Abstract
3-Deoxyglucosone reductase activity in the extracts of rat, pig and human livers was potently inhibited by aldehyde reductase inhibitors. The major species of 3-deoxyglucosone reductase purified from human and pig livers were biochemically and immunochemically identical with aldehyde reductase. The two enzymes and rat liver aldehyde reductase exhibited higher catalytic efficiency for 3-deoxyglucosone than for D-glucuronate, a representative substrate of aldehyde reductase.
Full text
PDF
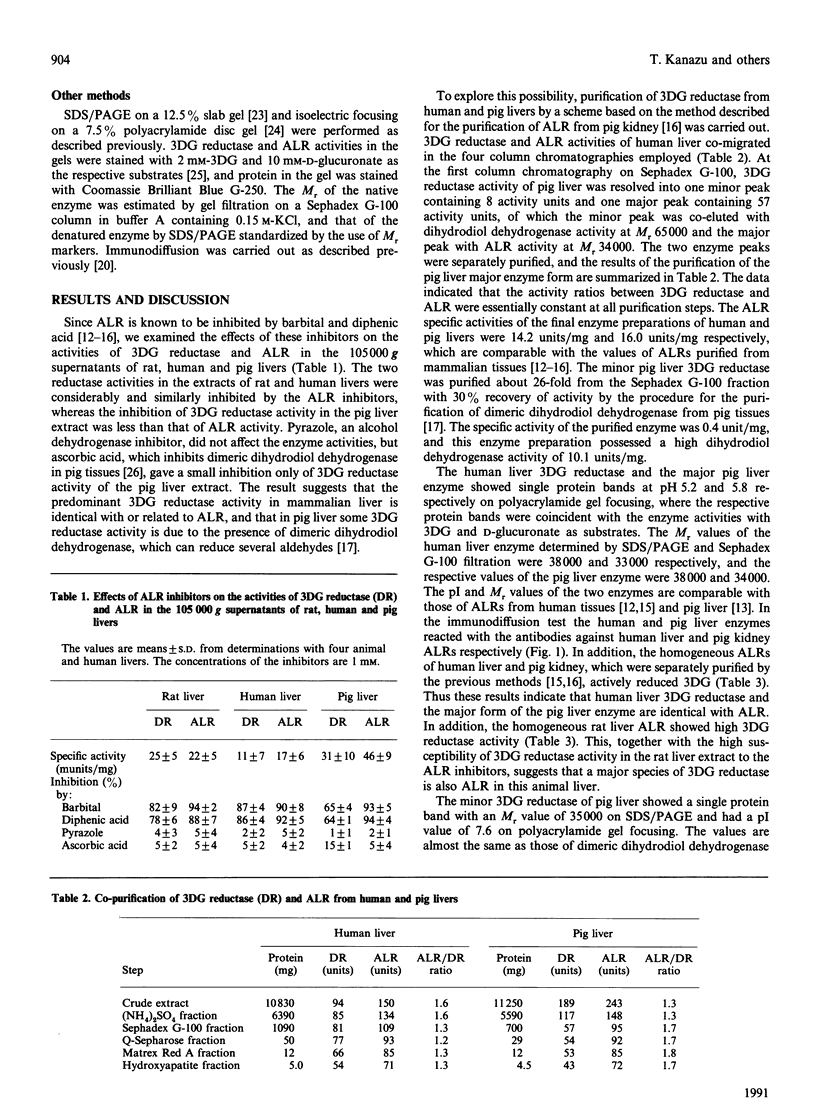

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Branlant G. Properties of an aldose reductase from pig lens. Comparative studies of an aldehyde reductase from pig lens. Eur J Biochem. 1982 Dec;129(1):99–104. doi: 10.1111/j.1432-1033.1982.tb07026.x. [DOI] [PubMed] [Google Scholar]
- Eble A. S., Thorpe S. R., Baynes J. W. Nonenzymatic glucosylation and glucose-dependent cross-linking of protein. J Biol Chem. 1983 Aug 10;258(15):9406–9412. [PubMed] [Google Scholar]
- Felsted R. L., Bachur N. R. Mammalian carbonyl reductases. Drug Metab Rev. 1980;11(1):1–60. doi: 10.3109/03602538008994021. [DOI] [PubMed] [Google Scholar]
- Felsted R. L., Gee M., Bachur N. R. Rat liver daunorubicin reductase. An aldo-keto reductase. J Biol Chem. 1974 Jun 25;249(12):3672–3679. [PubMed] [Google Scholar]
- Felsted R. L., Richter D. R., Bachur N. R. Rat liver aldehyde reductase. Biochem Pharmacol. 1977 Jun 15;26(12):1117–1124. doi: 10.1016/0006-2952(77)90054-5. [DOI] [PubMed] [Google Scholar]
- Flynn T. G. Aldehyde reductases: monomeric NADPH-dependent oxidoreductases with multifunctional potential. Biochem Pharmacol. 1982 Sep 1;31(17):2705–2712. doi: 10.1016/0006-2952(82)90123-x. [DOI] [PubMed] [Google Scholar]
- Hara A., Deyashiki Y., Nakagawa M., Nakayama T., Sawada H. Isolation of proteins with carbonyl reductase activity and prostaglandin-9-ketoreductase activity from chicken kidney. J Biochem. 1982 Dec;92(6):1753–1762. doi: 10.1093/oxfordjournals.jbchem.a134105. [DOI] [PubMed] [Google Scholar]
- Hara A., Harada T., Nakagawa M., Matsuura K., Nakayama T., Sawada H. Isolation from pig lens of two proteins with dihydrodiol dehydrogenase and aldehyde reductase activities. Biochem J. 1989 Dec 1;264(2):403–407. doi: 10.1042/bj2640403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara A., Shinoda M., Kanazu T., Nakayama T., Deyashiki Y., Sawada H. Inhibition of dimeric