Abstract
Background
Sub-phenotyping of acute respiratory distress syndrome (ARDS) could be useful for evaluating the severity of ARDS or predicting its responsiveness to given therapeutic strategies, but no studies have yet investigated the heterogeneity of patients with severe ARDS requiring veno-venous extracorporeal membrane oxygenation (V-V ECMO).
Methods
We conducted this retrospective multicenter observational study in adult patients with severe ARDS treated by V-V ECMO. We performed latent class analysis (LCA) for identifying sub-phenotypes of severe ARDS based on the radiological and clinical findings at the start of ECMO support. Multivariate Cox regression analysis was conducted to investigate the differences in mortality and association between the PEEP setting of ≥ 10 cmH2O and mortality by the sub-phenotypes.
Results
We identified three sub-phenotypes from analysis of the data of a total of 544 patients with severe ARDS treated by V-V ECMO, as follows: Dry type (n = 185; 34%); Wet type (n = 169; 31%); and Fibrotic type (n = 190; 35%). The 90-days in-hospital mortality risk was higher in the patients with the Fibrotic type than in those with the Dry type (adjusted hazard ratio [95% confidence interval] 1.75 [1.10–2.79], p = 0.019) or the Wet type (1.50 [1.02–2.23], p = 0.042). The PEEP setting of ≥ 10 cmH2O during the first 3 days of ECMO decreased the 90-days in-hospital mortality risk only in patients with the Wet type, and not in those with the Dry or Fibrotic type. A significant interaction effect was observed between the Wet type and the PEEP setting of ≥ 10 cmH2O in relation to the 90-day in-hospital mortality (pinteraction = 0.036).
Conclusions
The three sub-phenotypes showed different mortality rates and different relationships between higher PEEP settings in the early phase of V-V ECMO and patient outcomes. Our data suggest that we may need to change our management approach to patients with severe ARDS during V-V ECMO according to their clinical sub-phenotype.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-024-05143-3.
Keywords: Severe acute respiratory distress syndrome, Veno-venous extracorporeal membrane oxygenation, Computed tomography, In-hospital mortality, Latent class analysis
Background
Patients with acute respiratory distress syndrome (ARDS), a life-threatening lung injury characterized by inflammatory pulmonary edema, sometimes require mechanical support by veno-venous extracorporeal membrane oxygenation (V-V ECMO). Although V-V ECMO is helpful in improving their oxygenation and decarboxylation, their hospital mortality rate is greater than 40% [1, 2], which suggests that we may need to develop better strategies for the management of V-V ECMO in order to improve their outcomes.
Recently, the heterogeneity of ARDS was addressed by different management strategies by sub-phenotyping. One of the most frequent classifications, based on latent class analysis (LCA) using clinical and plasma biomarker data, divides patients into a hyper-inflammatory or hypo-inflammatory sub-phenotype. Several studies have shown the usefulness of this sub-phenotyping for evaluating the severity of ARDS [3, 4] or predicting its responsiveness to given therapeutic strategies [5, 6] in a general population of ARDS patients. However, there are no reports of studies conducted to investigate sub-phenotypes focusing on severe ARDS patients requiring V-V ECMO, the most severely ill subpopulation of ARDS patients with a high risk of death.
The most appropriate ventilator setting for patients with severe ARDS requiring V-V ECMO remains unclear [7]. Because oxygenation and CO2 removal are mainly accomplished by ECMO rather than by mechanical ventilation (MV) and the PEEP setting can be adjusted without limiting oxygenation, the optimal positive end-expiratory pressure (PEEP) setting for patients requiring V-V ECMO support is more controversial than it is for ARDS patients not requiring V-V ECMO [8]. While a recent international guideline recommends a PEEP setting of ≥ 10 cmH2O during ECMO [9], the Consensus Conference 2014 recommends that “mechanical ventilation be adjusted to minimize the plateau pressure, while administering a minimum positive expiratory pressure” [10]. We hypothesized that the optimal PEEP setting differs according to the subgroups of severe ARDS patients that require V-V ECMO and that effective sub-phenotyping could be helpful in identifying the optimal PEEP for each subgroup.
Previously, we developed a database of patients with severe ARDS receiving V-V ECMO, namely, the Japan Chest CT for ARDS Requiring V-V ECMO Registry (J-CARVE registry), including data from 24 institutions across Japan [11]. The J-CARVE includes chest computed tomography (CT) imaging data, as well as data on comorbidities and laboratory data at the start of ECMO support. Undoubtedly, chest CT findings are helpful for understanding the pathophysiology of ARDS [12, 13], and we hypothesized that patients with severe ARDS requiring V-V ECMO could be more effectively classified based on a radiological findings. Hence, the aim of this study was to identify sub-phenotypes of severe ARDS requiring V-V ECMO based on the radiological and clinical parameters at the start of ECMO support.
Methods
Study design
This study was conducted using the J-CARVE registry, a retrospective database of patients with severe ARDS on V-V ECMO, that includes the chest CT images of the patients at the start of ECMO. It was registered in the University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR) before the start of data collection (UMIN000048709). The details of the registry are described in our previous report [11]. Briefly, the registry includes data on the basic demographics and comorbidities of the patients, laboratory data, MV settings and the measured values, treatment, outcomes, and anonymized chest CT data at the time of initiation of ECMO. This study was conducted with the approval of the Institutional Review Board of Hiroshima University Hospital (E-2768), which waived the need to obtain informed consent from the patients, to ensure participant anonymity as stipulated in the Japanese government guidelines.
