Abstract
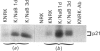
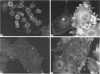
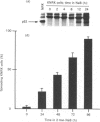
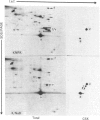
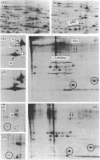
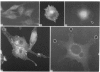
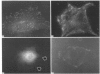
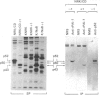
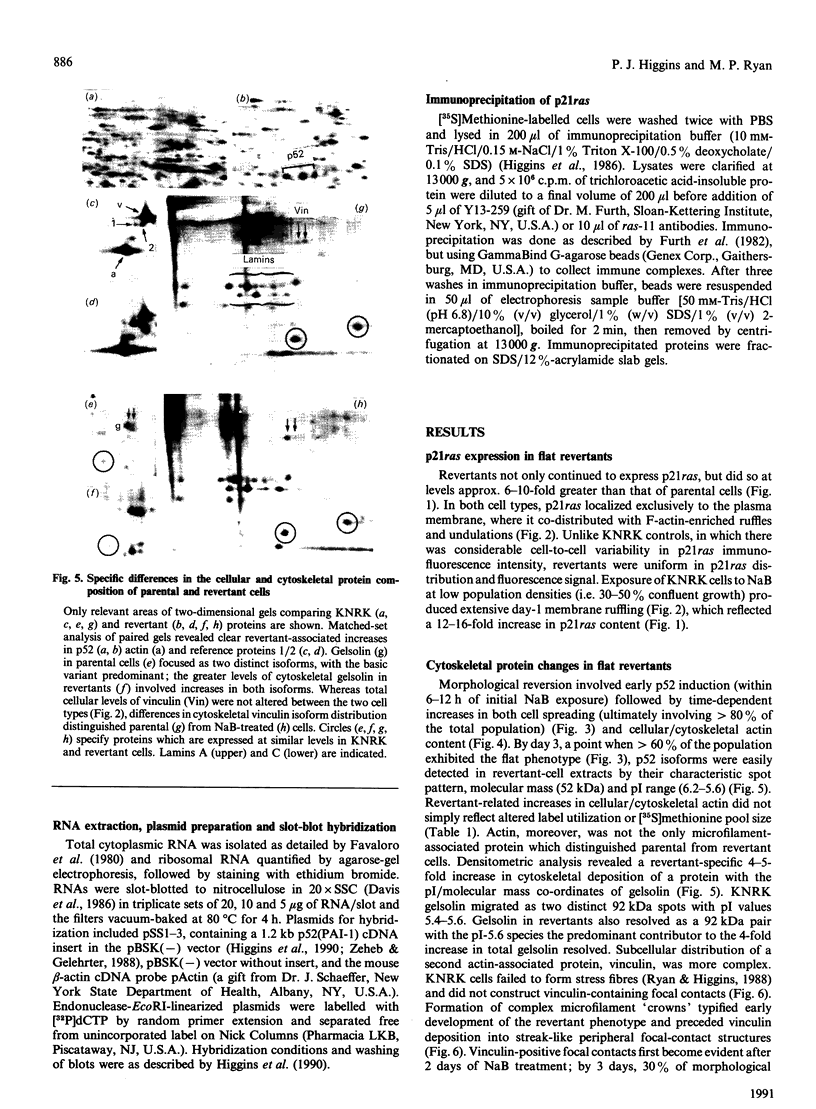
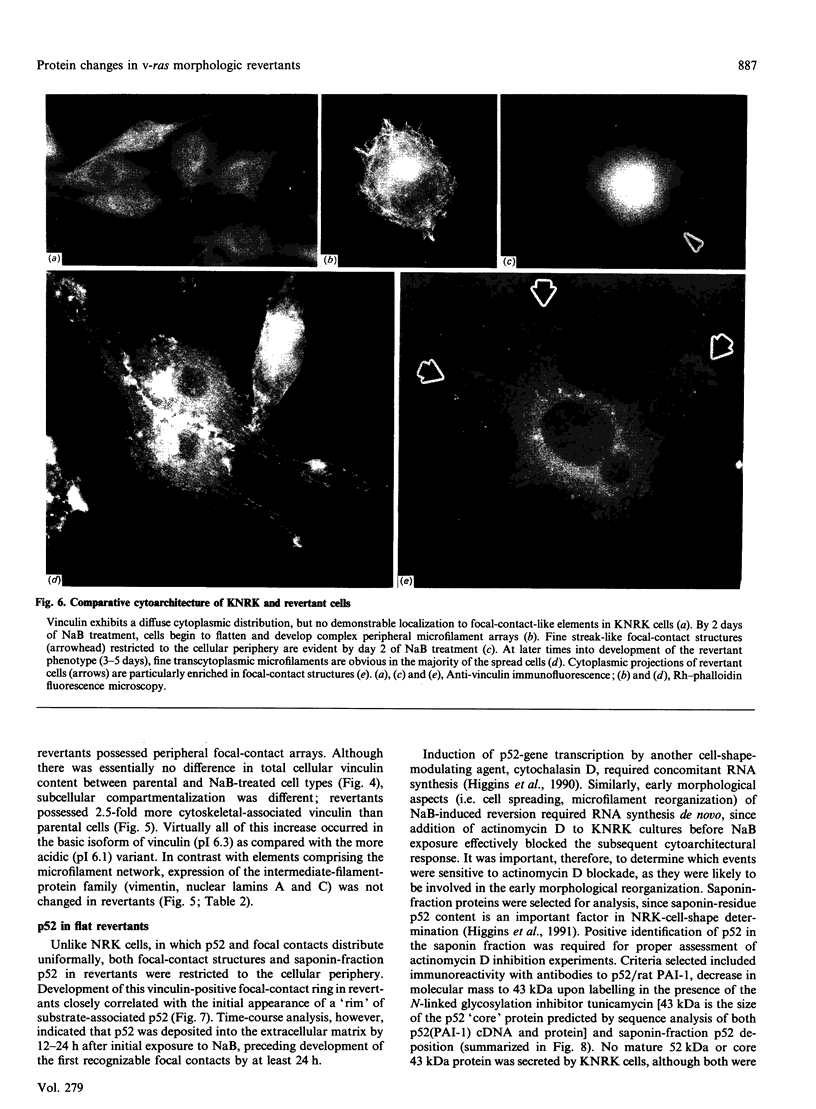
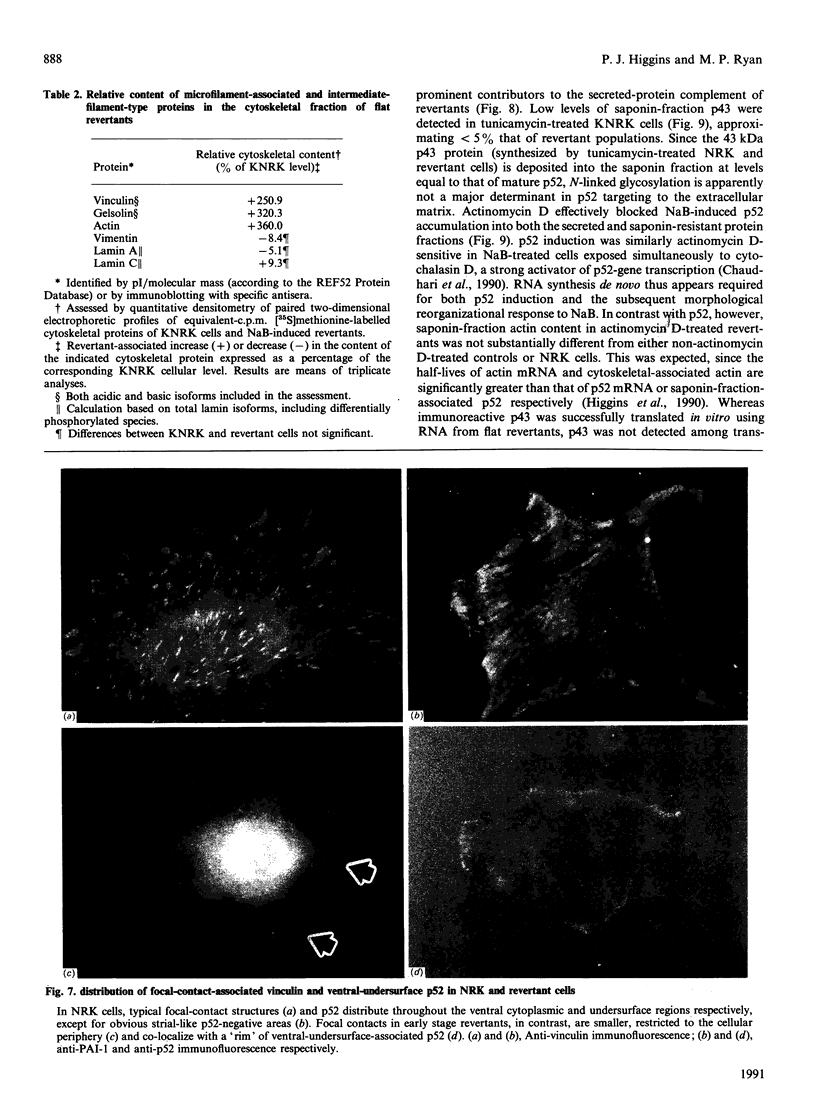
Flat revertants of v-ras-transformed (KNRK) rat kidney cells, which express elevated levels of p21ras protein, were generated to high efficiencies with sodium butyrate (NaB). Overall protein synthesis in revertants was not different from parental cells, although changes were evident in expression and distribution of specific microfilament-associated cytoskeletal proteins. Quantitative two-dimensional electrophoresis revealed revertant-associated 3-4-fold increases in cytoskeletal deposition of the microfilament-associated proteins gelsolin and vinculin correlating with microfilament reorganization and focal-contact formation respectively. Similar increases in actin content were evident at both the total-cellular- and cytoskeletal-associated-protein levels. In contrast, intermediate-filament family elements (vimentin, lamins) remained unaltered. The only unique protein resolved in flat revertants was p52, a 52 kDa extracellular-matrix-associated protein previously identified as plasminogen-activator inhibitor type 1 (PAI-1). p52(PAI-1) expression was induced early during generation of the revertant phenotype and preceded development of focal-contact structures. NaB-induced p52(PAI-1) synthesis and generation of early morphological reversion in KNRK cells required ongoing RNA synthesis, since exposure to actinomycin D before addition of NaB inhibited both events. p52(PAI-1) induction by NaB was regulated at the level of mRNA abundance; in contrast, actin mRNA levels were the same in parental and revertant cells, suggesting that the increased actin content which typified the revertant phenotype was due to augmented actin microfilament stability.
