Abstract
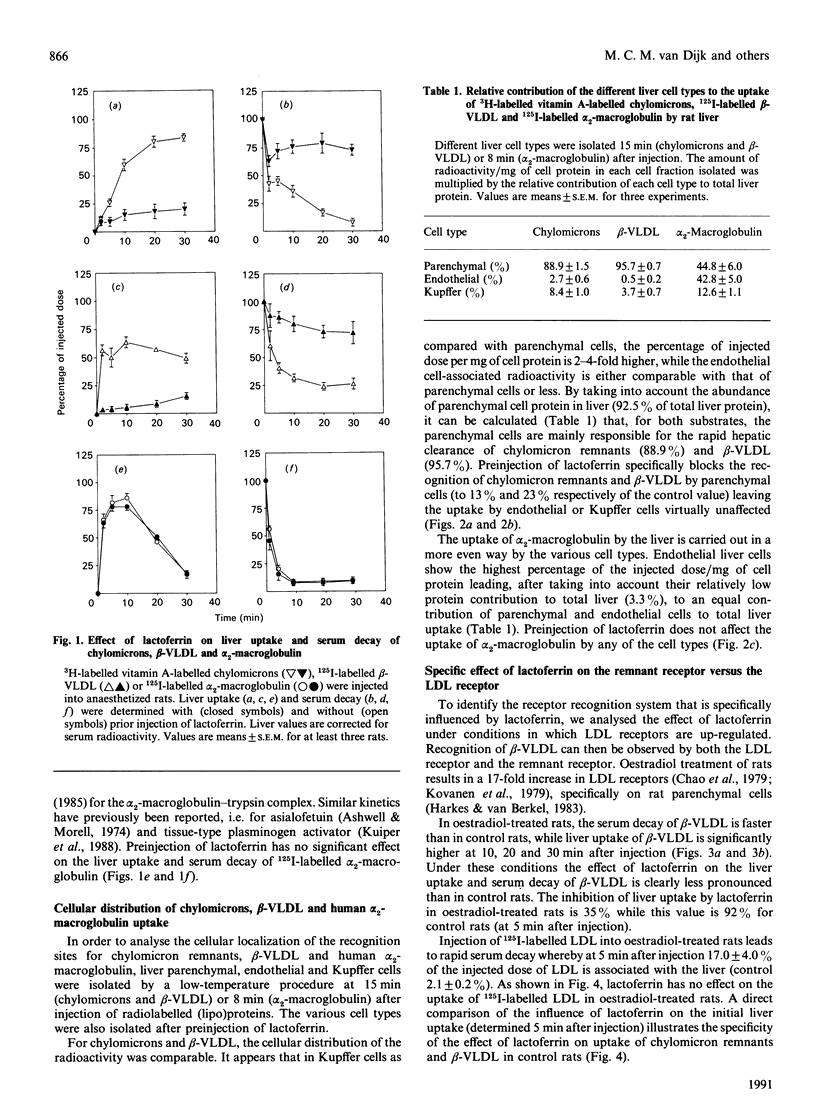
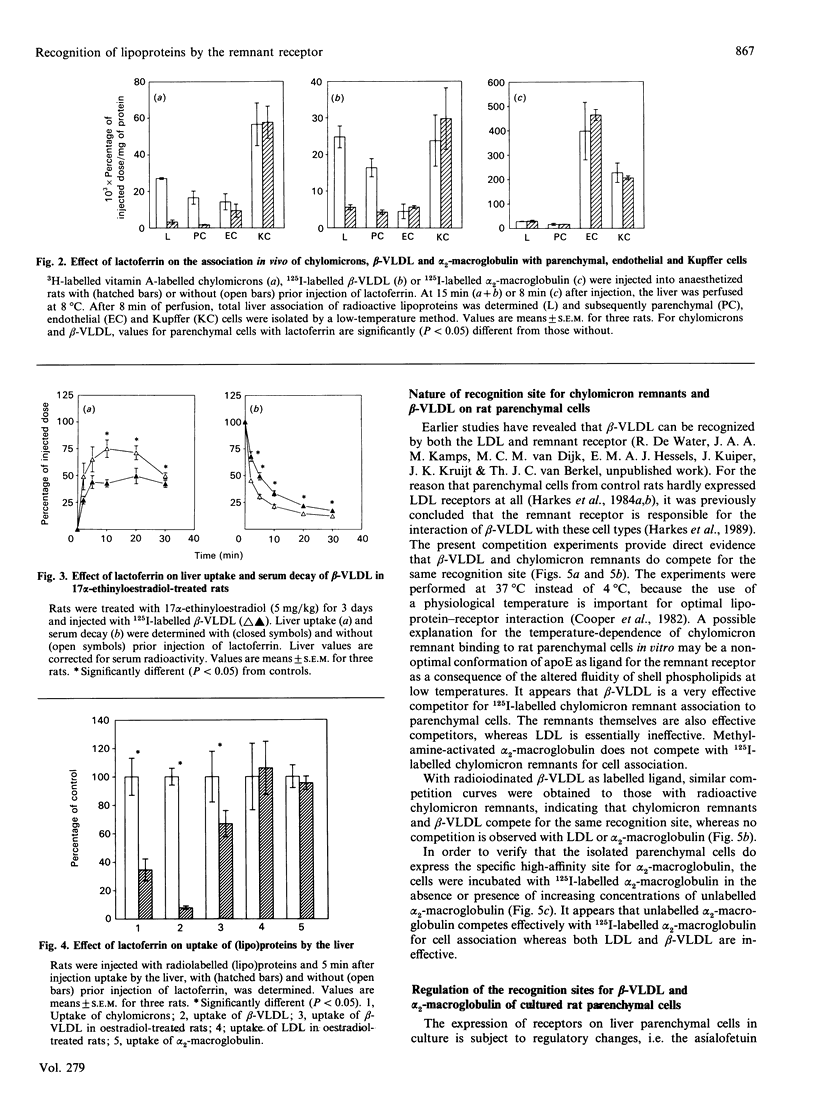
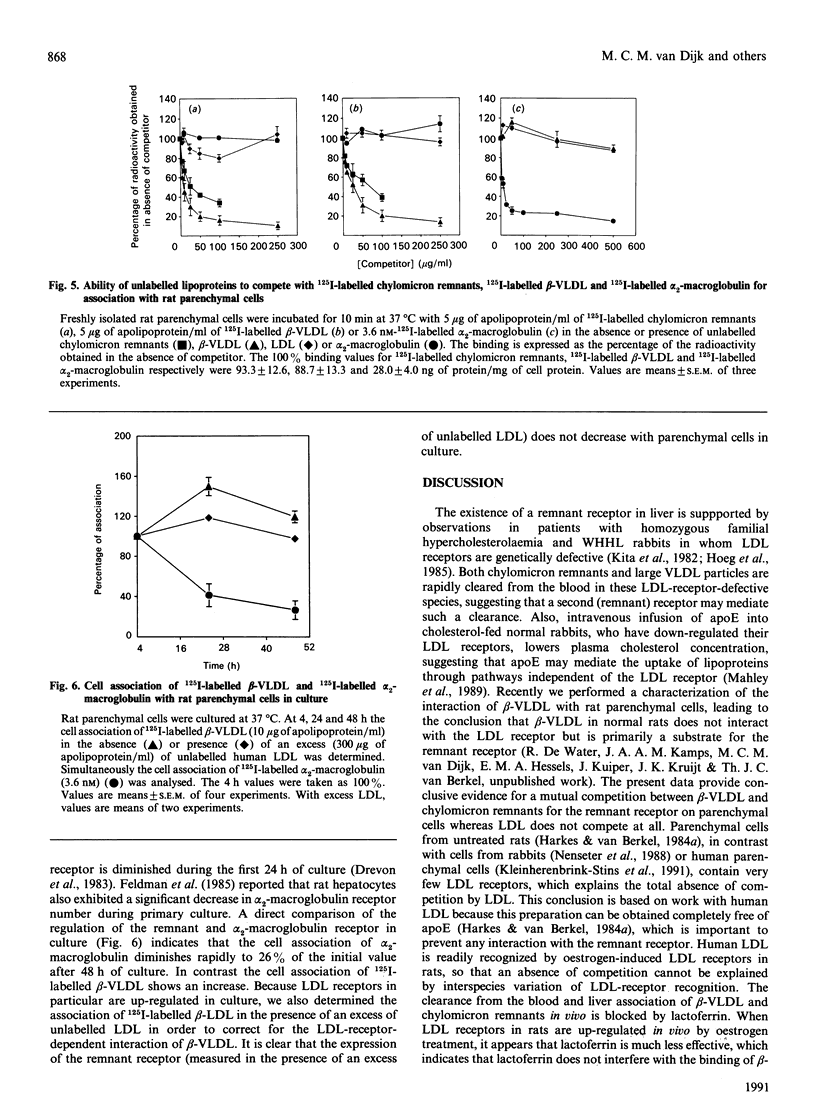
The uptake in vivo of chylomicrons and beta-migrating very-low-density lipoprotein (beta-VLDL) by rat liver, which is primarily carried out by parenchymal cells, is inhibited, 5 min after injection, to respectively 35 and 8% of the control values after preinjection of lactoferrin. The decrease in the uptake of lipoproteins by the liver caused by lactoferrin is a specific inhibition of uptake by parenchymal cells. Competition studies in vitro demonstrate that chylomicron remnants and beta-VLDL compete for the same recognition site on parenchymal cells. Data obtained in vivo together with the competition studies performed in vitro indicate that chylomicron remnants and beta-VLDL interact specifically with the same remnant receptor. Hepatic uptake of 125I-labelled-alpha 2-macroglobulin in vivo, mediated equally by parenchymal and endothelial cells, is not decreased by preinjection of lactoferrin and no effect on the parenchymal-cell-mediated uptake is found. In vitro, alpha 2-macroglobulin and chylomicron remnants or beta-VLDL show no cross-competition. Culturing of parenchymal cells for 24-48 h leads to a decrease in the cell association of alpha 2-macroglobulin to 26% of the initial value, while the cell association of beta-VLDL with the remnant receptor is not influenced. It is concluded that beta-VLDL and chylomicron remnants are recognized by a specific remnant receptor on parenchymal liver cells, while uptake of alpha 2-macroglobulin by liver is carried out by a specific receptor system (presumably involving the LDL-receptor-related protein) which shows properties that are distinct from those of the remnant receptor.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arbeeny C. M., Rifici V. A. The uptake of chylomicron remnants and very low density lipoprotein remnants by the perfused rat liver. J Biol Chem. 1984 Aug 10;259(15):9662–9666. [PubMed] [Google Scholar]
- Ashwell G., Morell A. G. The role of surface carbohydrates in the hepatic recognition and transport of circulating glycoproteins. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):99–128. doi: 10.1002/9780470122860.ch3. [DOI] [PubMed] [Google Scholar]
- Beisiegel U., Weber W., Ihrke G., Herz J., Stanley K. K. The LDL-receptor-related protein, LRP, is an apolipoprotein E-binding protein. Nature. 1989 Sep 14;341(6238):162–164. doi: 10.1038/341162a0. [DOI] [PubMed] [Google Scholar]
- Bijsterbosch M. K., Ziere G. J., Van Berkel T. J. Lactosylated low density lipoprotein: a potential carrier for the site-specific delivery of drugs to Kupffer cells. Mol Pharmacol. 1989 Sep;36(3):484–489. [PubMed] [Google Scholar]