dihydrodiol dehydrogenases of rabbit and pig lens by ascorbic acid. Biochem J. 1991 Apr 1;275(Pt 1):121–126. doi: 10.1042/bj2750121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding J. J. Nonenzymatic covalent posttranslational modification of proteins in vivo. Adv Protein Chem. 1985;37:247–334. doi: 10.1016/s0065-3233(08)60066-2. [DOI] [PubMed] [Google Scholar]
- Hayase F., Nagaraj R. H., Miyata S., Njoroge F. G., Monnier V. M. Aging of proteins: immunological detection of a glucose-derived pyrrole formed during maillard reaction in vivo. J Biol Chem. 1989 Mar 5;264(7):3758–3764. [PubMed] [Google Scholar]
- Hayashi H., Fujii Y., Watanabe K., Urade Y., Hayaishi O. Enzymatic conversion of prostaglandin H2 to prostaglandin F2 alpha by aldehyde reductase from human liver: comparison to the prostaglandin F synthetase from bovine lung. J Biol Chem. 1989 Jan 15;264(2):1036–1040. [PubMed] [Google Scholar]
- Kato H., Hayase F., Shin D. B., Oimomi M., Baba S. 3-Deoxyglucosone, an intermediate product of the Maillard reaction. Prog Clin Biol Res. 1989;304:69–84. [PubMed] [Google Scholar]
- Kato H., van Chuyen N., Shinoda T., Sekiya F., Hayase F. Metabolism of 3-deoxyglucosone, an intermediate compound in the Maillard reaction, administered orally or intravenously to rats. Biochim Biophys Acta. 1990 Jul 20;1035(1):71–76. doi: 10.1016/0304-4165(90)90175-v. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liang Z. Q., Hayase F., Kato H. Purification and characterization of NADPH-dependent 2-oxoaldehyde reductase from porcine liver. A self-defence enzyme preventing the advanced stage of the Maillard reaction. Eur J Biochem. 1991 Apr 23;197(2):373–379. doi: 10.1111/j.1432-1033.1991.tb15921.x. [DOI] [PubMed] [Google Scholar]
- Monnier V. M., Cerami A. Non-enzymatic glycosylation and browning of proteins in diabetes. Clin Endocrinol Metab. 1982 Jul;11(2):431–452. doi: 10.1016/s0300-595x(82)80023-6. [DOI] [PubMed] [Google Scholar]
- Nakayama T., Hara A., Yashiro K., Sawada H. Reductases for carbonyl compounds in human liver. Biochem Pharmacol. 1985 Jan 1;34(1):107–117. doi: 10.1016/0006-2952(85)90108-x. [DOI] [PubMed] [Google Scholar]
- Nakayama T., Sawada H., Deyashiki Y., Kanazu T., Hara A., Shinoda M., Matsuura K., Bunai Y., Ohya I. Distribution of dimeric dihydrodiol dehydrogenase in pig tissues and its role in carbonyl metabolism. Adv Exp Med Biol. 1991;284:187–196. doi: 10.1007/978-1-4684-5901-2_21. [DOI] [PubMed] [Google Scholar]
