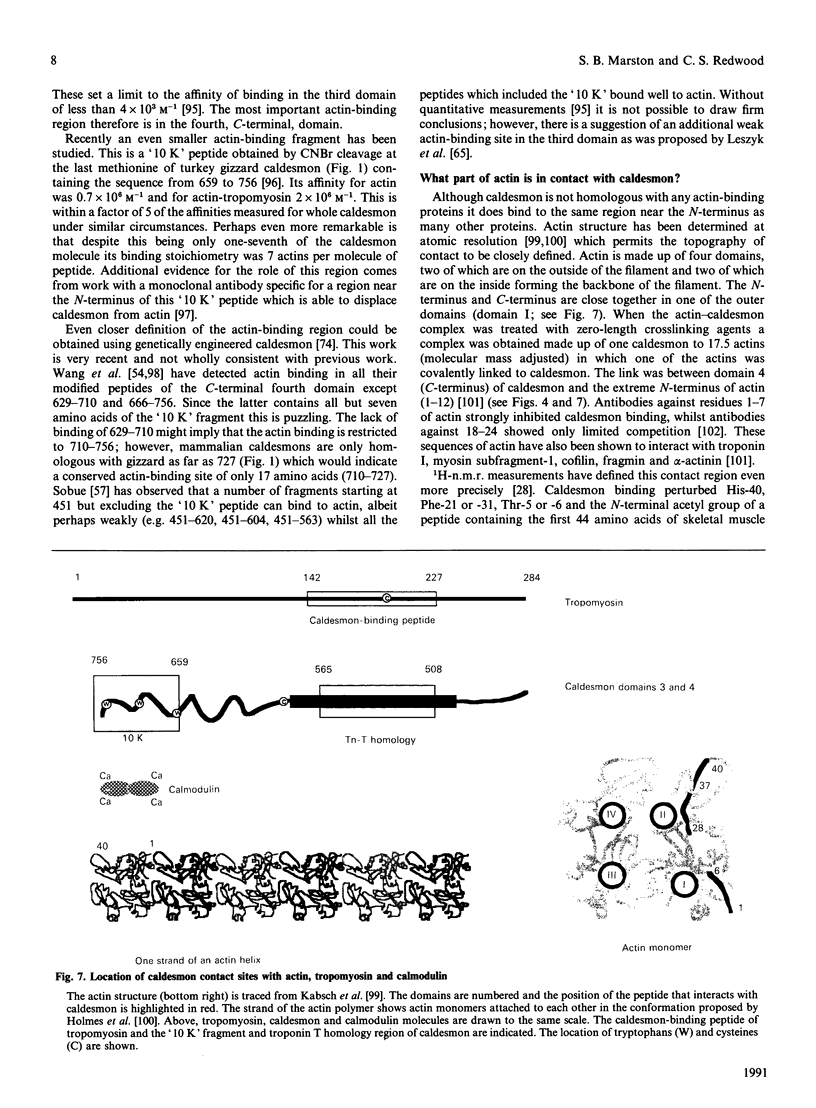
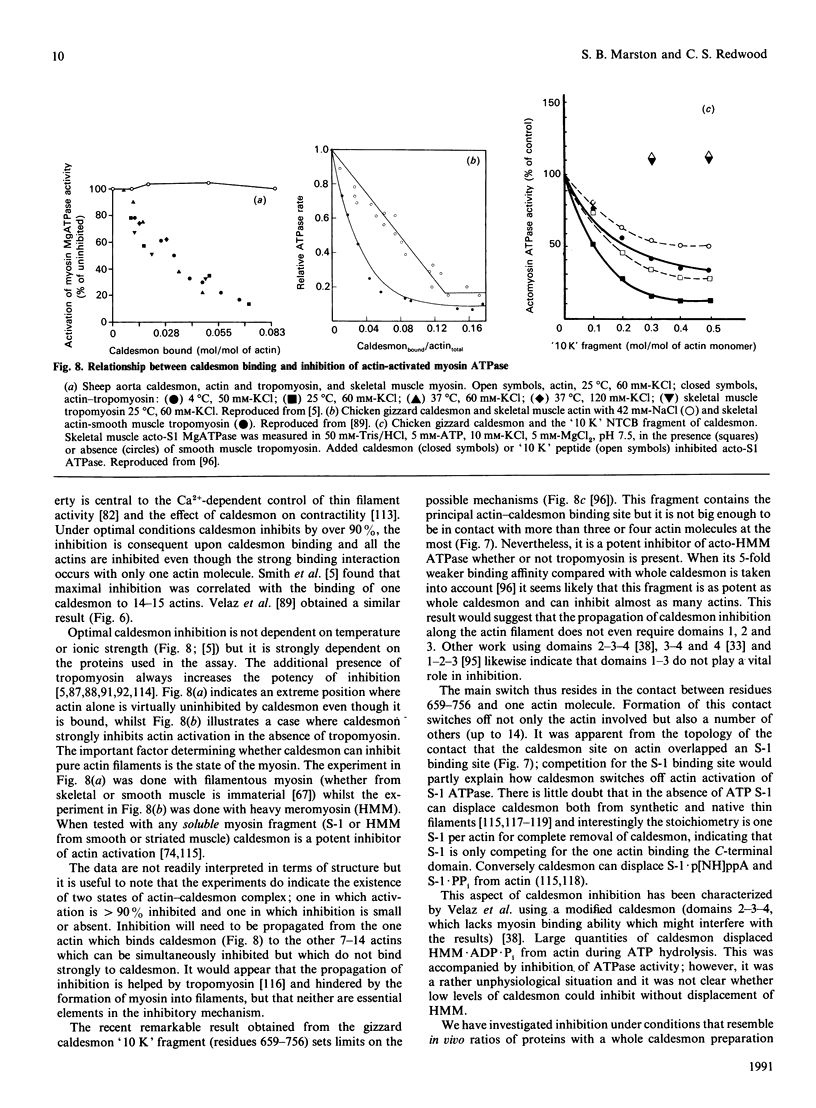
Full text
PDF


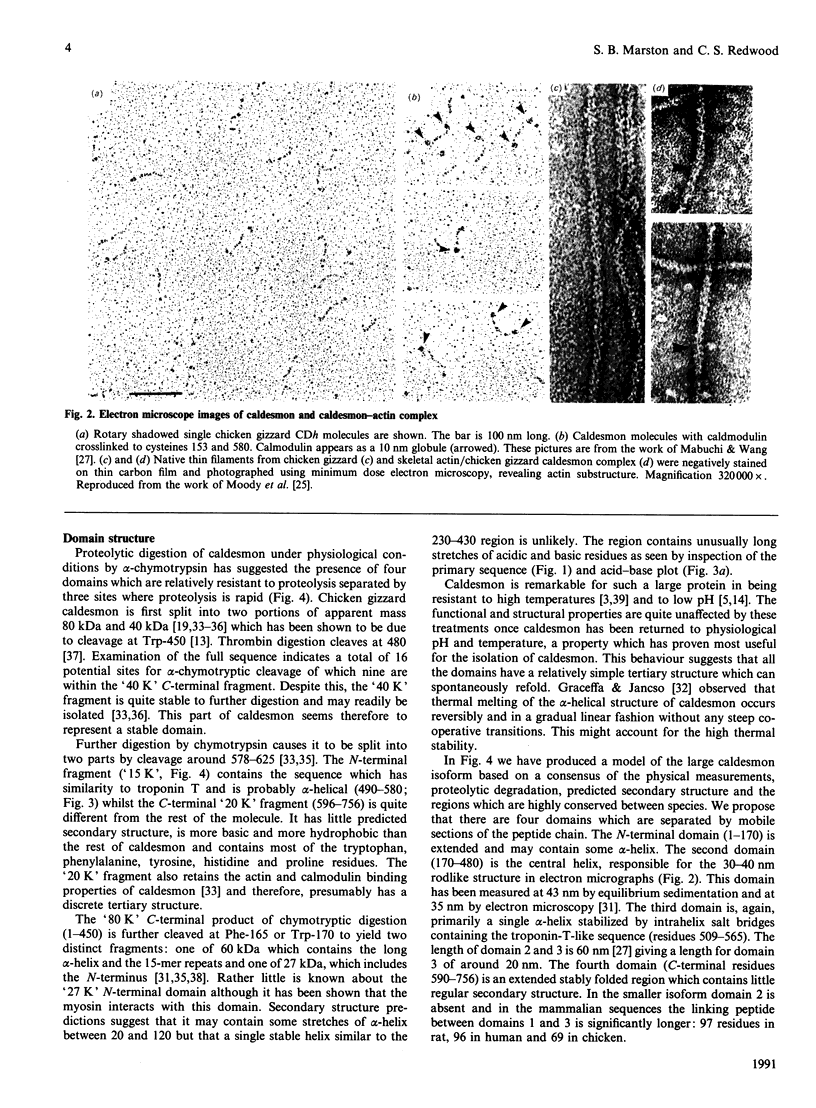







Images in this article