- Blomhoff R., Eskild W., Kindberg G. M., Prydz K., Berg T. Intracellular transport of endocytosed chylomicron [3H]retinyl ester in rat liver parenchymal cells. Evidence for translocation of a [3H]retinoid from endosomes to endoplasmic reticulum. J Biol Chem. 1985 Nov 5;260(25):13566–13570. [PubMed] [Google Scholar]
- Blomhoff R., Helgerud P., Rasmussen M., Berg T., Norum K. R. In vivo uptake of chylomicron [3H]retinyl ester by rat liver: evidence for retinol transfer from parenchymal to nonparenchymal cells. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7326–7330. doi: 10.1073/pnas.79.23.7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blouin A., Bolender R. P., Weibel E. R. Distribution of organelles and membranes between hepatocytes and nonhepatocytes in the rat liver parenchyma. A stereological study. J Cell Biol. 1977 Feb;72(2):441–455. doi: 10.1083/jcb.72.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986 Apr 4;232(4746):34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- CASTER W. O., SIMON A. B., ARMSTRONG W. D. Evans blue space in tissues of the rat. Am J Physiol. 1955 Nov;183(2):317–321. doi: 10.1152/ajplegacy.1955.183.2.317. [DOI] [PubMed] [Google Scholar]
- Casteleijn E., van Rooij H. C., van Berkel T. J., Koster J. F. Mechanism of glucagon stimulation of fructose-1,6-bisphosphatase in rat hepatocytes. Involvement of a low-Mr activator. FEBS Lett. 1986 Jun 9;201(2):193–197. doi: 10.1016/0014-5793(86)80607-x. [DOI] [PubMed] [Google Scholar]
- Chao Y. S., Windler E. E., Chen G. C., Havel R. J. Hepatic catabolism of rat and human lipoproteins in rats treated with 17 alpha-ethinyl estradiol. J Biol Chem. 1979 Nov 25;254(22):11360–11366. [PubMed] [Google Scholar]
- Cooper A. D., Erickson S. K., Nutik R., Shrewsbury M. A. Characterization of chylomicron remnant binding to rat liver membranes. J Lipid Res. 1982 Jan;23(1):42–52. [PubMed] [Google Scholar]
- Davidsen O., Christensen E. I., Gliemann J. The plasma clearance of human alpha 2-macroglobulin-trypsin complex in the rat is mainly accounted for by uptake into hepatocytes. Biochim Biophys Acta. 1985 Jul 30;846(1):85–92. doi: 10.1016/0167-4889(85)90113-2. [DOI] [PubMed] [Google Scholar]
- Drevon C. A., Tolleshaug H., Carlander B., Berg T. Metabolism of asialoglycoproteins in cultured rat hepatocytes: evidence for receptor mediated uptake and degradation which is not feed-back regulated. Int J Biochem. 1983;15(6):827–833. doi: 10.1016/0020-711x(83)90154-4. [DOI] [PubMed] [Google Scholar]
