Abstract
Background
Patients with diabetes on dialysis experience wide variations in glucose levels and an increased risk of hypoglycaemia. Due to the inaccuracies of HbA1c in dialysis patients, JBDS-IP and KDIGO recommend the use of continuous glucose monitoring (CGM). We conducted a systematic review to examine the current evidence for CGM use and its impact on clinical outcomes in patients with diabetes on dialysis.
Methods
A search of MEDLINE(R) ALL, Ovid Emcare, Journals@Ovid Full Text and Embase databases were conducted. Clinical or observational trials in adults with Type 1(T1D) or Type 2 (T2D) diabetes on dialysis and CGM intervention reporting on glycaemic outcomes were included.
Results
Of the 936 citations identified, 49 duplicates were removed. 887 citations were screened by title and abstract. 9 full texts were reviewed and a further 7 excluded due to duplications or failure to meet to selection criteria. Data was extracted for 2 studies, both prospective before-and-after interventional studies with no control group. Joubert et al. (2015) showed results for 15 participants with T1D. Mean CGM glucose level decreased from 8.37mmol/L at baseline to 7.7mmol/L at the end of the CGM period (p < 0.05) while HbA1c decreased from 6.9 to 6.5% (p < 0.05) during the same period. Mean CGM was lower on dialysis days (7.68mmol/L vs. 7.8mmol/L, p < 0.05). Képénékian et al. (2014) reported on data from 29 T2D patients. Following a 3 month CGM-adapted insulin regimen, HbA1c decreased from 8.4% at baseline to 7.6% (p < 0.01) by the end of study. Mean CGM values decreased from 9.9mmol/L to 8.9mmol/L (p = 0.05) and the frequency of glucose values > 10mmol/L decreased from 41 to 30% (p < 0.05), without a significant increase in hypoglycaemia frequency. Both studies were deemed to be of ‘good’ quality.
Conclusion
Evidence demonstrating the benefits of CGM in patients with diabetes receiving dialysis is lacking. There is a need for well-designed randomised controlled trials to ascertain the benefits of this technology in this patient group.
Trail registration
PROSPERO registration number: CRD42023371635, https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=371635.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12882-024-03763-z.
Keywords: Dialysis, Diabetes mellitus, Diabetic kidney disease, Haemodialysis, Peritoneal dialysis, Systematic review
Key learning points
What is known
Due to the inaccuracies of HbA1c in dialysis patients, JBDS-IP and KDIGO recommend the use of CGM. Despite this, there is limited evidence for the clinical benefits of CGM in patients on dialysis.
What this study adds
We undertook this systematic review to examine the current evidence around the use of CGM and its effects in patients with diabetes and ESRD on dialysis.
Two before-and-after studies have been conducted regarding the use of CGM in dialysis patients met out selection criteria.
Potential impact
This study highlights the limited evidence surrounding CGM use in dialysis patients, with no large-scale randomised control trials available to demonstrate glycaemic or clinical outcomes.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12882-024-03763-z.
Introduction
Chronic kidney disease (CKD) is a major global health problem accounting for an estimated 5 to 10 million annual deaths worldwide [1, 2]. Diabetes is a leading cause of CKD and end-stage renal disease (ESRD). Diabetes-related CKD affects nearly 40% of people with diabetes and is also associated with an increased risk of cardiovascular death (UK Kidney Association 2020). Although the benefits of intensive glycaemic control are less apparent in advanced stages of CKD, improved glycaemic control in patients on dialysis has been shown to improve survival [3–6]. Achieving optimal glycaemic control in patients with CKD, however, has added challenges due to increased insulin resistance, increased hepatic gluconeogenesis, impaired intracellular glucose metabolism, decreased insulin clearance and decreased insulin secretion [7]. These factors may be further potentiated by metabolic acidosis contributing to wide fluctuations in blood glucose levels and exogenous insulin requirements [8, 9]. Further, both peritoneal and haemodialysis have been associated with wide variability in blood glucose patterns dependent on the dialysate used as well as membrane factors [10, 12]. Diabetes patients on dialysis not only experience high glucose variability leading to increased risk of hypoglycaemia but also have impaired awareness of hypoglycaemia [13, 14].
