Abstract
Background
The integration of high-risk human papilloma virus (HPV) DNA into the human genome has been implicated in cervical carcinogenesis and head and neck squamous cell cancer. However, its role in lung squamous cell carcinoma is not well understood. In addition, tuberculosis (TB) and lung cancer(LC) share similar clinical symptoms and imaging features, increasing the risk of misdiagnosis.
Case Presentation
The patient presented with a 16-month history of hemoptysis, chest pain, and occasional fatigue, without fever, chills, or history of mechanical damage or autoimmune diseases. Examination revealed normal vital signs and laboratory parameters, except for a positive interferon-gamma release assay indicating tuberculosis infection. Bronchoscopic examinations identified congestion and edema of the tracheal wall, along with a tiny lesion in the right wall of the trachea. She had been misdiagnosed with tuberculosis. However, the diagnosis of squamous cell carcinoma was eventually confirmed by endoscopic biopsy. The patient’s macrogenomic second-generation sequencing (mNGS) of the bronchoscopic biopsy specimen was positive for HPV-16.The patient’s sex partner tested positive for HPV-16 in penile scrapings, indicating HPV transmission through oral sex.
Conclusions
This case highlights the potential for HPV infection acquired through oral sex to lead to lung squamous cell carcinoma. It emphasizes the importance of considering HPV-associated malignancies in patients with respiratory symptoms who engage in oral sexual behaviors.
Keywords: Human papilloma virus, Lung squamous cell carcinoma, Tuberculosis
Background
Pulmonary tuberculosis and lung cance are both common diseases with poor prognosis and high mortality worldwide. The radiological features and clinical symptoms of both diseases are sometimes very similar [1, 2]. In clinical practice, patients with cancer are sometimes misdiagnosed as having TB, leading to delays in diagnosis and treatment. High-risk HPV, especially HPV-16 and HPV-18, caused virtually all cervical cancers and a substantial proportion of anogenital (vulvar, vagina, penile, and anal) cancers as well as an increasing fraction of oropharyngeal cancers, but the association between HPV infection and lung cancer remains controversial [3]. Here, we report a case of squamous cell carcinoma of the trachea initially misdiagnosed as tracheal tuberculosis and explore the role of HPV infection and gene integration in its pathogenesis.
Case presentation
A 38-year-old woman attended our hospital with a 16-month history of Intermittent cough and hemoptysis of unknown etiology. The patient also present with fatigue and occasional chest pains. She felt feverish, but her temperature was within the normal range.She had no history of smoking or alcohol consumption and no occupational history related to dust exposure. No history of mechanical or physical damage or autoimmune diseases was reported.
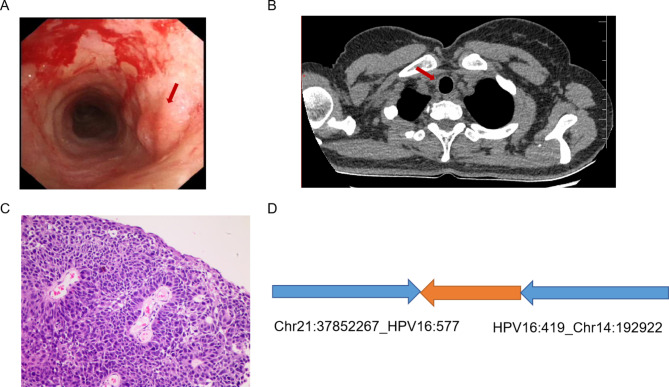
On examination, we found the patient to be generally well; her blood pressure was 121/60 mm Hg, heart rate 95 beats per min, oxygen saturation 98% room air, body temperature 36.5°C. Laboratory investigations demonstrated normal complete blood count, coagulation indicators, and infection parameters, except for a positive interferon-gamma release assay result indicating tuberculosis infection. Computed Tomography (CT) of the Chest showed no significant abnormality, and sputum and bronchoalveolar lavage mycobacteria cultures as well as sputum Xpert MTB/RIF were negative. Bronchoscopic examinations were conducted to identify the bleeding point. We found the tracheal walls in the upper and mid-portions of the trachea exhibit congestion, edema, roughness, and locally visible diffuse protrusive hyperplasia, which is prone to bleeding upon touch.” (Fig. 1); whereas the rest of the trachea was normal, with patent lumens without stenosis, and no neoplasm is observed. The routine microbiological examinations were performed on bronchoalveolar lavage fluid (BALF) specimen, and no bacteria and fungi were identified. A CT scan of the chest confirmed the presence of a tiny lesion in the right wall of trachea.
Fig. 1.