- Feldman S. R., Ney K. A., Gonias S. L., Pizzo S. V. In vitro binding and in vivo clearance of human alpha 2-macroglobulin after reaction with endoproteases from four different classes. Biochem Biophys Res Commun. 1983 Jul 29;114(2):757–762. doi: 10.1016/0006-291x(83)90845-8. [DOI] [PubMed] [Google Scholar]
- Feldman S. R., Rosenberg M. R., Ney K. A., Michalopoulos G., Pizzo S. V. Binding of alpha 2-macroglobulin to hepatocytes: mechanism of in vivo clearance. Biochem Biophys Res Commun. 1985 Apr 30;128(2):795–802. doi: 10.1016/0006-291x(85)90117-2. [DOI] [PubMed] [Google Scholar]
- Fredrickson D. S., Levy R. I., Lindgren F. T. A comparison of heritable abnormal lipoprotein patterns as defined by two different techniques. J Clin Invest. 1969 Nov;47(11):2446–2457. doi: 10.1172/JCI105927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groot P. H., Van Berkel T. J., Van Tol A. Relative contributions of parenchymal and non-parenchymal (sinusoidal) liver cells in the uptake of chylomicron remnants. Metabolism. 1981 Aug;30(8):792–797. doi: 10.1016/0026-0495(81)90025-1. [DOI] [PubMed] [Google Scholar]
- Harkes L., Van Berkel J. C. Quantitative role of parenchymal and non-parenchymal liver cells in the uptake of [14C]sucrose-labelled low-density lipoprotein in vivo. Biochem J. 1984 Nov 15;224(1):21–27. doi: 10.1042/bj2240021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkes L., Van Berkel T. J. In vivo characteristics of a specific recognition site for LDL on non-parenchymal rat liver cells which differs from the 17 alpha-ethinyl estradiol-induced LDL receptor on parenchymal liver cells. Biochim Biophys Acta. 1984 Jul 6;794(2):340–347. doi: 10.1016/0005-2760(84)90165-6. [DOI] [PubMed] [Google Scholar]
- Harkes L., van Berkel T. J. Cellular localization of the receptor-dependent and receptor-independent uptake of human LDL in the liver of normal and 17 alpha-ethinyl estradiol-treated rats. FEBS Lett. 1983 Apr 5;154(1):75–80. doi: 10.1016/0014-5793(83)80878-3. [DOI] [PubMed] [Google Scholar]
- Harkes L., van Duijne A., van Berkel T. J. Interaction of beta-very-low-density lipoproteins with rat liver cells. Eur J Biochem. 1989 Mar 1;180(1):241–248. doi: 10.1111/j.1432-1033.1989.tb14639.x. [DOI] [PubMed] [Google Scholar]
- Herz J., Hamann U., Rogne S., Myklebost O., Gausepohl H., Stanley K. K. Surface location and high affinity for calcium of a 500-kd liver membrane protein closely related to the LDL-receptor suggest a physiological role as lipoprotein receptor. EMBO J. 1988 Dec 20;7(13):4119–4127. doi: 10.1002/j.1460-2075.1988.tb03306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeg J. M., Demosky S. J., Jr, Gregg R. E., Schaefer E. J., Brewer H. B., Jr Distinct hepatic receptors for low density lipoprotein and apolipoprotein E in humans. Science. 1985 Feb 15;227(4688):759–761. doi: 10.1126/science.2982214. [DOI] [PubMed] [Google Scholar]
