Abstract
Background
The effectiveness and adverse effects of coenzyme Q10 for heart failure remain unclear owing to small sample sizes and variations in the quality of existing studies in literature.
Methods
The databases of EMBASE, PubMed, Web of Science, CINAHL databases, Scopus, Cochrane Central Register of Controlled Trials, VIP, Wanfang, and CNKI were searched for randomized controlled trials on the coenzyme Q10-assisted treatment of heart failure. Relevant literature was retrieved, data were extracted, and the risk of bias of the included studies was evaluated by two investigators independently using the Review Manager 5.4 software and the STATA 15 software.
Results
In total, 33 studies were included in this meta-analysis, which showed that all-cause mortality [RR = 0.64, 95% CI (0.48, 0.85), P = 0.002; GRADE: moderate quality], hospitalization for heart failure [RR = 0.50, 95% CI (0.37, 0.67), P < 0.00001; GRADE: moderate quality], New York Heart Association classification [MD = − 0.29, 95% CI (− 0.39, − 0.19), P < 0.00001; GRADE: low quality], and brain natriuretic peptide level [MD = − 91.97, 95% CI (− 103.11, − 80.83), P < 0.00001; GRADE: low quality] were lower in the coenzyme Q10 group than in the control group. Meanwhile, left ventricular ejection fraction [MD = 0.51, 95% CI (0.31, 0.71), P < 0.00001; GRADE: low quality] and 6-min walk test result [MD = 31.70, 95% CI (19.96, 43.43), P < 0.00001; GRADE: moderate quality] were better than those in the control group.
Conclusions
According to the existing evidence, coenzyme Q10 reduces all-cause mortality, hospitalization for heart failure, New York Heart Association classification, and brain natriuretic peptide level and improves left ventricular ejection fraction and 6-min walk test result in those with heart failure without major adverse effects.
Trial registration
This study protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO, http://www.crd.york.ac.uk/prospero), with the registration number CRD42023493184.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12872-024-04232-z.
Keywords: Heart failure, Safety outcomes, Coenzyme Q10, Meta analysis, Randomized controlled trial
Introduction
Heart failure, a complex syndrome resulting from heart abnormalities, impairs heart function and manifests in symptoms like breathlessness and fatigue, along with signs of fluid buildup [1, 2]. As a prevalent syndrome affecting millions globally, it poses substantial health and economic burdens, with costs projected to escalate [3–6]. Characterized by impaired cardiac function and recurrent exacerbations, it carries a significant mortality risk, particularly for hospitalized patients [7]. The condition involves disrupted ATP production and calcium imbalance, leading to oxidative stress and mitochondrial damage, further compounded by overactive sympathetic responses [8–12].
In the past few decades, the treatment of heart failure has mainly relied on β-blockers, ACE inhibitors, and AT1 antagonists to reduce excessive neural and fluid activation and alleviate cardiac burden [13, 14]. Despite alleviating symptoms, these interventions have limited success in improving death and readmission rates [15]. The regulation of cardiac energy constitutes a novel therapeutic approach. Therapies that prevent myocardial energy consumption may play a role in the treatment and management of heart failure. As both an electron transporter and antioxidant, coenzyme Q10 boosts mitochondrial ATP production, which increases myocardial contractility [16, 17]. At present, it is used for heart failure treatment in some studies.
According to meta-analyses of randomized controlled trials (RCTs), coenzyme Q10 improves left ventricular ejection fraction (LVEF) regardless of New York Heart Association (NYHA) class [18–19]. In addition, two systematic reviews of coenzyme Q10 in heart failure reported a reduction in mortality rates [20, 21], whereas one did not [22]. Despite not being the primary treatment method for heart failure, coenzyme Q10 has been proven safe and effective. In spite of this, its efficacy and adverse reactions remain unclear due to small sample sizes and variable quality of existing studies. Thus, the present study conducted a meta-analysis to evaluate the effectiveness and safety of using coenzyme Q10 to treat patients with heart failure and provide evidence-based guidelines.
Materials and methods
The study protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO, http://www.crd.york.ac.uk/prospero), with the registration number CRD42023493184.
Search strategy
To identify RCTs investigating the effect of coenzyme Q10 in patients with heart failure, the databases of EMBASE, PubMed, Web of Science, CINAHL, Scopus, Cochrane Central Register of Controlled Trials, VIP, Wanfang, and CNKI were searched. Next, coenzyme Q10 and heart failure concept groups were developed using medical subject headings and keywords from PubMed. Without applying any additional filters or limits, the Cochrane RCT filter for PubMed was combined with concept groups for coenzyme Q10 and heart failure. Up to April 20, 2024, all the abovementioned databases were searched by two researchers using the search strategies listed in appendix 1, and disputed areas were referred to a third researcher for resolution. To report this systematic review and meta-analysis, the recommendations in the PRISMA statement were followed [23].
Inclusion and exclusion criteria
Study design Studies published in medical journals on the effect of coenzyme Q10 on heart failure were retrieved. In order to reduce the bias of interpretation, only Chinese and English studies were included.
Participants Patients with heart failure aged > 18 years, regardless of their race, nationality, duration of illness, or LVEF were included.
Interventions The experimental group that received coenzyme Q10 as an adjuvant therapy with conventional heart failure treatment was included. For control, patients with heart failure who received only conventional treatment with or without placebo were included. In both groups, the relevant drugs were administered at any dosage for a minimum period of 1 month.
Outcomes The primary outcomes were all-cause mortality and hospitalization for heart failure. The secondary outcomes included LVEF, NYHA classification, brain natriuretic peptide (BNP) level, 6-min walk test (6MWT), and adverse events.
Exclusion criteria Non-Chinese and non-English language studies and duplicate studies were excluded. Moreover, studies without full text and with incomplete data were not considered. Finally, non-RCTs were excluded. Studies in phase1, 2 and 3 trials, observational studies, retrospective studies, reviews, and letters were excluded.
Study selection
Two authors independently screened the titles and abstracts of RCTs that used coenzyme Q10 treatment for patients with heart failure. The shortlisted literature was assessed by reading the title and abstract, and after excluding irrelevant literature, the full text was read to determine final inclusion. Any differences included in the decision were discussed and resolved after reaching a consensus. The kappa agreement index was used to evaluate the level of agreement between the two authors.
Data extraction
A self-developed data extraction form was used to extract the following data: basic information about the included studies, baseline characteristics of the study participants, specific information about the interventions, duration of treatment, and outcome indicators.