Full text
PDF
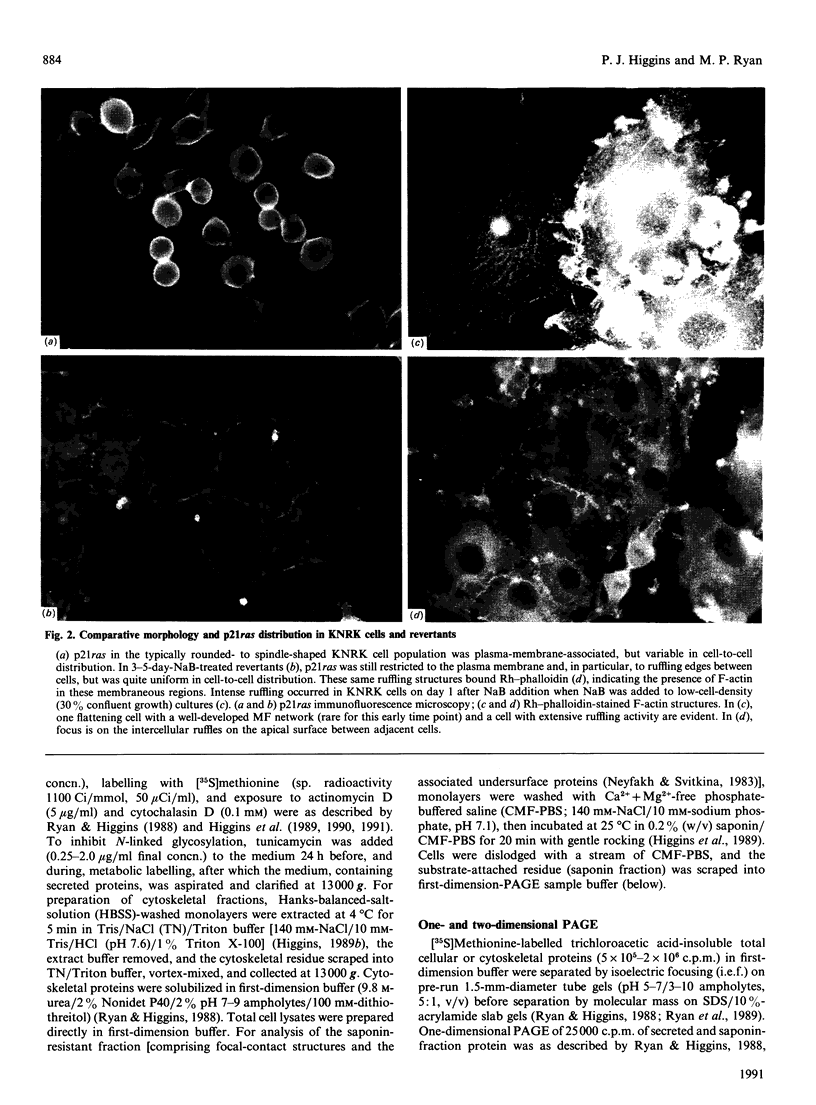
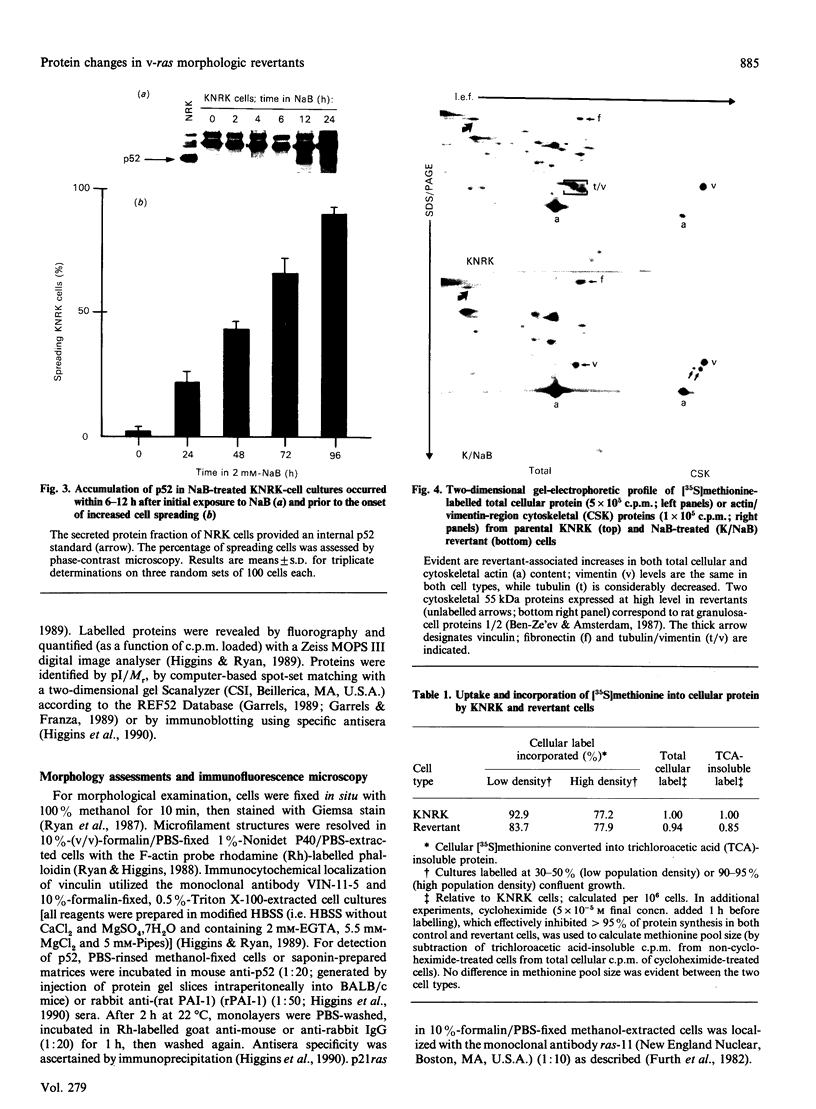


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altenburg B. C., Via D. P., Steiner S. H. Modification of the phenotype of murine sarcoma virus-transformed cells by sodium butyrate. Effects on morphology and cytoskeletal elements. Exp Cell Res. 1976 Oct 15;102(2):223–231. doi: 10.1016/0014-4827(76)90036-7. [DOI] [PubMed] [Google Scholar]
- Banyard M. R., Medveczyk C. J., Tellam R. L. Microfilament organization correlates with increased cellular content of gelsolin. Exp Cell Res. 1990 Mar;187(1):180–183. doi: 10.1016/0014-4827(90)90135-w. [DOI] [PubMed] [Google Scholar]
- Bar-Sagi D., Feramisco J. R. Induction of membrane ruffling and fluid-phase pinocytosis in quiescent fibroblasts by ras proteins. Science. 1986 Sep 5;233(4768):1061–1068. doi: 10.1126/science.3090687. [DOI] [PubMed] [Google Scholar]
- Bar-Sagi D., Suhan J. P., McCormick F., Feramisco J. R. Localization of phospholipase A2 in normal and ras-transformed cells. J Cell Biol. 1988 May;106(5):1649–1658. doi: 10.1083/jcb.106.5.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ze'ev A., Amsterdam A. In vitro regulation of granulosa cell differentiation. Involvement of cytoskeletal protein expression. J Biol Chem. 1987 Apr 15;262(11):5366–5376. [PubMed] [Google Scholar]
- Berridge M. J., Galione A. Cytosolic calcium oscillators. FASEB J. 1988 Dec;2(15):3074–3082. doi: 10.1096/fasebj.2.15.2847949. [DOI] [PubMed] [Google Scholar]
- Cohen R. L., Niclas J., Lee W. M., Wun T. C., Crowley C. W., Levinson A. D., Sadler J. E., Shuman M. A. Effects of cellular transformation on expression of plasminogen activator inhibitors 1 and 2. Evidence for independent regulation. J Biol Chem. 1989 May 15;264(14):8375–8383. [PubMed] [Google Scholar]
- Declerck P. J., De Mol M., Alessi M. C., Baudner S., Pâques E. P., Preissner K. T., Müller-Berghaus G., Collen D. Purification and characterization of a plasminogen activator inhibitor 1 binding protein from human plasma. Identification as a multimeric form of S protein (vitronectin). J Biol Chem. 1988 Oct 25;263(30):15454–15461. [PubMed] [Google Scholar]