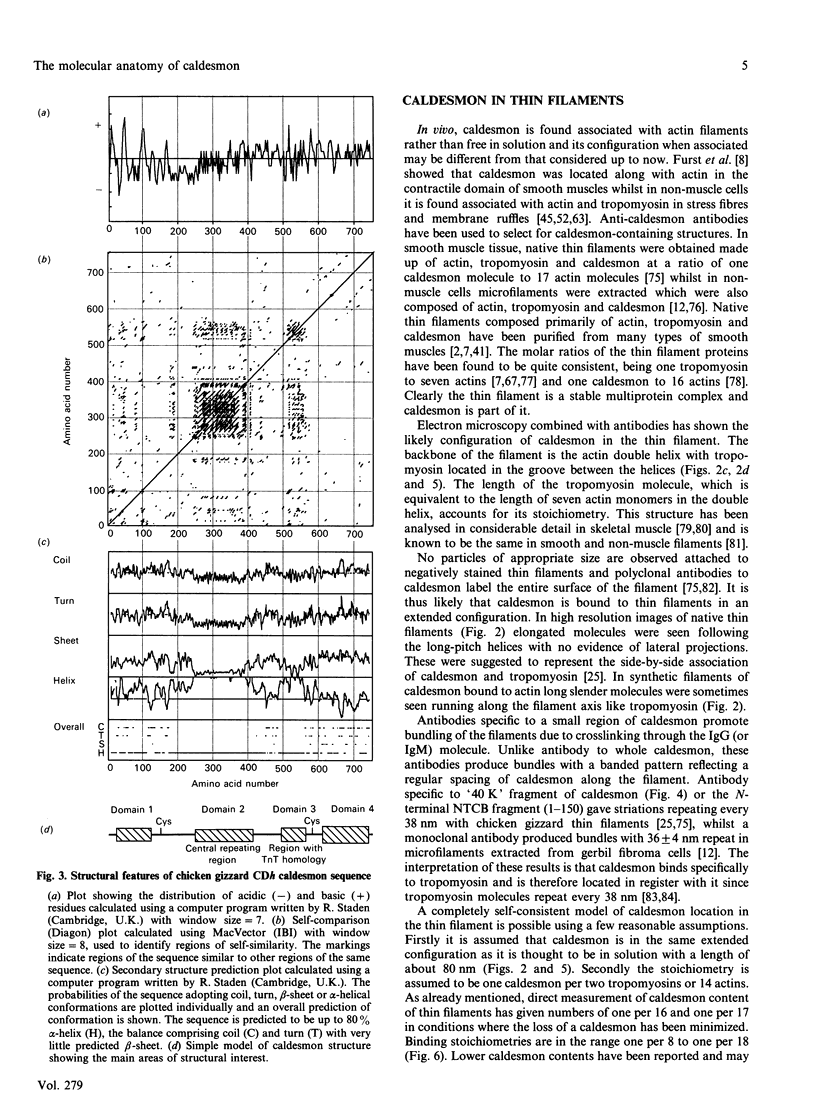
Selected References
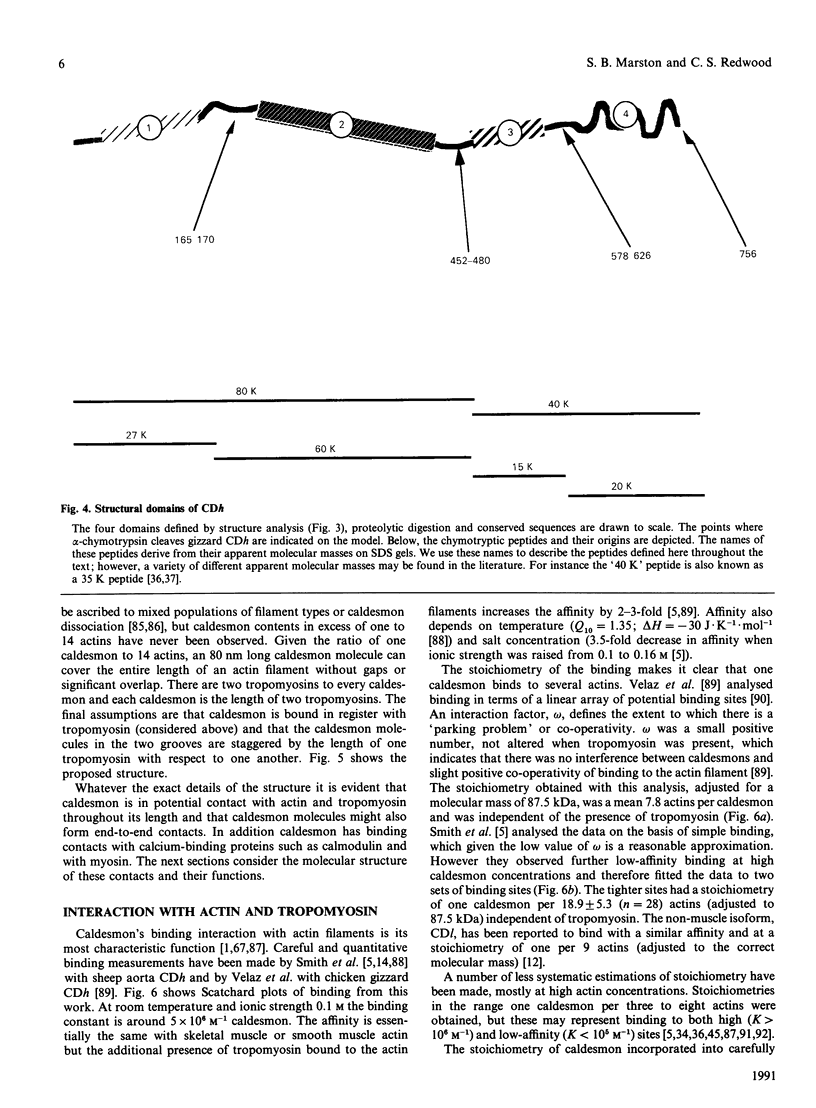
These references are in PubMed. This may not be the complete list of references from this article.