Data analysis
Meta-analysis was performed using the Review Manager 5.4 software and the STATA 15 software. For count data, the relative risk (RR) was used as the effect indicator and for measurement data, the mean difference (MD) or standardized mean difference was used. Statistically significant differences were assessed using the point estimates and 95% confidence intervals (CIs). Meanwhile, heterogeneity among the included studies was analyzed using the χ2 test (test level: α = 0.1), and I2 quantification was used to estimate its magnitude. If there was no statistical heterogeneity among the study results, a fixed-effects model was used for meta-analysis. When statistical heterogeneity was detected between the study results, the source of heterogeneity was further analyzed, and after excluding the impact of significant clinical heterogeneity, randomization was performed. A subgroup analysis, sensitivity analysis, or descriptive analysis was performed when there was evident clinical heterogeneity. The meta-analysis test level was set at α = 0.05. The publication bias was assessed according to the funnel plot, the Begg’s test and the Egger’s test. To explore the robustness of the pooled results, sensitivity analysis was carried out using the leave-one-out method.
Risk of bias assessment
Two evaluators independently assessed the risk of bias of the included RCTs using the Cochrane Handbook 5.1.0 risk of bias assessment tool [24]. A third party was consulted when necessary to ensure the accuracy of the final study results. Seven points were followed to assess quality: (1) random sequence generation, (2) allocation concealment, (3) blinding of participants and personnel, (4) blinding of outcome assessment, (5) incomplete outcome data, (6) selective reporting; and (7) other biases. According to the criteria of “low risk of bias,” “unknown risk of bias,” and “high risk of bias,” the quality of the included studies was comprehensively assessed.
Certainty of evidence
GRADE (Grading Recommendations, Assessment, Development, and Evaluation) was used to rate the certainty of evidence for each outcome. The GRADE assessment was carried out using the GRADEpro Guideline Development Tool. Through this approach, bias, inconsistency, indirectness, imprecision, and other considerations (e.g. publication bias) are ranked as “high”, “moderate”, “low,” or “very low.”
Results
Search results
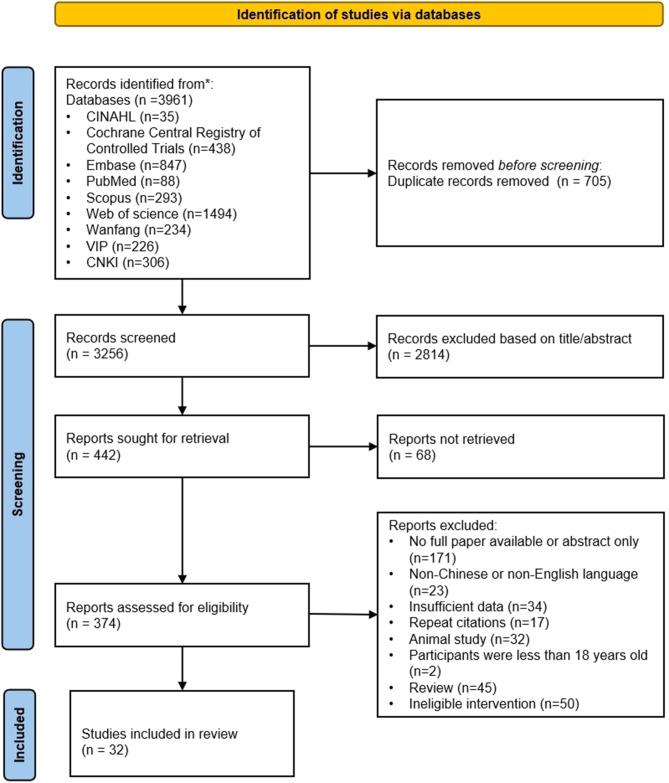
In total, 3961 relevant records were obtained through the electronic search. Of these, 705 duplicate records were removed using the Endnote X9 software. The remaining 3256 records were assessed based on the title or abstract, resulting in the exclusion of 2814 ineligible records. Ultimately, 32 RCTs were included after reviewing the full-text of the remaining 386 records (Fig. 1). The two authors had a high agreement in study selection and data integration (kappa value = 0.825).
Fig. 1.
Literature search flow diagram
Study characteristics
The 32 RCTs included 3763 patients with heart failure: 1,898 cases in the treatment group and 1,845 cases in the control group. Among the studies, the maximum sample size was 322 cases [25] and the minimum was 6 [26]. While 22 studies were conducted in Asia [27–48], 1 study recruited participants from Europe, Australia, and Asia [49]. In the intervention group, coenzyme Q10 was combined with conventional treatment. In the control group, conventional treatment was used, with placebo in addition to conventional treatment in 12 studies [25, 26, 28, 39, 44, 48–54]. Except one study [39] that involved nasal drops, coenzyme Q10 was administered orally in all others. The characteristics of the included studies are shown in Table 1.
Table 1.
Characteristics of included studies
| No. | study | Country | Sample size (EXP/CON) |
Age(year) (EXP/CON) |
Baseline LVEF (%)(EXP/CON) | Intervention | Control | Intervention duration | Outcomes | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Permanetter et al. (1992) | Germany | 15/10 | 52 ± 9 | NR | CoQ10(33.3 mg, 3 times/day) + conventional therapy | conventional therapy alone | 4months | ④ | NR |
| 2 | Morisco et al. (1993) | Denmark | 319/322 |
26∽89/ 30∽88 |
NR | coenzyme Q10 50 mg twice or 3 times daily + conventional therapy | Placebo + conventional therapy | 12months | ①② | NR |
| 3 | Morisco et al. (1994) | Italy | 6/6 | 49.8 ± 6.7 | 29 ± 11 | CoQ10(50 mg, 3 times/day) + conventional therapy | Placebo + conventional therapy | 1month | ③⑥ | 12months |
| 4 | Hofman-Bang et al. (1995) | Denmark | 79/79 | 61 ± 10 | 22 ± 10 | CoQ10(100 mg, 1 time/day) + conventional therapy | placebo + conventional therapy | 3months | ①③⑥ | 6months |
| 5 | Li & Li (1999) | China | 56/68 | NR | NR | CoQ10(10∽20mg, 3 times/day) + conventional therapy | conventional therapy alone | 1month | ⑦ | 12months |
| 6 | Munkholm et al. (1999) | Denmark | 11/11 |
43∽73/ 39∽75 |
31 ± 5/ 26 ± 6 |
oral coenzyme Q10 100 mg twice daily + conventional therapy | placebo + conventional therapy | 3months | ①③ | NR |
| 7 | Watson et al. (1999) | Australia | 30/30 | 55 ± 11 | 26 ± 6 | CoQ10(33 mg, 3 times/day) + conventional therapy | placebo + conventional therapy | 3months | ③ | NR |
| 8 | Khatta et al. (2000) | America | 28/27 | 67 | 27/30 | oral coenzyme Q10 200 mg/day + conventional therapy | placebo + conventional therapy | 1months | ①③ | 6months |