Haemoglobin A1c (HbA1c) is the established gold standard indicator for assessing long-term glucose control in diabetes. Studies worldwide, however, highlight the inaccuracy of HbA1c as a measure of long-term glucose control in patients on dialysis. Elevated blood urea nitrogen level, iron deficiency and metabolic acidosis may falsely increase HbA1c [15–17] while reduced red blood cell lifespan and use of erythropoiesis-stimulating agents can increase the proportion of young non-glycated erythrocytes leading to falsely low HbA1c [18, 19].
Given these limitations with HbA1c, the Joint British Diabetes Societies for Inpatient Care (JBDS-IP) and Kidney Diseases: Improving Global Outcomes (KDIGO) Diabetes Work Group both recommend the use of continuous glucose monitoring (CGM) in patients on dialysis for assessing glycaemic control, particularly in those treated with insulin or sulfonylurea, have loss of hypoglycaemia awareness or are at high risk of recurrent hypoglycaemia [20, 21]. In 2019, an international consensus report was published outlining CGM targets for the time in ranges for both the general diabetes population (including type 1 and 2 diabetes) as well as a second group of older and high-risk diabetic patients, including those on dialysis [22]. Continuous glucose monitoring metrics offer a wealth of advantages, including improved glycemic control, enhanced quality of life and a reduction in the risk of diabetes-related complications. These metrics provide valuable insights for both patients and healthcare providers, enabling better-informed treatment decisions and a more personalised approach to diabetes management.
Despite these recommendations, there is limited evidence for the clinical benefits of CGM in patients on dialysis. We undertook this systematic review to examine the current evidence around the use of CGM and its effects on patients with diabetes and ESRD on dialysis.
Methods
This systematic review was undertaken in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and was registered with. PROSPERO (registration number CRD42023371635).
Search strategy
A comprehensive search of literature was was conducted on MEDLINE(R) ALL, Ovid Emcare, Journals@Ovid Full Text and Embase databases. Search terms used across databases were consistent and included ’type 1 diabetes’, ’type 2 diabetes’,’end stage renal disease’, ’dialysis’. The search term for flash/intermittently scanned and continuous glucose monitoring included ’flash’, ’libre’, ’dexcom’. Alternative spellings as well as common abbreviations in relation to the topic were also included in the search (supplementary Tables 1 and 3).
Searches were limited to articles published in the English language since 1995 until March 2023. This is to ensure that any work completed leading-up to the Food and Drug Administration (FDA) approval for the first CGM system in 1999 may be included.
Inclusion and exclusion criteria
We included clinical trials and observational studies involving (a) adults aged 18 and over, (b) diagnosed with diabetes mellitus (c) have ESRD and (d) receiving long term dialysis (participants), (e) where CGM-intervention was trialed (intervention) and (f) impact of glycaemic index was assessed (outcome). The full selection criteria are displayed on Table 1.
Table 1.
Selection criteria
| Inclusion criteria: | Exclusion criteria: | |
|---|---|---|
| Population |
Adults aged 18 and over People with diabetes mellitus and end stage kidney disease on long term dialysis (peritoneal or haemodialysis) Receiving glucose lowering medication |
Chronic kidney disease not receiving dialysis Acute Kidney injury |
| Study design |
Randomised clinical trials, clinical trials and observational studies. Minimum follow up duration of 6 weeks Minimum sample size 10 participants |
Other study designs: case study, case series and review articles |
| Intervention | Continuous Glucose Monitoring (isCGM and rtCGM) | |
| Outcomes |
Primary outcomes: overall glycaemic control determined by Time in Range (TIR), Time Above Range (TAR) and Time Below Range (TBR) Secondary outcomes: estimated HbA1c, episodes of severe hypoglycemia, number of hospitalisations, patient compliance with glucose scanning, quality of life |
Other outcome measures |
| Others |
Published in English language Papers published in peer-reviewed-journals |
Articles published before year 1999 |
Study selection
The search results identified were loaded on Rayyan™, a highly effective and user-friendly web- based platform for systematic review management. It helps in facilitating efficient collaboration among researchers and simplifying the screening and selection of relevant studies.
Stage 1: After removing duplicates, all identified studies were screened by title and abstract independently by two authors. Stage 2: Full text was requested for all papers fulfilling the inclusion criteria in stage 1 and were further screened by two reviewers to identify articles suitable for data-extraction. Disagreements between the reviewers were resolved by discussion to reach a consensus. Where there was no resolution, the papers were independently reviewed by a third reviewer to assess suitability for inclusion.
