Abstract
Background
A Chronic Kidney Disease (CKD) Epidemiology Collaboration (EPI) formula not including a Black race coefficient has been recently developed and is now recommended in the US. The new (2021) equation was shown to yield higher estimated glomerular filtration rate (eGFR) values than the old (2009) one in a non-Black general population sample, thus reclassifying a significant number of individuals to a better eGFR category. However, reclassified individuals were previously shown to have a lower risk of progression to end-stage kidney disease, but higher adjusted risks for all-cause death and morbidity and mortality from cardiovascular disease than those not reclassified. This study evaluated the prognostic impact of switching from the 2009 to the 2021 CKD-EPI equation in non-Black individuals with type 2 diabetes.
Methods
The Renal Insufficiency And Cardiovascular Events (RIACE) was a prospective cohort study enrolling 15,773 Caucasian patients in 19 Italian centers in 2006–2008. Cardiometabolic risk profile, treatments, complications, and comorbidities were assessed at baseline and eGFR was calculated with the two equations. Vital status was retrieved on 31 October 2015 for 15,656 participants (99.3%).
Results
With the 2021 equation, the eGFR value increased in all patients, except for 293 individuals with a 2009 eGFR ≥ 105 ml·min− 1·1.73 m− 2. The median difference was 4.10 ml·min− 1·1.73 m− 2 and was higher in males, older individuals and those in the G2 category. Reclassification decreased the percentage of patients with reduced eGFR from 17.28 to 13.96% and with any CKD from 36.23 to 34.03%. Reclassified individuals had better cardiometabolic risk profile and lower prevalence of complications and use of medications than non-reclassified individuals. Risk of death versus the 2009 G1 category was lower for reclassified than non-reclassified participants in all eGFR categories and, particularly, in each 2009 eGFR category, though difference was significant only in the G4-G5 category. The Receiver Operator Characteristic curves were statistically, but not clinically different with the two equations.
Conclusion
Changing from the 2009 to the 2021 CKD-EPI equation results in higher eGFR and lower CKD prevalence, with a lower risk of death in reclassified patients with an eGFR < 30 ml·min− 1·1.73 m− 2, but virtually no impact on mortality prediction.
Trial registration: ClinicalTrials.gov, NCT00715481, retrospectively registered 15 July, 2008.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-024-02450-5.
Keywords: All-cause mortality, Chronic kidney disease epidemiology collaboration equation, Estimated glomerular filtration rate, Race, Type 2 diabetes
Background
Glomerular filtration rate (GFR) is widely used for diagnostic, prognostic, and therapeutic purposes [1]. A GFR cut-off of 60 ml·min− 1·1.73 m− 2 is in fact a criterion for diagnosing chronic kidney disease (CKD) and, on a population level, for calculating CKD incidence and prevalence. Moreover, GFR thresholds are used for nephrologist referral and dialysis or transplant planning as well as for clinical trial eligibility. The level of eGFR is also used for predicting risk of CKD progression to end-stage kidney disease (ESKD) and, in epidemiological studies, to assess the association with adverse renal and cardiovascular outcomes. Finally, GFR serves as a guide for medication initiation, discontinuation, and dosing as well as for utilization of contrast media for imaging procedures.
The gold standard for measuring GFR is plasma or urinary clearance of an exogenous filtration marker [2, 3], which however is a cumbersome procedure that cannot be routinely performed [4]. For this reason, several equations have been developed for estimating GFR from serum levels of endogenous filtration markers such a creatinine and cystatin C [5]. The currently recommended creatinine-based equation is the 2009 Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula [6], which estimates GFR using the variables age, sex, and race (Black versus non-Black), in addition to creatinine [7]. However, the evidence supporting the introduction of race as a correction factor for muscle mass has been questioned [8], and it is now widely accepted that race is a social, not a biological construct [9]. Therefore, the inclusion of this variable has been recently questioned [10, 11], leading to the development of a new (2021) CKD-EPI formula that does not include a race coefficient and includes refitted coefficients for age, sex and creatinine [12]. The 2021 CKD-EPI equation was shown to underestimate measured GFR in Blacks and to overestimate it in non-Blacks; moreover, in non-Blacks, bias versus measured GFR was larger than with the 2009 CKD-EPI equation [12]. Nevertheless, due to issues with inequities, the 2021 CKD-EPI equation has been recommended for immediate implementation in the US [13]. With the exception of UK [14], this has not yet been the case in Europe, where a much greater proportion of the population is non-Black, though the percentage of Black individuals is increasing due to the increasing migration flows [15].
A recent report from a Swedish general population sample, mostly consisting of non-black people, showed that changing from the 2009 to the 2021 CKD-EPI equation increased estimated GFR (eGFR), thus reclassifying a significant number of individuals to a better eGFR category and decreasing the prevalence of eGFR categories G3a-G5 from 5.1 to 3.8% [16]. However, reclassified individuals had a lower risk of progression to end-stage kidney disease (ESKD), but higher adjusted risks for all-cause death and morbidity and mortality from cardiovascular disease (CVD) than those who were not reclassified [16]. This might be a matter of concern as people reclassified to a better eGFR category would receive a less aggressive treatment despite a worse prognosis.
In the present analysis, we focused on people with type 2 diabetes, because there are virtually no data on this population that is at high risk for CKD and mortality. To this end, we used the Renal Insufficiency And Cardiovascular Events (RIACE) cohort of Caucasian individuals with type 2 diabetes for assessing the prognostic impact of estimating GFR with the 2021 CKD-EPI equation in a non-Black population. In particular, we evaluated the effect on CKD prevalence and staging, risk of death from any cause, and mortality prediction.
Methods
Design
The RIACE Italian Multicenter Study was an observational, prospective, cohort study on the impact of eGFR on morbidity and mortality in individuals with type 2 diabetes [17]. The study was conducted in accordance with the Declaration of Helsinki. The research protocol was approved by the ethics committees of participating centers. Participants provided an informed consent.
Participants
The RIACE enrolled 15,773 Caucasian patients with type 2 diabetes, consecutively attending 19 hospital-based, tertiary referral Diabetes Clinics of the National Health Service throughout Italy, most of them in the years 2006–2008 (first patients 6 October 2005 - last patient 17 December 2008). Exclusion criteria were dialysis or renal transplantation.
Baseline data
Baseline data were collected using a standardized protocol across participating centers; results from different laboratories/methods were standardized by comparison with values detected in test samples at the reference laboratory of the Coordinating Center [17].
Participants underwent a structured interview to collect the following information: current age, smoking status, known diabetes duration, severe co-morbidities, and current treatments including glucose-, lipid-, and blood pressure (BP)-lowering therapies.
Body mass index (BMI) was calculated from weight and height, whereas estimated waist circumference (eWC) was calculated from log-transformed BMI values [18]. Then, BP was measured with a sphygmomanometer with the patients seated with the arm at the heart level.
Hemoglobin A1c (HbA1c) was measured by HPLC using DCCT-aligned methods, whereas triglycerides and total and HDL cholesterol were determined in fasting blood samples by standard colorimetric enzymatic methods. Then, LDL cholesterol concentration was estimated using the Friedewald formula.
The presence of CKD was assessed by measuring albuminuria and serum creatinine, as previously detailed [17, 19]. Briefly, albumin excretion rate (AER) was obtained from 24-hour urine collections or calculated from albumin-to-creatinine ratio in early-morning, first-voided urine samples; albumin concentration in urines was measured by immunonephelometry or immunoturbidimetry, in the absence of interfering clinical conditions. Serum (and urine) creatinine was measured by the modified Jaffe method, traceable to IDMS, and GFR was estimated using both the 2009 [7] and the 2021 [12] CKD-EPI equations (Table S1). Based on albuminuria and eGFR values, participants were then stratified according to the Kidney Disease Improving Global Outcomes (KDIGO) classification [6, 20].