| 9 | Keogh et al. (2003) | Australia | 79/79 | 62 ± 7/61 ± 9 | NR | oral coenzyme Q10 150 mg/day + conventional therapy | placebo + conventional therapy | 3months | ④⑥ | 3months |
| 10 | Belardinelli et al. (2006) | Italy | 21/21 | 59 ± 9 | 37 ± 7 | CoQ10(100 mg, 3 times/day) + conventional therapy | placebo + conventional therapy | 1month | ③ | NR |
| 11 | Adarsh et al. (2008) | India | 46/41 | 24.4∽77.5 | NR | CoQ10(100 mg, 2 times/day) + conventional therapy | placebo + conventional therapy | 14.5months | ① | 27.5months |
| 12 | Feng et al. (2010) | China | 109/92 |
69 ± 7/ 68 ± 9 |
30.7 ± 6.3/ 31.2 ± 5.8 |
CoQ10(10 mg, 3 times/day) + conventional therapy | conventional therapy alone | 24months | ①③⑥ | 24months |
| 13 | Pei et al. (2010) | China | 62/66 | 62.47 ± 6.48 | 36 ± 4 | CoQ10(10 mg, 3 times/day) + conventional therapy | conventional therapy alone | 12months | ①③ | 12months |
| 14 | Bi (2012) | China | 27/27 | 49.5 ± 12.5 |
25.77 ± 12.59/ 26.33 ± 10.43 |
CoQ10(10 mg, 3 times/day) + conventional therapy | conventional therapy alone | 3weeks | ③④ | NR |
| 15 | Yao et al. (2012) | China | 50/50 | 62 ± 8/61 ± 9 | 36 ± 8 | CoQ10(100 mg, 1 time/day) + conventional therapy | conventional therapy alone | 3months | ③④⑥ | NR |
| 16 | Yang(2013) | China | 100/100 | 68.4 ± 13.85 | NR | CoQ10(10 mg, 3 times/day) + conventional therapy | conventional therapy alone | 2months | ① | 12months |
| 17 | Mortensen et al. (2014) | Multicenter | 202/218 |
62.3 ± 12/ 62.3 ± 11 |
31 ± 10 | oral coenzyme Q10 100 mg 3 times daily + conventional therapy | placebo with standard HF therapy | 26.5months | ①②③⑦ | NR |
| 18 | Wu (2014) | China | 35/35 |
84.5 ± 12.2/ 86.6 ± 14.3 |
NR | CoQ10(10 mg, 3 times/day) + conventional therapy | Placebo + conventional therapy | 2months | ①⑦ | NR |
| 19 | Zhao et al. (2015) | China | 62/66 | 63 ± 7/62 ± 6 | 36 ± 4 | oral coenzyme Q10 30 mg/day + conventional therapy | conventional therapy alone | 12months | ①③ | NR |
| 20 | Ping et al. (2015) | China | 61/61 | 58.3 ± 4.7 |
36.82 ± 8.53/ 37.19 ± 7.96 |
CoQ10(10 mg, 3 times/day) + conventional therapy | conventional therapy alone | 3months | ③⑥ | NR |
| 21 | Zhang (2015) | China | 28/28 |
65.4 ± 10.4/ 63.1 ± 10.2 |
NR | CoQ10(10 mg, 3 times/day) + conventional therapy | conventional therapy alone | 6months | ⑥⑦ | NR |
| 22 | Zhang (2016) | China | 30/30 | 38∽83 |
29 ± 7/ 31 ± 8 |
CoQ10(10 mg, 3 times/day) + conventional therapy | conventional therapy alone | 3months | ②③④⑥ | NR |
| 23 | Mareev (2017) | Russia | 101/47 | NR | 39.3 | coenzyme Q10 nasal drops (90 mg/day = equivalent 225 mg/day for liposoluble tablets) + conventional therapy | placebo + conventional therapy | 6months | ③⑤ | NR |
| 24 | Sobirin et al. (2019) | Indonesia | 15/15 | 62 ± 8 | 55/58 | oral coenzyme Q10 100 mg 3 times/day + conventional therapy | conventional therapy alone | 1month | ③ | 14months |
| 25 | Gan & Hu (2019) | China | 30/30 |
57.5 ± 6.1/ 58.4 ± 6.7 |
35.1 ± 4.0/ 34.5 ± 3.9 |
CoQ10(30 mg, 1 time/day) + conventional therapy | conventional therapy alone | 12months | ③ | NR |
| 26 | Jiang (2019) | China | 100/100 |
61.9 ± 5.6/ 61.3 ± 5.9 |
32.78 ± 3.40/ 33.58 ± 3.76 |
CoQ10(10 mg, 3 times/day) + conventional therapy | conventional therapy alone | 1month | ③⑥⑦ | NR |
| 27 | Wu et al. (2019) | China | 34/33 |
55.28 ± 12.53/ 55.12 ± 12.12 |
31.86 ± 6.53/ 31.86 ± 6.38 |
CoQ10(10 mg, 3 times/day) + conventional therapy | conventional therapy alone | 2months | ③⑥⑦ | NR |
| 28 | Kawashima et al. (2020) | Japan | 10/10 | 70 ± 9 | 34.5 ± 4.0 | ubiquinol 200 mg twice daily (400 mg/day) + conventional therapy | placebo + conventional therapy | 3months | ③⑤⑦ | 3months |
| 29 | Liu (2020) | China | 59/59 |
58.67 ± 7.28/ 58.43 ± 7.55 |
33.47 ± 3.82/ 32.67 ± 3.59 |
CoQ10(10 mg, 3 times/day) + conventional therapy | conventional therapy alone | 1month | ③⑥⑦ | NR |
| 30 | Wan et al. (2021) | China | 30/30 |
63.52 ± 3.76/ 63.41 ± 3.70 |
38.25 ± 3.19 | CoQ10(20 mg, 3 times/day) + conventional therapy | conventional therapy alone | 1.5months | ③⑥⑦ | NR |
| 31 | Zheng et al. (2021) | China | 44/34 |
62.0 ± 3.9/ 62.8 ± 3.5 |
29 | CoQ10(10 mg, 3 times/day) + conventional therapy | conventional therapy alone | 3months | ③ | NR |
| 32 | Samuel et al. (2022) | Israel | 19/20 | 75.4 ± 9.48 |
59.1 ± 6.1/ 59.3 ± 6.1 |
Treatment in the CoQ10 arm consisted of 100 mg three times daily + conventional therapy | placebo + conventional therapy | 4months | ③ | 4months |
Note EXP, Experimental group; CON, Control group; NR, Not report; ①All-cause mortality; ②Hospitalization for heart failure; ③LVEF (%); ④NYHA classification; ⑤BNP (pg/mL); ⑥6MWT; ⑦Adverse events
Risk of bias of included studies
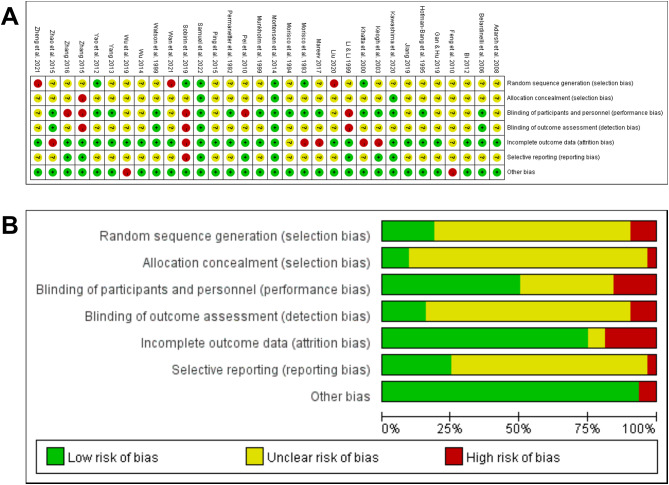
Two investigators independently assessed the risk of bias in the included studies. There was insufficient information in most studies, which made a comprehensive assessment of the risk of bias difficult. Of the 32 studies, only 6 reported randomization methods [25, 32, 40, 48, 49, 53] and only 3 reported allocation concealment [44, 48, 49]. Meanwhile, 16 studies reported participant and personnel blinding to random assignment [25, 26, 32, 33, 36, 39, 44, 48–56]. The total number of cases in one study did not correspond to the number of grouped cases [29], whereas multiple groups were analyzed in another study, of which two data sets were used in this investigation [43]. Figure 2A and B show the risk of bias of included studies.