Participants
Adults (aged ≥ 18 years) with severe ARDS who were initiated on V-V ECMO support between January 2012 and December 2022 at any of 24 intensive care units (ICUs) were included in this study. Severe ARDS was diagnosed on the basis of the Berlin criteria (P/F ratio ≤ 100) [14]. Patients were excluded, if they had not undergone chest CT examination at the start of V-V ECMO support (within a time window of 3 days from the start of the V-V ECMO support). Patients with extra-pulmonary ARDS were also excluded, because of significant differences in the characteristics, including the prognosis, between patients with extra-pulmonary and intra-pulmonary causes of ARDS [15, 16]. Also, we considered that more definitive therapies might be available for patients with extra-pulmonary ARDS than supportive ARDS management, which could have a significant influence on the prognosis. In addition, patients whose chest CT images were interpreted by radiologists as not showing any evidence of ARDS were also excluded from the study.
Interpretation of the chest CT images
All patients were deeply analgosedated, and, whenever necessary, neuromuscular blockade was used during the CT examination. The method of interpretation of the chest CT images is described in detail in our registry report [11]. The chest CT findings are summarized in sTable 1. Briefly, the findings were interpreted by two blinded reviewers who were randomly selected from among five Japanese board-certified radiologists. Any disagreements were resolved by a third reviewer in a blinded manner. Prior to the interpretation of any CT findings, the diagnosis of ARDS was confirmed radiologically, and patients whose CT images were interpreted by both radiologists as not showing any evidence of ARDS were excluded from further assessments.
Outcomes
The primary outcome was the 90-day in-hospital mortality (in-hospital mortality up to 90 days after the initiation of V-V ECMO support). As a secondary outcome, the rate of successful liberation from V-V ECMO, which was defined as liberation without the need for re-cannulation within 48 h, was also evaluated.
Statistical analyses
Data are shown as the mean ± standard deviation for continuous variables with parametric distributions, the median (25th-75th percentile) for continuous variables with non-parametric distributions and the counts and frequencies for categorical variables. An unpaired two-tailed Student t test or Mann–Whitney U test was used to compare two independent groups, as appropriate, for continuous variables. The chi-square test was used to compare the categorical variables. The method for imputation of missing values is described in eMethods in the online data supplement.
Predictive accuracies were evaluated by determining the AUC: The predictive accuracy for an AUC value of > 0.9 was regarded as excellent and that for a value of > 0.7 was regarded as acceptable [17]. LCA was conducted using radiological, laboratory and respiratory mechanics variables, as well as data on the basic comorbidities (age, sex, and body mass index [BMI]) that were available in our registry (sTable 1). The method for LCA is described in eMethods in the online data supplement.
The methods for the survival analyses were also described in eMethods in the online data supplement. Briefly, we plotted Kaplan–Meir survival curves with the log-rank test, and performed adjusted Cox proportional hazards regression analyses to compare the 90-day in-hospital mortality among the three sub-phenotypes of ARDS. As for the rate of successful liberation from V-V ECMO, Fine and Gray competing-risk regression was used. Adjusted Cox proportional hazards regression analyses were also performed to evaluate the effect of PEEP ≥ 10 cmH2O during the first 3 days of ECMO support on the 90-day in-hospital mortality.
All reported P-values are two-sided, and p < 0.05 was considered as being indicative of statistical significance. The Fine and Gray proportional hazards regression analysis and non-parametric missing value imputation analyses were performed using R version 4.3.2 (R Foundation for Statistical Computing, Vienna, Austria), and the rest of the analyses, including LCA were performed using JMP Pro 17.
Results
The flow diagram of patient recruitment for this study is shown in sFigure 1. Among the 697 patients admitted in any of the 24 participating ICUs in Japan, 153 were excluded, and the LCA for this study was conducted using the data of the remaining 544 patients. The time difference between chest CT examinations and the start of V-V ECMO support among the analyzed patients is shown in sFigure 2. In approximately 80% of all the analyzed patients, the CT examination had been performed within 24 h, and in 90%, it had been performed within 48 h.
First, the most appropriate number of classes was determined based on the value of Bayesian information criterion (BIC). Three classes showed the lowest values of BIC and entropy, and the decline in the values of Akaike information criterion (AIC) exhibited a more gradual transition around the number three (sFigure 3). We decided to apply a three-class latent model for this study, and named them the Dry, Wet and Fibrotic types based on the typical imaging findings of each. The chosen three-class latent model assigned 189 (34.7%) patients to the Dry type, 168 (30.9%) patients to the Wet type, and 187 (34.4%) patients to the Fibrotic type. We confirmed that the number of classes remained at three even when we used different imputation methods, and the AUC of the classification using different imputation methods for our main classification was approximately 0.95, which implies that the effect of the imputation method on the latent class modelling was small (sFigure 4).