- Egan S. E., Wright J. A., Jarolim L., Yanagihara K., Bassin R. H., Greenberg A. H. Transformation by oncogenes encoding protein kinases induces the metastatic phenotype. Science. 1987 Oct 9;238(4824):202–205. doi: 10.1126/science.3659911. [DOI] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Fujita H., Suzuki H., Kuzumaki N., Müllauer L., Ogiso Y., Oda A., Ebisawa K., Sakurai T., Nonomura Y., Kijimoto-Ochiai S. A specific protein, p92, detected in flat revertants derived from NIH/3T3 transformed by human activated c-Ha-ras oncogene. Exp Cell Res. 1990 Jan;186(1):115–121. doi: 10.1016/0014-4827(90)90217-x. [DOI] [PubMed] [Google Scholar]
- Furth M. E., Davis L. J., Fleurdelys B., Scolnick E. M. Monoclonal antibodies to the p21 products of the transforming gene of Harvey murine sarcoma virus and of the cellular ras gene family. J Virol. 1982 Jul;43(1):294–304. doi: 10.1128/jvi.43.1.294-304.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrels J. I., Franza B. R., Jr The REF52 protein database. Methods of database construction and analysis using the QUEST system and characterizations of protein patterns from proliferating and quiescent REF52 cells. J Biol Chem. 1989 Mar 25;264(9):5283–5298. [PubMed] [Google Scholar]
- Garrels J. I. The QUEST system for quantitative analysis of two-dimensional gels. J Biol Chem. 1989 Mar 25;264(9):5269–5282. [PubMed] [Google Scholar]
- Higgins P. J. Altered expression and distribution of the cytoskeletal-associated p35 protein in NIH 3T3 cells transformed with the Harvey sarcoma virus v-ras oncogene. Int J Biochem. 1989;21(6):609–617. doi: 10.1016/0020-711x(89)90379-0. [DOI] [PubMed] [Google Scholar]
- Higgins P. J., Chaudhari P., Ryan M. P. Cell-shape regulation and matrix protein p52 content in phenotypic variants of ras-transformed rat kidney fibroblasts. Functional analysis and biochemical comparison of p52 with proteins implicated in cell-shape determination. Biochem J. 1991 Feb 1;273(Pt 3):651–658. doi: 10.1042/bj2730651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins P. J., Ryan M. P. Biochemical localization of the transformation-sensitive 52 kDa (p52) protein to the substratum contact regions of cultured rat fibroblasts. Butyrate induction, characterization, and quantification of p52 in v-ras transformed cells. Biochem J. 1989 Jan 1;257(1):173–182. doi: 10.1042/bj2570173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins P. J., Ryan M. P., Chaudhari P. Cytochalasin D-mediated hyperinduction of the substrate-associated 52-kilodalton protein p52 in rat kidney fibroblasts. J Cell Physiol. 1989 May;139(2):407–417. doi: 10.1002/jcp.1041390225. [DOI] [PubMed] [Google Scholar]