Fig. 2.
Risk of bias summary and graph. Note A. Risk of bias summary; A. Risk of bias graph
Meta-analysis results
Primary outcomes
All-cause mortality
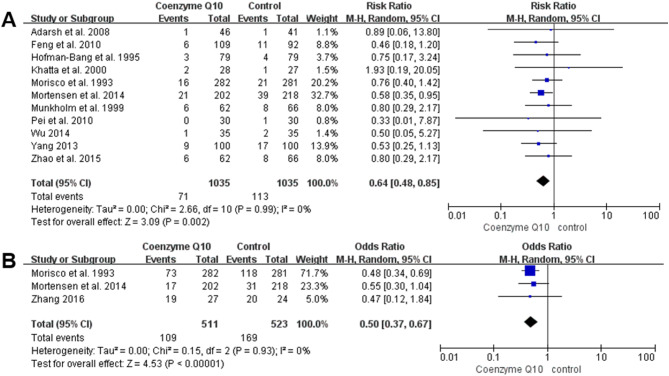
Eleven studies, with 2070 participants (1035 in the coenzyme Q10 group and 1035 in the control group), reported all-cause mortality [25, 28–30, 33, 34, 36, 49–51, 53]. Meta-analysis showed that all-cause mortality was significantly lower in the coenzyme Q10 group than in the control group [RR = 0.64, 95% CI (0.48, 0.85), P = 0.002; GRADE: moderate quality]. Figure 3A shows that there was no heterogeneity among the included studies (P = 0.99, I2 = 0%).
Fig. 3.
Forest plot of the effect of coenzyme Q10 on primary outcomes change. Note A. Forest Plot of the Effect of Coenzyme Q10 on All-cause Mortality Change; B. Forest Plot of the Effect of Coenzyme Q10 on Hospitalization for Heart Failure Change
Hospitalization for heart failure
Three studies reported hospitalization for heart failure as an outcome indicator [25, 38, 49]. A total of 1034 participants were included, 511 in the coenzyme Q10 group and 523 in the control group. As shown in Fig. 3B, there was no significant heterogeneity among the included studies (P = 0.93, I2 = 0%). The result showed that the coenzyme Q10 was able to reduce the hospitalization for heart failure compared to the control group. [RR = 0.50, 95% CI (0.37, 0.67), P < 0.00001; GRADE: moderate quality].
Secondary outcomes
Left ventricular ejection fraction (LVEF, %)
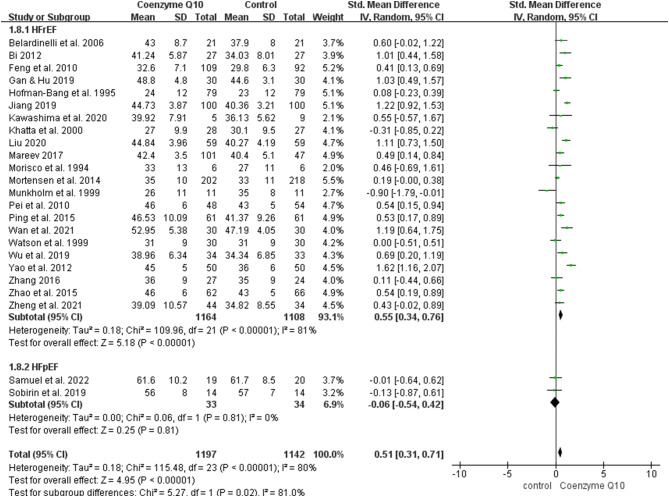
With regard to LVEF, 2339 patients from 24 RCTs were included (1197 in the coenzyme Q10 group and 1142 in the control group) [26, 29–32, 35, 36, 38–54]. Notably, the LVEF of patients with heart failure included in these studies was not statistically different at baseline. There was significant heterogeneity among the included studies (P < 0.00001, I2 = 80%), and a random-effects model was used for the meta-analysis. Patients with heart failure in the coenzyme Q10 group had a significantly better LVEF than those in the control group [MD = 0.51, 95% CI (0.31, 0.71), P < 0.00001; GRADE: low quality]. In the subgroup analysis by the baseline LVEF, 22 RCTs including heart failure with reduced ejection fraction (HFrEF) demonstrated that the coenzyme Q10 group had a significantly higher LVEF than the control group [MD = 0.55, 95% CI (0.34, 0.76), P < 0.00001]. (Fig. 4).
Fig. 4.