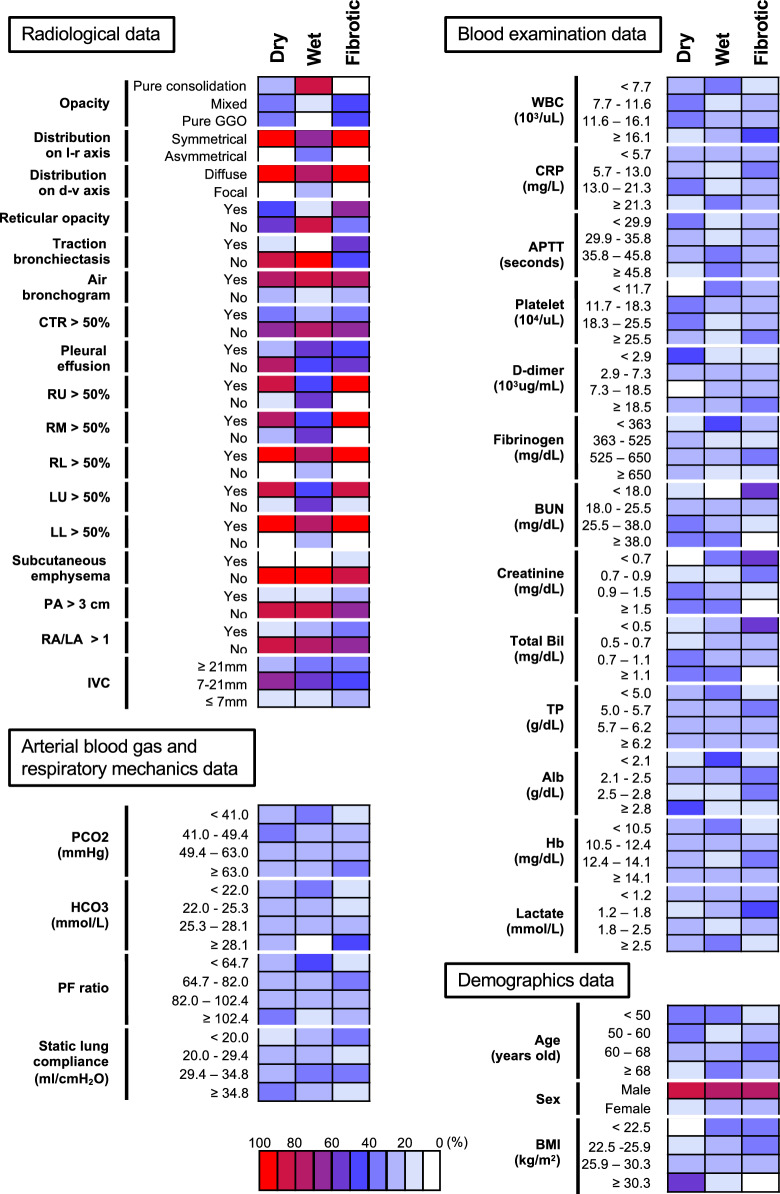
The categorical distribution of all variables used for the LCA and the baseline characteristics in each class are shown in Fig. 1 and Table 1, respectively. The median time from the start of oxygen supplementation to initiation of V-V ECMO support was 3.0 (1.0–6.0) days in patients with the Dry type, 2.0 (1.0–4.0) days in patients with the Wet type, and 7.0 (3.0–12.0) days in patients with the Fibrotic type (p < 0.001). The median values of the SOFA score, PF ratio, and C-reactive protein (CRP) at the start of ECMO support were 8.0 (5.0–11.0), 87.0 (65.2–109.2), and 11.8 (4.7–17.1) mg/L, respectively, in patients with the Dry type, 12.0 (9.0–14.0), 71.1 (58.0–89.8), and 17.0 (6.3–29.5) mg/L, respectively, in patients with the Wet type, and 10.0 (8.0–12.0), 86.0 (73.7–107.0), and 11.8 (6.2–20.1) mg/L, respectively, in patients with the Fibrotic type (p < 0.001 for all comparisons). The baseline characteristics of pairs of groups were compared in a sTable 2, 3, and 4. The differences in the ventilatory parameters among the patients with the three sub-phenotypes are shown in sTable 5.
Fig. 1.
Differences among the three sub-phenotypes in the categorized variables used for latent class analysis. The proportions of each categorized variable used for the latent class analysis among three sub-phenotypes are shown. Abbreviations: l-r, left–right; d-v, dorso-ventral; CTR, cardiothoracic ratio; RU, right upper lobe; RM, right middle lobe; RL, right lower lobe; LU, left upper lobe; LL, left lower lobe; PA, pulmonary artery size; RA / LA, right atrium / left atrium ratio; IVC, inferior vena cava; WBC, white blood cell; CRP, C-reactive protein; APTT, activated partial thromboplastin time; BUN, blood urea nitrogen; Bil, bilirubin; TP, total protein; Alb, albumin; Hb, hemoglobin; BMI, body mass index
Table 1.
Baseline characteristics of all the subjects prior to ECMO initiation
| All patients (n = 544) | Dry (n = 189) | Wet (n = 168) | Fibrotic (n = 187) | |
|---|---|---|---|---|
| Age, y | 60.0 (50.0–68.0) | 56.0 (48.5–64.0) | 60.5 (46.0–71.0) | 62.0 (56.0–68.0) |
| Sex, male, n (%) | 412 (75.7) | 156 (82.5) | 123 (73.2) | 133 (71.1) |
| BMI, kg/m2a | 25.9 (22.5–30.3) | 30.4 (25.9–34.2) | 24.0 (20.8–28.4) | 24.5 (21.3–27.6) |
| Past medical history | ||||
| Chronic kidney disease, n (%) | 43 (7.9) | 6 (3.2) | 25 (14.9) | 12 (6.4) |
| COLD, n (%) | 79 (14.5) | 27 (14.3) | 22 (13.1) | 30 (16.0) |
| Interstitial lung disease, n (%) | 23 (4.2) | 5 (2.7) | 5 (3.0) | 13 (7.0) |
| Interval from the start of MV to ECMO initiation, days | 2.0 (1.0–4.0) | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) | 2.0 (1.0–7.0) |
| Interval from the start of MV to ECMO initiation > 7 days, n (%) | 88 (16.2) | 18 (9.5) | 19 (11.3) | 51 (27.3) |
| Interval from the start of oxygen supplementation to ECMO initiation, daysb | 3.0 (2.0–8.0) | 3.0 (1.0–6.0) | 2.0 (1.0–4.0) | 7.0 (3.0–12.0) |
| Primary etiology of ARDS, n (%) | ||||
| Bacterial pneumonia | 99 (18.2) | 11 (5.8) | 61 (36.3) | 27 (14.4) |
| Viral pneumonia | 312 (57.4) | 155 (82.0) | 53 (31.6) | 104 (55.6) |
| Other pneumonia | 133 (24.4) | 23 (12.2) | 54 (32.1) | 56 (30.0) |
| SOFA score at ECMO initiation | 10.0 (7.0–12.0) | 8.0 (5.0–11.0) | 12.0 (9.0–14.0) | 10.0 (8.0–12.0) |
| P/F ratio just before ECMO initiation | 89.3 ± 36.7 | 94.5 ± 40.5 | 77.7 ± 30.2 | 94.5 ± 35.7 |
| CRP, mg/L | 14.8 ± 11.5 | 12.2 ± 8.8 | 18.7 ± 14.4 | 14.0 ± 10.0 |
| Murray lung injury scorec | 3.25 (2.75–3.50) | 3.25 (3.00–3.50) | 3.00 (2.75–3.50) | 3.25 (2.75–3.50) |
| Use of neuromuscular blockers prior to ECMO initiation, n (%) | 223 (41.0) | 79 (41.8) | 67 (39.9) | 77 (41.2) |
| Prone positioning prior to ECMO initiation, n (%) | 92 (16.9) | 41 (21.7) | 15 (8.9) | 36 (19.3) |
Data are presented as the medians and interquartile ranges (25th-75th percentile), mean ± standard deviation or as absolute frequencies with percentages