- Higgins P. J., Ryan M. P., Zeheb R., Gelehrter T. D., Chaudhari P. p52 induction by cytochalasin D in rat kidney fibroblasts: homologies between p52 and plasminogen activator inhibitor type-1. J Cell Physiol. 1990 May;143(2):321–329. doi: 10.1002/jcp.1041430216. [DOI] [PubMed] [Google Scholar]
- Higgins P. J., Silverstone A. E., Bueti C., Pizzi V. F., Melamed M. R., Lipkin M., Traganos F. Expression of murine gamma fetal antigen in adult hematopoietic tissue and during induced differentiation of Friend erythroleukemia cells. J Natl Cancer Inst. 1986 May;76(5):885–893. [PubMed] [Google Scholar]
- Ishikawa R., Yamashiro S., Matsumura F. Differential modulation of actin-severing activity of gelsolin by multiple isoforms of cultured rat cell tropomyosin. Potentiation of protective ability of tropomyosins by 83-kDa nonmuscle caldesmon. J Biol Chem. 1989 May 5;264(13):7490–7497. [PubMed] [Google Scholar]
- Laiho M., Keski-Oja J. Growth factors in the regulation of pericellular proteolysis: a review. Cancer Res. 1989 May 15;49(10):2533–2553. [PubMed] [Google Scholar]
- Laiho M., Saksela O., Keski-Oja J. Transforming growth factor-beta induction of type-1 plasminogen activator inhibitor. Pericellular deposition and sensitivity to exogenous urokinase. J Biol Chem. 1987 Dec 25;262(36):17467–17474. [PubMed] [Google Scholar]
- Lassing I., Lindberg U. Specificity of the interaction between phosphatidylinositol 4,5-bisphosphate and the profilin:actin complex. J Cell Biochem. 1988 Jul;37(3):255–267. doi: 10.1002/jcb.240370302. [DOI] [PubMed] [Google Scholar]
- Leavitt J., Kakunaga T. Expression of a variant form of actin and additional polypeptide changes following chemical-induced in vitro neoplastic transformation of human fibroblasts. J Biol Chem. 1980 Feb 25;255(4):1650–1661. [PubMed] [Google Scholar]
- Ledwith B. J., Manam S., Kraynak A. R., Nichols W. W., Bradley M. O. Antisense-fos RNA causes partial reversion of the transformed phenotypes induced by the c-Ha-ras oncogene. Mol Cell Biol. 1990 Apr;10(4):1545–1555. doi: 10.1128/mcb.10.4.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura F., Lin J. J., Yamashiro-Matsumura S., Thomas G. P., Topp W. C. Differential expression of tropomyosin forms in the microfilaments isolated from normal and transformed rat cultured cells. J Biol Chem. 1983 Nov 25;258(22):13954–13964. [PubMed] [Google Scholar]
