Abstract
Background
Females with diminished ovarian reserve (DOR) have significantly lower cumulative live birth rates (CLBRs) than females with normal ovarian reserve. A subset of young infertile patients, whose ovarian reserve is declining but has not yet met the POSEIDON criteria for DOR, has not received the attention it merited. These individuals have not been identified in a timely manner prior to the initiation of assisted reproductive technology (ART), leading to suboptimal clinical pregnancy outcomes. We categorized this overlooked cohort as the “high-risk DOR” group.
Objective
The primary aim of this study was to identify high-risk DOR patients through anti-Mullerian hormone (AMH) and antral follicle counts (AFCs).
Methods
A total of 10037 young women (≤ 35 years old) who underwent their first initial oocyte aspiration cycle at a single reproductive medicine center were included and further classified into three groups, based on the thresholds for AMH and AFC established through receiver operating characteristic (ROC) analysis and in alignment with the POSEIDON criteria. Two ROC analyses were performed to identify the cutoff values of AMH and AFC to obtain one viable embryo (one top-quality embryo or one viable blastocyst). The cutoffs of ROC were measured by sensitivity and specificity. The primary outcome was the cumulative live birth rate (CLBR) per oocyte aspiration cycle. The secondary outcomes included the number of oocytes retrieved and the number of viable embryos formed. Pearson’s chi-square tests were conducted to compare the clinical outcomes among the three groups. Furthermore, univariate logistic regression analyses were performed to investigate the associations between ovarian reserve and clinical outcomes. All of the above comparisons between the high-risk DOR and NOR were further confirmed by propensity score matching (PSM) (1:1 nearest-neighbor matching, with a caliper width of 0.02).
Results
According to the ROC analyses and POSEIDON criteria, the present study identified a population of high-risk DOR patients (1.20 ng/mL < AMH values < 2.50 ng/mL, with 6 ≤ AFC ≤ 10; n = 682), and their outcomes were further compared to those of DOR patients (positive control, AMH values ≤ 1.2 ng/mL, and/or AFC ≤ 5; n = 1153) and of NOR patients (negative control, 2.5 ng/mL ≤ AMH values ≤ 5.5 ng/mL, and 11 ≤ AFC ≤ 20; n = 2649). Patients in the high-risk DOR group had significantly lower CLBRs than those in the NOR group (p < 0.001) but higher CLBRs than those in the DOR group (p < 0.001). Logistic regression further demonstrated that high-risk DOR was associated with a lower likelihood of cumulative live birth chance (OR 0.401, 95% CI: 0.332–0.486, p < 0.001) than NOR was, with a greater likelihood of cumulative live birth chance (OR 1.911, 95% CI:1.558–2.344, p < 0.001) than DOR was. To investigate the effects of embryo development stage, the outcomes of D3 embryos and blastocysts were analyzed separately. Significant differences in pregnancy outcomes were detected only in D3 embryo ET cycles among the three groups (high-risk DOR vs. NOR, all p < 0.05; DOR vs. NOR, all p < 0.05). DOR/high-risk DOR did not influence the pregnancy loss rates or pregnancy outcomes (clinical pregnancy rates and ongoing pregnancy rates) per positive HCG cycle (all p > 0.05). After PSM, the differences in ovarian response and pregnancy outcomes between the high-risk DOR and NOR groups were consistent with the results before PSM.
Conclusion(s)
Our study revealed that the CLBR of the high-risk DOR patients was significantly lower than that of females with normal ovarian reserve and greater than that of females with DOR. The values of AMH ranging from 1.2 to 2.5 and AFC ranging from 6 to 10 appeared to constitute meaningful thresholds in females with mildly reduced ovarian reserve.
Keywords: Anti-mullerian hormone (AMH), Antral follicle count (AFC), Diminished ovarian reserve (DOR), Cumulative live birth rate (CLBR)
Introduction
Diminished ovarian reserve (DOR) and poor ovarian response (POR) to stimulation, which are used to indicate poor predictive performance, are increasing, but intractable issues in reproductive medicine [1, 2]. Hu et al. have reported that approximately 6.3% of infertile women younger than 35 years were diagnosed with DOR or POR [3]. Up to one-third of patients with DOR suffer a POR to ovarian stimulation resulting in cycle cancellation and a reduced chance of a live birth [4]. Currently, age, baseline follicular stimulating hormone (FSH), anti-Mullerian hormone (AMH), antral follicle count (AFC), and inhibin-B have been widely applied in clinical practice and used to predict ovarian reserve [1, 5–7]. DOR/POR manifests with a reduced AFC, and/or increased serum FSH and/or decreased AMH [2]. According to the POSEIDON standard, AMH, AFC and the number of oocytes retrieved after ovarian stimulation, are the most commonly used biomarkers for determining the ovarian reserve and ovarian response to stimulation [8]. Females with DOR must resort to assisted reproductive technology (ART), such as in-vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI).