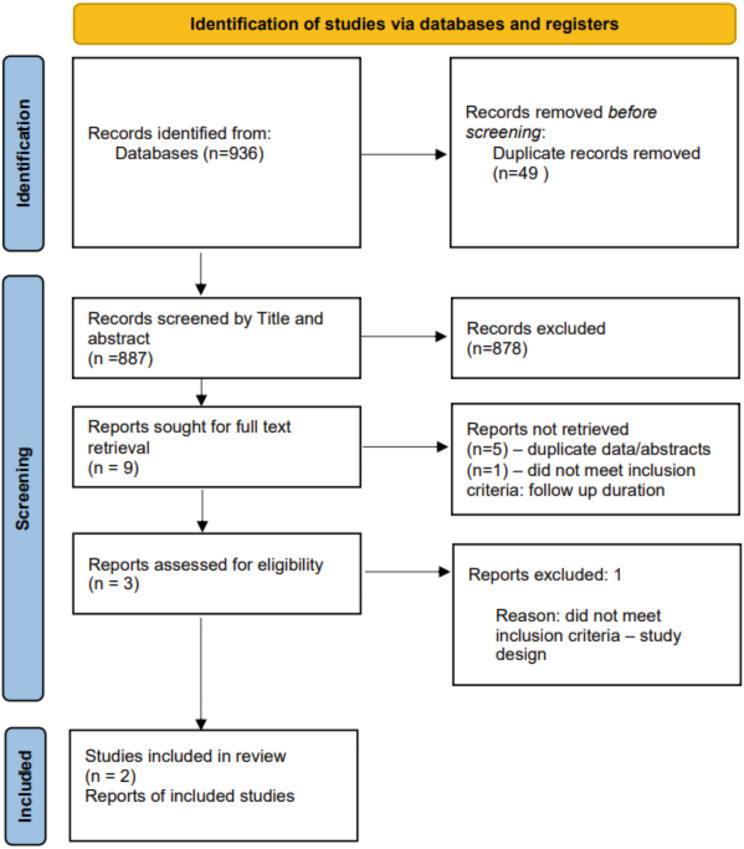
Study selection process is summarised in the PRISMA chart.
Data extraction
Data extraction of the included studies was carried out by two independent reviewers. The data extracted included: study location, population, diabetes type, dialysis type, baseline characteristics, number of participants, intervention, comparator, outcomes and duration of study (Table 2).
Table 2.
Clinical and demographic characteristics of included studies
| Author (year) | Country | Participant characteristics | Age | Male to female ratio | Baseline characteristics | Sample size | Comparator | Intervention | Outcome - Primary | Outcome - Secondary | Follow up duration |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Joubert (2015) | France | Patients with diabetes type 1 or 2. Haemodialysis (4 hours three times a week) | 60.9 +/- 14.8 years | 8:7 | BMI 29.9, HbA1c 51 | 15 | self-monitoring blood glucose | 5 day CGM at 2 week intervals, antidiabetic drug adjusted accordingly | Change in CGM readings | Diabetic treatment changes and quality of life, AUC and MAGE, HbA1c levels | Two 6 week periods |
| Képénékian (2014) | France | Patients with type 2 diabetes on treatment with insulin, HbA1c > 47.5, maintenance dialysis more than 3 months | 65.7 +/- 9.4 years | 19:9 | BMI 33.3, HbA1c 68 | 28 | baseline CGM and HbA1c readings | CGM adapted basal-bolus insulin regimen | Change in HbA1c | Body weight, insulin requirements, symptomatic hypoglycaemia and CGM parameters | 3 months |
Assessment of methodological quality
To minimise bias in this systematic review, we utilised the Quality Assessment for Before-After Studies with No Control Group tool, as designed by the National Heart, Lung, and Blood Institute [23]. We customised this tool and included 11 out of 12 questions focussing on internal validity of study, as we felt question 8 was not applicable for the intervention. Selected papers were critically appraised by two independent reviewers and any disagreements between the reviewers were resolved by discussion (supplementary Table 2).