- Myrdal S. E., Auersperg N. p21ras. Heterogeneous localization in transformed cells. Exp Cell Res. 1985 Aug;159(2):441–450. doi: 10.1016/s0014-4827(85)80017-3. [DOI] [PubMed] [Google Scholar]
- Newman M. J., Lane E. A., Iannotti A. M., Nugent M. A., Pepinsky R. B., Keski-Oja J. Characterization and purification of a secreted plasminogen activator inhibitor (PAI-1) induced by transforming growth factor-beta 1 in normal rat kidney (NRK) cells: decreased PAI-1 expression in transformed NRK cells. Endocrinology. 1990 Jun;126(6):2936–2946. doi: 10.1210/endo-126-6-2936. [DOI] [PubMed] [Google Scholar]
- Neyfakh A. A., Jr, Svitkina T. M. Isolation of focal contact membrane using saponin. Exp Cell Res. 1983 Dec;149(2):582–586. doi: 10.1016/0014-4827(83)90369-5. [DOI] [PubMed] [Google Scholar]
- Pöllänen J., Hedman K., Nielsen L. S., Danø K., Vaheri A. Ultrastructural localization of plasma membrane-associated urokinase-type plasminogen activator at focal contacts. J Cell Biol. 1988 Jan;106(1):87–95. doi: 10.1083/jcb.106.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pöllänen J., Saksela O., Salonen E. M., Andreasen P., Nielsen L., Danø K., Vaheri A. Distinct localizations of urokinase-type plasminogen activator and its type 1 inhibitor under cultured human fibroblasts and sarcoma cells. J Cell Biol. 1987 Apr;104(4):1085–1096. doi: 10.1083/jcb.104.4.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rheinwald J. G., Jorgensen J. L., Hahn W. C., Terpstra A. J., O'Connell T. M., Plummer K. K. Mesosecrin: a secreted glycoprotein produced in abundance by human mesothelial, endothelial, and kidney epithelial cells in culture. J Cell Biol. 1987 Feb;104(2):263–275. doi: 10.1083/jcb.104.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan M. P., Borenfreund E., Higgins P. J. Butyrate-induced cytoarchitectural reorganization of Mallory body-containing rat hepatic tumor cells. J Natl Cancer Inst. 1987 Sep;79(3):555–567. [PubMed] [Google Scholar]
- Ryan M. P., Borenfreund E., Higgins P. J. Cytoarchitectural analysis of epithelial sheets formed in vitro by hepatic tumor cells possessing defined intermediate-sized filament cytoskeletal abnormalities. Am J Pathol. 1989 Feb;134(2):447–456. [PMC free article] [PubMed] [Google Scholar]