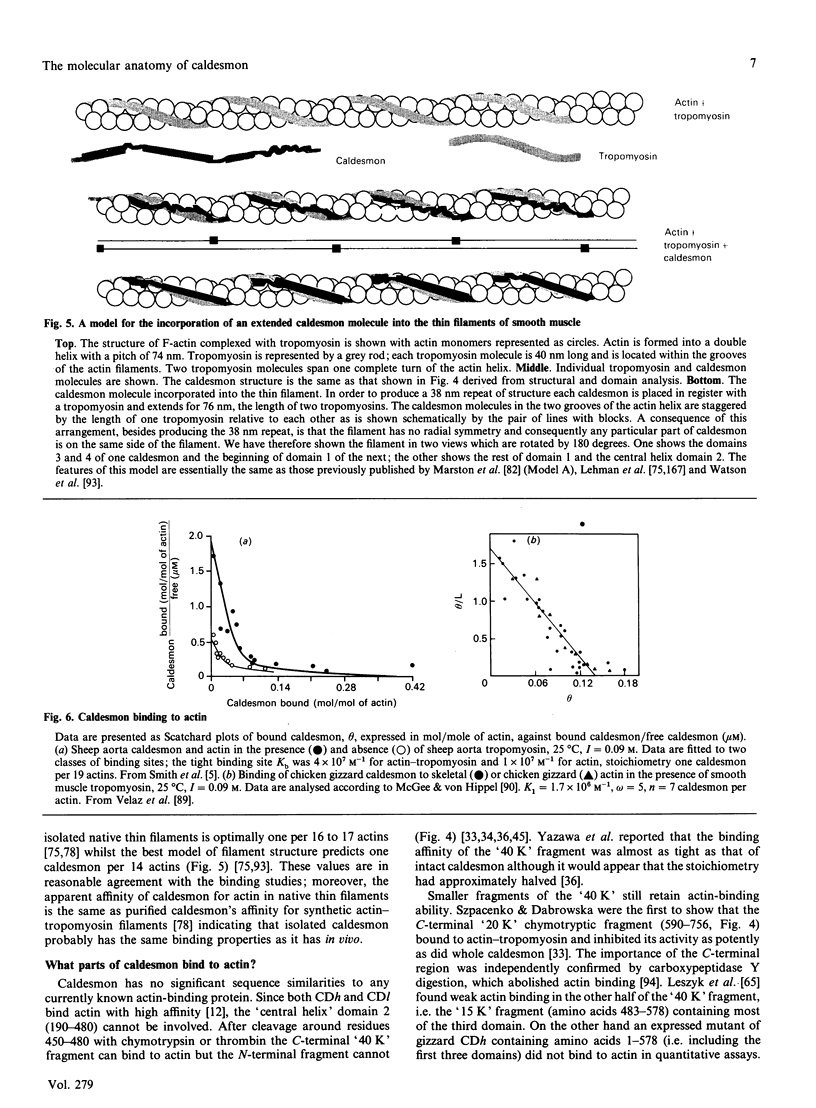
- Abougou J. C., Hagiwara M., Hachiya T., Terasawa M., Hidaka H., Hartshorne D. J. Phosphorylation of caldesmon. FEBS Lett. 1989 Nov 6;257(2):408–410. doi: 10.1016/0014-5793(89)81583-2. [DOI] [PubMed] [Google Scholar]
- Adam L. P., Haeberle J. R., Hathaway D. R. Phosphorylation of caldesmon in arterial smooth muscle. J Biol Chem. 1989 May 5;264(13):7698–7703. [PubMed] [Google Scholar]
- Adam L. P., Milio L., Brengle B., Hathaway D. R. Myosin light chain and caldesmon phosphorylation in arterial muscle stimulated with endothelin-1. J Mol Cell Cardiol. 1990 Sep;22(9):1017–1023. doi: 10.1016/0022-2828(90)91041-5. [DOI] [PubMed] [Google Scholar]
- Adams S., DasGupta G., Chalovich J. M., Reisler E. Immunochemical evidence for the binding of caldesmon to the NH2-terminal segment of actin. J Biol Chem. 1990 Nov 15;265(32):19652–19657. [PubMed] [Google Scholar]
- Ball E. H., Kovala T. Mapping of caldesmon: relationship between the high and low molecular weight forms. Biochemistry. 1988 Aug 9;27(16):6093–6098. doi: 10.1021/bi00416a039. [DOI] [PubMed] [Google Scholar]
- Bartegi A., Fattoum A., Dagorn C., Gabrion J., Kassab R. Isolation, characterization and immunocytochemical localization of caldesmon-like protein from molluscan striated muscle. Eur J Biochem. 1989 Nov 20;185(3):589–595. doi: 10.1111/j.1432-1033.1989.tb15154.x. [DOI] [PubMed] [Google Scholar]
- Bartegi A., Fattoum A., Derancourt J., Kassab R. Characterization of the carboxyl-terminal 10-kDa cyanogen bromide fragment of caldesmon as an actin-calmodulin-binding region. J Biol Chem. 1990 Sep 5;265(25):15231–15238. [PubMed] [Google Scholar]
- Bartegi A., Fattoum A., Kassab R. Cross-linking of smooth muscle caldesmon to the NH2-terminal region of skeletal F-actin. J Biol Chem. 1990 Feb 5;265(4):2231–2237. [PubMed] [Google Scholar]
- Bennett P. M., Marston S. B. Calcium regulated thin filaments from molluscan catch muscles contain a caldesmon-like regulatory protein. J Muscle Res Cell Motil. 1990 Aug;11(4):302–312. doi: 10.1007/BF01766668. [DOI] [PubMed] [Google Scholar]
- Birukov K. G., Shirinsky V. P., Vorotnikov A. V., Gusev N. B. Competitive binding of the troponin T-specific pool of caldesmon antibodies and tropomyosin to skeletal troponin T and smooth muscle caldesmon. FEBS Lett. 1990 Mar 26;262(2):263–265. doi: 10.1016/0014-5793(90)80206-x. [DOI] [PubMed] [Google Scholar]
- Bonet-Kerrache A. A., Walsh M. P. Chemical modification of the sole histidine residue of smooth muscle caldesmon. FEBS Lett. 1991 Apr 9;281(1-2):81–84. doi: 10.1016/0014-5793(91)80363-8. [DOI] [PubMed] [Google Scholar]
- Bretscher A., Lynch W. Identification and localization of immunoreactive forms of caldesmon in smooth and nonmuscle cells: a comparison with the distributions of tropomyosin and alpha-actinin. J Cell Biol. 1985 May;100(5):1656–1663. doi: 10.1083/jcb.100.5.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A. Smooth muscle caldesmon. Rapid purification and F-actin cross-linking properties. J Biol Chem. 1984 Oct 25;259(20):12873–12880. [PubMed] [Google Scholar]
- Bryan J. Caldesmon, acidic amino acids and molecular weight determinations. J Muscle Res Cell Motil. 1989 Apr;10(2):95–96. doi: 10.1007/BF01739964. [DOI] [PubMed] [Google Scholar]
- Bryan J., Imai M., Lee R., Moore P., Cook R. G., Lin W. G. Cloning and expression of a smooth muscle caldesmon. J Biol Chem. 1989 Aug 15;264(23):13873–13879. [PubMed] [Google Scholar]
- Bryan J., Lee R. Sequence of an avian non-muscle caldesmon. J Muscle Res Cell Motil. 1991 Aug;12(4):372–375. doi: 10.1007/BF01738592. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D., Cheek T. R., Norman K. M. Identification of a secretory granule-binding protein as caldesmon. Nature. 1986 Jan 2;319(6048):68–70. doi: 10.1038/319068a0. [DOI] [PubMed] [Google Scholar]
- Bárány M., Rokolya A., Bárány K. Absence of calponin phosphorylation in contracting or resting arterial smooth muscle. FEBS Lett. 1991 Feb 11;279(1):65–68. doi: 10.1016/0014-5793(91)80252-x. [DOI] [PubMed] [Google Scholar]
- Cande W. Z., Tooth P. J., Kendrick-Jones J. Regulation of contraction and thick filament assembly-disassembly in glycerinated vertebrate smooth muscle cells. J Cell Biol. 1983 Oct;97(4):1062–1071. doi: 10.1083/jcb.97.4.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanni P., Cavallini P., Ratti E., Gaviraghi G., Dalla Libera L. Caldesmon: anomalous electrophoretic behaviour in polyacrylamide gel. Biochem Biophys Res Commun. 1989 Apr 14;160(1):174–180. doi: 10.1016/0006-291x(89)91637-9. [DOI] [PubMed] [Google Scholar]
- Chalovich J. M., Cornelius P., Benson C. E. Caldesmon inhibits skeletal actomyosin subfragment-1 ATPase activity and the binding of myosin subfragment-1 to actin. J Biol Chem. 1987 Apr 25;262(12):5711–5716. [PubMed] [Google Scholar]
- Chalovich J. M., Hemric M. E., Velaz L. Regulation of ATP hydrolysis by caldesmon. A novel change in the interaction of myosin with actin. Ann N Y Acad Sci. 1990;599:85–99. doi: 10.1111/j.1749-6632.1990.tb42367.x. [DOI] [PubMed] [Google Scholar]
- Cooke P. H., Fay F. S., Craig R. Myosin filaments isolated from skinned amphibian smooth muscle cells are side-polar. J Muscle Res Cell Motil. 1989 Jun;10(3):206–220. doi: 10.1007/BF01739811. [DOI] [PubMed] [Google Scholar]
- Craig R., Megerman J. Assembly of smooth muscle myosin into side-polar filaments. J Cell Biol. 1977 Dec;75(3):990–996. doi: 10.1083/jcb.75.3.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross R. A., Cross K. E., Small J. V. Salt dependent dimerisation of caldesmon. FEBS Lett. 1987 Jul 27;219(2):306–310. doi: 10.1016/0014-5793(87)80241-7. [DOI] [PubMed] [Google Scholar]
- Dabrowska R., Goch A., Gałazkiewicz B., Osińska H. The influence of caldesmon on ATPase activity of the skeletal muscle actomyosin and bundling of actin filaments. Biochim Biophys Acta. 1985 Sep 27;842(1):70–75. doi: 10.1016/0304-4165(85)90295-8. [DOI] [PubMed] [Google Scholar]