The presence of diabetic retinopathy (DR) was assessed in each center by an expert ophthalmologist by dilated fundoscopy [21]. Patients were then classified as having no DR, non-advanced DR (including mild or moderate non-proliferative DR), or advanced DR (including severe non-proliferative DR, proliferative DR, or diabetic macular edema). DR grade was assigned based on the worse eye.
Previous major adverse CVD events, including myocardial infarction, stroke, foot ulcer, gangrene and non-traumatic amputation, and cerebrovascular, carotid, and lower limb revascularization, were adjudicated based on hospital discharge records by an ad hoc committee in each center [22].
All-cause mortality
The vital status of study participants on 31 October 2015 was verified by interrogating the Italian Health Card database (http://sistemats1.sanita.finanze.it/wps/portal/), which provides updated and reliable information on all current Italian residents [23].
Statistical analysis
Data are expressed as mean ± SD or median (interquartile range) for continuous variables, and number of cases (percentage) for categorical variables. For continuous variables, the Kolmogorov-Smirnov test was used to determine if variables were normally distributed; if not, logarithmic conversion was performed before regression analyses. Continuous variables were compared using the Student’s t-test (or one-way ANOVA) and Mann-Whitney test (or Kruskal-Wallis’s test) for parametric and non-parametric data, respectively, whereas the χ2 test was applied to categorical variables. None of the variables had missing values.
The eGFR distributions for the two equations were calculated in the whole cohort and in pre-specified subgroups using kernel density estimation, a nonparametric technique that provides a better estimation of the probability density function than traditional histogram [24]. Since the coefficients of age, sex and creatinine differ between the two equations, their influence on eGFR increase was assessed by calculating for each individual the eGFR change (ΔeGFR) from the 2009 to the 2021 CKD-EPI equation and plotting it against age, sex and the 2009 eGFR level. The level of agreement between the two equations were estimated using Bland-Altman plots, Lin’s concordance correlation, and linear weighted Cohen’s kappa.
The number and percentage of participants in each eGFR and KDIGO category with the 2009 CKD-EPI equation that were reclassified with the 2021 CKD-EPI equation to another rGFR category were then calculated. As nobody was reclassified to a worse eGFR category, the term “reclassified” is hereinafter used for “reclassified to a better eGFR category. The baseline clinical features of reclassified versus non-reclassified participants were compared either in the whole cohort (excluding individuals falling in the G1 category, who could not be reclassified to a better eGFR category) or separately in those with a 2009 eGFR 60–90 or < 60 ml·min− 1·1.73m− 2.
For survival analysis, the index date was the date of the baseline visit when participants were enrolled into the study and the end of follow-up was the date of the census (31 October 2015) or, for those who died, the date of death. Kaplan-Meier survival probabilities for all-cause mortality were estimated for reclassified and non-reclassified participants in each eGFR category and differences were analyzed with the Log rank statistic. The hazard ratios (HRs) and their 95% confidence intervals (CIs) were estimated by Cox proportional hazards regression with backward selection of variables, separately for reclassified and non-reclassified participants in each eGFR category, using the 2009 G1 category as reference. The backward variable selection method was chosen to reduce the chances of overfitting the data and make the linear regression model more interpretable. These analyses were unadjusted (model 1) or adjusted for baseline age (model 2) or age and other CVD risk factors (i.e., sex, smoking status, diabetes duration, HbA1c, BMI, triglycerides, total and HDL cholesterol, systolic and diastolic BP, anti-hyperglycemic, lipid-lowering, and anti-hypertensive treatment) and complications/comorbidities (albuminuria, DR grade, any CVD, and any comorbidity) (model 3). The analyses were repeated for reclassified versus non-reclassified participants in each eGFR category and further adjusted for the 2009 CKD-EPI eGFR level on top of model 2 (model 2a) and 3 (model 3a). Finally, Cox proportional hazards regression analyses according to KDIGO categories were run separately for the 2009 and 2021 CKD-EPI equations, using the category G1A1a as reference and adjusting as in models 1–3 (except for albuminuria); the G2 category was split in G2a and G2b (75–89 and 60–74 ml·min− 1·1.73 m− 2, respectively), as previously reported [20].
Receiver Operator Characteristic (ROC) curves were plotted and areas under ROC curves were calculated, using all-cause mortality at the end of follow-up as dependent variable and the CKD-EPI 2009 or 2021 eGFR values (as continuous variables) as predictors. Moreover, the Youden’s J statistic was used to assess the cut-off point with the maximum “J” index, where J = sensitivity + specificity– 100.
Tests were two sided, and a p value < 0.05 was considered statistically significant. Data entry and statistical analyses were performed using SPSS version 26.0 (SPSS, Chicago, IL, USA) and MedCalc version 22.014 (MedCalc Software Ltd, Ostend, Belgium).
Results
Level and distribution of eGFR
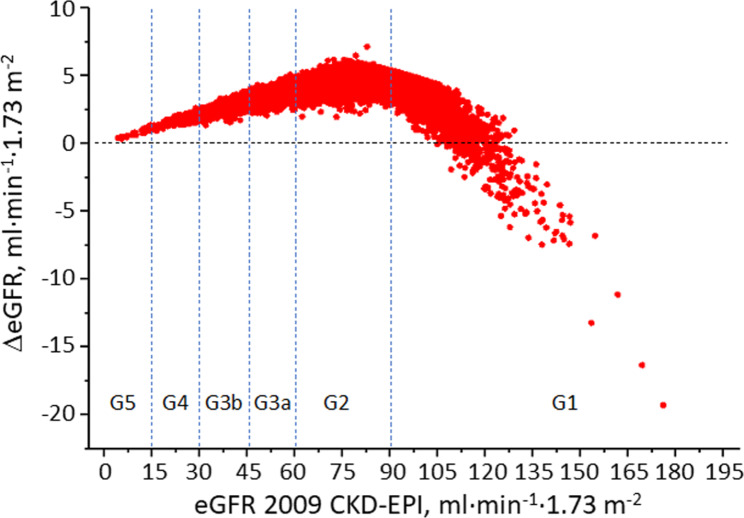
The distributions of eGFR using the 2009 and 2021 CKD-EPI creatinine-based equations are shown in Figure S1; values with both equations were not normally distributed (Kolmogorov-Smirnov test, p < 0.0001). The use of the 2021 equation resulted in a statistically significant higher eGFR compared with the 2009 equation, with a median ΔeGFR of 4.1 ml·min− 1·1.73 m− 2 (Table S2), and a correlation coefficient r of 0.998 (p < 0.0001). With the 2021 equation, all participants with a 2009 eGFR level < 105 ml·min− 1·1.73 m− 2 had a higher eGFR value, whereas among the 1,373 with a 2009 eGFR level ≥ 105 ml·min− 1·1.73 m− 2, 293 (21.3%) had a lower eGFR value (Fig. 1). Density distributions were similar in males and females, with larger ΔeGFR in males; moreover, ΔeGFR was smallest in younger individuals and those with a 2009 eGFR < 30 ml·min− 1·1.73 m− 2 (G4-5) and largest for older individuals and those with a 2009 eGFR of 60–89 ml·min− 1·1.73 m− 2 (G2) (Table S2). Limits of agreement with Bland-Altman analysis were 1.14–6.54 ml·min− 1·1.73 m− 2 and varied according to sex and age and eGFR categories; moreover, rho and к were 0.98, and 0.83, indicating a substantial and strong agreement, respectively, and remained in these ranges in most subgroups (not shown).
Fig. 1.
