Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA. Molecular portraits of human breast tumours. nature 2000, 406(6797):747–752. [DOI] [PubMed]
- 58.Pereira B, Billaud M, Almeida R. RNA-binding proteins in cancer: old players and new actors. Trends cancer. 2017;3(7):506–28. [DOI] [PubMed] [Google Scholar]
- 59.Obeng EA, Stewart C, Abdel-Wahab O. Altered RNA processing in cancer pathogenesis and therapy. Cancer Discov. 2019;9(11):1493–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Parsons J, Harrison H, Kedward T, Fullwood P, Cabral S, Spence K, Barden D, Haworth J, Watson J, Tsafou K. Proteomics of Patient-derived Breast Tumours Identifies a Pro-migratory Osteomodulin-Cyclin Dependent Kinase 1 Axis which Drives Bone Metastasis. bioRxiv 2023.
- 61.Aseervatham J. Cytoskeletal remodeling in cancer. Biology. 2020;9(11):385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ong MS, Deng S, Halim CE, Cai W, Tan TZ, Huang RY-J, Sethi G, Hooi SC, Kumar AP, Yap CT. Cytoskeletal proteins in cancer and intracellular stress: a therapeutic perspective. Cancers. 2020;12(1):238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yamaguchi H, Wyckoff J, Condeelis J. Cell migration in tumors. Curr Opin Cell Biol. 2005;17(5):559–64. [DOI] [PubMed] [Google Scholar]
- 64.Garcia-Martin R, Brandao BB, Thomou T, Altindis E, Kahn CR. Tissue differences in the exosomal/small extracellular vesicle proteome and their potential as indicators of altered tissue metabolism. Cell Rep 2022, 38(3). [DOI] [PMC free article] [PubMed]
- 65.Rao N, Van Wolferen M, Gracanin A, Bhatti S, Krol M, Holstege F, Mol J. Gene expression profiles of progestin-induced canine mammary hyperplasia and spontaneous mammary tumors. J Physiol Pharmacol. 2009;60(Suppl 1):73–84. [PubMed] [Google Scholar]
- 66.Pöschel A, Beebe E, Kunz L, Amini P, Guscetti F, Malbon A, Markkanen E. Identification of disease-promoting stromal components by comparative proteomic and transcriptomic profiling of canine mammary tumors using laser-capture microdissected FFPE tissue. Neoplasia. 2021;23(4):400–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cong L, Maishi N, Annan DA, Young MF, Morimoto H, Morimoto M, Nam J-M, Hida Y, Hida K. Inhibition of stromal biglycan promotes normalization of the tumor microenvironment and enhances chemotherapeutic efficacy. Breast Cancer Res. 2021;23(1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang Y, Xu H, Zhu B, Qiu Z, Lin Z. Systematic identification of the key candidate genes in breast cancer stroma. Cell Mol Biol Lett. 2018;23(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee K-H, Park H-M, Son K-H, Shin T-J, Cho J-Y. Transcriptome signatures of canine mammary gland tumors and its comparison to human breast cancers. Cancers. 2018;10(9):317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ratajczak MZ. J Ratajczak 2020 Extracellular microvesicles/exosomes: discovery, disbelief, acceptance, and the future? Leukemia 34 12 3126–35. [DOI] [PMC free article] [PubMed]
- 71.Perez-Riverol Y, Bai J, Bandla C, García-Seisdedos D, Hewapathirana S, Kamatchinathan S, Kundu DJ, Prakash A, Frericks-Zipper A, Eisenacher M. The PRIDE database resources in 2022: a hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 2022;50(D1):D543–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE [71] partner repository with the dataset identifier PXD046033 and 10.6019/PXD046033. Reviewer account details:
Username: reviewer_pxd046033@ebi.ac.uk.
Password: dMG0adD3.