Lung squamous carcinogenesis caused by HPV infection. (A) Bronchoscopic examination shows the congestion and edema of the tracheal wall close to the tracheal entrance. (B) CT angiogram shows the presence of a tiny lesion in the right wall of trachea. (C) Pathological examination of the biopsy confirms the squamous cell carcinoma. (D) Integrated HPV DNA segments in clonal integration event
Metagenomic next-generation sequencing (mNGS) found the presence of HPV-16. Analysis of samples of biopsies taken during bronchoscopy also showed positive results for HPV-16 by mNGS. Further histological examination revealed squamous-cell carcinoma that were positive for p40, and CK5/6, while negative for CKpan, TTF-1, and Napsin-A (figure). Enhanced CT of the neck and enhanced nuclear magnetic resonance imaging of the head did not show any obvious metastatic signs, suggesting that it was in the early stage of squamous carcinoma of the trachea. Notably, her cervical specimens were negative for HPV infection, while positive HPV-16 result was noted in penile scrapings of her sex partner, indicating HPV transmission through oral sex. The Nanopore long-read sequencing revealed HPV-human integration events in this HPV-16-postivie case, and bioinformatic analysis revealed that the biopsy sample was detected with one integration event, causing a complex rearrangement involving chromosomes 14 and 21 (figure). The integration of HPV potentially led to increased expression level of chromodomain helicase DNA binding protein 8 (CHD8) on chromosome 14. CHD8, thereby driving lung squamous carcinogenesis in the tracheal epithelium. We started the patient on radiotherapy. A chest CT scan showed a decrease in the size of the lesion.Our regular follow-up of this patient continued until one year after her diagnosis, and the patient reported that she was recovering well, with a reduction in the size of the lesion and a significant reduction in haemoptysis.
Discussion and conclusion
This patient, presenting with clinical manifestations such as hemoptysis and fatigue, along with a positive result in the interferon-gamma release assay, was previously misdiagnosed with tracheal tuberculosis. This case serves as a reminder to clinicians that the possibility of lung cancer should always be considered in patients with tuberculosis-like symptoms but negative etiological findings, and diagnoses should be made with caution.
The role of HPV in lung cancer has been controversial. Several studies have reported a high prevalence of HPV in lung cancer patients, thus demonstrating that HPV may be a major risk factor for lung cancer and that HPV infection increases the risk of developing lung cancer [4–6]. However, there is also some evidence that the association between HPV infection and lung carcinogenesis is opportunistic [7] or absent [8–10]. A prospective cross-sectional study showed that human papillomavirus was not associated with non-small cell lung cancer [11]. The most recent Mendelian randomisation analyses exploring whether HPV infection is causally associated with lung cancer risk showed that no significant causal association was observed between lung cancer and its subtypes lung squamous cell carcinoma and lung adenocarcinoma and HPV infection [12]. Some analyses even pointed to HPV18 as a risk factor for lung cancer, whereas chronic HPV16 infection appeared to be an important protective factor for lung cancer [13]. These conflicting findings require further studies to elucidate this association.
This case may serve as one piece of evidence supporting the notion that HPV infection can lead to the development of lung cancer. The potential role of HPV as an independent carcinogen in non-smoking individuals warrants further investigation [14]. Smoking is widely recognized as the most crucial risk factor for lung cancer, with other risk factors including exposure to radon, occupational hazards, and so forth [15]. The patient, a relatively young female, has no history of smoking and no occupational exposure to dust. The presence of HPV in non-small cell lung cancer among never-smokers suggests that the virus may play a role in the carcinogenesis of this disease [16]. Furthermore, there are three primary hypotheses regarding the pathogenic mechanism of HPV in the lungs: spread via the bloodstream from cervical lesions to the lungs, resulting in microscopic abrasions; unprotected oral sex; and infection through inhalation of air [3]. Given that both the patient’s bronchoscopic biopsy sample and her sexual partner’s penile smear tested positive for HPV-16, combined with the patient’s current medical history and sexual history, there is a strong indication that HPV infection was transmitted to the trachea through oral sex, ultimately leading to lung squamous cell carcinoma. Moreover, HPV infection is usually considered to be more associated with advanced lung cancer [17, 18], with fewer reports of earlier stages. This reinforces the value of this case in the early squamous stage.
Dysregulation of viral gene expression and host genomic instability play a central role in virus-mediated carcinogenesis in a large number of studies on cervical cancer. Critical events such as gene integration may lead to dysregulated viral and host gene expression [19]. Furthermore, HPV integration is facilitated by repair processes activated in chromosomally unstable cells, a hallmark of human cancer [20]. However, there is a lack of studies targeting HPV integration sites in lung cancer. The Nanopore long-read sequencing revealed HPV-human integration events in this HPV-16-postivie case, and bioinformatic analysis revealed that the biopsy sample was detected with one integration event, causing a complex rearrangement involving chromosomes 14 and 21.
Integration of high-risk HPV DNA into the human genome is regarded as an important driving force in cervical carcinogenesis, as well as in head and neck squamous cell cancer. In this report, an HPV infection can spread to the trachea via oral sex, leading to a lung squamous cell cancer. Unprotected oral sexual behaviors with the HPV carrier plays a critical role in transmission of this virus, thus resulting in HPV-associated head and neck, as well as lung squamous cell cancer. Although rare, patients with respiratory symptoms who have oral sexual behaviors should be examined for the presence of HPV, and the HPV-associated lung squamous cell cancer cannot be ruled out.
Acknowledgements
Not applicable.