- Ryan M. P., Higgins P. J. Cytoarchitecture of Kirsten sarcoma virus-transformed rat kidney fibroblasts: butyrate-induced reorganization within the actin microfilament network. J Cell Physiol. 1988 Oct;137(1):25–34. doi: 10.1002/jcp.1041370104. [DOI] [PubMed] [Google Scholar]
- Ryan M. P., Higgins P. J. Sodium-n-butyrate induces secretion and substrate accumulation of p52 in Kirsten sarcoma virus-transformed rat kidney fibroblasts. Int J Biochem. 1989;21(1):31–37. doi: 10.1016/0020-711x(89)90024-4. [DOI] [PubMed] [Google Scholar]
- Samid D., Flessate D. M., Friedman R. M. Interferon-induced revertants of ras-transformed cells: resistance to transformation by specific oncogenes and retransformation by 5-azacytidine. Mol Cell Biol. 1987 Jun;7(6):2196–2200. doi: 10.1128/mcb.7.6.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarén J. F., Bravo R. Immediate induction of a 45 K secreted glycoprotein by serum and growth factors in quiescent mouse 3T3 cells. Two-dimensional gel analysis. Exp Cell Res. 1987 Feb;168(2):494–506. doi: 10.1016/0014-4827(87)90022-x. [DOI] [PubMed] [Google Scholar]
- Sistonen L., Keski-Oja J., Ulmanen I., Hölttä E., Wikgren B. J., Alitalo K. Dose effects of transfected c-Ha-rasVal 12 oncogene in transformed cell clones. Exp Cell Res. 1987 Feb;168(2):518–530. doi: 10.1016/0014-4827(87)90024-3. [DOI] [PubMed] [Google Scholar]
- Stossel T. P. From signal to pseudopod. How cells control cytoplasmic actin assembly. J Biol Chem. 1989 Nov 5;264(31):18261–18264. [PubMed] [Google Scholar]
- White J. E., Tsan M. F., Phillips P. G., Higgins P. J. The substrate-associated protein p45 of porcine endothelial cells: multiple isoforms, cytoskeletal-like properties and induction by hyperoxic stress. Int J Biochem. 1990;22(10):1159–1164. doi: 10.1016/0020-711x(90)90115-j. [DOI] [PubMed] [Google Scholar]
- Yamada H., Omata-Yamada T., Wakabayashi-Ito N., Carter S. G., Lengyel P. Isolation of recessive (mediator-) revertants from NIH 3T3 cells transformed with a c-H-ras oncogene. Mol Cell Biol. 1990 Apr;10(4):1822–1827. doi: 10.1128/mcb.10.4.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C. L., Tsai M. H., Stacey D. W. Cellular ras activity and phospholipid metabolism. Cell. 1988 Jan 15;52(1):63–71. doi: 10.1016/0092-8674(88)90531-4. [DOI] [PubMed] [Google Scholar]
- Zeheb R., Gelehrter T. D. Cloning and sequencing of cDNA for the rat plasminogen activator inhibitor-1. Gene. 1988 Dec 20;73(2):459–468. doi: 10.1016/0378-1119(88)90510-0. [DOI] [PubMed] [Google Scholar]