Data synthesis and analysis
Our primary outcome was change in diabetes status. We defined change in diabetes status as change in HbA1c or other continuous glucose monitoring glycaemic index like time in range. We also assessed the impact of CGMs on change in diabetic medications, body weight, hypoglycaemic episodes and quality of life. Due to limited number of studies and heterogeneity of study design, we restricted our analysis to descriptive analysis.
Results
Search yield
Our initial search yielded 936 papers, of which 49 were duplicates and hence removed. The remaining 887 papers 9 papers were identified after screening on title and abstract. Full texts of these 9 papers were subsequently evaluated, of which a further 7 studies were exlcuded as they were either duplicates/abstracts or did not meet inclusion criteria (Fig. 1). Two remaining studies were included for data extraction and analysis (Table 2).
Fig. 1.
PRISMA flow diagram. (PRISMA = preferred reporting items for systematic reviews and meta-analyses)
Study characteristics and quality
The characteristics of the included studies are summarised on Table 2.
Both included studies were prospective, before-and-after interventional study designs with no control group or blinding. They were published around the same period and from France. Sample size of both studies was small and had a follow up duration of 12 weeks. Both studies had similar participant selection criteria, baseline characteristics and demographic features. Neither study selected participants based on blood sugar control and all patients meeting the selection criteria in the given time period were recruited.
Joubert et al. [24] included participants with type 1 and secondary diabetes; treated on diet alone or diet and insulin. Joubert et al. described the DIALYDIAB study which was split into two 6-week periods. During the first 6 weeks, patients self-monitored blood glucose (SMBG) 3 to 6 times a day on their own glucometer devices. For the second half of the study, a 5-day blinded CGM was performed at 2-week interval using the iPRO CGM® (Medtronic). Both the SMBG and CGM results were reviewed remotely by diabetes experts and proposed treatment changes such as insulin dose adjustment and/or oral hypoglycaemic agents.
Képénékian et al. [25] included patients with type 2 diabetes on insulin. In the study by Képénékian et al., CGM Navigator® was initiated at the beginning of a dialysis session and continued at home and subsequent dialysis sessions. Insulin doses were titrated to obtain an optimal glycaemic control, with dose adjusted by a doctor based on the CGM readings. CGM and HbA1c readings at 3 months were measured. A total of 54 hours of CGM were obtained at baseline and after 3 months.
Both studies were deemed to be of ‘good’ quality as they reported well defined question, defined eligibility criteria and population included representing the population of interest, defined outcome measures.
Study outcomes
In the study by Joubert et al., [24] 18 patients were recruited. 15 results were analysed with complete CGM readings. 3 patients were not included in analysis due to incomplete data. Mean CGM glucose level was 8.3mmol/L at baseline, 8.2mmol/L at the end of the SMBG period and 7.7mmol/l at the end of the CGM period (p < 0.05). Glucose AUC > 10mmol/L decreased significantly from 0.9 to 0.4mmol/L/day (p < 0.05) while the AUC for glucose < 3.3mmol/L did not change. HbA1c decreased from 6.85% at baseline to 6.46% at the end of the 12 week period (p < 0.05). Treatment adaptation was higher during the CGM period compared to the SMBG period (2.1 vs. 1.4 respectively, p < 0.05) due to a higher number of treatment regimen changes during the CGM period, such as addition of short acting insulin or establishing a different treatment pattern for days with and without dialysis. Mean blood sugar on CGM was lower on dialysis days (7.6mmol/L) vs. non dialysis days (7.8mmol/L, p < 0.05). Joubert et al. assessed quality of life using a 32 item questionnaire but the results were not reported in the publication.
Képénékian et al. [25] recruited 38 patients and reported results of 28 patients who completed the study. After 3 months with a CGM-adapted basal bolus insulin regimen, HbA1c significantly decreased from 8.4 ± 1.0% (65 ± 1 mmol/mol) to 7.6 ± 1.0% (60 ± 11 mmol/mol; p < 0.01). There was a significant reduction in mean CGM glucose values (9.9 ± 1.9 to 8.9 ± 2.1 mmol/L; p = 0.05) and frequency of glucose value > 10mmol (41.3–30.1%; p < 0.05) over the duration of the study, without an increase in the frequency of hypoglycaemia. There was a modest but statistically significant increase in daily insulin requirement by the end of the study compared to baseline (70 ± 51IU/d to 82 ± 77IU/d (p < 0.001) without notable increase in body weight. 10 patients were excluded during the follow up period and data not included in analysis (6 withdrawal of consent, 1 death from severe respiratory insufficiency, 2 failed to comply with treatment regimen and 1 spontaneous change of their previous insulin regimen).