BMI body mass index, COLD chronic obstructive lung disease, MV mechanical ventilation, ECMO extracorporeal membrane oxygenation, ARDS acute respiratory distress syndrome, SOFA score sequential organ failure assessment score, PF ratio PaO2/FIO2 ratio, CRP C-reactive protein
aMissing value = 3. bMissing value = 4. Missing value = 5
Representative CT images from patients with the three classes are shown in sFigure 5. In general, the CT images of patients with the Dry type tended to show diffusely distributed pure ground-glass opacities or a mixed pattern of pulmonary opacities without fibroproliferative changes (less characteristics of lung edema), those of patients with the Wet type showed a pure consolidation pattern of pulmonary opacities with pleural effusion, but no fibroproliferative changes (typical characteristics of lung edema, “wet lung”). On the other hand, the Fibrotic type was associated with fibroproliferative changes on the chest CT (e.g., reticular opacities, traction bronchiectasis, etc.), which were rarely observed in the other two types. While the intensity of the opacities was similar between the Fibrotic and Dry types, signs of right ventricular overload (e.g., pulmonary artery diameter > 3 cm and right atrium/left atrium ratio of > 1) tended to be seen in the Fibrotic type, but not the Dry type.
The top 15 variables which were important for the classification into the three phenotypes are shown in sFigure 6. Multi-dimensional scaling using these 15 variables showed a high incidence of reticular opacities and traction bronchiectasis and a poor nutritional status (lower values of creatinine and albumin in the serum) in patients with the Fibrotic type. The Dry type tended to show no reticular opacities or traction bronchiectasis, normal serum levels of total protein, albumin, hemoglobin, and d-dimers, but an increased BMI. The Wet type also tended to show no reticular opacities or traction bronchiectasis, consolidations, ≤ 50% opacification of the left upper, right upper, and right middle lobe, focal distribution on the dorso-ventral axis, increased serum creatine levels, and decreased serum bicarbonate levels.
In order to confirm that our classification was not completely dependent on the disease severity, interval from intubation to initiation of ECMO support, or the PEEP setting at the time of the CT, we evaluated the predictive accuracies of these variables for our classification. The AUC values were around 0.50 to 0.70, most of which fell outside acceptable predictive accuracy levels (sFigure 7). Also, in order to investigate whether our classification was mainly dependent on the radiological or laboratory/respiratory mechanics data, we performed LCA separately using only radiological data and only laboratory/respiratory mechanics data. The AUCs of the classification obtained using only radiological data for predicting our full-model classification were 0.66 (for the Dry type), 0.88 (for the Wet type), and 0.78 (for the Fibrotic type). The AUCs of the classification based on the laboratory/respiratory mechanics data were 0.81 (for the Dry type), 0.71 (for the Wet type), and 0.77 (for the Fibrotic type) (sFigure 8).
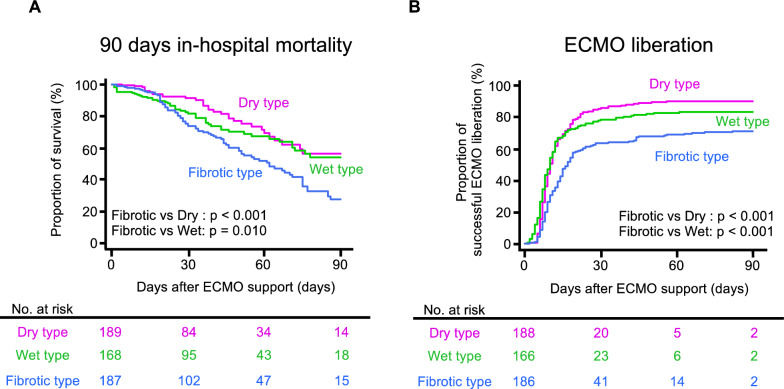
The outcomes of the three patient groups are summarized in sTable 6. The log rank test on the Kaplan–Meier curves showed that the mortality was higher in the patients with the Fibrotic type than in the patients with the other two sub-phenotypes (vs. the Dry type, p < 0.001; vs. the Wet type, p = 0.010); also, the probability of successful liberation from ECMO was lower in the patients with the Fibrotic type (vs. both the other types, p < 0.001) (Fig. 2). Multivariate Cox regression analyses showed that patients classified into the Fibrotic type showed a higher 90-day in-hospital mortality risk than those classified into the Dry type (adjusted hazard ratio [95% confidence interval] 1.75 [1.10–2.79], p = 0.019) or Wet type (1.50 [1.02–2.23], p = 0.042) (sTable 7), as well as a lower probability of successful liberation from ECMO (0.63 [0.49–0.83], p < 0.001, and 0.67 [0.50–0.89], p = 0.005) (sTable 8).