- Huettinger M., Retzek H., Eder M., Goldenberg H. Characteristics of chylomicron remnant uptake into rat liver. Clin Biochem. 1988 Apr;21(2):87–92. doi: 10.1016/s0009-9120(88)80093-6. [DOI] [PubMed] [Google Scholar]
- Imber M. J., Pizzo S. V. Clearance and binding of two electrophoretic "fast" forms of human alpha 2-macroglobulin. J Biol Chem. 1981 Aug 10;256(15):8134–8139. [PubMed] [Google Scholar]
- Jaeckle S., Brady S. E., Havel R. J. Membrane binding sites for plasma lipoproteins on endosomes from rat liver. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1880–1884. doi: 10.1073/pnas.86.6.1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A. L., Hradek G. T., Hornick C., Renaud G., Windler E. E., Havel R. J. Uptake and processing of remnants of chylomicrons and very low density lipoproteins by rat liver. J Lipid Res. 1984 Nov;25(11):1151–1158. [PubMed] [Google Scholar]
- Kita T., Goldstein J. L., Brown M. S., Watanabe Y., Hornick C. A., Havel R. J. Hepatic uptake of chylomicron remnants in WHHL rabbits: a mechanism genetically distinct from the low density lipoprotein receptor. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3623–3627. doi: 10.1073/pnas.79.11.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinherenbrink-Stins M. F., van de Boom J. H., Schouten D., Roholl P. J., Niels van der Heyde M., Brouwer A., van Berkel T. J., Knook D. L. Visualization of the interaction of native and modified lipoproteins with parenchymal, endothelial and Kupffer cells from human liver. Hepatology. 1991 Jul;14(1):79–90. doi: 10.1002/hep.1840140114. [DOI] [PubMed] [Google Scholar]
- Koo C., Wernette-Hammond M. E., Innerarity T. L. Uptake of canine beta-very low density lipoproteins by mouse peritoneal macrophages is mediated by a low density lipoprotein receptor. J Biol Chem. 1986 Aug 25;261(24):11194–11201. [PubMed] [Google Scholar]
- Kovanen P. T., Brown M. S., Goldstein J. L. Increased binding of low density lipoprotein to liver membranes from rats treated with 17 alpha-ethinyl estradiol. J Biol Chem. 1979 Nov 25;254(22):11367–11373. [PubMed] [Google Scholar]
- Kowal R. C., Herz J., Goldstein J. L., Esser V., Brown M. S. Low density lipoprotein receptor-related protein mediates uptake of cholesteryl esters derived from apoprotein E-enriched lipoproteins. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5810–5814. doi: 10.1073/pnas.86.15.5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowal R. C., Herz J., Weisgraber K. H., Mahley R. W., Brown M. S., Goldstein J. L. Opposing effects of apolipoproteins E and C on lipoprotein binding to low density lipoprotein receptor-related protein. J Biol Chem. 1990 Jun 25;265(18):10771–10779. [PubMed] [Google Scholar]