Forest plot of the effect of coenzyme Q10 on left ventricular ejection fraction (LVEF) change
NYHA classification
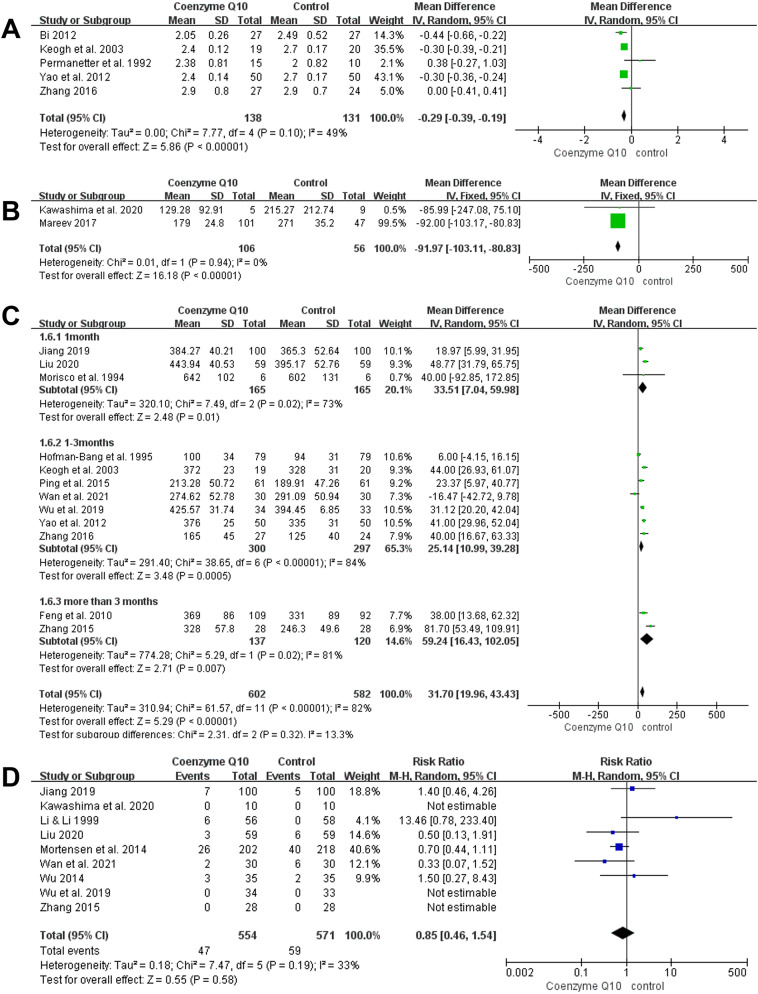
Five studies were included in this outcome index, covering 269 patients with heart failure (138 in the coenzyme Q10 group and 131 in the control group) [31, 32, 38, 55, 56]. The results showed that patients in the coenzyme Q10 group had a significantly lower NYHA classification than those in the control group [MD = − 0.29, 95% CI (− 0.39, − 0.19), P < 0.00001; GRADE: low quality; Fig. 5A]. Heterogeneity among the included studies was low (P = 0.10, I2 = 49%).
Fig. 5.
Forest plots of the effect of coenzyme Q10 on NYHA classification, BNP, 6MWT, adverse events change. Note A. Forest Plots of the Effect of Coenzyme Q10 on NYHA Classification; B. Forest Plots of the Effect of Coenzyme Q10 on BNP Change; C. Forest Plots of the Effect of Coenzyme Q10 on 6MWT Change; D. Forest Plots of the Effect of Coenzyme Q10 on adverse events Change
BNP (peg/mL)
Two RCTs reported BNP as an outcome indicator, including 162 participants (106 in the coenzyme Q10 group and 56 in the control group) [39, 44]. There was no significant heterogeneity among the included studies (P = 0.94, I2 = 0%), and BNP was significantly lower in the coenzyme Q10 group than in the control group [MD = − 91.97, 95% CI (− 103.11, − 80.83), P < 0.00001; GRADE: low quality; Fig. 5B].
6MWT
In total, 12 studies evaluated 6MWT in 1184 patients with heart failure, with 602 in the coenzyme Q10 group and 582 in the control group [26, 29, 32, 35, 37, 38, 42, 43, 45, 46, 50, 55]. As the included studies were highly heterogeneous (P < 0.00001, I2 = 82%), a random-effects model was applied. Compared with the control group, patients with heart failure taking coenzyme Q10 had significantly longer 6MWTs [MD = 31.70, 95% CI (19.96, 43.43), P < 0.00001; GRADE: moderate quality]. Afterward, based on the length of the course of treatment, three groups were analyzed: ≤1-month group, 1–3-month group, and > 3-month group. Figure 5C illustrates that regardless of the distances of the course of treatment, 6MWT distances were longer in patients with heart failure who received coenzyme Q10.
Adverse events
The incidence of adverse events was reported in nine studies [27, 34, 37, 42–46, 49]. A total of 1125 patients were included: 554 in the coenzyme Q10 group and 571 in the control group. Some of the adverse reactions were deep vein thrombosis, stroke, myocardial infarction, and arrhythmia. According to Fig. 5D, heterogeneity among the included studies was small (P = 0.19, I2 = 33%; GRADE: moderate quality). Meanwhile, the results were inconclusive for the risk of adverse reactions between the CoQ10 and control groups [RR = 0.85, 95% CI (0.46, 1.54), P = 0.58].
Publication bias assessment and sensitivity analysis
Funnel plots were analyzed to detect publication bias, which indicated that the included studies were evenly distributed on the left and right sides of the combined effect value line, suggesting low publication bias. At the same time, the results of both the Begg’s test and the Egger’s test showed no significant publication bias (Appendix 2). A leave-one-out sensitivity analysis was performed on the outcomes of meta-analyses. Exclusion of individual studies did not lead to significant changes in the direction or magnitude of the combined estimates, indicating that the results were reliable (Appendix 3).
Discussion
This meta-analysis, incorporating 32 randomized controlled trials and 3,763 heart failure patients, reveals promising outcomes for coenzyme Q10 supplementation in conjunction with conventional therapy.
This study has shown that coenzyme Q10 reduced all-cause mortality and hospitalization for heart failure as well as increased LVEF in patients with heart failure [17–19], crucial for improving patient outcomes and reducing healthcare utilization. Moreover, a previous meta-analysis showed that coenzyme Q10 improved the NYHA classification of patients with heart failure, in line with a similar improvement found in the current study [21]. Coenzyme Q10 is a cofactor in the mitochondrial enzyme complex and participates in oxidative phosphorylation in the respiratory chain [57, 58]. In the absence of coenzyme Q10, ATP production is reduced and heart failure may be aggravated because of increased myocardial wall pressure. This, in turn, increases energy demand, resulting in imbalanced supply and demand [59]. Previous research has shown that there is a negative correlation between coenzyme Q10 level and the exacerbation of heart failure symptoms in patients with heart failure [20]. Therefore, the supplementation of coenzyme Q10 may reduce major adverse cardiovascular events and improve heart failure symptoms.
In addition to being a biomarker of cardiac disease, BNP is used as a surrogate marker for heart failure, acute coronary syndrome, and myocardial infarction. Research has shown that it plays a significant role in stratifying heart failure severity. To some extent, the decrease of BNP level reflects the improvement of heart function [60]. The present study showed that coenzyme Q10 significantly decreased BNP levels in patients with heart failure, similar to previous research [44]. 6MWT, which examines exercise tolerance, was chosen in this study, as it is a simple and inexpensive test that is well tolerated by patients with heart failure and is considered useful in their management [61]. The results of this meta-analysis suggested that no matter how long patients with heart failure were treated with coenzyme Q10, 6MWT distances were longer and points to its utility in promoting functional independence and daily activity among heart failure patients. Despite the moderate quality of the evidence supporting this conclusion, it deserves clinical attention. Safety data, showing no increase in adverse events, reinforces coenzyme Q10’s tolerability as an adjunct therapy, important in the primary care setting where patient safety is paramount, especially for those with complex health conditions.