Author contributions
PY and RL wrote the manuscript. ZW and MG revised the manuscript. ZL and DMB directly accessed and verified the data reported in the manuscript. YP and SL isolated the bacterium and provided microbiological input. YW conducted DNA sequencing and provided bioinformatic analysis.
Funding
This study was funded in part by the Capital’s Funds for Health Improvement and Research [grant number 2022-2-1041] and Leading Talents of Beijing Tongzhou District High-level Talents Development Support Program [grant number YHLD2019011].
Data availability
All data contained in this study can be obtained from the corresponding author under reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rongmei Liu and Zhuoran Wu contributed equally to this work.
References
- 1.Lang S, Sun J, Wang X, et al. Asymptomatic pulmonary tuberculosis mimicking lung cancer on imaging: a retrospective study. Exp Ther Med. 2017;14(3):2180–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh VK, Chandra S, Kumar S, Pangtey G, Mohan A, Guleria R. A common medical error: lung cancer misdiagnosed as sputum negative tuberculosis. Asian Pac J Cancer Prev. 2009;10(3):335–8. [PubMed] [Google Scholar]
- 3.Cao F, Li YZ, Zhang DY, et al. Human papillomavirus infection and the risk of cancer at specific sites other than anogenital tract and oropharyngeal region: an umbrella review. EBioMedicine. 2024;104:105155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Syrjänen K. Detection of human papillomavirus in lung cancer: systematic review and meta-analysis. Anticancer Res. 2012;32(8):3235–50. [PubMed] [Google Scholar]
- 5.Shikova E, Ivanova Z, Alexandrova D, Shindov M, Lekov A. Human papillomavirus prevalence in lung carcinomas in Bulgaria. Microbiol Immunol. 2017;61(10):427–32. [DOI] [PubMed] [Google Scholar]
- 6.Karnosky J, Dietmaier W, Knuettel H, et al. HPV and lung cancer: a systematic review and meta-analysis. Cancer Rep (Hoboken). 2021;4(4):e1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coissard CJ, Besson G, Polette MC, Monteau M, Birembaut PL, Clavel CE. Prevalence of human papillomaviruses in lung carcinomas: a study of 218 cases. Mod Pathol. 2005;18(12):1606–9. [DOI] [PubMed] [Google Scholar]
- 8.Colombara DV, Manhart LE, Carter JJ, et al. Absence of an association of human polyomavirus and papillomavirus infection with lung cancer in China: a nested case-control study. BMC Cancer. 2016;16:342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He F, Xiong W, Yu F, et al. Human papillomavirus infection maybe not associated with primary lung cancer in the Fujian population of China. Thorac Cancer. 2020;11(3):561–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anantharaman D, Gheit T, Waterboer T, et al. No causal association identified for human papillomavirus infections in lung cancer. Cancer Res. 2014;74(13):3525–34. [DOI] [PubMed] [Google Scholar]
- 11.Oyouni A. Human papillomavirus in cancer: infection, disease transmission, and progress in vaccines. J Infect Public Health. 2023;16(4):626–31. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, Xu Z, Zhang Z, Wang X, Dong M. No genetic causal association between human papillomavirus and lung cancer risk: a bidirectional two-sample mendelian randomization analysis. Trials. 2024;25(1):582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun J, Xiang J, An Y, et al. Unveiling the Association between HPV and Pan-cancers: a bidirectional two-sample mendelian randomization study. Cancers (Basel). 2023;15(21):5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wee JT, Poh SS. The most important questions in cancer research and clinical oncology: question 1. Could the vertical transmission of human papilloma virus (HPV) infection account for the cause, characteristics, and epidemiology of HPV-positive oropharyngeal carcinoma, non-smoking east Asian female lung adenocarcinoma, and/or east Asian triple-negative breast carcinoma. Chin J Cancer. 2017;36(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corrales L, Rosell R, Cardona AF, Martín C, Zatarain-Barrón ZL, Arrieta O. Lung cancer in never smokers: the role of different risk factors other than tobacco smoking. Crit Rev Oncol Hematol. 2020;148:102895. [DOI] [PubMed] [Google Scholar]
- 16.Hasegawa Y, Ando M, Kubo A, et al. Human papilloma virus in non-small cell lung cancer in never smokers: a systematic review of the literature. Lung Cancer. 2014;83(1):8–13. [DOI] [PubMed] [Google Scholar]
- 17.Li M, Deng F, Qian LT, et al. Association between human papillomavirus and EGFR mutations in advanced lung adenocarcinoma. Oncol Lett. 2016;12(3):1953–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li M, Zhang XL, Deng F, et al. Involvement of TP53 and TP16 expression in human papillomavirus-associated non-small cell lung cancer. Oncol Lett. 2016;12(5):3330–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Senapati R, Senapati NN, Dwibedi B. Molecular mechanisms of HPV mediated neoplastic progression. Infect Agent Cancer. 2016;11:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oyervides-Muñoz MA, Pérez-Maya AA, Rodríguez-Gutiérrez HF, et al. Understanding the HPV integration and its progression to cervical cancer. Infect Genet Evol. 2018;61:134–44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data contained in this study can be obtained from the corresponding author under reasonable request.