Fig. 2.
Differences in the outcomes among the three sub-phenotypes. The 90-day in-hospital mortality (A) and veno-venous extracorporeal membrane oxygenation (V-V ECMO) liberation ratio (B) were compared among the three sub-phenotypes using the log-rank test. Four patients were excluded because they had missing values about the time of ECMO liberation
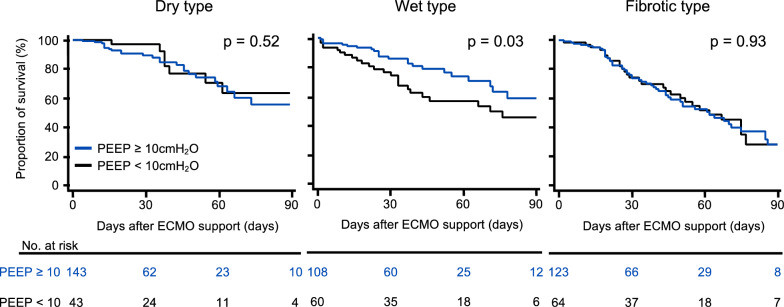
Then, we evaluated the effect of PEEP settings of ≥ 10 cmH2O during the first 3 days of ECMO in the three sub-phenotypes. Differences in the ventilatory parameters among the sub-phenotypes for ≥ 10 cmH2O and < 10 cmH2O of PEEP setting groups were shown in sTable 9 and 10. Kaplan–Meier curves showed that the value of PEEP setting of ≥ 10 cmH2O reduced the 90-day in-hospital mortality only in patients with the Wet type (Fig. 3). Multivariate Cox regression analyses showed that the PEEP value of ≥ 10 cmH2O was associated with a decreased risk of 90-day in-hospital mortality in patients with the Wet type (0.48 [0.25–0.92], p = 0.026) (sTable 11). We performed multivariate Cox regression analysis to determine the interaction effect between the Wet type and the PEEP setting of ≥ 10 cmH2O in relation to the 90-day in-hospital mortality, and the results revealed a statistically significant interaction effect (pinteraction = 0.036) (sTable 12).
Fig. 3.
Differences in the effect of high positive end-expiratory pressure values after the start of V-V ECMO support among the three sub-phenotypes. The 90-day in-hospital mortality was compared between patients who received PEEP ≥ 10 cmH2O and < 10 cmH2O after the start of veno-venous extracorporeal membrane oxygenation (V-V ECMO) in each sub-phenotype using the log-rank test. Three patients in the Dry type were excluded because of missing PEEP values. Abbreviations: PEEP, positive end-expiratory pressure
Discussion
In a multicenter study performed with the participation of 24 institutions across Japan, where CT examinations are conducted frequently than in other countries [18], we conducted LCA using a combination of the radiological findings and laboratory data, and successfully identified three sub-phenotypes among patients with severe ARDS requiring V-V ECMO. We also found that patients classified into the Fibrotic type showed higher mortality as compared with those classified into the Dry or Wet type, while the patients classified into the Wet type showed a significant association between the PEEP setting of ≥ 10 cmH2O in the early phase and better outcome. This is the first study to report sub-phenotyping the patients with severe ARDS requiring V-V ECMO.
In this study, we identified 3 classes, although historically, only two sub-phenotypes have been described in previous literature [3]. This discrepancy may be explained by the difference between the timing of the classification. Our study aimed to sub-phenotype at the initiation of V-V ECMO, while previous studies did at the early phase after the onset of ARDS. In fact, our data included some patients who received V-V ECMO support in the late phase, which may be the reason why we identified the different number of phenotypes from that identified in previous studies. Also, our study included radiological data for LCA, while previous studies mainly used for sub-phenotyping of ARDS patients have mainly used laboratory data and respiratory mechanics data (except a small retrospective study [19]). Undoubtedly, radiological data have the potential to add significant findings, such as fibroproliferative changes, which could explain why we identified an additional phenotype, namely, the Fibrotic type. Interestingly, classification using only radiological findings and that using only laboratory and respiratory mechanics data did not perfectly match the full-model classification, which indicates that each of the radiological findings and laboratory data/respiratory mechanics significantly contributed to the sub-phenotyping.
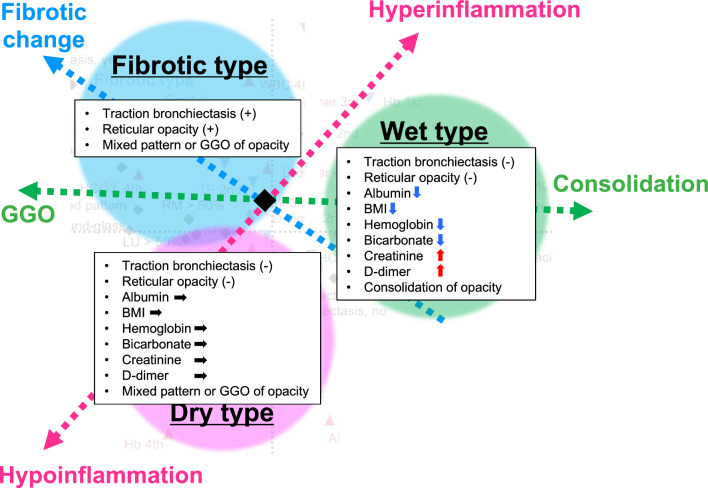
The described multi-dimensional scaling using the top 15 important variables suggests that the differences among three types can be mainly explained by three domains; inflammatory response, intensity of opacities, and presence/absence of fibrotic changes (Fig. 4). Six of the seven selected laboratory and comorbidity variables have been reported as important factors for classification into the hyper- and hypo-inflammatory sub-phenotypes in previous studies (lower values of serum albumin, BMI, hemoglobin, and bicarbonate, and higher values of serum creatinine and d-dimer) [3, 20, 21]. Given together that increased ARDS severity and higher CRP levels in the patients in the Wet type [22–24], the Wet type may share the characteristics of the hyper-inflammatory type. On the other hands, the Dry type may share the characteristics of the hypo-inflammatory type.