- Dingus J., Hwo S., Bryan J. Identification by monoclonal antibodies and characterization of human platelet caldesmon. J Cell Biol. 1986 May;102(5):1748–1757. doi: 10.1083/jcb.102.5.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draetta G. Cell cycle control in eukaryotes: molecular mechanisms of cdc2 activation. Trends Biochem Sci. 1990 Oct;15(10):378–383. doi: 10.1016/0968-0004(90)90235-4. [DOI] [PubMed] [Google Scholar]
- Egelman E. H. The structure of F-actin. J Muscle Res Cell Motil. 1985 Apr;6(2):129–151. doi: 10.1007/BF00713056. [DOI] [PubMed] [Google Scholar]
- Fatigati V., Murphy R. A. Actin and tropomyosin variants in smooth muscles. Dependence on tissue type. J Biol Chem. 1984 Dec 10;259(23):14383–14388. [PubMed] [Google Scholar]
- Fujii T., Imai M., Rosenfeld G. C., Bryan J. Domain mapping of chicken gizzard caldesmon. J Biol Chem. 1987 Feb 25;262(6):2757–2763. [PubMed] [Google Scholar]
- Fujii T., Machino K., Andoh H., Satoh T., Kondo Y. Calcium-dependent control of caldesmon-actin interaction by S100 protein. J Biochem. 1990 Jan;107(1):133–137. doi: 10.1093/oxfordjournals.jbchem.a122996. [DOI] [PubMed] [Google Scholar]
- Fujii T., Ozawa J., Ogoma Y., Kondo Y. Interaction between chicken gizzard caldesmon and tropomyosin. J Biochem. 1988 Nov;104(5):734–737. doi: 10.1093/oxfordjournals.jbchem.a122542. [DOI] [PubMed] [Google Scholar]
- Fujita H., Ishimura K., Ban T., Kurosumi M., Sobue K., Kakiuchi S. Immunocytochemical demonstration of caldesmon and actin in thyroid glands of rats. Cell Tissue Res. 1984;237(2):375–377. doi: 10.1007/BF00217161. [DOI] [PubMed] [Google Scholar]
- Fürst D. O., Cross R. A., De Mey J., Small J. V. Caldesmon is an elongated, flexible molecule localized in the actomyosin domains of smooth muscle. EMBO J. 1986 Feb;5(2):251–257. doi: 10.1002/j.1460-2075.1986.tb04206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gałazkiewicz B., Belagyi J., Dabrowska R. The effect of caldesmon on assembly and dynamic properties of actin. Eur J Biochem. 1989 May 15;181(3):607–614. doi: 10.1111/j.1432-1033.1989.tb14767.x. [DOI] [PubMed] [Google Scholar]
- Gałazkiewicz B., Mossakowska M., Osińska H., Dabrowska R. Polymerization of G-actin by caldesmon. FEBS Lett. 1985 May 6;184(1):144–149. doi: 10.1016/0014-5793(85)80671-2. [DOI] [PubMed] [Google Scholar]
- Glukhova M. A., Frid M. G., Koteliansky V. E. Developmental changes in expression of contractile and cytoskeletal proteins in human aortic smooth muscle. J Biol Chem. 1990 Aug 5;265(22):13042–13046. [PubMed] [Google Scholar]
- Glukhova M. A., Kabakov A. E., Frid M. G., Ornatsky O. I., Belkin A. M., Mukhin D. N., Orekhov A. N., Koteliansky V. E., Smirnov V. N. Modulation of human aorta smooth muscle cell phenotype: a study of muscle-specific variants of vinculin, caldesmon, and actin expression. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9542–9546. doi: 10.1073/pnas.85.24.9542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glukhova M. A., Kabakov A. E., Ornatsky O. I., Vasilevskaya T. D., Koteliansky V. E., Smirnov V. N. Immunoreactive forms of caldesmon in cultivated human vascular smooth muscle cells. FEBS Lett. 1987 Jun 29;218(2):292–294. doi: 10.1016/0014-5793(87)81064-5. [DOI] [PubMed] [Google Scholar]
- Graceffa P. Evidence for interaction between smooth muscle tropomyosin and caldesmon. FEBS Lett. 1987 Jun 22;218(1):139–142. doi: 10.1016/0014-5793(87)81034-7. [DOI] [PubMed] [Google Scholar]
- Graceffa P., Wang C. L., Stafford W. F. Caldesmon. Molecular weight and subunit composition by analytical ultracentrifugation. J Biol Chem. 1988 Oct 5;263(28):14196–14202. [PubMed] [Google Scholar]
- Hai C. M., Murphy R. A. Cross-bridge phosphorylation and regulation of latch state in smooth muscle. Am J Physiol. 1988 Jan;254(1 Pt 1):C99–106. doi: 10.1152/ajpcell.1988.254.1.C99. [DOI] [PubMed] [Google Scholar]
- Hayashi K., Fujio Y., Kato I., Sobue K. Structural and functional relationships between h- and l-caldesmons. J Biol Chem. 1991 Jan 5;266(1):355–361. [PubMed] [Google Scholar]
- Hayashi K., Kanda K., Kimizuka F., Kato I., Sobue K. Primary structure and functional expression of h-caldesmon complementary DNA. Biochem Biophys Res Commun. 1989 Oct 16;164(1):503–511. doi: 10.1016/0006-291x(89)91748-8. [DOI] [PubMed] [Google Scholar]
- Hayashi K., Yamada S., Kanda K., Kimizuka F., Kato I., Sobue K. 35 kDa fragment of h-caldesmon conserves two consensus sequences of the tropomyosin-binding domain in troponin T. Biochem Biophys Res Commun. 1989 May 30;161(1):38–45. doi: 10.1016/0006-291x(89)91556-8. [DOI] [PubMed] [Google Scholar]
- Hemric M. E., Chalovich J. M. Characterization of caldesmon binding to myosin. J Biol Chem. 1990 Nov 15;265(32):19672–19678. [PMC free article] [PubMed] [Google Scholar]
- Hemric M. E., Chalovich J. M. Effect of caldesmon on the ATPase activity and the binding of smooth and skeletal myosin subfragments to actin. J Biol Chem. 1988 Feb 5;263(4):1878–1885. [PubMed] [Google Scholar]
- Holmes K. C., Popp D., Gebhard W., Kabsch W. Atomic model of the actin filament. Nature. 1990 Sep 6;347(6288):44–49. doi: 10.1038/347044a0. [DOI] [PubMed] [Google Scholar]
- Horiuchi K. Y., Chacko S. Caldesmon inhibits the cooperative turning-on of the smooth muscle heavy meromyosin by tropomyosin-actin. Biochemistry. 1989 Nov 14;28(23):9111–9116. doi: 10.1021/bi00449a023. [DOI] [PubMed] [Google Scholar]
- Horiuchi K. Y., Chacko S. Interaction between caldesmon and tropomyosin in the presence and absence of smooth muscle actin. Biochemistry. 1988 Nov 1;27(22):8388–8393. doi: 10.1021/bi00422a014. [DOI] [PubMed] [Google Scholar]
- Horiuchi K. Y., Miyata H., Chacko S. Modulation of smooth muscle actomyosin ATPase by thin filament associated proteins. Biochem Biophys Res Commun. 1986 May 14;136(3):962–968. doi: 10.1016/0006-291x(86)90426-2. [DOI] [PubMed] [Google Scholar]
- Horiuchi K. Y., Samuel M., Chacko S. Mechanism for the inhibition of acto-heavy meromyosin ATPase by the actin/calmodulin binding domain of caldesmon. Biochemistry. 1991 Jan 22;30(3):712–717. doi: 10.1021/bi00217a019. [DOI] [PubMed] [Google Scholar]