Both these studies did not report on measures such as Time in Range (TIR), Time Above Range (TAR) and Time Below Range (TBR).
Discussion
Our systematic review found scanty but supportive evidence relating to the use of continuous glucose monitoring in patients with diabetes on dialysis. To our knowledge, this is the first systematic review undertaken to define the benefits of CGM in patients with diabetes on dialysis. In our review, we found only two studies that analysed the impact of CGM on glycaemic indices in patients with diabetes on dialysis highlighting the limited evidence available in this field. Both studies identified in this review have shown that CGM can significantly reduce mean serum glucose concentrations and HbA1c, without an increased risk of hypoglycaemia.
Assessment of glycaemic control in patients with dialysis presents considerable challenges. Due to the limitations in pharmacological options, many patients with diabetes on dialysis are likely to be on insulin therapy, exposing them to risk of glycaemic variability. Presence of multi-morbidity, reduced awareness of hypoglycaemia and fluctuations in blood glucose levels during dialysis and non-dialysis days further aggravates the problem [26, 27]. The usefulness of conventional measures of glycaemic control such as HbA1c and fructosamine is also limited in this patient group.
In recent years, the availability of CGM devices (continuous and intermittently scanned) has emerged as useful tools to assess glycaemic control and overcome some of the above limitations. As a result, many guidelines now recommend the use of CGM for assessing glycaemic control in patients on dialysis [1, 21]. Despite the recommendations and theoretical benefits of CGM use in the dialysis population, the data available to support the use of CGM is limited.
To date, studies involving CGM have either focused on glycaemic variability on dialysis and non-dialysis days or have been accuracy studies evaluating CGM with other measures of glucose control such as capillary glucose testing or HbA1c. We found only two studies that evaluated the clinical benefits of CGM in patients on dialysis. The benefits of CGM in patients with type 1 and type 2 diabetes not on dialysis has been demonstrated in several studies. An observational study conducted by Deshmukh, et al. [28] of 10,370 users across 102 hospitals across the UK showed that flash CGM use resulted in improved glycaemic control, hypoglycaemic awareness, reduced diabetes-related distress and reduced hospital admissions compared with baseline data. They reported improved glycaemic control in participants with higher baseline HbA1c and in those who had a greater number of scans of their device per day. In this study 97% of participants had type 1 diabetes with the other forms of diabetes making up the remainder. Similar findings were reported from a meta-analysis of flash CMG use in participants with type 1 and type 2 diabetes, showing significant and sustained change in HbA1c [29]. However, neither studies commented on participants’ renal function or need for renal replacement therapy. FLASH-UK is a randomised control trial of participants with type 1 diabetes, comparing the psychological and glycaemic outcomes of flash CGM with SMBG. This will provide valuable data regarding the utility and efficacy of the flash CGM to guide further recommendations; however, those who are on dialysis or are considered pre-dialysis were excluded from the trial [30]. Although, it is widely perceived that patients with diabetes on dialysis may similarly benefit from CGM, the lack of studies that support CGM use is a concern and highlights the need for well-designed studies in this cohort.
CGM provides a summary data of TIR, TAR and TBR as measures of glycaemic variability. This can be used to ascertain overall glucose control, as well as trends and hypoglycaemic risk to guide treatment. The international consensus report recommended specific CGM targets for the dialysis population [22]. The goal is to maintain more than 50% time within target range (3.9-10.0mmol/L) for more than 12 hours a day. The reasoning behind a reduced time within range target and an increased mean plasma glucose level is to avoid hypoglycaemia in this high-risk group [22]. Interestingly, the studies included in our systematic review did not report on these measures. However, both studies reported improvements in mean glucose levels and HbA1c over the study duration. CGM also enabled better treatment adjustment without significant increase in hypoglycaemia. Similar results were noted in an 8 day trial of closed loop CGM insulin delivery, which has also significantly increased the time spent in the target glucose range compared to conventional blood glucose monitoring, with a lower mean sensor glucose level and no increase in the number of hypoglycaemia episodes [31]. Whether these results can be replicated in larger cohorts and over a longer period of time remains to be seen.