While recognizing the variable study quality and need for more rigorous research, our findings offer primary care physicians a basis for discussing coenzyme Q10 supplementation with suitable heart failure patients. Incorporating shared decision-making, physicians can weigh the potential benefits against the current evidence, tailoring recommendations to each patient’s circumstances. Ultimately, coenzyme Q10 emerges as a supplementary option, warranting cautious optimism and further exploration within the context of comprehensive heart failure management strategies.
Considering the importance of nonpharmacologic therapy for patients with heart failure, this study sought to explore how more data on nonpharmacologic therapy might affect outcomes. Unfortunately, few articles described non-pharmacologic treatments during the intervention, and even fewer reported whether surgically treated patients with heart failure were included. For example, one study advised patients to follow a low-fat diet during the trial, but no exercise program was provided [55]. Some studies did not make baseline comparisons related to non-pharmacological treatment for heart failure patients, such as comparing only their exercise capacity, while ignoring their exercise habits, such as their duration and frequency [49, 50]. In light of the important role exercise-based cardiac rehabilitation programs play in treating heart failure patients, further refinement is needed. Patients with heart failure were given coenzyme Q10 at doses ranging from 30 mg to 400 mg per day in the studies included. There was no determination of the minimum and optimal doses for coenzyme Q10 use, and further dose-response analyses will be required in the future.
This study significantly enhances knowledge on coenzyme Q10 (CoQ10) use in heart failure patients. By integrating recent trials with a large combined participant pool, we provide a robust update on CoQ10’s effectiveness and safety, with broadened applicability of the results. A key novelty lies in the inclusion of Chinese RCTs, addressing a demographic gap in prior reviews and deepening the global comprehension of CoQ10 benefits across diverse populations. We meticulously examined CoQ10 impact on vital clinical measures, presenting a comprehensive view of its therapeutic prowess. These outcomes reinforce CoQ10 potential as a safe and efficacious supplement, accentuating its role in bolstering cardiac function and mitigating the disease’s debilitating effects.
To overcome potential limitations, rigorous actions were undertaken. These included conducting a comprehensive literature search focusing on high-quality randomized controlled trials, implementing strict inclusion and exclusion criteria, assessing risk of bias systematically, performing subgroup and sensitivity analyses to address heterogeneity, and other measures. However, there are still some unresolved limitations. First, heterogeneity among studies related to LVEF and 6MWT was high among the outcomes included. Although subgroup analysis based on the baseline LVEF and the length of the course of treatment had been performed, the sources of heterogeneity could not be identified. It was possible, however, that heterogeneity arose from differences in drug tolerance, different environments, and differences in how the indices were measured in different patients in addition to differences in the severity of their disease. Unfortunately, additional subgroups could not be analyzed owing to the lack of relevant data. Moreover, the dosage or duration of coenzyme Q10 administration was not uniform across studies, which may have affected the reliability of the results. In addition, accessibility issues hindered the search for grey literature, which is one of the limitations of this study. Finally, the risk of bias assessment of the included studies revealed that most were of low quality and methodologically flawed. To validate the results of the present study, additional high-quality RCTs are needed in the future.
Conclusions
In summary, current evidence suggested that adjuvant coenzyme Q10 therapy in patients with heart failure not only reduced all-cause mortality, hospitalization for heart failure, NYHA classification, and BNP levels but also improved LVEF and 6MWT. However, owing to the different severities of heart failure, long gap between studies, heterogeneity among the study populations, errors in the test results of each unit, and differences in the treatment dosages and courses, additional high-quality, large-sample, long-term follow-up clinical studies are needed to validate these conclusions.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
It is with great gratitude that we thank the authors of the included publications, especially their corresponding authors, for providing data to this study.
Abbreviations
- ATP
Adenosine triphosphate
- ROS
Reactive oxygen species
- RCTs
Randomized controlled trials
- LVEF
Left ventricular ejection fraction
- NYHA
New York Heart Association
- BNP
Brain natriuretic peptide
- 6MWT
6-minute walk test
- RR
Relative risk
- MD
Mean difference
- SMD
Standardized mean difference
- HFrEF
Heart failure with reduced ejection fraction
Author contributions
Designing this study: Z. G.; H. Y.; J. X.; L. X. Performed this study: J. X.; L. X.; X.Y.; H. S. Analyzing the data: J. X.; L. X.; C. C.; B. Y. Drafted the article: J. X.; L. X.; X.Y.; H. S.; C. C.; B. Y. Revised the article critically for important intellectual content: Z.G.; H. Y. All authors reviewed the manuscript.
Funding
This work was supported by the Jiangsu Provincial Medical Innovation Team (CXTDA2017019).
Data availability
The original data used during the current study can be obtained by contacting the corresponding author.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jiayi Xu and Luwei Xiang contributed equally to this work and should be considered co-first authors.
Contributor Information
Hongfang Ye, Email: 13915969627@163.com.
Zejuan Gu, Email: jassicagu@163.com.