- Ikebe M., Reardon S. Binding of caldesmon to smooth muscle myosin. J Biol Chem. 1988 Mar 5;263(7):3055–3058. [PubMed] [Google Scholar]
- Ikebe M., Reardon S., Scott-Woo G. C., Zhou Z., Koda Y. Purification and characterization of calmodulin-dependent multifunctional protein kinase from smooth muscle: isolation of caldesmon kinase. Biochemistry. 1990 Dec 25;29(51):11242–11248. doi: 10.1021/bi00503a013. [DOI] [PubMed] [Google Scholar]
- Itoh T., Ikebe M., Kargacin G. J., Hartshorne D. J., Kemp B. E., Fay F. S. Effects of modulators of myosin light-chain kinase activity in single smooth muscle cells. Nature. 1989 Mar 9;338(6211):164–167. doi: 10.1038/338164a0. [DOI] [PubMed] [Google Scholar]
- Jiang M. J., Morgan K. G. Agonist-specific myosin phosphorylation and intracellular calcium during isometric contractions of arterial smooth muscle. Pflugers Arch. 1989 Apr;413(6):637–643. doi: 10.1007/BF00581814. [DOI] [PubMed] [Google Scholar]
- Kabsch W., Mannherz H. G., Suck D., Pai E. F., Holmes K. C. Atomic structure of the actin:DNase I complex. Nature. 1990 Sep 6;347(6288):37–44. doi: 10.1038/347037a0. [DOI] [PubMed] [Google Scholar]
- Kakiuchi R., Inui M., Morimoto K., Kanda K., Sobue K., Kakiuchi S. Caldesmon, a calmodulin-binding, F actin-interacting protein, is present in aorta, uterus and platelets. FEBS Lett. 1983 Apr 18;154(2):351–356. doi: 10.1016/0014-5793(83)80181-1. [DOI] [PubMed] [Google Scholar]
- Kamm K. E., Stull J. T. Regulation of smooth muscle contractile elements by second messengers. Annu Rev Physiol. 1989;51:299–313. doi: 10.1146/annurev.ph.51.030189.001503. [DOI] [PubMed] [Google Scholar]
- Kamm K. E., Stull J. T. The function of myosin and myosin light chain kinase phosphorylation in smooth muscle. Annu Rev Pharmacol Toxicol. 1985;25:593–620. doi: 10.1146/annurev.pa.25.040185.003113. [DOI] [PubMed] [Google Scholar]
- Kargacin G. J., Ikebe M., Fay F. S. Peptide modulators of myosin light chain kinase affect smooth muscle cell contraction. Am J Physiol. 1990 Aug;259(2 Pt 1):C315–C324. doi: 10.1152/ajpcell.1990.259.2.C315. [DOI] [PubMed] [Google Scholar]
- Katayama E. Assignment of the positions of chymotryptic fragments and cysteinyl groups in the primary structure of caldesmon in relation to a conformational change. J Biochem. 1989 Dec;106(6):988–993. doi: 10.1093/oxfordjournals.jbchem.a122987. [DOI] [PubMed] [Google Scholar]
- Katayama E., Horiuchi K. Y., Chacko S. Characteristics of the myosin and tropomyosin binding regions of the smooth muscle caldesmon. Biochem Biophys Res Commun. 1989 May 15;160(3):1316–1322. doi: 10.1016/s0006-291x(89)80147-0. [DOI] [PubMed] [Google Scholar]
- Kenney R. E., Hoar P. E., Kerrick W. G. The relationship between ATPase activity, isometric force, and myosin light-chain phosphorylation and thiophosphorylation in skinned smooth muscle fiber bundles from chicken gizzard. J Biol Chem. 1990 May 25;265(15):8642–8649. [PubMed] [Google Scholar]
- Kossmann T., Fürst D., Small J. V. Structural and biochemical analysis of skinned smooth muscle preparations. J Muscle Res Cell Motil. 1987 Apr;8(2):135–144. doi: 10.1007/BF01753989. [DOI] [PubMed] [Google Scholar]
- Krishnan G., Altekar W. An unusual class I (Schiff base) fructose-1,6-bisphosphate aldolase from the halophilic archaebacterium Haloarcula vallismortis. Eur J Biochem. 1991 Jan 30;195(2):343–350. doi: 10.1111/j.1432-1033.1991.tb15712.x. [DOI] [PubMed] [Google Scholar]
- Labeit S., Barlow D. P., Gautel M., Gibson T., Holt J., Hsieh C. L., Francke U., Leonard K., Wardale J., Whiting A. A regular pattern of two types of 100-residue motif in the sequence of titin. Nature. 1990 May 17;345(6272):273–276. doi: 10.1038/345273a0. [DOI] [PubMed] [Google Scholar]
- Lash J. A., Sellers J. R., Hathaway D. R. The effects of caldesmon on smooth muscle heavy actomeromyosin ATPase activity and binding of heavy meromyosin to actin. J Biol Chem. 1986 Dec 5;261(34):16155–16160. [PubMed] [Google Scholar]
- Lehman W. Caldesmon association with smooth muscle thin filaments isolated in the presence and absence of calcium. Biochim Biophys Acta. 1986 Jan 23;885(1):88–90. doi: 10.1016/0167-4889(86)90042-x. [DOI] [PubMed] [Google Scholar]
- Lehman W., Craig R., Lui J., Moody C. Caldesmon and the structure of smooth muscle thin filaments: immunolocalization of caldesmon on thin filaments. J Muscle Res Cell Motil. 1989 Apr;10(2):101–112. doi: 10.1007/BF01739966. [DOI] [PubMed] [Google Scholar]
- Lehman W., Moody C., Craig R. Caldesmon and the structure of vertebrate smooth muscle thin filaments. A minireview. Ann N Y Acad Sci. 1990;599:75–84. doi: 10.1111/j.1749-6632.1990.tb42366.x. [DOI] [PubMed] [Google Scholar]
- Lehman W., Sheldon A., Madonia W. Diversity in smooth muscle thin filament composition. Biochim Biophys Acta. 1987 Jul 24;914(1):35–39. doi: 10.1016/0167-4838(87)90158-0. [DOI] [PubMed] [Google Scholar]
- Lehman W., Szent-Györgyi A. G. Regulation of muscular contraction. Distribution of actin control and myosin control in the animal kingdom. J Gen Physiol. 1975 Jul;66(1):1–30. doi: 10.1085/jgp.66.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leszyk J., Mornet D., Audemard E., Collins J. H. Amino acid sequence of a 15 kilodalton actin-binding fragment of turkey gizzard caldesmon: similarity with dystrophin, tropomyosin and the tropomyosin-binding region of troponin T. Biochem Biophys Res Commun. 1989 Apr 14;160(1):210–216. doi: 10.1016/0006-291x(89)91642-2. [DOI] [PubMed] [Google Scholar]
- Leszyk J., Mornet D., Audemard E., Collins J. H. Caldesmon structure and function: sequence analysis of a 35 kilodalton actin- and calmodulin-binding fragment from the C-terminus of the turkey gizzard protein. Biochem Biophys Res Commun. 1989 May 15;160(3):1371–1378. doi: 10.1016/s0006-291x(89)80155-x. [DOI] [PubMed] [Google Scholar]
- Levine B. A., Moir A. J., Audemard E., Mornet D., Patchell V. B., Perry S. V. Structural study of gizzard caldesmon and its interaction with actin. Binding involves residues of actin also recognised by myosin subfragment 1. Eur J Biochem. 1990 Nov 13;193(3):687–696. doi: 10.1111/j.1432-1033.1990.tb19388.x. [DOI] [PubMed] [Google Scholar]