The lack of data on the use of CGM in this patient population has been emphasised by Bomholt et al., [32] who have also highlighted the practical challenges of the daily use of CGM in the dialysis population, such as the need for sensor application and replacement, interpretation and actioning of the data by the individual. For those with limited dexterity and cognition, the handing of the sensor and use of the software such as on a smartphone may be difficult. Periodic use of CGM has also been suggested, to be performed by clinical staff to aid adjustment of anti-diabetic treatments. Emphasis on patient education and support from diabetes specialised nurse/educators are therefore needed for successful initiation and implementation of this technology in this patient cohort [33].
Limitations
In this systematic review, we included both randomised and observational studies to capture as much evidence as possible. However, we only included studies with a minimum of 10 patients and a follow up duration of at least 6 weeks. This was to ensure we assess the utility of CGM over a reasonable period. As a result, we excluded studies with smaller sample sizes and shorter follow ups. The two studies in this review were both before-and-after trials, with the same patient group evaluated pre and post intervention. This study design invariably carries a potential risk of carry over effect from the initial treatment to the CGM treatment period. Further, due to the nature of these studies, it was not possible to incorporate any blinding and although the qualities of both trial designs were good, the absence of a randomised control design requires a cautious interpretation of the findings.It is also worth noting that the focus of this review was primarily on glycaemic measures and we did not extend the review for other non-glycaemic outcomes and hence unable to comment on these benefits.
Conclusion
Assessment of glycaemic control in patients with dialysis remains a major challenge. CGM promises to be a useful alternative to currently used methods such as SMBG and HbA1c and offers both efficacy and convenience. Our study shows that, despite the suggested clinical benefits, the data on CGM use in dialysis patients is scarce, with no large-scale randomised control trials available to demonstrate glycaemic or clinical outcomes. More research is required to better understand the benefits of CGM in the dialysis population. Adequately powered randomised controlled studies comparing SMBG with CGM, focused on glycaemic and non-glycaemic outcomes, particularly quality of life and diabetes distress are urgently needed to ascertain the potential benefits of these interventions and support widespread implementation.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Author contributions
Y.Z., P.S., V.S, M.K, J.B and S.B were involved in the conception and design of the study. Y.Z., P.S., K.G., J.B. conducted the literature search and contributed to the screening and recording of results. Y.Z. wrote the first draft of the manuscript and all authors reviewed and edited the paper. All authors approve the final version of the manuscript.
Funding
The authors declare no financial support or assistance relevant to this research.
Data availability
All data generated or analysed during this study are included in this published article.
Declarations
Guarantor statement
S.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior presentation
Poster presentation at 6th Joint Meeting of ABCD & UKKA 2023, Birmingham, UK, 11th October 2023.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bikbov B, Purcell CA, Levey AS, Smith M, Abdoli A, Abebe M, Adebayo OM, Afarideh M, Agarwal SK, Agudelo-Botero M, Ahmadian E. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the global burden of Disease Study 2017. Lancet. 2020;395(10225):709–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luyckx VA, Tonelli M, Stanifer JW. The global burden of kidney disease and the sustainable development goals. Bull World Health Organ. 2018;96(6):414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shurraw S, Hemmelgarn B, Lin M, Majumdar SR, Klarenbach S, Manns B, Bello A, James M, Turin TC, Tonelli M. Alberta Kidney Disease Network. Association between glycemic control and adverse outcomes in people with diabetes mellitus and chronic kidney disease: a population-based cohort study. Arch Intern Med. 2011;171(21):1920–7. [DOI] [PubMed] [Google Scholar]