- Krempler F., Kostner G. M., Friedl W., Paulweber B., Bauer H., Sandhofer F. Lipoprotein binding to cultured human hepatoma cells. J Clin Invest. 1987 Aug;80(2):401–408. doi: 10.1172/JCI113086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper J., Kamps J. A., Van Berkel T. J. Induction of ornithine decarboxylase in rat liver by phorbol ester is mediated by prostanoids from Kupffer cells. J Biol Chem. 1989 Apr 25;264(12):6874–6878. [PubMed] [Google Scholar]
- Kuiper J., Otter M., Rijken D. C., van Berkel T. J. Characterization of the interaction in vivo of tissue-type plasminogen activator with liver cells. J Biol Chem. 1988 Dec 5;263(34):18220–18224. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lonberg-Holm K., Reed D. L., Roberts R. C., Hebert R. R., Hillman M. C., Kutney R. M. Three high molecular weight protease inhibitors of rat plasma. Isolation, characterization, and acute phase changes. J Biol Chem. 1987 Jan 5;262(1):438–445. [PubMed] [Google Scholar]
- Lund H., Takahashi K., Hamilton R. L., Havel R. J. Lipoprotein binding and endosomal itinerary of the low density lipoprotein receptor-related protein in rat liver. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9318–9322. doi: 10.1073/pnas.86.23.9318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley R. W., Holcombe K. S. Alterations of the plasma lipoproteins and apoproteins following cholesterol feeding in the rat. J Lipid Res. 1977 May;18(3):314–324. [PubMed] [Google Scholar]
- Mahley R. W., Weisgraber K. H., Hussain M. M., Greenman B., Fisher M., Vogel T., Gorecki M. Intravenous infusion of apolipoprotein E accelerates clearance of plasma lipoproteins in rabbits. J Clin Invest. 1989 Jun;83(6):2125–2130. doi: 10.1172/JCI114126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFARLANE A. S. Efficient trace-labelling of proteins with iodine. Nature. 1958 Jul 5;182(4627):53–53. doi: 10.1038/182053a0. [DOI] [PubMed] [Google Scholar]
- Mjos O. D., Faergeman O., Hamilton R. L., Havel R. J. Characterization of remnants produced during the metabolism of triglyceride-rich lipoproteins of blood plasma and intestinal lymph in the rat. J Clin Invest. 1975 Sep;56(3):603–615. doi: 10.1172/JCI108130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moestrup S. K., Kaltoft K., Sottrup-Jensen L., Gliemann J. The human alpha 2-macroglobulin receptor contains high affinity calcium binding sites important for receptor conformation and ligand recognition. J Biol Chem. 1990 Jul 25;265(21):12623–12628. [PubMed] [Google Scholar]