- Lewin B. Driving the cell cycle: M phase kinase, its partners, and substrates. Cell. 1990 Jun 1;61(5):743–752. doi: 10.1016/0092-8674(90)90181-d. [DOI] [PubMed] [Google Scholar]
- Lim S. S., Tu Z. H., Lemanski L. F. Anti-troponin-T monoclonal antibody crossreacts with all muscle types. J Muscle Res Cell Motil. 1984 Oct;5(5):515–526. doi: 10.1007/BF00713258. [DOI] [PubMed] [Google Scholar]
- Lin J. J., Lin J. L., Davis-Nanthakumar E. J., Lourim D. Monoclonal antibodies against caldesmon, a Ca++/calmodulin- and actin-binding protein of smooth muscle and nonmuscle cells. Hybridoma. 1988 Jun;7(3):273–288. doi: 10.1089/hyb.1988.7.273. [DOI] [PubMed] [Google Scholar]
- Litchfield D. W., Ball E. H. Phosphorylation of caldesmon77 by protein kinase C in vitro and in intact human platelets. J Biol Chem. 1987 Jun 15;262(17):8056–8060. [PubMed] [Google Scholar]
- Liu Y. C., Storm D. R. Regulation of free calmodulin levels by neuromodulin: neuron growth and regeneration. Trends Pharmacol Sci. 1990 Mar;11(3):107–111. doi: 10.1016/0165-6147(90)90195-e. [DOI] [PubMed] [Google Scholar]
- Lynch W. P., Riseman V. M., Bretscher A. Smooth muscle caldesmon is an extended flexible monomeric protein in solution that can readily undergo reversible intra- and intermolecular sulfhydryl cross-linking. A mechanism for caldesmon's F-actin bundling activity. J Biol Chem. 1987 May 25;262(15):7429–7437. [PubMed] [Google Scholar]
- Lynch W., Bretscher A. Purification of caldesmon. Methods Enzymol. 1986;134:37–42. doi: 10.1016/0076-6879(86)34073-4. [DOI] [PubMed] [Google Scholar]
- Mabuchi K., Wang C. L. Electron microscopic studies of chicken gizzard caldesmon and its complex with calmodulin. J Muscle Res Cell Motil. 1991 Apr;12(2):145–151. doi: 10.1007/BF01774033. [DOI] [PubMed] [Google Scholar]
- Mak A. S., Smillie L. B. Structural interpretation of the two-site binding of troponin on the muscle thin filament. J Mol Biol. 1981 Jul 5;149(3):541–550. doi: 10.1016/0022-2836(81)90486-1. [DOI] [PubMed] [Google Scholar]
- Mak A. S., Watson M. H., Litwin C. M., Wang J. H. Phosphorylation of caldesmon by cdc2 kinase. J Biol Chem. 1991 Apr 15;266(11):6678–6681. [PubMed] [Google Scholar]
- Makuch R., Walsh M. P., Dabrowska R. Location of the calmodulin- and actin-binding domains at the C-terminus of caldesmon. FEBS Lett. 1989 Apr 24;247(2):411–414. doi: 10.1016/0014-5793(89)81381-x. [DOI] [PubMed] [Google Scholar]
- Malencik D. A., Ausio J., Byles C. E., Modrell B., Anderson S. R. Turkey gizzard caldesmon: molecular weight determination and calmodulin binding studies. Biochemistry. 1989 Oct 3;28(20):8227–8233. doi: 10.1021/bi00446a039. [DOI] [PubMed] [Google Scholar]
- Marston S. B. A tight-binding interaction between smooth-muscle native thin filaments and heavy meromyosin in the presence of MgATP. Biochem J. 1989 Apr 1;259(1):303–306. doi: 10.1042/bj2590303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston S. B., Lehman W. Caldesmon is a Ca2+-regulatory component of native smooth-muscle thin filaments. Biochem J. 1985 Nov 1;231(3):517–522. doi: 10.1042/bj2310517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston S. B., Redwood C. S., Lehman W. Reversal of caldesmon function by anti-caldesmon antibodies confirms its role in the calcium regulation of vascular smooth muscle thin filaments. Biochem Biophys Res Commun. 1988 Aug 30;155(1):197–202. doi: 10.1016/s0006-291x(88)81068-4. [DOI] [PubMed] [Google Scholar]
- Marston S. B., Smith C. W. Purification and properties of Ca2+-regulated thin filaments and F-actin from sheep aorta smooth muscle. J Muscle Res Cell Motil. 1984 Oct;5(5):559–575. doi: 10.1007/BF00713261. [DOI] [PubMed] [Google Scholar]
- Marston S. B., Smith C. W. The thin filaments of smooth muscles. J Muscle Res Cell Motil. 1985 Dec;6(6):669–708. doi: 10.1007/BF00712237. [DOI] [PubMed] [Google Scholar]
- Marston S. B., Trevett R. M., Walters M. Calcium ion-regulated thin filaments from vascular smooth muscle. Biochem J. 1980 Feb 1;185(2):355–365. doi: 10.1042/bj1850355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston S. B. What is latch? New ideas about tonic contraction in smooth muscle. J Muscle Res Cell Motil. 1989 Apr;10(2):97–100. doi: 10.1007/BF01739965. [DOI] [PubMed] [Google Scholar]
- Marston S. Aorta caldesmon inhibits actin activation of thiophosphorylated heavy meromyosin Mg2+-ATPase activity by slowing the rate of product release. FEBS Lett. 1988 Sep 26;238(1):147–150. doi: 10.1016/0014-5793(88)80245-x. [DOI] [PubMed] [Google Scholar]
- Marston S. Calcium ion-dependent regulation of uterine smooth muscle thin filaments by caldesmon. Am J Obstet Gynecol. 1989 Jan;160(1):252–257. doi: 10.1016/0002-9378(89)90131-2. [DOI] [PubMed] [Google Scholar]
- Marston S. Stoichiometry and stability of caldesmon in native thin filaments from sheep aorta smooth muscle. Biochem J. 1990 Dec 1;272(2):305–310. doi: 10.1042/bj2720305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura F., Lin J. J. Visualization of monoclonal antibody binding to tropomyosin on native smooth muscle thin filaments by electron microscopy. J Mol Biol. 1982 May 5;157(1):163–171. doi: 10.1016/0022-2836(82)90520-4. [DOI] [PubMed] [Google Scholar]
- McGhee J. D., von Hippel P. H. Theoretical aspects of DNA-protein interactions: co-operative and non-co-operative binding of large ligands to a one-dimensional homogeneous lattice. J Mol Biol. 1974 Jun 25;86(2):469–489. doi: 10.1016/0022-2836(74)90031-x. [DOI] [PubMed] [Google Scholar]
- Mills J. S., Walsh M. P., Nemcek K., Johnson J. D. Biologically active fluorescent derivatives of spinach calmodulin that report calmodulin target protein binding. Biochemistry. 1988 Feb 9;27(3):991–996. doi: 10.1021/bi00403a023. [DOI] [PubMed] [Google Scholar]
- Moody C. J., Marston S. B., Smith C. W. Bundling of actin filaments by aorta caldesmon is not related to its regulatory function. FEBS Lett. 1985 Oct 21;191(1):107–112. doi: 10.1016/0014-5793(85)81003-6. [DOI] [PubMed] [Google Scholar]