- 4.Oomichi T, Emoto M, Tabata T, Morioka T, Tsujimoto Y, Tahara H, Shoji T, Nishizawa Y. Impact of glycemic control on survival of diabetic patients on chronic regular hemodialysis: a 7-year observational study. Diabetes Care. 2006;29(7):1496–500. [DOI] [PubMed] [Google Scholar]
- 5.Morioka T, Emoto M, Tabata T, Shoji T, Tahara H, Kishimoto H, Ishimura E, Nishizawa Y. Glycemic control is a predictor of survival for diabetic patients on hemodialysis. Diabetes Care. 2001;24(5):909–13. [DOI] [PubMed] [Google Scholar]
- 6.Yoo DE, Park JT, Oh HJ, Kim SJ, Lee MJ, Shin DH, Han SH, Yoo TH, Choi KH, Kang SW. Good glycemic control is associated with better survival in diabetic patients on peritoneal dialysis: a prospective observational study. PLoS ONE. 2012;7(1):e30072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rahhal MN, Gharaibeh NE, Rahimi L, Ismail-Beigi F. Disturbances in insulin–glucose metabolism in patients with advanced renal disease with and without diabetes. J Clin Endocrinol Metabolism. 2019;104(11):4949–66. [DOI] [PubMed] [Google Scholar]
- 8.Kovesdy CP, Sharma K, Kalantar-Zadeh K. Glycemic control in diabetic CKD patients: where do we stand? Am J Kidney Dis. 2008;52(4):766–77. [DOI] [PubMed] [Google Scholar]
- 9.Jin YP, Su XF, Yin GP, Xu XH, Lou JZ, Chen JJ, Zhou Y, Lan J, Jiang B, Li Z, Lee KO. Blood glucose fluctuations in hemodialysis patients with end stage diabetic nephropathy. J Diabetes Complicat. 2015;29(3):395–9. [DOI] [PubMed] [Google Scholar]
- 10.Li PK, Culleton BF, Ariza A, Do JY, Johnson DW, Sanabria M, Shockley TR, Story K, Vatazin A, Verrelli M, Alex WY. Randomized, controlled trial of glucose-sparing peritoneal dialysis in diabetic patients. J Am Soc Nephrology: JASN. 2013;24(11):1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ling J, Ng JK, Chan JC, Chow E. Use of continuous glucose monitoring in the assessment and management of patients with diabetes and chronic kidney disease. Front Endocrinol. 2022;13:869899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sudha MJ, Salam HS, Viveka S, Udupa AL. Assessment of changes in insulin requirement in patients of type 2 diabetes mellitus on maintenance hemodialysis. J Nat Sci Biology Med. 2017;8(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qayyum A, Chowdhury TA, Oei EL, Fan SL. Use of continuous glucose monitoring in patients with diabetes mellitus on peritoneal dialysis: correlation with glycated hemoglobin and detection of high incidence of unaware hypoglycemia. Blood Purif. 2016;41(1–3):18–24. [DOI] [PubMed] [Google Scholar]
- 14.Habte-Asres HH, Jiang Y, Rosenthal M, Wheeler DC. Burden of impaired awareness of hypoglycemia in people with diabetes undergoing hemodialysis. BMJ Open Diabetes Res Care. 2024;12(1):e003730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flückiger R, Harmon W, Meier W, Loo S, Gabbay KH. Hemoglobin carbamylation in uremia. N Engl J Med. 1981;304(14):823–7. [DOI] [PubMed] [Google Scholar]
- 16.De Marchi S, Cecchin E, Camurri C, Quaia P, Raimondi A, Donadon W, Lippi U, Tesio F. Origin of glycosylated hemoglobin Al in chronic renal failure. Int J Artif Organs. 1983;6(2):77–82. [PubMed] [Google Scholar]
- 17.Christy AL, Manjrekar PA, Babu RP, Hegde A, Rukmini MS. Influence of iron deficiency anemia on hemoglobin A1c levels in diabetic individuals with controlled plasma glucose levels. Iran Biomed J. 2014;18(2):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdel-Aziz MM, Shaltout IF, Al-Jarhi UM, Alshewi YT, Elalfy MM. HbA1c reliability in patients with diabetes on regular hemodialysis before and after erythropoietin therapy. Egypt J Intern Med. 2013;25:70–4. [Google Scholar]
- 19.Nakao T, Matsumoto H, Okada T, Han M, Hidaka H, YOSHINO M, SHINO T, YAMADA C, NAGAOKA Y. Influence of erythropoietin treatment on hemoglobin A1c levels in patients with chronic renal failure on hemodialysis. Intern Med. 1998;37(10):826–30. [DOI] [PubMed] [Google Scholar]
- 20.Diabetes UK. Joint British Diabetes Societies for Inpatient Care (JBDS-IP) Clinical Guideline Management of adults with diabetes on dialysis [Internet]. 2022.[cited 2022 Dec8].