Fig. 4.
Three-axis schema to explain the differences among the three sub-phenotypes. Based on the multi-dimensional scaling according to the top 15 important variables (see sFig. 6B), we assumed that the differences among the three types could be explained by their positions along three axes: inflammatory response, intensity of opacities, and presence/absence of fibrotic changes. Namely, the Dry type may share the characteristics of the hypo-inflammatory type and tends to show a GGO pattern on the chest CT. The Wet type may share the characteristics of the hyper-inflammatory type and tends to show a pure consolidation pattern on the chest CT. The Fibrotic type tends to show a GGO pattern with fibrotic changes on the chest CT
In the present study, there was both a longer interval from the start of MV to ECMO and a higher proportion with an interval from the start of MV to ECMO initiation > 7 days in the Fibrotic type. A longer duration of MV support with a high plateau pressure can result in ventilator-induced lung injury (VILI), which can exacerbate the lung fibrosis [25]. Although the time from ARDS onset may not be completely matched with the progression of fibroproliferative change [26] and our classification did not completely depend on this interval (as shown in sFigure 7), the differences in the duration of MV before ECMO initiation may have contributed to the results of our LCA. It would be of great interest to investigate the relationship between the pathophysiological phase of ARDS and our clinical sub-phenotypes.
The PEEP setting of ≥ 10 cmH2O during the early phase of ECMO was associated with improved in-hospital survival in the patients classified into the Wet type, but not in those classified into the Dry or Fibrotic type. It may be explained by the characteristics of the hyper-inflammatory type in the Wet type, which has been reported to show a higher responsiveness to higher PEEP values in patients with severe ARDS not needing ECMO support [3]. Also, consolidation opacity in the Wet type may indicate nonaerated or poorly aerated alveoli which can be opened by high PEEP [27], although further studies would be needed.
There were several limitations of this study. First, the values of PEEP and presence of spontaneous breathing at the time of the chest CT examination were varied, which can influence the interpretation of the opacities on chest CT [28]. However, we believe that the contribution of the PEEP value at the time of the chest CT examination was minimal for our overall classification, because the values of PEEP could not predict our classification with acceptable accuracy (all AUC values were < 0.60). Second, the data used to conduct our analysis were limited to the variables that were available in our J-CARVE registry. For example, no data on fluid balance, which may have substantially influenced the patients’ outcomes, or respiratory mechanics variables after ECMO support, except dynamic driving pressure and PEEP, were included in our registry. Third, we categorized all continuous laboratory variables in this study, which could have influenced our results. Fourth, there were differences between the major etiologies of ARDS in our study and previous reports of large cohort studies [29], which could possibly be explained by the differences in the periods when the studies were conducted, that is, according to whether the studies included COVID-19 patients or not. The etiological differences among sub-phenotypes may have influenced the results of LCA. We need to confirm that the sub-phenotypes in our study can be also identified in different datasets in the future. Fifth, our study was conducted in a large sample of patients in Japan, and we observed some differences between our study and reports from other registries [29–31], such as the lower percentage of patients who received neuromuscular blockers and prone positioning prior to the initiation of ECMO. Sixth, the mechanism underlying the beneficial effects of PEEP ≥ 10 cmH2O on the mortality has not been evaluated in this study. The differences between the ventilator setting during ECMO across participating hospitals in our study may have influenced the results of the survival analysis at PEEP values ≥ 10 cmH2O. Finally, although all participating hospitals followed the guidelines for the management before and during ECMO support, they depended on the clinical preference at each participating hospital. In particular, the titration method for PEEP values is unclear and may be different across participating hospitals, because the most appropriate method has not been established. We need to further investigate the effect of these differences in the future.
Conclusions
The three sub-phenotypes showed different mortality rates and different relationships between higher PEEP settings in the early phase of V-V ECMO and patient outcomes. Our data suggest that we may need to change our management approach to patients with severe ARDS during V-V ECMO according to their clinical sub-phenotype.
Supplementary Information
Acknowledgements
We acknowledge and honor all of our team members who consistently put themselves in harm’s way during the COVID-19 pandemic. We dedicate this manuscript to them, as their vital contribution to knowledge about COVID-19 and sacrifices on the behalf of patients made it possible. We also want to thank Keigo Narita, Hidenori Mitani, Shota Kondo, and Shogo Maeda for interpretating chest CT scans.
J-CARVE registry group authors: Jun Hamaguchi9; Kazuki Matsumura9; Kenji Fujizuka10; Yoshihiro Hagiwara11; Ryuichi Nakayama12; Naofumi Bunya12; Junichi Maruyama13; Takayuki Ogura11; Mitsunobu Nakamura10; Keiki Shimizu9; Mamoru Masuda10. 9 Department of Critical Care and Emergency Medicine, Tokyo Metropolitan Tama Medical Center, Tokyo, Japan; 10 Advanced Medical Emergency Department and Critical Care Center, Japan Red Cross Maebashi Hospital, Maebashi, Japan; 11 Department of Emergency Medicine and Critical Care Medicine, SAISEIKAI Utsunomiya Hospital, Utsunomiya, Japan; 12 Department of Emergency Medicine, Sapporo Medical University, Sapporo, Japan; 13 Department of Emergency medicine and Critical care, Fukuoka University Hospital, Fukuoka, Japan.