ΔeGFR between the 2009 to the 2021 CKD-EPI equation in each participant. ΔeGFR = eGFR change
CKD prevalence and staging
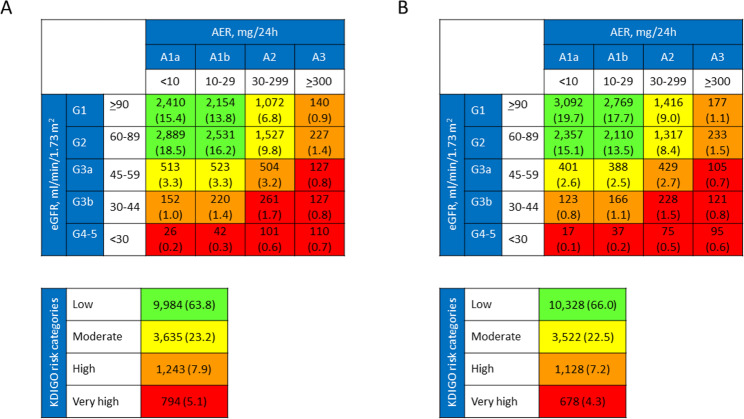
A total of 2,431 individuals (15.5%) in the whole cohort and 753 individuals (27.8%) among those with an eGFR < 60 ml·min− 1·1.73 m− 2 (G3a-5) were reclassified with the 2021 CKD-EPI equation (Table 1). The extent of reclassification was lowest from G4-5 to G3b category (19.7%) and highest from G3a to G2 category (31.3%). As 521 individuals were upgraded to G2, the number of participants with an eGFR < 60 ml·min− 1·1.73 m− 2 decreased from 2,706 (17.28%) to 2,185 (13.96%). Reclassified participants were only slightly younger, more frequently males, and had shorter diabetes duration, lower HbA1c, BMI, eWC, triglycerides, non-HDL cholesterol, albuminuria, and prevalence of dyslipidemia, hypertension, insulin, lipid lowering, anti-hypertensive, anti-coagulant, and anti-platelet treatment, advanced DR, CVD (any and by vascular bed), and higher HDL cholesterol and, by definition, eGFR (Table 2). Distribution of participants with the two equations and reclassification with the 2021 formula across KDIGO categories are shown in Fig. 2 and Table S3, respectively. The extent of reclassification was lowest from G4-5A1b to G3bA1b (11.9%) and highest from G3aA1b to G2A1b (37.1%). Since 344 individuals were upgraded from G3aA1 to G2A1, the number of participants with any CKD decreased from 5,672 (36.23%) to 5,328 (34.03%) and that of participants at very high risk from 794 (5.1%) to 678 (4.3%).
Table 1.
Reclassification of participants across eGFR categories with the 2021 CKD-EPI eGFR equation
| eGFR categories with the 2009 CKD-EPI equation (ml·min− 1·1.73 m− 2) | eGFR categories with the 2021 CKD-EPI equation (ml·min− 1·1.73 m− 2) | Total | ||||
|---|---|---|---|---|---|---|
| G1 ≥ 90 | G2 60–89 | G3a 45–59 | G3b 30–44 | G4-5 < 30 | ||
| G1 ≥ 90 | 5,776 (100) | 5,776 (36.9) | ||||
| G2 60–89 | 1,678 (23.4) | 5,496 (76.5) | 7,174 (45.8) | |||
| G3a 45–59 | 521 (31.3) | 1,146 (68.8) | 1,667 (10.6) | |||
| G3b 30–44 | 177 (23.3) | 583 (76.7) | 760 (4.9) | |||
| G4-5 < 30 | 55 (19.7) | 224 (80.3) | 279 (1.8) | |||
| Total | 7,454 (47.6) | 6,017 (38.4) | 1,323 (8.5) | 638 (4.1) | 224 (1.4) | 15,656 (100) |
| Reclassifed | 1,678 (23.4) | 753 (27.8) | 753 (27.8) | |||
Data are expressed as number of cases (percentage) of reclassified (black cells) and non-reclassified (white cells) participants in each 2009 eGFR category and among individuals with a 2009 eGFR 60–89 and < 60 ml·min− 1·1.73 m− 2. eGFR = estimated glomerular filtration rate; CKD-EPI = Chronic Kidney Disease Epidemiology Collaboration
Table 2.
Clinical features of reclassified and non-reclassified individuals with the 2021 CKD-EPI eGFR equation
| Variable | eGFR with the 2009 CKD-EPI equation | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| < 90 ml·min− 1·1.73 m− 2 (G2-G5; n = 9,880) | 60–89 ml·min− 1·1.73 m− 2 (G2; n = 7,174) | < 60 ml·min− 1·1.73 m− 2 (G3a-G5; n = 2,706) | |||||||
| Reclassified | Non-reclassified | p | Reclassified | Non-reclassified | p | Reclassified | Non-reclassified | p | |
| n (%) | 2,431 (24.6) | 7,449 (75.4) | 1,678 (23.4) | 5,496 (76.6) | 753 (27.8) | 1,953 (72.2) | |||
| Age, years | 70.2 ± 8.2 | 70.6 ± 9.1 | < 0.0001 | 68.6 ± 7.5 | 69.5 ± 8.9 | < 0.0001 | 74.0 ± 8.30 | 73.8 ± 8.7 | 0.586 |
| Gender, n (%) | 0.005 | 0.041 | 0.007 | ||||||
| Females | 1,008 (41.5) | 3,334 (44.8) | 663 (39.5) | 2,326 (42.3) | 345 (45.8) | 1,008 (51.6) | |||
| Males | 1,423 (58.5) | 4,115 (55.2) | 1,015 (60.5) | 3,170 (57.7) | 408 (54.2) | 945 (48.4) | |||
| Smoking status, n (%) | 0.270 | 0.335 | 0.452 | ||||||
| Never | 1,378 (56.7) | 4,317 (58.0) | 949 (56.6) | 3,156 (57.4) | 429 (57.0) | 1,161 (59.4) | |||
| Former | 731 (30.1) | 2,234 (30.0) | 491 (29.3) | 1,637 (29.8) | 240 (31,9) | 597 (30.6) | |||
| Current | 322 (13.2) | 808 (12.0) | 238 (14.2) | 703 (12.8) | 84 (11.2) | 105 (10.0) | |||
| Diabetes duration, years | 14.6 ± 10.6 | 15.1 ± 10.7 | 0.032 | 13.4 ± 10.0 | 14.3 ± 10.4 | 0.001 | 17.1 ± 11.3 | 17.3 ± 11.1 | 0.788 |
| HbA1c, % | 7.47 ± 1.41 | 7.58 ± 1.48 | 0.002 | 7.40 ± 1.36 | 7.53 ± 1.45 | 0.001 | 7.65 ± 1.51 | 7,72 ± 1.56 | 0.242 |
| (mmol·mol− 1) | (58.2 ± 15.5) | (59.4 ± 16.2) | (57.3 ± 14.9) | (58.8 ± 15.8) | (60.1 ± 16.5) | (60.9 ± 17.1) | |||
| BMI, kg 2− 2 | 28.5 ± 4.7 | 28.9 ± 5.0 | < 0.0001 | 28.40 ± 4.70 | 28.81 ± 4.91 | 0.002 | 28.68 ± 4.78 | 29.29 ± 5.19 | 0.006 |
| eWC, cm | 101.6 ± 9.6 | 102.4 ± 10.0 | < 0.0001 | 101.4 ± 9.7 | 102.2 ± 9.9 | 0.006 | 101.8 ± 9.6 | 102.9 ± 10.4 | 0.011 |
| Triglycerides, mmol·l− 1 | 1.29 (0.93–1.79) | 1.37 (1.00-1.94) | < 0.0001 | 1.22 (0.89–1.70) | 1.33 (0.96–1.84) | < 0.0001 | 1.45 (1.07–2.01) | 1.53 (1.14–2.17) | 0.005 |
| Total cholesterol, mmol·l− 1 | 4.74 ± 0.97 | 4.77 ± 0.99 | 0.145 | 4.72 ± 0.95 | 4.77 ± 0.97 | 0.063 | 4.78 ± 1.02 | 4.78 ± 1.06 | 0.968 |
| HDL cholesterol, mmol·l− 1 | 1.30 ± 0.35 | 1.28 ± 0.35 | 0.041 | 1.32 ± 0.35 | 1.30 ± 0.35 | 0.016 | 1.25 ± 0.36 | 1.23 ± 0.36 | 0.420 |