An increasing number of studies have focused on the pregnancy outcomes of women with DOR/POR who resort to IVF/ICSI. Some studies have shown that advanced female patients with DOR have low pregnancy rates and high rates of pregnancy loss, as well as decreased live birth rates [9, 10]. Some studies have demonstrated that in young patients with either prestimulation evidence of DOR or POR to stimulation, there is no qualitative decline in oocyte performance and there are no negative effects on pregnancy outcomes [11, 12]. Among young women (< 35 years old) with DOR, the mean cumulative live birth rate (CLBR) per oocyte aspiration cycle was lower than 36.1% [6, 13]. A low CLBR increases the mental, physical, and economic burdens on this young population of patients [12]. However, the pregnancy outcomes of young infertile patients whose ovarian reserve is declining but has not yet met the POSEIDON criteria for DOR, termed high-risk DOR in the present study, have not been investigated.
To alleviate the heavy burden of infertile young women, we aimed to identify a young population of high-risk DOR patients through AMH and AFC, and further used pregnancy outcomes to test the fertility of the above suspected subfertile females. The CLBR is considered a preferable measure of the success of IVF/ICSI treatment [14], and thus CLBR served as the primary pregnancy outcome in the present study. Women with normal ovarian reserve were regarded as the negative control group, and those with DOR were regarded as the positive control group. Additionally, the outcomes were compared between high-risk DOR and patients with normal ovarian reserve after PSM, controlling for confounding variables, including age, duration of infertility, BMI and infertility type.
Methods
Study population and design
This was a retrospective cohort study performed at the reproductive medicine center of Wen Zhou Medical University. This study was approved by the Review of Ethics Committee in Clinical Research of the First Affiliated Hospital of Wenzhou Medical University (protocol number, KY2023-R224). We included all women (≤ 35 years of age) who underwent autologous IVF/ICSI and whose first oocyte aspiration cycles occurred from January 2018 to March 2023 (n = 11008). The initial included patients were aged 20–35 years and had a normal BMI (19–24 kg/m2) before ovarian stimulation. Females with endometriosis, basic endocrine diseases (diabetes, thyroid disease etc.), immune diseases, or genetic disorders were excluded. Males with genetic disorders, or whose sperms were retrieved by operations were excluded. A total of 10037 patients were ultimately enrolled. The frozen-thawed embryo transfer (FET) outcomes were followed up until December 31, 2023.
Patient grouping
To identify the young population of high-risk DOR patients, two receiver operating characteristic (ROC) curve analyses were performed: first, ROC curve analysis of the number of oocytes retrieved for predicting one viable embryo obtained (one top-quality embryo or one viable blastocyst), and second, ROC curves of the number of oocytes retrieved for predicting the cutoffs of AMH and AFC. According to our ROC analysis and POSEIDON criteria, young patients were further included and categorized into three groups: (1) DOR patients with AMH values ≤ 1.2 ng/mL and/or with AFC ≤ 5 (according to POSEIDON criteria) were selected as the positive control group, as these patients were most likely to have true depletion of ovarian reserve (DOR group, n = 1153); (2) patients with AMH values and AFC, which were below the thresholds of ROC analyses in the present study but did not reach the values of POSEIDON criteria, were selected as the study group, as these patients were likely to have mild but not severe depletion of ovarian reserve (high-risk DOR group, n = 682); and (3) patients with AMH values ranging from 2.5 to 5.5 ng/mL and with AFC ranging from 11 to 20 were selected as the negative control group, as these patients were most likely to have normal ovarian reserve (NOR group, n = 2649).