- Moody C., Lehman W., Craig R. Caldesmon and the structure of smooth muscle thin filaments: electron microscopy of isolated thin filaments. J Muscle Res Cell Motil. 1990 Apr;11(2):176–185. doi: 10.1007/BF01766496. [DOI] [PubMed] [Google Scholar]
- Moreno S., Nurse P. Substrates for p34cdc2: in vivo veritas? Cell. 1990 May 18;61(4):549–551. doi: 10.1016/0092-8674(90)90463-o. [DOI] [PubMed] [Google Scholar]
- Ngai P. K., Walsh M. P. Inhibition of smooth muscle actin-activated myosin Mg2+-ATPase activity by caldesmon. J Biol Chem. 1984 Nov 25;259(22):13656–13659. [PubMed] [Google Scholar]
- Ngai P. K., Walsh M. P. Properties of caldesmon isolated from chicken gizzard. Biochem J. 1985 Sep 15;230(3):695–707. doi: 10.1042/bj2300695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngai P. K., Walsh M. P. The effects of phosphorylation of smooth-muscle caldesmon. Biochem J. 1987 Jun 1;244(2):417–425. doi: 10.1042/bj2440417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neil K. T., DeGrado W. F. How calmodulin binds its targets: sequence independent recognition of amphiphilic alpha-helices. Trends Biochem Sci. 1990 Feb;15(2):59–64. doi: 10.1016/0968-0004(90)90177-d. [DOI] [PubMed] [Google Scholar]
- Owada M. K., Hakura A., Iida K., Yahara I., Sobue K., Kakiuchi S. Occurrence of caldesmon (a calmodulin-binding protein) in cultured cells: comparison of normal and transformed cells. Proc Natl Acad Sci U S A. 1984 May;81(10):3133–3137. doi: 10.1073/pnas.81.10.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S., Rasmussen H. Carbachol-induced protein phosphorylation changes in bovine tracheal smooth muscle. J Biol Chem. 1986 Nov 25;261(33):15734–15739. [PubMed] [Google Scholar]
- Parry D. A. Analysis of the amino acid sequence of a tropomyosin-binding fragment from troponin-T. J Mol Biol. 1981 Feb 25;146(2):259–263. doi: 10.1016/0022-2836(81)90435-6. [DOI] [PubMed] [Google Scholar]
- Pearlstone J. R., Smillie L. B. Binding of troponin-T fragments to several types of tropomyosin. Sensitivity to Ca2+ in the presence of troponin-C. J Biol Chem. 1982 Sep 25;257(18):10587–10592. [PubMed] [Google Scholar]
- Pritchard K., Marston S. B. Ca(2+)-dependent regulation of vascular smooth-muscle caldesmon by S.100 and related smooth-muscle proteins. Biochem J. 1991 Aug 1;277(Pt 3):819–824. doi: 10.1042/bj2770819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard K., Marston S. B. Ca2+-calmodulin binding to caldesmon and the caldesmon-actin-tropomyosin complex. Its role in Ca2+ regulation of the activity of synthetic smooth-muscle thin filaments. Biochem J. 1989 Feb 1;257(3):839–843. doi: 10.1042/bj2570839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen H., Takuwa Y., Park S. Protein kinase C in the regulation of smooth muscle contraction. FASEB J. 1987 Sep;1(3):177–185. [PubMed] [Google Scholar]
- Redwood C. S., Marston S. B., Bryan J., Cross R. A., Kendrick-Jones J. The functional properties of full length and mutant chicken gizzard smooth muscle caldesmon expressed in Escherichia coli. FEBS Lett. 1990 Sep 17;270(1-2):53–56. doi: 10.1016/0014-5793(90)81233-e. [DOI] [PubMed] [Google Scholar]
- Riseman V. M., Lynch W. P., Nefsky B., Bretscher A. The calmodulin and F-actin binding sites of smooth muscle caldesmon lie in the carboxyl-terminal domain whereas the molecular weight heterogeneity lies in the middle of the molecule. J Biol Chem. 1989 Feb 15;264(5):2869–2875. [PubMed] [Google Scholar]
- Scott-Woo G. C., Walsh M. P. Autophosphorylation of smooth-muscle caldesmon. Biochem J. 1988 Jun 1;252(2):463–472. doi: 10.1042/bj2520463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy S., Choi J. K., Bagrodia S., Copeland T. D., Maller J. L., Shalloway D. Purified maturation promoting factor phosphorylates pp60c-src at the sites phosphorylated during fibroblast mitosis. Cell. 1989 Jun 2;57(5):763–774. doi: 10.1016/0092-8674(89)90791-5. [DOI] [PubMed] [Google Scholar]
- Shirinsky V. P., Biryukov K. G., Vorotnikov A. V., Gusev N. B. Caldesmon150, caldesmon77 and skeletal muscle troponin T share a common antigenic determinant. FEBS Lett. 1989 Jul 17;251(1-2):65–68. doi: 10.1016/0014-5793(89)81429-2. [DOI] [PubMed] [Google Scholar]
- Shirinsky V. P., Bushueva T. L., Frolova S. I. Caldesmon-calmodulin interaction. Study by the method of protein intrinsic tryptophan fluorescence. Biochem J. 1988 Oct 1;255(1):203–208. [PMC free article] [PubMed] [Google Scholar]
- Skripnikova E. V., Gusev N. B. Interaction of smooth muscle caldesmon with S-100 protein. FEBS Lett. 1989 Nov 6;257(2):380–382. doi: 10.1016/0014-5793(89)81577-7. [DOI] [PubMed] [Google Scholar]
- Smith C. W., Marston S. B. Disassembly and reconstitution of the Ca2+-sensitive thin filaments of vascular smooth muscle. FEBS Lett. 1985 May 6;184(1):115–119. doi: 10.1016/0014-5793(85)80665-7. [DOI] [PubMed] [Google Scholar]
- Smith C. W., Pritchard K., Marston S. B. The mechanism of Ca2+ regulation of vascular smooth muscle thin filaments by caldesmon and calmodulin. J Biol Chem. 1987 Jan 5;262(1):116–122. [PubMed] [Google Scholar]
- Sobue K., Fujio Y. Significances of two different Mr caldesmons. Adv Exp Med Biol. 1989;255:325–335. doi: 10.1007/978-1-4684-5679-0_36. [DOI] [PubMed] [Google Scholar]
- Sobue K., Muramoto Y., Fujita M., Kakiuchi S. Purification of a calmodulin-binding protein from chicken gizzard that interacts with F-actin. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5652–5655. doi: 10.1073/pnas.78.9.5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobue K., Takahashi K., Wakabayashi I. Caldesmon150 regulates the tropomyosin-enhanced actin-myosin interaction in gizzard smooth muscle. Biochem Biophys Res Commun. 1985 Oct 30;132(2):645–651. doi: 10.1016/0006-291x(85)91181-7. [DOI] [PubMed] [Google Scholar]
- Stafford W. F., Jancso A., Graceffa P. Caldesmon from rabbit liver: molecular weight and length by analytical ultracentrifugation. Arch Biochem Biophys. 1990 Aug 15;281(1):66–69. doi: 10.1016/0003-9861(90)90413-s. [DOI] [PubMed] [Google Scholar]