| Non-HDL cholesterol, mmol·l− 1 | 3.44 ± 0.93 | 3.49 ± 0.95 | 0.020 | 3.40 ± 0.92 | 3.47 ± 0.93 | 0.004 | 2.77 ± 0.84 | 2.74 ± 0.84 | 0.409 |
| LDL cholesterol, mmol·l− 1 | 2.76 ± 0.85 | 2.77 ± 0.85 | 0.878 | 2.76 ± 0.84 | 2.78 ± 0.84 | 0.518 | 3.53 ± 0.96 | 3.55 ± 1.00 | 0.752 |
| Dyslipidaemia, n (%) | 1,955 (80.4) | 6,241 (83.8) | < 0.0001 | 1,331 (79.3) | 4,605 (83.8) | < 0.0001 | 624 (82.9) | 1,636 (83.8) | 0.572 |
| Systolic BP, mmHg | 139.4 ± 17.9 | 139.5 ± 18.5 | 0.864 | 139.2 ± 17.4 | 139.5 ± 18.2 | 0.570 | 139.9 ± 19.0 | 139.5 ± 19.3 | 0.636 |
| Diastolic BP, mmHg | 78.3 ± 9.4 | 78.4 ± 9.5 | 0.679 | 78.6 ± 9.2 | 78.7 ± 9.4 | 0.752 | 77.6 ± 9.7 | 77.5 ± 10.0 | 0.855 |
| Pulse pressure, mmHg | 61.1 ± 15.7 | 61.1 ± 16.3 | 0.961 | 60.6 ± 15.2 | 60.8 ± 15.9 | 0.635 | 62.4 ± 16.9 | 62.1 ± 17.2 | 0.671 |
| Hypertension, n (%) | 2,110 (86.8) | 6,648 (89.2) | 0.001 | 1,417 (84.4) | 4,801 (87.4) | 0.002 | 693 (92.0) | 1,847 (94.6) | 0.014 |
| Anti-hyperglycaemic treatment, n (%) | 0.001 | 0.036 | < 0.0001 | ||||||
| Lifestyle only | 317 (13.0) | 948 (12.7) | 232 (13.8) | 780 (14.2) | 85 (11.3) | 168 (8.6) | |||
| Non-insulin | 1,526 (62.8) | 4,412 (59.2) | 1,097 (65.4) | 3,418 (62.2) | 429 (57.0) | 994 (50.9) | |||
| Insulin | 588 (22.5) | 2,089 (28.0) | 349 (20.8) | 1,298 (23.6) | 239 (31.7) | 791 (40.5) | |||
| Lipid-lowering treatment, n (%) | 1,142 (47.0) | 3,749 (50.3) | 0.004 | 741 (44.2) | 2,673 (48.6) | 0.001 | 401 (53.3) | 1,076 (55.1) | 0.389 |
| Anti-hypertensive treatment, n (%) | 1,806 (74.3) | 5,856 (78.6) | < 0.0001 | 1,177 (70.1) | 4,099 (74.6) | < 0.0001 | 629 (83.5) | 1757 (90.0) | < 0.0001 |
| Anti-platelet treatment, n (%) | 1,052 (43.3) | 3,432 (46.1) | 0.016 | 667 (39.7) | 2,336 (42.5) | 0.045 | 385 (51.1) | 1,096 (56.1) | 0.019 |
| Anti-coagulant treatment, n (%) | 116 (4.8) | 439 (5.9) | 0.037 | 48 (2.9) | 263 (4.8) | 0.001 | 68 (9.0) | 176 (9.0) | 0.988 |
| Albuminuria, mg/24 hours | 14.0 (7.0-34.6) | 15.0 (6.9–42.3) | 0.020 | 12.3 (6.5–27.0) | 12.9 (6.2–30.0) | 0.204 | 20.6 (9.5–77.5) | 27.0 (9.8-119.6) | 0.002 |
| Serum creatinine, µmol/l | 87.0 ± 34.1 | 98.8 ± 37.9 | < 0.0001 | 74.7 ± 10.2 | 86.0 ± 12.3 | < 0.0001 | 112.2 ± 38.4 | 134.5 ± 57.5 | < 0.0001 |
| eGFR (2009 CKD-EPI), ml·min− 1·1.73 m− 2 | 76.7 ± 17.0 | 65.9 ± 15.8 | < 0.0001 | 87.5 ± 1.5 | 73.9 ± 7.12 | < 0.0001 | 52.6 ± 9.0 | 43.4 ± 10.9 | < 0.0001 |
| eGFR (2021 CKD-EPI), ml·min− 1·1.73 m− 2 | 81.2 ± 17.6 | 70.0 ± 16.6 | < 0.0001 | 92.4 ± 1.4 | 78.4 ± 7.4 | < 0.0001 | 56.2 ± 9.6 | 46.4 ± 11.6 | < 0.0001 |
| DR, n (%) | 0.021 | 0.512 | 0.001 | ||||||
| No | 1,871 (77.0) | 5,591 (75.1) | 1,324 (78.9) | 4,294 (78.1) | 547 (72.64) | 1,297 (66.41) | |||
| Non-advanced | 333 (13.7) | 1,013 (13.6) | 215 (12.8) | 696 (12.7) | 118 (15.67) | 317 (16.23) | |||
| Advanced | 227 (9.3) | 845 (11.3) | 139 (8.3) | 506 (9.2) | 88 (11.69) | 339 (17.36) | |||
| CVD, n (%) | |||||||||
| Any | 585 (24.1) | 2,160 (29.0) | < 0.0001 | 325 (19.4) | 1,363 (24.8) | < 0.0001 | 260 (34.5) | 797 (40.8) | 0.003 |
| Acute myocardial infarction | 281 (11.6) | 1,047 (14.1) | 0.002 | 152 (9.1) | 658 (12.0) | 0.001 | 129 (17.1) | 389 (19.9) | 0.099 |
| Coronary revascularization | 238 (9.8) | 930 (12.5) | < 0.0001 | 130 (7.7) | 588 (10.7) | < 0.0001 | 108 (14.3) | 342 (17.5) | 0.047 |
| Any coronary event | 381 (15.7) | 1,424 (19.1) | < 0.0001 | 210 (12.5) | 895 (16.3) | < 0.0001 | 171 (22.7) | 529 (27.1) | 0.020 |
| Stroke | 88 (3.6) | 326 (4.4) | 0.106 | 48 (2.9) | 207 (3.8) | 0.079 | 40 (5.3) | 119 (6.1) | 0.439 |
| Carotid revascularization | 134 (5.5) | 543 (7.3) | 0.003 | 57 (3.4) | 325 (5.9) | < 0.0001 | 77 (10.2) | 218 (11.2) | 0.484 |
| Any cerebrovascular event | 210 (8.6) | 815 (10.9) | 0.001 | 102 (6.1) | 500 (9.1) | < 0.0001 | 108 (14.3) | 315 (16.1) | 0.251 |
| Ulcer/gangrene/amputation | 84 (3.5) | 343 (4.6) | 0.021 | 42 (2.5) | 190 (3.5) | 0.053 | 42 (5.6) | 153 (7.8) | 0.042 |
| Lower limb revascularization | 81 (3.3) | 293 (3.9) | 0.177 | 37 (2.2) | 167 (3.0) | 0.072 | 44 (5.8) | 126 (6.5) | 0.559 |
| Any peripheral event | 151 (6.2) | 559 (7.5) | 0.032 | 74 (4.4) | 325 (5.9) | 0.019 | 77 (10.2) | 234 (12.0) | 0.199 |
| Comorbidities, n (%) | |||||||||
| Any | 456 (18.8) | 1,398 (18.8) | 0.991 | 297 (17.7) | 953 (17.3) | 0.734 | 159 (21.1) | 445 (22.8) | 0.350 |
| COPD | 110 (4.5) | 365 (4.9) | 0.453 | 63 (3.8) | 213 (3.9) | 0.821 | 47 (6.2) | 152 (7.8) | 0.169 |
| Liver disease | 209 (8.6) | 646 (8.7) | 0.909 | 139 (8.3) | 455 (8.3) | 0.995 | 70 (9.3) | 191 (9.8) | 0.702 |
| Cancer | 177 (7.3) | 554 (7.4) | 0.798 | 120 (7.2) | 390 (7.1) | 0.938 | 57 (7.6) | 164 (8.4) | 0.481 |
Data are expressed as mean ± SD or median (interquartile range), for continuous variables, and number of cases (percentage), for categorical variables. eGFR = estimated glomerular filtration rate; CKD-EPI = Chronic Kidney Disease Epidemiology Collaboration; HbA1c = haemoglobin A1c; BMI = body mass index; eWC = estimated waist circumference; BP = blood pressure; DR = diabetic retinopathy; CVD = cardiovascular disease; COPD = chronic obstructive pulmonary disease
Fig. 2.