References
- 1.Tanai E, Frantz S. Pathophysiology of heart failure. Compr Physiol. 2015;6(1):187–214. [DOI] [PubMed] [Google Scholar]
- 2.Ziaeian B, Fonarow GC. Epidemiology and aetiology of heart failure. Nat Reviews Cardiol. 2016;13(6):368–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet (London England). 2018;392(10159):1789–858. [DOI] [PMC free article] [PubMed]
- 4.Bragazzi NL, Zhong W, Shu J, Abu Much A, Lotan D, Grupper A, Younis A, Dai H. Burden of heart failure and underlying causes in 195 countries and territories from 1990 to 2017. Eur J Prev Cardiol. 2021;28(15):1682–90. [DOI] [PubMed] [Google Scholar]
- 5.Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circulation Heart Fail. 2013;6(3):606–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, et al. Heart Disease and Stroke Statistics-2021 update: a Report from the American Heart Association. Circulation. 2021;143(8):e254–743. [DOI] [PubMed] [Google Scholar]
- 7.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–200. [DOI] [PubMed] [Google Scholar]
- 8.Aimo A, Castiglione V, Borrelli C, Saccaro LF, Franzini M, Masi S, Emdin M, Giannoni A. Oxidative stress and inflammation in the evolution of heart failure: from pathophysiology to therapeutic strategies. Eur J Prev Cardiol. 2020;27(5):494–510. [DOI] [PubMed] [Google Scholar]
- 9.Kumar AA, Kelly DP, Chirinos JA. Mitochondrial dysfunction in heart failure with preserved ejection fraction. Circulation. 2019;139(11):1435–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaanine AH, Joyce LD, Stulak JM, Maltais S, Joyce DL, Dearani JA, Klaus K, Nair KS, Hajjar RJ, Redfield MM. Mitochondrial morphology, Dynamics, and function in human pressure overload or ischemic heart Disease with preserved or reduced ejection fraction. Circulation Heart Fail. 2019;12(2):e005131. [DOI] [PubMed] [Google Scholar]
- 11.Cao M, Yuan W, Peng M, Mao Z, Zhao Q, Sun X, Yan J. Role of CyPA in cardiac hypertrophy and remodeling. Biosci Rep 2019, 39(12). [DOI] [PMC free article] [PubMed]
- 12.Boyman L, Karbowski M, Lederer WJ. Regulation of mitochondrial ATP production: ca(2+) signaling and Quality Control. Trends Mol Med. 2020;26(1):21–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Senni M, Gavazzi A, Gheorghiade M, Butler J. Heart failure at the crossroads: moving beyond blaming stakeholders to targeting the heart. Eur J Heart Fail. 2015;17(8):760–3. [DOI] [PubMed] [Google Scholar]
- 14.Brum PC, Bacurau AV, Medeiros A, Ferreira JC, Vanzelli AS, Negrão CE. Aerobic exercise training in heart failure: impact on sympathetic hyperactivity and cardiac and skeletal muscle function. Brazilian J Med Biol Res = Revista brasileira de pesquisas medicas e Biologicas. 2011;44(9):827–35. [DOI] [PubMed] [Google Scholar]
- 15.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice guidelines. J Am Coll Cardiol. 2013;62(16):e147–239. [DOI] [PubMed] [Google Scholar]
- 16.Di Lorenzo A, Iannuzzo G, Parlato A, Cuomo G, Testa C, Coppola M, D’Ambrosio G, Oliviero DA, Sarullo S, Vitale G et al. Clinical evidence for Q10 Coenzyme Supplementation in Heart failure: from energetics to functional improvement. J Clin Med 2020, 9(5). [DOI] [PMC free article] [PubMed]
- 17.Dabbaghi Varnousfaderani S, Musazadeh V, Ghalichi F, Kavyani Z, Razmjouei S, Faghfouri AH, Ahrabi SS, Seyyed Shoura SM, Dehghan P. Alleviating effects of coenzyme Q10 supplements on biomarkers of inflammation and oxidative stress: results from an umbrella meta-analysis. Front Pharmacol. 2023;8(14):1191290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sander S, Coleman CI, Patel AA, Kluger J, White CM. The impact of coenzyme Q10 on systolic function in patients with chronic heart failure. J Card Fail. 2006;12(6):464–72. [DOI] [PubMed] [Google Scholar]
- 19.Fotino AD, Thompson-Paul AM, Bazzano LA. Effect of coenzyme Q₁₀ supplementation on heart failure: a meta-analysis. Am J Clin Nutr. 2013;97(2):268–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al Saadi T, Assaf Y, Farwati M, Turkmani K, Al-Mouakeh A, Shebli B, Khoja M, Essali A, Madmani ME. Coenzyme Q10 for heart failure. Cochrane Database Syst Rev. 2021;2021(2):Cd008684. [DOI] [PMC free article] [PubMed]
- 21.Rosenfeldt F, Hilton D, Pepe S, Krum H. Systematic review of effect of coenzyme Q10 in physical exercise, hypertension and heart failure. Biofactors. 2003;18(1–4):91–100. [DOI] [PubMed] [Google Scholar]
- 22.Madmani ME, Yusuf Solaiman A, Tamr Agha K, Madmani Y, Shahrour Y, Essali A, Kadro W. Coenzyme Q10 for heart failure. Cochrane Database Syst Rev. 2014;2014(6):Cd008684. [DOI] [PubMed]
- 23.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ (Clinical Res ed). 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morisco C, Trimarco B, Condorelli M. Effect of coenzyme Q10 therapy in patients with congestive heart failure: a long-term multicenter randomized study. Clin Investigator. 1993;71(8 Suppl):S134–136. [DOI] [PubMed] [Google Scholar]
- 26.Morisco C, Nappi A, Argenziano L, Sarno D, Fonatana D, Imbriaco M, Nicolai E, Romano M, Rosiello G, Cuocolo A. Noninvasive evaluation of cardiac hemodynamics during exercise in patients with chronic heart failure: effects of short-term coenzyme Q10 treatment. Mol Aspects Med. 1994;15:s155–163. [DOI] [PubMed] [Google Scholar]
- 27.Li N, Li H. The efficacy of coenzyme Q10 in the treatment of heart failure. J Practical Med. 1999;15(8):638. [Google Scholar]
- 28.Adarsh K, Kaur H, Mohan V. Coenzyme Q10 (CoQ10) in isolated diastolic heart failure in hypertrophic cardiomyopathy (HCM). Biofactors. 2008;32(1–4):145–9. [DOI] [PubMed] [Google Scholar]
- 29.Feng Z, Bai T, Zhang L, Li X, Wang H, Wu R, Liu Y. Clinical Observation on 206 cases CHF treated with CoQ10 combined with routine therapy. Hebei Med. 2010;16(10):1184–7. [Google Scholar]
- 30.Pei D, Zhao Q, Okello E, Yu S, Zhang Y. Effect of coenzyme Q10 administration on the incidence of atrial fibrillation in patients with chronic heart failure. Chin J Cardiac Pacing Electrophysiol. 2010;24(5):423–5. [Google Scholar]
- 31.Bi J. Clinical Observation of Coenzyme Q10 on the efficacy of dilated cardiomyopathy patients with heart failure. Practical J Cardiac Cereb Pneumal Vascular Disease. 2012;20(11):1813–4. [Google Scholar]
- 32.Yao X, Li H, Gao Y, Dong J. Coenzyme Q10 for chronic heart failure:a randomized,double-blind,placebo-controlled trial. Chin J Multiple Organ Dis Elder. 2012;11(6):435–7. [Google Scholar]
- 33.Yang D. Clinical observation of coenzyme Q10 in reducing the occurrence of major adverse cardiovascular events in patients with chronic heart failure. Chin J Clin Ration Drug Use. 2013;6(30):19–19. [Google Scholar]
- 34.Wu Y. Coenzyme Q10 for the elderly with chronic heart failure efficacy and safety analysis. Chin J Geriatric Care. 2014;12(3):42–3.
- 35.Ping G, Zidan L, Shufen L. Clinical observation of coenzyme Q10 in the treatment of heart failure. Mod Diagnosis Treat. 2015;26(13):2983–4. [Google Scholar]
- 36.Zhao Q, Kebbati AH, Zhang Y, Tang Y, Okello E, Huang C. Effect of coenzyme Q10 on the incidence of atrial fibrillation in patients with heart failure. J Invest Medicine: Official Publication Am Federation Clin Res. 2015;63(5):735–9. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Q. Observations on the efficacy of coenzyme Q10 in chronic heart failure. Med Front. 2015;5(35):131. [Google Scholar]
- 38.Zhang X. Effect of coenzyme Q10 on QT interval and QT variability in patients with ischemic heart failure Knowledge of cardiovascular disease prevention and treatment 2016(1):98–100.