Treatment protocol
Women were treated with either the gonadotrophin-releasing hormone (GnRH) agonist protocol, the GnRH antagonist protocol, or the progesterone-primed ovarian protocol for pituitary downregulation. Briefly, in the GnRH agonist protocol, 0.8–3.75 mg depot of gonadotropin-releasing hormone (GnRH) agonist (Diphereline, Ipsen Pharma Biotech, France) was administered in the midluteal phase of the preceding cycle. In the GnRH antagonist protocol, 0.25 mg of cetrorelix (Cetrotide, Merck, Germany) was administered daily from the day the leading follicle was larger than 14 mm until the human chorionic gonadotrophin (hCG) triggering day. Tablet medroxyprogesterone acetate (MPA) (Meprates, Serum Institute of India) 10 mg once daily was started from the second day of menses or from the luteal period until the day of ovulation trigger. Human menopausal gonadotrophin or recombinant follicle-stimulating hormone (FSH) was administered daily to stimulate follicular development, and the starting dose was based on the female’s body mass index (BMI), baseline AFC and AMH. Recombinant human choriogonadotropin alfa (Ovidrel, 250 ug) was used to trigger oocyte maturation and ovulation with a leading mature follicle > 18 mm, or at least 2 follicles > 17 mm, or at least 3 follicles > 16 mm. Transvaginal ultrasound-guided oocyte pickup was performed approximately 36 h after hCG injection. Oocytes were inseminated using in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) depending on semen parameters. Embryo transfer (ET) was performed on day 3 or 5 post aspiration and luteal support was provided with 40 mg of daily intramuscular injections of progesterone and 20 mg of dydrogesterone twice daily from the day of oocytes retrieved. All the embryos were cryopreserved via vitrification. Embryo quality was assessed following the system of morphological assessment described by Simon et al. [15]. Top-quality D3 embryos were defined as those with less than 20% anucleate fragments with equally or slightly unequally sized blastomeres. All viable blastocysts were defined as top-quality embryos.
For FET cycles, hormonal replacement therapy was administered. In all cases, patients were orally administered 2 mg of estradiol valerates (Progynova; Bayer-Schering Pharma AG, Germany) twice daily, which was increased after 4 days to 3 mg twice daily. Transvaginal ultrasound monitoring (SonoScape S15; Kaili Biomedical Technology, China) was performed to assess endometrial thickness approximately 10 days after starting oral estradiol to measure the thickness and pattern of the endometrium. Progesterone was added if the endometrial thickness was greater than 7 mm. If the endometrial thickness was less than 7 mm, estrogen was continuously increased to 4 mg twice daily with/without vaginal administration of micronized E2 (Femoston; Abbott, the Netherlands) for another 5‒7 days. Day 3 embryos or blastocysts were warmed and transferred 3 or 5 days after progesterone administration, respectively. A serum pregnancy test was performed at 11‒13 days after ET in fresh IVF/ICSI cycles. Ultrasound scans were carried out at 6 and 8 weeks of gestation to confirm clinical pregnancy if the serum beta-hCG level was > 10 mIU/mL.
Definitions
CLBR per oocyte aspiration cycle was the primary outcome. The secondary outcomes included the parameters of ovarian response (number of oocytes retrieved, metaphase II (MII) oocytes retrieved, top-quality D3 embryos, viable D3 embryos and viable blastocysts), and pregnancy outcomes per ET (live birth rates, clinical pregnancy rates, ongoing pregnancy rates, and pregnancy loss rates). The CLBR per initiated cycle included all live birth events from the fresh ET and all frozen-thawed ET events derived from this initial cycle. Normal fertilization was confirmed as the presence of two pronuclei on day 1. Pregnancy was defined as a serum beta-hCG level > 10 mIU/mL at 11‒13 days following ET. Clinical pregnancy was defined as an intrauterine gestational sac on transvaginal ultrasound. Ongoing pregnancy was defined as gestation lasting for more than 12 weeks. Live birth was defined as delivering a live baby at ≥ 28 weeks of gestation.
Propensity score matching
PSM was used to control for confounding variables, including age, duration of infertility, BMI and infertility type. The high-risk DOR group was matched by propensity score with the NOR group at a ratio of 1:1 to those in the NOR group and the matching tolerance was set to 0.02. A case-control analysis was subsequently conducted to analyze follicle development and oocyte performance during oocyte aspiration cycles, and the outcomes of fresh ET and FET cycles between the high-risk DOR group and NOR group.