Distribution of participants across KDIGO categories with eGFR calculated with the 2009 (A) and the 2021 (B) CKD-EPI equations. KDIGO = Kidney Disease Improving Global Outcomes; eGFR = estimated glomerular filtration rate; CKD-EPI = Chronic Kidney Disease Epidemiology Collaboration
Risk of death from any cause and mortality prediction
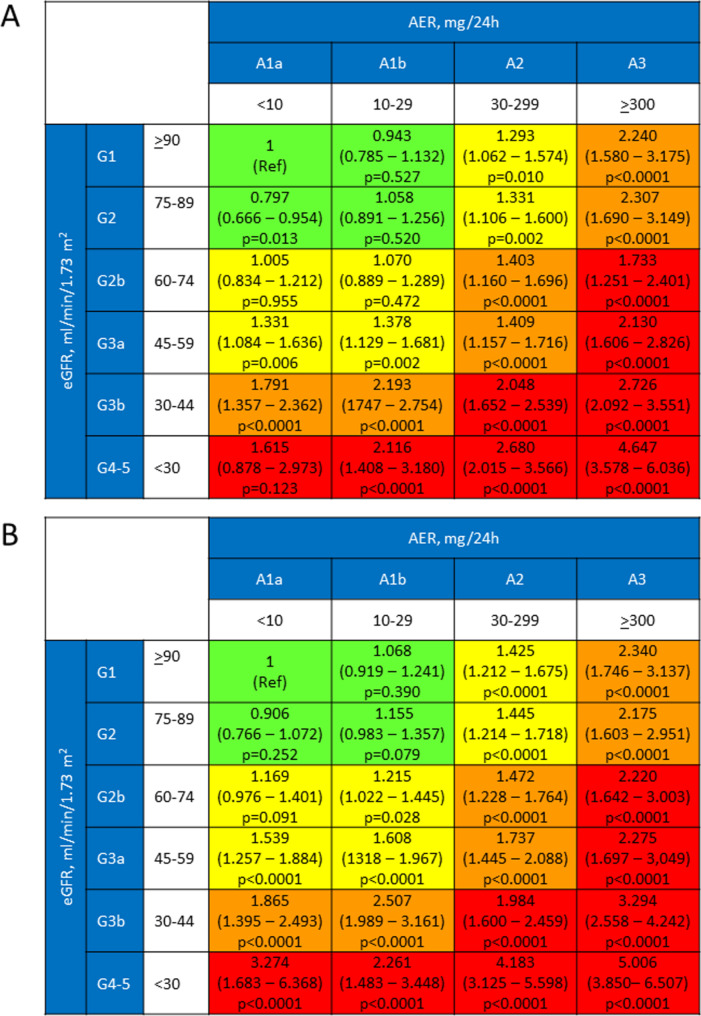
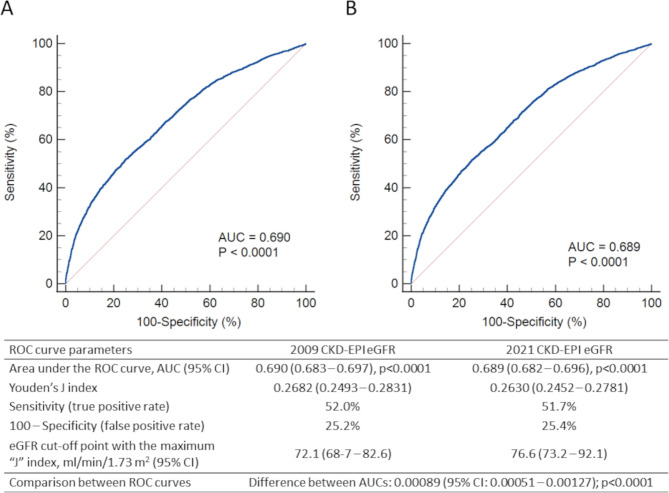
As previously reported, valid information on vital status was retrieved for 15,656 participants (99.3%); of these individuals, 12,054 (76.99%) were alive, whereas 3,602 (23.01%) had deceased (follow-up duration: 7.42 ± 2.05 years; death rate: 31.02 per 1,000 person-years [20, 25]. When using the 2009 G1 category as reference, the HR for reclassified participants was lower than that for non-reclassified participants in each 2009 eGFR category (Table S4). When using each 2009 eGFR category as reference, risk of death was lower for reclassified versus non-reclassified individuals in all categories, though difference was significant only in the G4-G5 category (Table 3). When mortality risk was analyzed according to KDIGO categories, a significantly higher risk was observed in patients with normal or mildly increased albuminuria (A1a and A1b, respectively) only for a 2009 eGFR < 60 ml·min− 1·1.73 m− 2. In contrast, when using the 2021 CKD-EPI equation, the HR was significantly higher also in the G2bA1b category, i.e., in participants with an eGFR < 75 ml·min− 1·1.73 m− 2 and an AER 10–29 mg/24 h (Fig. 3). There was a statistically, but not clinically significant difference between the areas under the curve with the two equations, with similar Jouden’s J statistic (Fig. 4).
Table 3.