Author contributions
MN had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: Nishikimi, Shime, Ohshimo Acquisition, analysis, or interpretation of data: Nishikimi, Anzai, Fukumoto, Kyo, Awai, Takahashi, Ohshimo Drafting of the manuscript: Nishikimi, Anzai (statistical part), Ohshimo Critical revision of the manuscript for important intellectual content: Ohshimo, Bellani, Liu Takahashi, Shime, Statistical analysis: Nishikimi, Anzai, Takahashi.
Funding
This work was supported by JSPS KAKENHI (Grant Numbers JP 22K09120) and the TSUCHIYA MEMORIAL MEDICAL FOUNDATION, a Grant-in-aid for multicenter clinical research from Japanese Association for Acute Medicine, and Japan Agency for Medical Research and Development (AMED, Grant Numbers JP23fk0108654).
Availability of the data and materials
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to partcipate
This study was approved by the Institutional Review Boards of Hiroshima University Hospital.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mitsuaki Nishikimi, Email: m0528332626@yahoo.co.jp.
J-CARVE registry group:
Jun Hamaguchi, Kazuki Matsumura, Kenji Fujizuka, Yoshihiro Hagiwara, Ryuichi Nakayama, Naofumi Bunya, Junichi Maruyama, Takayuki Ogura, Mitsunobu Nakamura, Keiki Shimizu, and Mamoru Masuda
References
- 1.Friedrichson B, Mutlak H, Zacharowski K, Piekarski F. Insight into ECMO, mortality and ARDS: a nationwide analysis of 45,647 ECMO runs. Crit Care. 2021;25:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbaro RP, Odetola FO, Kidwell KM, Paden ML, Bartlett RH, Davis MM, Annich GM. Association of hospital-level volume of extracorporeal membrane oxygenation cases and mortality. Analysis of the extracorporeal life support organization registry. Am J Respir Crit Care Med. 2015;191:894–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calfee CS, Delucchi K, Parsons PE, Thompson BT, Ware LB, Matthay MA, Network NA. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med. 2014;2:611–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson JG, Calfee CS. ARDS Subphenotypes: understanding a heterogeneous syndrome. Crit Care. 2020;24:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sinha P, Furfaro D, Cummings MJ, Abrams D, Delucchi K, Maddali MV, He J, Thompson A, Murn M, Fountain J, Rosen A, Robbins-Juarez SY, Adan MA, Satish T, Madhavan M, Gupta A, Lyashchenko AK, Agerstrand C, Yip NH, Burkart KM, Beitler JR, Baldwin MR, Calfee CS, Brodie D, O’Donnell MR. Latent class analysis reveals COVID-19-related acute respiratory distress syndrome subgroups with differential responses to corticosteroids. Am J Respir Crit Care Med. 2021;204:1274–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Famous KR, Delucchi K, Ware LB, Kangelaris KN, Liu KD, Thompson BT, Calfee CS, Network A. Acute respiratory distress syndrome subphenotypes respond differently to randomized fluid management strategy. Am J Respir Crit Care Med. 2017;195:331–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The Extracorporeal Life Support Organization, Extracorporeal Life Support Guidelines, Patient Care Practice Guidelines. In: Editor (ed)^(eds) Book The Extracorporeal Life Support Organization, Extracorporeal Life Support Guidelines, Patient Care Practice Guidelines. City, pp.
- 8.Qadir N, Sahetya S, Munshi L, Summers C, Abrams D, Beitler J, Bellani G, Brower RG, Burry L, Chen JT, Hodgson C, Hough CL, Lamontagne F, Law A, Papazian L, Pham T, Rubin E, Siuba M, Telias I, Patolia S, Chaudhuri D, Walkey A, Rochwerg B, Fan E. An update on management of adult patients with acute respiratory distress syndrome: an official american thoracic society clinical practice guideline. Am J Respir Crit Care Med. 2024;209:24–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tonna JE, Abrams D, Brodie D, Greenwood JC, Rubio Mateo-Sidron JA, Usman A, Fan E. Management of adult patients supported with venovenous extracorporeal membrane oxygenation (VV ECMO): guideline from the extracorporeal life support organization (ELSO). ASAIO J. 2021;67:601–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richard C, Argaud L, Blet A, Boulain T, Contentin L, Dechartres A, Dejode JM, Donetti L, Fartoukh M, Fletcher D, Kuteifan K, Lasocki S, Liet JM, Lukaszewicz AC, Mal H, Maury E, Osman D, Outin H, Richard JC, Schneider F, Tamion F. Extracorporeal life support for patients with acute respiratory distress syndrome: report of a Consensus Conference. Ann Intensive Care. 2014;4:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishikimi M, Ohshimo S, Fukumoto W, Hamaguchi J, Matsumura K, Fujizuka K, Hagiwara Y, Nakayama R, Bunya N, Maruyama J, Abe T, Anzai T, Ogata Y, Naito H, Amemiya Y, Ikeda T, Yagi M, Furukawa Y, Taniguchi H, Yagi T, Katsuta K, Konno D, Suzuki G, Kawasaki Y, Hattori N, Nakamura T, Kondo N, Kikuchi H, Kai S, Ichiyama S, Awai K, Takahashi K, Shime N, group JCr. Chest CT findings in severe acute respiratory distress syndrome requiring V-V ECMO: J-CARVE registry. J Intensive Care. 2024;12:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheard S, Rao P, Devaraj A. Imaging of acute respiratory distress syndrome. Respir Care. 2012;57:607–12. [DOI] [PubMed] [Google Scholar]
- 13.Zompatori M, Ciccarese F, Fasano L. Overview of current lung imaging in acute respiratory distress syndrome. Eur Respir Rev. 2014;23:519–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Force ADT, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–33. [DOI] [PubMed] [Google Scholar]
- 15.Pelosi P, D’Onofrio D, Chiumello D, Paolo S, Chiara G, Capelozzi VL, Barbas CS, Chiaranda M, Gattinoni L. Pulmonary and extrapulmonary acute respiratory distress syndrome are different. Eur Respir J Suppl. 2003;42:48s–56s. [DOI] [PubMed] [Google Scholar]
- 16.Luo L, Shaver CM, Zhao Z, Koyama T, Calfee CS, Bastarache JA, Ware LB. Clinical predictors of hospital mortality differ between direct and indirect ARDS. Chest. 2017;151:755–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mandrekar JN. Receiver operating characteristic curve in diagnostic test assessment. J Thorac Oncol. 2010;5:1315–6. [DOI] [PubMed] [Google Scholar]
- 18.OECD database. In: Editor (ed)^(eds) Book OECD database. City, pp.