- 39.Mareev VY, Minina YV, Mareev YV. Coenzyme Q-10 in treatment of patients with heart failure: results Russian multicenter double blind placebo controlled study. Eur J Heart Fail. 2017;19:56. [Google Scholar]
- 40.Sobirin MA, Herry Y, Sofia SN, Uddin I, Rifqi S, Tsutsui H. Effects of coenzyme Q10 supplementation on diastolic function in patients with heart failure with preserved ejection fraction. Drug Discoveries Ther. 2019;13(1):38–46. [DOI] [PubMed] [Google Scholar]
- 41.Gan L, Hu L. Observations on the effects of coenzyme Q10 combined with valsartan in the treatment of chronic heart failure. Fam Med. 2019;2019(10):184.
- 42.Jiang Z. Clinical study of coenzyme Q10 combined with valsartan in treatment of chronic heart failure. Drugs Clin. 2019;34(2):346–50. [Google Scholar]
- 43.Wu B, Su C, Liu Z, Chen P, Huang S, Li M, Xu J. Clinical observation of Qiliqiangxin capsule combined with coenzyme q10 in the treatment of chronic heart failure. J Guangdong Med Coll. 2019;37(6):662–5. [Google Scholar]
- 44.Kawashima C, Matsuzawa Y, Konishi M, Akiyama E, Suzuki H, Sato R, Nakahashi H, Kikuchi S, Kimura Y, Maejima N, et al. Ubiquinol improves endothelial function in patients with heart failure with reduced ejection fraction: a Single-Center, randomized double-blind placebo-controlled crossover pilot study. Am J Cardiovasc Drugs: Drugs Devices Other Interventions. 2020;20(4):363–72. [DOI] [PubMed] [Google Scholar]
- 45.Liu Y. Effects of coenzyme Q10 combined with angiotensin II receptor antagonist on cardiac function and 6min walking distance in patients with chronic heart failure. J Hubei Univ Sci Technology(Medical Sciences). 2020;34(2):122–4. [Google Scholar]
- 46.Wan X, Yang G, Zhou Q. Effect of trimetazidine combined with coenzyme Q10 on cardiac function in patients with chronic heart failure. Contemp Med. 2021;27(31):4–6. [Google Scholar]
- 47.Zheng D, Lin C, Zhao X. Effects of Sacubitril Valsartan combined with Coenzyme Q10 in the treatment of patients with chronic heart failure. China J Pharm Econ. 2021;16(10):66–6871. [Google Scholar]
- 48.Samuel TY, Hasin T, Gotsman I, Weitzman T, Ben Ivgi F, Dadon Z, Asher E, Amir O, Glikson M, Alcalai R, et al. Coenzyme Q10 in the treatment of Heart failure with preserved ejection fraction: a prospective, randomized, Double-Blind, placebo-controlled trial. Drugs R D. 2022;22(1):25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mortensen SA, Rosenfeldt F, Kumar A, Dolliner P, Filipiak KJ, Pella D, Alehagen U, Steurer G, Littarru GP. The effect of coenzyme Q10 on morbidity and mortality in chronic heart failure: results from Q-SYMBIO: a randomized double-blind trial. JACC Heart Fail. 2014;2(6):641–9. [DOI] [PubMed] [Google Scholar]
- 50.Hofman-Bang C, Rehnqvist N, Swedberg K, Wiklund I, Aström H. Coenzyme Q10 as an adjunctive in the treatment of chronic congestive heart failure. The Q10 Study Group. J Card Fail. 1995;1(2):101–7. [DOI] [PubMed] [Google Scholar]
- 51.Munkholm H, Hansen HH, Rasmussen K. Coenzyme Q10 treatment in serious heart failure. Biofactors. 1999;9(2–4):285–9. [DOI] [PubMed] [Google Scholar]
- 52.Watson PS, Scalia GM, Galbraith A, Burstow DJ, Bett N, Aroney CN. Lack of effect of coenzyme Q on left ventricular function in patients with congestive heart failure. J Am Coll Cardiol. 1999;33(6):1549–52. [DOI] [PubMed] [Google Scholar]
- 53.Khatta M, Alexander BS, Krichten CM, Fisher ML, Freudenberger R, Robinson SW, Gottlieb SS. The effect of coenzyme Q10 in patients with congestive heart failure. Ann Intern Med. 2000;132(8):636–40. [DOI] [PubMed] [Google Scholar]
- 54.Belardinelli R, Muçaj A, Lacalaprice F, Solenghi M, Seddaiu G, Principi F, Tiano L, Littarru GP. Coenzyme Q10 and exercise training in chronic heart failure. Eur Heart J. 2006;27(22):2675–81. [DOI] [PubMed] [Google Scholar]
- 55.Keogh A, Fenton S, Leslie C, Aboyoun C, Macdonald P, Zhao YC, Bailey M, Rosenfeldt F. Randomised double-blind, placebo-controlled trial of coenzyme Q, therapy in class II and III systolic heart failure. Heart Lung Circ. 2003;12(3):135–41. [DOI] [PubMed] [Google Scholar]
- 56.Permanetter B, Rössy W, Klein G, Weingartner F, Seidl KF, Blömer H. Ubiquinone (coenzyme Q10) in the long-term treatment of idiopathic dilated cardiomyopathy. Eur Heart J. 1992;13(11):1528–33. [DOI] [PubMed] [Google Scholar]
- 57.Piepoli MF, Guazzi M, Boriani G, Cicoira M, Corrà U, Dalla Libera L, Emdin M, Mele D, Passino C, Vescovo G et al. Exercise intolerance in chronic heart failure: mechanisms and therapies. Part I.Eur J Cardioasc Prev Rehail. 2010;17(6):637–42. [DOI] [PubMed]
- 58.Kramer F, Sabbah HN, Januzzi JJ, Zannad F, van Peter J, Schelbert EB, Kim RJ, Milting H, Vonk R. Redefining the role of biomarkers in heart failure trials: expert consensus document. Heart Fail Rev. 2017; 22(3):263–77. [DOI] [PubMed]
- 59.Zhou B, Tian RJTJ. Mitochondrial dysfunction in pathophysiology of heart failure. J Clin Investig. 2018;128(9):3716–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li N, Wang JA. Brain natriuretic peptide and optimal management of heart failure. J Zhejiang Univ Sci B. 2005;6(9):877–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guazzi M, Dickstein K, Vicenzi M, Arena R. Six-minute walk test and cardiopulmonary exercise testing in patients with chronic heart failure: a comparative analysis on clinical and prognostic insights. Circulation Heart Fail. 2009;2(6):549–55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original data used during the current study can be obtained by contacting the corresponding author.