Numbers and percentages of deaths, Kaplan-Meier estimates, and hazard ratios for all-cause mortality for non-reclassified and reclassified participants with the 2021 CKD-EPI eGFR equation by Cox proportional hazards regression with backward selection of variables using the by Cox proportional hazards regression with backward selection of variables for all-cause mortality for non-reclassified versus reclassified participants with the 2021 CKD-EPI eGFR equation using the corresponding 2009 CKD-EPI eGFR category as reference
| eGFR category | n | Deaths n (%) |
Kaplan-Meier log rank, p |
Cox proportional hazards regression with backward selection of variables Hazard ratio (95% confidence interval) and p value |
||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 2a | Model 3 | Model 3a | ||||
| G2 | 1,678 vs. 5,496 | 341 (20.3%) vs. 1312 (23.9%) |
9.009 p = 0.003 |
0.83 (0.74–0.94) p = 0.003 |
0.96 (0.85–1.09) p = 0.524 |
1.08 (0.92–1.26) p = 0.362 |
0.98 (0.87–1.11) p = 0.796 |
1.04 (0.89–1.22) p = 0.616 |
| G3a | 521 vs. 1,146 | 186 (35.7%) vs. 466 (41.7%) |
3.881 p = 0.049 |
0.84 (0.71–0.99) p = 0.049 |
0.84 (0.71–0.99) p = 0.049 |
1.23 (0.94–1.61) p = 0.136 |
0.89 (0.75–1.06) p = 0.190 |
1.18 (0.90–1.56) p = 0.229 |
| G3b | 177 vs. 583 | 95 (53.7%) vs. 339 (58.1%) |
0.666 p = 0.414 |
0.91 (0.72–1.14) p = 415 |
0.86 (0.68–1.07) p = 0.177 |
0.99 (0.73–1.35) p = 0.945 |
0.95 (0.75–1.20) p = 0.661 |
1.03 (0.75–1.43) p = 0.836 |
| G4-5 | 55 vs. 224 | 24 (43.6%) vs. 163 (72.8%) |
11.604 p < 0.0001 |
0.48 (0.31–0.74) p < 0.0001 |
0.45 (0.29–0.69) p < 0.0001 |
0.60 (0.37–0.97) p = 0.039 |
0.44 (0.28–0.71) p < 0.0001 |
0.56 (0.33–0.95) p = 0.031 |
Model 1: not adjusted; model 2: adjusted for age; model 3: further adjusted for CVD risk factors and complications/comorbidities, i.e., age, sex, smoking status, diabetes duration, HbA1c, BMI, triglycerides, total cholesterol and HDL-cholesterol, systolic and diastolic BP, anti-hyperglycemic, lipid-lowering, and anti-hypertensive treatment, albuminuria categories, DR grade, any CVD, and any comorbidity; model 2a: adjusted as in model 2 plus the 2009 CKD-EPI eGFR level; model 3a: adjusted as in model 3 plus 2009 CKD-EPI eGFR level. CKD-EPI = Chronic Kidney Disease Epidemiology Collaboration; eGFR = estimated glomerular filtration rate; HbA1c = haemoglobin A1c; BMI = body mass index; BP = blood pressure; DR = diabetic retinopathy; CVD = cardiovascular disease
Fig. 3.
Survival analysis by Cox proportional hazard regression, adjusted for multiple confounders, according to KDIGO risk categories and subcategories using the eGFR values calculated with the 2009 (A) and the 2021 (B) CKD-EPI equations. KDIGO = Kidney Disease Improving Global Outcomes; eGFR = estimated glomerular filtration rate; CKD-EPI = Chronic Kidney Disease Epidemiology Collaboration
Fig. 4.
ROC curves for prediction of all-cause mortality according to the eGFR values calculated with the 2009 (A) and 2021 (B) CKD-EPI equations. ROC = Receiver Operator Characteristic; eGFR = estimated glomerular filtration rate; CKD-EPI = Chronic Kidney Disease Epidemiology Collaboration. e Epidemiology Collaboration
Discussion
This study analyzed the impact of switching from the 2009 to the 2021 CDK-EPI equation for estimating GFR in the non-Black cohort of people with type 2 diabetes from the RIACE Italian Multicenter Study. On the one hand, it confirmed that, in these individuals, the new, no-race formula yield higher eGFR values than the old one and, hence, reclassifies a significant number of them to a better eGFR category with consequent reduction of the proportion of those with impaired eGFR or any CKD. On the other hand, this analysis provided important new information on the prognostic implications of using the new CKD-EPI equation in a non-Black population of individuals with type 2 diabetes.
The 4.1 ml·min− 1·1.73 m− 2 median increase in eGFR, with higher increments in males, older individuals and those in the G2 category, is consistent with the 3.9 ml·min− 1·1.73 m− 2 median increase, with similar differences across subgroups, reported in a Swedish, predominantly White general population sample [16]. Another study in adult people with CKD from British Columbia, Canada reported a lower increase in eGFR (2.7 ml·min− 1·1.73 m− 2) [26], which however is similar to that observed in Jordanian individuals with type 2 diabetes (2.1 ml·min− 1·1.73 m− 2) [27] and in the RIACE participants with CKD. The extent of reclassification is also similar to that reported in individuals with diabetes in the Jordanian (20%) and the Swedish study (15.5% and 27.8% versus 15.3% and 32.4% of all participants and those with an eGFR < 60 ml·min− 1·1.73 m− 2, respectively), though different from that observed in the whole Swedish cohort (9.9% and 36.2%, respectively) [16] and in Jordanian individuals with type 2 diabetes (2.1 ml·min− 1·1.73 m− 2) [27]. Likewise, the 3.3% reduction in the prevalence of eGFR < 60 ml·min− 1·1.73 m− 2 is almost identical to that reported in the diabetic subgroup in the Swedish study (3.2%) [16], but higher than that observed in non-Black individuals from the general population, ranging from 1.3% [16, 28] to 1.6% [12], likely due to the higher burden from CKD in people with diabetes.
More importantly, in the Swedish study, risks for ESKD, all-cause death, and morbidity and mortality from CVD were lower in reclassified versus non-reclassified individuals in the unadjusted analysis, but became higher, except for ESKD, when adjusting for the 2009 eGFR level and remained significantly, though modestly higher (+ 4–11%) in individuals with a 2009 eGFR ≥ 30 ml·min− 1·1.73 m− 2 when further adjusting for age [16]. Furthermore, an analysis of the predominantly Caucasian Northeastern Italian general population from the Initiative on Nephropathy, of relevance to public health, which is Chronic, possibly in its Initial stages, and carries a Potential risk of major clinical End-points (INCIPE) study showed that the mortality risk of individuals reclassified to non-CKD was more similar to non-reclassified confirmed CKD than to confirmed non-CKD individuals [29]. These findings would imply that reclassification to a better eGFR category might be “harmful” by causing delayed care, less aggressive treatment and late referral of individuals with a risk of death that is not lower, if anything, than that of people remaining in the lower eGFR category [8]. However, in the INCIPE study, reclassified individuals to non-CKD were compared with n confirmed non-CKD group that included not only people with confirmed G2 category but also the much larger sample of those with confirmed G 1 category, who have at low risk of death [29]. Moreover, in the Swedish study, the increased mortality risk of reclassified individuals was attributed to the fact that, despite higher eGFR values, they were older and, consequently, had a higher prevalence of comorbidities and use of medications than non-reclassified individuals. However, these differences were likely due to the fact that the non-reclassified group inappropriately included individuals falling in the G1 category with the 2009 equation, who cannot be reclassified to a better eGFR category and were not considered when comparing the two groups for outcomes [16]. Conversely, our study showed that individuals reclassified to a better eGFR category with the 2021 equation had lower mortality risk across all eGFR categories than those who were not reclassified, though the difference was statistically significant only in those with a 2009 eGFR < 30 ml·min− 1·1.73 m− 2. The lower risk of death of reclassified individuals was in keeping with the better cardiometabolic risk profile and the lower prevalence of complications and treatments, indicating that reclassification with the new equation is appropriate and “safe” by moving to a better eGFR category those who are relatively healthier and leaving in the lower eGFR category those who are relatively sicker. These findings are consistent with those of a recent report form the Danish general population showing that people in the CKD range with the new equation had a higher mortality rate than those with the old Eq. (8.8% versus 7.9%), likely due to reclassification to the G2 category of 24.2% of individuals who were at lower risk [28].