- Ozeki S., Kato T., Holtzer M. E., Holtzer A. The kinetics of chain exchange in two-chain coiled coils: alpha alpha- and beta beta-tropomyosin. Biopolymers. 1991 Jul;31(8):957–966. doi: 10.1002/bip.360310805. [DOI] [PubMed] [Google Scholar]
- Peinado J., López-Soriano F. J., Argilés J. M. Gas chromatographic method for the estimation of acetone and its metabolites in biological samples. J Chromatogr. 1987 Apr 10;415(2):372–376. doi: 10.1016/s0378-4347(00)83229-6. [DOI] [PubMed] [Google Scholar]
- Reddy C. C., Swan J. S., Hamilton G. A. myo-Inositol oxygenase from hog kidney. I. Purification and characterization of the oxygenase and of an enzyme complex containing the oxygenase and D-glucuronate reductase. J Biol Chem. 1981 Aug 25;256(16):8510–8518. [PubMed] [Google Scholar]
- Sawada H., Hara A., Hayashibara M., Nakayama T. Guinea pig liver aromatic aldehyde-ketone reductases identical with 17 beta-hydroxysteroid dehydrogenase isozymes. J Biochem. 1979 Oct;86(4):883–892. doi: 10.1093/oxfordjournals.jbchem.a132620. [DOI] [PubMed] [Google Scholar]
- Sawada H., Hara A., Hayashibara M., Nakayama T., Usui S. Immunological identification of soluble carbonyl reductase with testosterone 17 beta-dehydrogenase (NADP+) in guinea pig liver and kidney. Biochim Biophys Acta. 1984 Jun 29;799(3):322–325. doi: 10.1016/0304-4165(84)90278-2. [DOI] [PubMed] [Google Scholar]
- Szwergold B. S., Kappler F., Brown T. R. Identification of fructose 3-phosphate in the lens of diabetic rats. Science. 1990 Jan 26;247(4941):451–454. doi: 10.1126/science.2300805. [DOI] [PubMed] [Google Scholar]
- Wermuth B., Monder C. Aldose and aldehyde reductase exhibit isocorticosteroid reductase activity. Eur J Biochem. 1983 Mar 15;131(2):423–426. doi: 10.1111/j.1432-1033.1983.tb07280.x. [DOI] [PubMed] [Google Scholar]
- Wermuth B., Münch J. D., von Wartburg J. P. Purification and properties of NADPH-dependent aldehyde reductase from human liver. J Biol Chem. 1977 Jun 10;252(11):3821–3828. [PubMed] [Google Scholar]
- Wirth H. P., Wermuth B. Immunohistochemical localisation of aldehyde and aldose reductase in human tissues. Prog Clin Biol Res. 1985;174:231–239. [PubMed] [Google Scholar]
- Zagalak B., Frey P. A., Karabatsos G. L., Abeles R. H. The stereochemistry of the conversion of D and L 1,2-propanediols to propionaldehyde. J Biol Chem. 1966 Jul 10;241(13):3028–3035. [PubMed] [Google Scholar]