- Sundaralingam M., Drendel W., Greaser M. Stabilization of the long central helix of troponin C by intrahelical salt bridges between charged amino acid side chains. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7944–7947. doi: 10.1073/pnas.82.23.7944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland C., Walsh M. P. Phosphorylation of caldesmon prevents its interaction with smooth muscle myosin. J Biol Chem. 1989 Jan 5;264(1):578–583. [PubMed] [Google Scholar]
- Szpacenko A., Dabrowska R. Functional domain of caldesmon. FEBS Lett. 1986 Jul 7;202(2):182–186. doi: 10.1016/0014-5793(86)80683-4. [DOI] [PubMed] [Google Scholar]
- Szpacenko A., Wagner J., Dabrowska R., Rüegg J. C. Caldesmon-induced inhibition of ATPase activity of actomyosin and contraction of skinned fibres of chicken gizzard smooth muscle. FEBS Lett. 1985 Nov 11;192(1):9–12. doi: 10.1016/0014-5793(85)80032-6. [DOI] [PubMed] [Google Scholar]
- Taggart M. J., Marston S. B. The effects of vascular smooth muscle caldesmon on force production by 'desensitised' skeletal muscle fibres. FEBS Lett. 1988 Dec 19;242(1):171–174. doi: 10.1016/0014-5793(88)81009-3. [DOI] [PubMed] [Google Scholar]
- Takagi T., Yazawa M., Ueno T., Suzuki S., Yagi K. Amino acid sequence studies on cyanogen bromide peptides of chicken caldesmon which bind to calmodulin. J Biochem. 1989 Nov;106(5):778–783. doi: 10.1093/oxfordjournals.jbchem.a122930. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Ohta H., Kanda K., Tanaka T., Hidaka H., Sobue K. Phosphorylation of high-Mr caldesmon by protein kinase C modulates the regulatory function of this protein on the interaction between actin and myosin. Eur J Biochem. 1990 Mar 30;188(3):495–500. doi: 10.1111/j.1432-1033.1990.tb15427.x. [DOI] [PubMed] [Google Scholar]
- Tansey M. G., Hori M., Karaki H., Kamm K. E., Stull J. T. Okadaic acid uncouples myosin light chain phosphorylation and tension in smooth muscle. FEBS Lett. 1990 Sep 17;270(1-2):219–221. doi: 10.1016/0014-5793(90)81272-p. [DOI] [PubMed] [Google Scholar]
- Ueki N., Sobue K., Kanda K., Hada T., Higashino K. Expression of high and low molecular weight caldesmons during phenotypic modulation of smooth muscle cells. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9049–9053. doi: 10.1073/pnas.84.24.9049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umekawa H., Hidaka H. Phosphorylation of caldesmon by protein kinase C. Biochem Biophys Res Commun. 1985 Oct 15;132(1):56–62. doi: 10.1016/0006-291x(85)90987-8. [DOI] [PubMed] [Google Scholar]
- Velaz L., Hemric M. E., Benson C. E., Chalovich J. M. The binding of caldesmon to actin and its effect on the ATPase activity of soluble myosin subfragments in the presence and absence of tropomyosin. J Biol Chem. 1989 Jun 5;264(16):9602–9610. [PubMed] [Google Scholar]
- Velaz L., Ingraham R. H., Chalovich J. M. Dissociation of the effect of caldesmon on the ATPase activity and on the binding of smooth heavy meromyosin to actin by partial digestion of caldesmon. J Biol Chem. 1990 Feb 15;265(5):2929–2934. [PubMed] [Google Scholar]
- Vorotnikov A. V., Gusev N. B. Some properties of duck gizzard caldesmon. Biochem J. 1991 Jan 1;273(Pt 1):161–163. doi: 10.1042/bj2730161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorotnikov A. V., Shirinsky V. P., Gusev N. B. Phosphorylation of smooth muscle caldesmon by three protein kinases: implication for domain mapping. FEBS Lett. 1988 Aug 29;236(2):321–324. doi: 10.1016/0014-5793(88)80047-4. [DOI] [PubMed] [Google Scholar]
- Walsh M. P., Bridenbaugh R., Hartshorne D. J., Kerrick W. G. Phosphorylation-dependent activated tension in skinned gizzard muscle fibers in the absence of Ca2+. J Biol Chem. 1982 Jun 10;257(11):5987–5990. [PubMed] [Google Scholar]
- Walsh M. P., Sutherland C. A model for caldesmon in latch-bridge formation in smooth muscle. Adv Exp Med Biol. 1989;255:337–346. doi: 10.1007/978-1-4684-5679-0_37. [DOI] [PubMed] [Google Scholar]
- Wang C. L. Photocrosslinking of calmodulin and/or actin to chicken gizzard caldesmon. Biochem Biophys Res Commun. 1988 Oct 31;156(2):1033–1038. doi: 10.1016/s0006-291x(88)80948-3. [DOI] [PubMed] [Google Scholar]
- Wang C. L., Wang L. W., Xu S. A., Lu R. C., Saavedra-Alanis V., Bryan J. Localization of the calmodulin- and the actin-binding sites of caldesmon. J Biol Chem. 1991 May 15;266(14):9166–9172. [PubMed] [Google Scholar]
- Watson M. H., Kuhn A. E., Novy R. E., Lin J. J., Mak A. S. Caldesmon-binding sites on tropomyosin. J Biol Chem. 1990 Nov 5;265(31):18860–18866. [PubMed] [Google Scholar]
- Yamashiro-Matsumura S., Matsumura F. Characterization of 83-kilodalton nonmuscle caldesmon from cultured rat cells: stimulation of actin binding of nonmuscle tropomyosin and periodic localization along microfilaments like tropomyosin. J Cell Biol. 1988 Jun;106(6):1973–1983. doi: 10.1083/jcb.106.6.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro S., Yamakita Y., Hosoya H., Matsumura F. Phosphorylation of non-muscle caldesmon by p34cdc2 kinase during mitosis. Nature. 1991 Jan 10;349(6305):169–172. doi: 10.1038/349169a0. [DOI] [PubMed] [Google Scholar]
- Yamashiro S., Yamakita Y., Ishikawa R., Matsumura F. Mitosis-specific phosphorylation causes 83K non-muscle caldesmon to dissociate from microfilaments. Nature. 1990 Apr 12;344(6267):675–678. doi: 10.1038/344675a0. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M., Hamada Y., Katsuragawa Y., Imamura M., Mikawa T., Masaki T. Complete primary structure of vertebrate smooth muscle myosin heavy chain deduced from its complementary DNA sequence. Implications on topography and function of myosin. J Mol Biol. 1987 Nov 20;198(2):143–157. doi: 10.1016/0022-2836(87)90302-0. [DOI] [PubMed] [Google Scholar]
- Yazawa M., Yagi K., Sobue K. Isolation and characterization of a calmodulin binding fragment of chicken gizzard caldesmon. J Biochem. 1987 Nov;102(5):1065–1073. doi: 10.1093/oxfordjournals.jbchem.a122144. [DOI] [PubMed] [Google Scholar]
- der Terrossian E., Deprette C., Cassoly R. Caldesmon is present in human and pig erythrocytes. Biochem Biophys Res Commun. 1989 Mar 15;159(2):395–401. doi: 10.1016/0006-291x(89)90004-1. [DOI] [PubMed] [Google Scholar]