Due to differences in risk profile between reclassified and non-reclassified individuals, differences in mortality among KDIGO categories were more marked with the new formula compared with the old one. This was particularly relevant for participants with mildly elevated albuminuria falling in the G2b category with the 2021 equation, who showed a significantly increased risk of death, at variance with the 2009 equation. This finding is in keeping with a large body of epidemiological surveys showing that risk for all-cause and CVD mortality, CVD events and ESKD starts to increase for an eGFR < 75 ml·min− 1·1.73 m− 2 [30–33]. This is also consistent with the 78 ml·min− 1·1.73 m− 2 eGFR threshold below which the CVD prevalence was found to increase in the RIACE cohort [22].
Strength of our study include the large sample size, the completeness of baseline and follow-up data and the assessment of a wide range of clinical parameters which allowed accounting for several confounders. However, there are several limitations. First, the lack of information on the causes of death did not allow detecting differences in CVD versus non-CVD deaths. Second, the lack of cystatin C measurements did not allow comparing the performance of the creatinine-based and the combined creatinine-cystatin C 2021 CKD-EPI equations. Third, the new anti-hyperglycemic drugs reducing mortality by providing cardiorenal protection [34] were not available at the time of enrolment and their use was very limited during the follow-up. Fourth, results may have been affected by unmeasured confounders that can affect mortality. Fifth, the study findings may not be applicable to the general ambulatory population with type 2 diabetes, but only to individuals attending outpatients diabetes clinics in Italy. Finally, potential limitations concerning non-centralized measurements have been extensively addressed in previous publication [17, 18, 22].
In conclusion, using the new CKD-EPI equation in non-Black individuals with type 2 diabetes resulted in higher eGFR values and, hence, lower CKD prevalence, due to reclassification of a significant proportion of them to a better eGFR category. Reclassification appears appropriate as reclassified people showed a lower risk of death than those who were not reclassified, especially if they were in the low eGFR range, and differences in mortality among eGFR and KDIGO categories were more marked using the new formula, though mortality prediction was similar with the two equations. These findings suggest that the 2021 CKD-EPI formula can be safely implemented also in predominantly non-Black populations, though further studies are needed to evaluate the effect of switching to the new formula on prognostic, diagnostic, and therapeutic issues either in the general population or in people with type 2 diabetes.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Additional file 1. The RIACE Study Group. List of the RIACE Investigators.
Additional file 2: Table S1. The 2009 and 2021 CKD-EPI equations for calculating eGFR.
Additional file 3: Table S2. Comparison of the 2009 and 2021 CKD-EPI equations for calculating eGFR in the whole cohort and in pre-specified subgroups.
Additional file 4: Table S3. Reclassification of participants across KDIGO categories with the 2021 CKD-EPI eGFR equation.
Additional file 5: Table S4. Hazard ratios for all-cause mortality for non-reclassified and reclassified participants with the 2021 CKD-EPI eGFR equation by Cox proportional hazards regression with backward selection of variables using the 2009 CKD-EPI G1 category as reference.
Additional file 6: Figure S1. Distribution of eGFR values using the 2009 and 2021 CKD-EPI equations.
Acknowledgements
The Authors thank the RIACE Investigators for participating in this study (see the complete list in the Additional file 1: The RIACE Study Group).
Abbreviations
- AER
Albumin excretion
- BMI
Body mass index
- BP
Blood pressure
- CI
Confidence interval
- CKD
Chronic kidney disease
- CKD-EPI
Chronic kidney disease epidemiology collaboration
- CVD
Cardiovascular disease
- DR
Diabetic retinopathy
- eGFR
Estimated glomerular filtration rate
- ΔeGFR
Estimated glomerular filtration rate change
- ESKD
End-stage kidney disease
- eWC
Estimated waist circumference
- GFR
Glomerular filtration rate
- HbA1c
Hemoglobin A1c
- HR
Hazard ratio
- KDIGO
Kidney disease improving global outcomes
- RIACE
Renal Insufficiency and Cardiovascular events
- ROC
Receiver operator characteristic
Author contributions
MG, MVi, GPe, AS, EO, and GPu conceived and designed the study. All authors contributed to data acquisition, analysis, or interpretation. GPu drafted the article and had full access to all the data and took responsibility for the integrity of data and accuracy of the data analysis in this study. MG, MVi, GPe, AS, EO, VG, EB, CF, RT, MVe, and AN revised the manuscript critically for essential intellectual content. All authors approved the submitted version of the manuscript and agreed to be personally accountable for the individual’s own contributions and to ensure that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Funding
This research was supported by the Research Foundation of the Italian Diabetes Society (Diabete Ricerca) and the Diabetes, Endocrinology and Metabolism (DEM) Foundation, and by unconditional grants from Eli-Lilly, Sigma-Tau, Takeda, Chiesi Farmaceutici, and Boehringer-Ingelheim. The funding sources had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki. The research protocol was approved by the ethics committee of the coordinating centre (Sant’Andrea Hospital, Rome, Italy) on 25 September 2006 (number 43/2006) and subsequently by the ethics committee of each participating centre. Participants provided an informed consent.
Consent for publication
Not applicable.
Competing interests
MG reports receiving consultant fees from Eli Lilly, and lecture fees from Eli Lilly, Merck Sharp & Dohme, and Novo Nordisk. MVi reports receiving lecture fees from MundiPharma and Novo Nordisk. GPe reports receiving consultant fees from Bayer and Eli Lilly, and lecture fees from AstraZeneca, Boerhinger Ingelheim, Eli-Lilly, Merck Sharp & Dohme, Mundipharma, Novo Nordisk, and Takeda. AS reports receiving consultant fees from Axxam, Bayer, and Novo Nordisk, and lecture fees from Eli Lilly, Novo Nordisk, and Sanofi-Aventis. EO reports receiving consultant fees from Eli Lilly and Novo Nordisk, and lecture fees from Astellas. VG reports receiving lecture fees from Abbot, Astra-Zeneca, Medtronic, Novo Nordisk, Sanofi-Aventis, Theras, and Vertex. EB reports receiving consultant fees from Abbott, Bayer, Becton Dickinson, Boehringer Ingelheim, Daiichi-Sankyo, Eli Lilly, and Novo Nordisk. CF reports receiving lecture fees from Abbot, Boehringer Ingelheim, Daiichi Sankyo, Eli Lilly, Merck Sharp & Dohme, Mundipharma, and Theras Lifetech. RT reports receiving consultant fees from AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme, Novo Nordisk, and Sanofi-Aventis, and lecture fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly, and Novo Nordisk. MVe reports receiving lecture fees from Lifescan and Novo Nordisk. AN reports receiving grants from Artsana, Astra-Zeneca, Eli Lilly, Novo Nordisk, and Sanofi Aventis and personal fees from Eli Lilly and Novo Nordisk. GPu reports receiving consultant fees from Abbot, Bayer, and Novo Nordisk, and lecture fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Mundipharma, and Novo Nordisk.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Levey AS, Grams ME, Inker LA. Uses of GFR and albuminuria level in acute and chronic kidney disease. N Engl J Med. 2022;386:2120–8. [DOI] [PubMed] [Google Scholar]
- 2.Warwick J, Holness J. Measurement of glomerular filtration rate. Semin Nucl Med. 2022;52:453–66. [DOI] [PubMed] [Google Scholar]
- 3.Traynor J, Mactier R, Geddes CC, Fox JG. How to measure renal function in clinical practice. BMJ. 2006;333:733–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function–measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–83. [DOI] [PubMed] [Google Scholar]
- 5.Florkowski CM, Chew-Harris JS. Methods of estimating GFR—different equations including CKD-EPI. Clin Biochem Rev. 2011;32:75–9. [PMC free article] [PubMed] [Google Scholar]
- 6.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 7.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams P. Retaining race in chronic kidney disease diagnosis and treatment. Cureus. 2023;15:e45054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delgado C, Baweja M, Burrows NR, Crews DC, Eneanya ND, Gadegbeku CA, et al. Reassessing the inclusion of race in diagnosing kidney diseases: an interim report from the NKF-ASN Task Force. J Am Soc Nephrol. 2021;32:1305–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sehgal AR. Race and the false Precision of glomerular filtration rate estimates. Ann Intern Med. 2020;173:1008–9. [DOI] [PubMed] [Google Scholar]
- 11.Gopalakrishnan C, Patorno E. Time to end the misuse of race in medicine: cases from nephrology. BMJ. 2021;375:n2435. [DOI] [PubMed] [Google Scholar]
- 12.Inker LA, Eneanya ND, Coresh J, Tighiouart H, Wang D, Sang Y, et al. New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med. 2021;385:1737–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delgado C, Baweja M, Crews DC, Eneanya ND, Gadegbeku CA, Inker LA, et al. A unifying approach for GFR estimation: recommendations of the NKF-ASN task force on reassessing the inclusion of race in diagnosing kidney disease. Am J Kidney Dis. 2022;79:268–e2881. [DOI] [PubMed] [Google Scholar]
- 14.Gama RM, Kalyesubula R, Fabian J, Mahalingasivam V. NICE takes ethnicity out of estimating kidney function. BMJ. 2021;374:n2159. [DOI] [PubMed] [Google Scholar]
- 15.Eurostat. Migration and migrant population statistics. Available online at https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Migration_and_migrant_population_statistics (accessed July 13, 2024).