- Nagelkerke J. F., Barto K. P., van Berkel T. J. In vivo and in vitro uptake and degradation of acetylated low density lipoprotein by rat liver endothelial, Kupffer, and parenchymal cells. J Biol Chem. 1983 Oct 25;258(20):12221–12227. [PubMed] [Google Scholar]
- Nenseter M. S., Blomhoff R., Drevon C. A., Kindberg G. M., Norum K. R., Berg T. Uptake of LDL in parenchymal and non-parenchymal rabbit liver cells in vivo. LDL uptake is increased in endothelial cells in cholesterol-fed rabbits. Biochem J. 1988 Sep 1;254(2):443–448. doi: 10.1042/bj2540443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redgrave T. G. Formation of cholesteryl ester-rich particulate lipid during metabolism of chylomicrons. J Clin Invest. 1970 Mar;49(3):465–471. doi: 10.1172/JCI106255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redgrave T. G., Roberts D. C., West C. E. Separation of plasma lipoproteins by density-gradient ultracentrifugation. Anal Biochem. 1975 May 12;65(1-2):42–49. doi: 10.1016/0003-2697(75)90488-1. [DOI] [PubMed] [Google Scholar]
- Redgrave T. G., Small D. M. Quantitation of the transfer of surface phospholipid of chylomicrons to the high density lipoprotein fraction during the catabolism of chylomicrons in the rat. J Clin Invest. 1979 Jul;64(1):162–171. doi: 10.1172/JCI109435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seglen P. O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Shore V. G., Shore B., Hart R. G. Changes in apolipoproteins and properties of rabbit very low density lipoproteins on induction of cholesteremia. Biochemistry. 1974 Apr 9;13(8):1579–1585. doi: 10.1021/bi00705a004. [DOI] [PubMed] [Google Scholar]
- Sottrup-Jensen L., Petersen T. E., Magnusson S. A thiol-ester in alpha 2-macroglobulin cleaved during proteinase complex formation. FEBS Lett. 1980 Dec 1;121(2):275–279. doi: 10.1016/0014-5793(80)80361-9. [DOI] [PubMed] [Google Scholar]
- Strickland D. K., Ashcom J. D., Williams S., Burgess W. H., Migliorini M., Argraves W. S. Sequence identity between the alpha 2-macroglobulin receptor and low density lipoprotein receptor-related protein suggests that this molecule is a multifunctional receptor. J Biol Chem. 1990 Oct 15;265(29):17401–17404. [PubMed] [Google Scholar]
- Van Tol A., Van 't Hooft F. M., Van Gent T. Discrepancies in the catabolic pathways of rat and human low density lipoproteins as revealed by partial hepatectomy in the rat. Atherosclerosis. 1978 Apr;29(4):449–457. doi: 10.1016/0021-9150(78)90173-9. [DOI] [PubMed] [Google Scholar]
- van Berkel T. J., Dekker C. J., Kruijt J. K., van Eijk H. G. The interaction in vivo of transferrin and asialotransferrin with liver cells. Biochem J. 1987 May 1;243(3):715–722. doi: 10.1042/bj2430715. [DOI] [PMC free article] [PubMed] [Google Scholar]