- 21.de Boer IH, Caramori ML, Chan JC, Heerspink HJ, Hurst C, Khunti K, Liew A, Michos ED, Navaneethan SD, Olowu WA, Sadusky T. KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2020;98(4):S1–15. [DOI] [PubMed] [Google Scholar]
- 22.Battelino T, Danne T, Bergenstal RM, Amiel SA, Beck R, Biester T, Bosi E, Buckingham BA, Cefalu WT, Close KL, Cobelli C. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42(8):1593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quality assessment tool for before-after studies with no control group. National Heart, Lung and Blood Institute. 2021 Jul < https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (last accessed 1st September 2023).
- 24.Joubert M, Fourmy C, Henri P, Ficheux M, Lobbedez T, Reznik Y. Effectiveness of continuous glucose monitoring in dialysis patients with diabetes: the DIALYDIAB pilot study. Diabetes Res Clin Pract. 2015;107(3):348–54. [DOI] [PubMed] [Google Scholar]
- 25.Képénékian L, Smagala A, Meyer L, Imhoff O, Alenabi F, Serb L, Fleury D, Dorey F, Krummel T, Le Floch JP, Chantrel F. Continuous glucose monitoring in hemodialyzed patients with type 2 diabetes: a multicenter pilot study. Clin Nephrol. 2014;82(4):240–6. [DOI] [PubMed] [Google Scholar]
- 26.Kazempour-Ardebili S, Lecamwasam VL, Dassanyake T, Frankel AH, Tam FW, Dornhorst A, Frost G, Turner JJ. Assessing glycemic control in maintenance hemodialysis patients with type 2 diabetes. Diabetes Care. 2009;32(7):1137–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gallieni M, De Salvo C, Lunati ME, Rossi A, D’Addio F, Pastore I, Sabiu G, Miglio R, Zuccotti GV, Fiorina P. Continuous glucose monitoring in patients with type 2 diabetes on hemodialysis. Acta Diabetol. 2021;58:975–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deshmukh H, Wilmot EG, Gregory R, Barnes D, Narendran P, Saunders S, Furlong N, Kamaruddin S, Banatwalla R, Herring R, Kilvert A. Effect of flash glucose monitoring on glycemic control, hypoglycemia, diabetes-related distress, and resource utilization in the Association of British Clinical Diabetologists (ABCD) nationwide audit. Diabetes Care. 2020;43(9):2153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Evans M, Welsh Z, Ells S, Seibold A. The impact of flash glucose monitoring on glycaemic control as measured by HbA1c: a meta-analysis of clinical trials and real-world observational studies. Diabetes Therapy. 2020;11:83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilmot EG, Evans M, Barnard-Kelly K, Burns M, Cranston I, Elliott RA, Gkountouras G, Kanumilli N, Krishan A, Kotonya C, Lumley S. Flash glucose monitoring with the FreeStyle Libre 2 compared with self-monitoring of blood glucose in suboptimally controlled type 1 diabetes: the FLASH-UK randomised controlled trial protocol. BMJ open. 2021;11(7):e050713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bally L, Gubler P, Thabit H, Hartnell S, Ruan Y, Wilinska ME, Evans ML, Semmo M, Vogt B, Coll AP, Stettler C. Fully closed-loop insulin delivery improves glucose control of inpatients with type 2 diabetes receiving hemodialysis. Kidney Int. 2019;96(3):593–6. [DOI] [PubMed] [Google Scholar]
- 32.Bomholt T, Kofod D, Nørgaard K, Rossing P, Feldt-Rasmussen B, Hornum M. Can the use of continuous glucose monitoring improve Glycemic Control in patients with type 1 and 2 diabetes receiving Dialysis? Nephron. 2023;147(2):91–6. [DOI] [PubMed] [Google Scholar]
- 33.Joseph K, Avari P, Goldet G, Edwards C, McCarthy S, Reed J, Duncan N, Hui E. The impact of diabetes specialist nurses’ in-reach service on people with diabetes on haemodialysis: a pilot study ‘education to protect tomorrow’. Diabet Med. 2024;41:e15306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article.