- 16.Fu EL, Coresh J, Grams ME, Clase CM, Elinder CG, Paik J, et al. Removing race from the CKD-EPI equation and its impact on prognosis in a predominantly white European population. Nephrol Dial Transpl. 2023;38:119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Penno G, Solini A, Bonora E, Fondelli C, Orsi E, Zerbini G, et al. Clinical significance of nonalbuminuric renal impairment in type 2 diabetes. J Hypertens. 2011;29:1802–9. [DOI] [PubMed] [Google Scholar]
- 18.Penno G, Solini A, Bonora E, Fondelli C, Orsi E, Zerbini G, et al. Gender differences in cardiovascular disease risk factors, treatments and complications in patients with type 2 diabetes: the RIACE Italian multicentre study. J Intern Med. 2013;274:176–91. [DOI] [PubMed] [Google Scholar]
- 19.Pugliese G, Solini A, Fondelli C, Trevisan R, Vedovato M, Nicolucci A, et al. Reproducibility of albuminuria in type 2 diabetic subjects. Findings from the Renal Insufficiency and Cardiovascular events (RIACE) study. Nephrol Dial Transpl. 2011;26:3950–4. [DOI] [PubMed] [Google Scholar]
- 20.Penno G, Solini A, Orsi E, Bonora E, Fondelli C, Trevisan R, et al. Non-albuminuric renal impairment is a strong predictor of mortality in individuals with type 2 diabetes: the Renal Insufficiency and Cardiovascular events (RIACE) Italian multicentre study. Diabetologia. 2018;61:2277–89. [DOI] [PubMed] [Google Scholar]
- 21.Orsi E, Solini A, Bonora E, Vitale M, Garofolo M, Fondelli C, et al. Retinopathy as an independent predictor of all-cause mortality in individuals with type 2 diabetes. Diabetes Metab. 2023;49:101413. [DOI] [PubMed] [Google Scholar]
- 22.Solini A, Penno G, Bonora E, Fondelli C, Orsi E, Arosio M, et al. Diverging association of reduced glomerular filtration rate and albuminuria with coronary and noncoronary events in patients with type 2 diabetes: the renal insufficiency and cardiovascular events (RIACE) Italian multicenter study. Diabetes Care. 2012;35:143–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orsi E, Solini A, Bonora E, Fondelli C, Trevisan R, Vedovato M, et al. Haemoglobin A1c variability is a strong, independent predictor of all-cause mortality in patients with type 2 diabetes. Diabetes Obes Metab. 2018;20:1885–93. [DOI] [PubMed] [Google Scholar]
- 24.Koutsoyiannis D. Replacing histogram with smooth empirical probability density function estimated by K-moments. Sci. 2022;4:50. [Google Scholar]
- 25.Penno G, Solini A, Bonora E, Orsi E, Fondelli C, Zerbini G, et al. Defining the contribution of chronic kidney disease to all-cause mortality in patients with type 2 diabetes: the Renal Insufficiency and Cardiovascular events (RIACE) Italian Multicenter Study. Acta Diabetol. 2018;55:603–12. [DOI] [PubMed] [Google Scholar]
- 26.Atiquzzaman M, Er L, Djurdjev O, Bevilacqua M, Elliott M, Birks PC, et al. Implications of implementing the 2021 CKD-EPI equation without race on managing patients with kidney disease in British Columbia, Canada. Kidney Int Rep. 2024;9:830–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farah RI, Alhajahjeh A, Al-Farahid O, Abuzaid H, Hiasat D, Rayyan R, et al. Comparison and evaluation of the 2009 and 2021 chronic kidney disease-epidemiological collaboration equations among Jordanian patients with type 2 diabetes mellitus. Acta Diabetol. 2024;61:169–80. [DOI] [PubMed] [Google Scholar]
- 28.Vestergaard SV, Heide-Jørgensen U, Birn H, Christiansen CF. Effect of the refitted race-free eGFR formula on the CKD prevalence and mortality in the Danish Population. Clin J Am Soc Nephrol. 2022;17:426–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferraro PM, Lombardi G, Gambaro G. Impact of the new, race-free CKD-EPI equation on prevalence and clinical outcomes of CKD in northeastern Italy: the INCIPE study. J Nephrol. 2022;35:1767–9. [DOI] [PubMed] [Google Scholar]
- 30.Fox CS, Matsushita K, Woodward M, Bilo HJ, Chalmers J, Heerspink HJ, et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet. 2012;380:1662–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsushita K, Coresh J, Sang Y, Chalmers J, Fox C, Guallar E, et al. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol. 2015;3:514–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey A, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int. 2011;79:1341–52. [DOI] [PubMed] [Google Scholar]
- 33.Gansevoort RT, Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, et al. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int. 2011;80:93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riley DR, Essa H, Austin P, Preston F, Kargbo I, Ibarburu GH, et al. All-cause mortality and cardiovascular outcomes with sodium-glucose co-transporter 2 inhibitors, glucagon-like peptide-1 receptor agonists and with combination therapy in people with type 2 diabetes. Diabetes Obes Metab. 2023;25:2897–909. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. The RIACE Study Group. List of the RIACE Investigators.
Additional file 2: Table S1. The 2009 and 2021 CKD-EPI equations for calculating eGFR.
Additional file 3: Table S2. Comparison of the 2009 and 2021 CKD-EPI equations for calculating eGFR in the whole cohort and in pre-specified subgroups.
Additional file 4: Table S3. Reclassification of participants across KDIGO categories with the 2021 CKD-EPI eGFR equation.
Additional file 5: Table S4. Hazard ratios for all-cause mortality for non-reclassified and reclassified participants with the 2021 CKD-EPI eGFR equation by Cox proportional hazards regression with backward selection of variables using the 2009 CKD-EPI G1 category as reference.
Additional file 6: Figure S1. Distribution of eGFR values using the 2009 and 2021 CKD-EPI equations.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.