- 19.Filippini DFL, Di Gennaro E, van Amstel RBE, Beenen LFM, Grasso S, Pisani L, Bos LDJ, Smit MR. Latent class analysis of imaging and clinical respiratory parameters from patients with COVID-19-related ARDS identifies recruitment subphenotypes. Crit Care. 2022;26:363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Redaelli S, von Wedel D, Fosset M, Suleiman A, Chen G, Alingrin J, Gong MN, Gajic O, Goodspeed V, Talmor D, Schaefer MS, Jung B. Inflammatory subphenotypes in patients at risk of ARDS: evidence from the LIPS-A trial. Intensive Care Med. 2023;49:1499–507. [DOI] [PubMed] [Google Scholar]
- 21.Sinha P, Delucchi KL, Thompson BT, McAuley DF, Matthay MA, Calfee CS, Network NA. Latent class analysis of ARDS subphenotypes: a secondary analysis of the statins for acutely injured lungs from sepsis (SAILS) study. Intensive Care Med. 2018;44:1859–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kitsios GD, Yang L, Manatakis DV, Nouraie M, Evankovich J, Bain W, Dunlap DG, Shah F, Barbash IJ, Rapport SF, Zhang Y, DeSensi RS, Weathington NM, Chen BB, Ray P, Mallampalli RK, Benos PV, Lee JS, Morris A, McVerry BJ. Host-response subphenotypes offer prognostic enrichment in patients with or at risk for acute respiratory distress syndrome. Crit Care Med. 2019;47:1724–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calfee CS, Delucchi KL, Sinha P, Matthay MA, Hackett J, Shankar-Hari M, McDowell C, Laffey JG, O’Kane CM, McAuley DF, Trials ICC. Acute respiratory distress syndrome subphenotypes and differential response to simvastatin: secondary analysis of a randomised controlled trial. Lancet Respir Med. 2018;6:691–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maddali MV, Churpek M, Pham T, Rezoagli E, Zhuo H, Zhao W, He J, Delucchi KL, Wang C, Wickersham N, McNeil JB, Jauregui A, Ke S, Vessel K, Gomez A, Hendrickson CM, Kangelaris KN, Sarma A, Leligdowicz A, Liu KD, Matthay MA, Ware LB, Laffey JG, Bellani G, Calfee CS, Sinha P, Investigators the ETG. Validation and utility of ARDS subphenotypes identified by machine-learning models using clinical data: an observational, multicohort, retrospective analysis. Lancet Respir Med. 2022;10:367–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cabrera-Benitez NE, Laffey JG, Parotto M, Spieth PM, Villar J, Zhang H, Slutsky AS. Mechanical ventilation-associated lung fibrosis in acute respiratory distress syndrome: a significant contributor to poor outcome. Anesthesiology. 2014;121:189–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marshall RP, Bellingan G, Webb S, Puddicombe A, Goldsack N, McAnulty RJ, Laurent GJ. Fibroproliferation occurs early in the acute respiratory distress syndrome and impacts on outcome. Am J Respir Crit Care Med. 2000;162:1783–8. [DOI] [PubMed] [Google Scholar]
- 27.Nieman GF, Gatto LA, Habashi NM. Impact of mechanical ventilation on the pathophysiology of progressive acute lung injury. J Appl Physiol. 2015;119:1245–61. [DOI] [PubMed] [Google Scholar]
- 28.Malbouisson LM, Muller JC, Constantin JM, Lu Q, Puybasset L, Rouby JJ, Group CTSAS. Computed tomography assessment of positive end-expiratory pressure-induced alveolar recruitment in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163:1444–50. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt M, Pham T, Arcadipane A, Agerstrand C, Ohshimo S, Pellegrino V, Vuylsteke A, Guervilly C, McGuinness S, Pierard S, Breeding J, Stewart C, Ching SSW, Camuso JM, Stephens RS, King B, Herr D, Schultz MJ, Neuville M, Zogheib E, Mira JP, Roze H, Pierrot M, Tobin A, Hodgson C, Chevret S, Brodie D, Combes A. Mechanical ventilation management during extracorporeal membrane oxygenation for acute respiratory distress syndrome. an international multicenter prospective cohort. Am J Respir Crit Care Med. 2019;200:1002–12. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt M, Stewart C, Bailey M, Nieszkowska A, Kelly J, Murphy L, Pilcher D, Cooper DJ, Scheinkestel C, Pellegrino V, Forrest P, Combes A, Hodgson C. Mechanical ventilation management during extracorporeal membrane oxygenation for acute respiratory distress syndrome: a retrospective international multicenter study. Crit Care Med. 2015;43:654–64. [DOI] [PubMed] [Google Scholar]
- 31.Hemmila MR, Rowe SA, Boules TN, Miskulin J, McGillicuddy JW, Schuerer DJ, Haft JW, Swaniker F, Arbabi S, Hirschl RB, Bartlett RH. Extracorporeal life support for severe acute respiratory distress syndrome in adults. Ann Surg. 2004;240:595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.