Statistical analysis
Statistical analysis was performed via SPSS 27.0 (SPSS IBM Corp., USA). If continuous variables were distributed normally, the data were expressed as the mean ± standard deviation (SD), and then analyzed by an independent-sample t test (groups = 2) or ANOVA (groups ≥ 3); if not, the data were presented as the median (quartile) and then analyzed by a nonparametric test. The numerical data were expressed as percentages and were analyzed by the chi-square test. ROC analyses were performed via Graphpad version 10 (GraphPad Software Inc., San Diego, CA). The cutoffs of ROC were measured by sensitivity and specificity. Univariate logistic regressions were performed to determine the group allocation affecting the outcomes of IVF/ICSI. Odds ratios (ORs) and 95% confidence intervals (95% CIs) were calculated to present the effects of the thresholds of AMH and AFC. A value of p < 0.05 was considered statistically significant.
Results
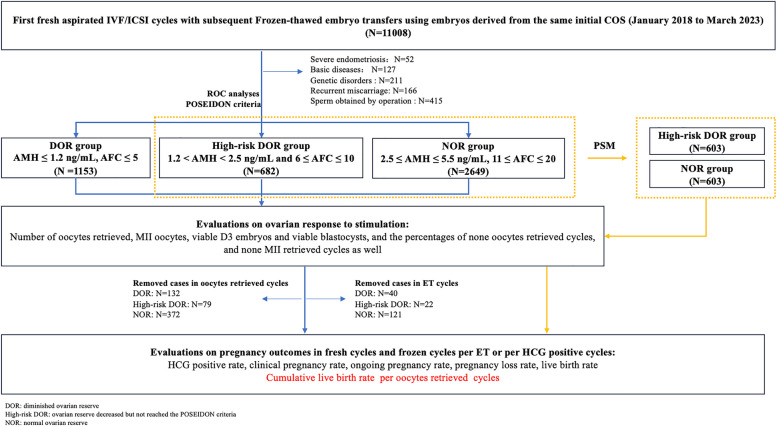
A total of 10037 patients (≤ 35 years old) from January 2018 to March 2023 were analyzed for ovarian reserve, including precycle parameters (AMH and AFC) and postcycle parameters (oocyte yield and embryo yield). According to ROC analyses and the POSEIDON criteria, 4484 patients were included and further divided into three groups (1153 patients in the DOR group, 682 patients in the high-risk DOR group, and 2649 patients in the NOR group). The FET outcomes were followed up to December 31, 2023. The outcomes of 5841 ET cycles were analyzed. PSM was conducted to further confirm the differences in ovarian response and pregnancy outcomes between the high-risk DOR group and NOR group. A profile summary of the study is shown in Fig. 1.
Fig. 1.
Flow chart of patient selection, grouping and comparisons
Patients grouped according to ROC curve analyses and POSEIDON criteria
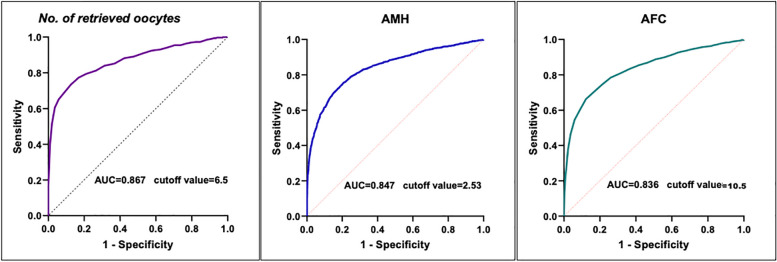
In the present study, the ROC curve (Fig. 2A) of the number of oocytes retrieved for predicting one viable embryo (one top-quality embryo yield or one viable blastocyst) indicated that the threshold of the number of oocytes retrieved was 6.5 (AUC = 0.867, standard error = 0.013, 95% confidence interval: 0.842–0.891, p < 0.001, sensitivity: 0.875, specificity: 0.733, Youden’s index: 0.608). Additionally, to obtain 6.5 oocytes, ROC curves of the AMH (Fig. 2B) and AFC (Fig. 2C) prediction models suggested that the cutoff of AMH was 2.53 ng/mL (AUC = 0.847, standard error = 0.006, 95% CI: 0.829–0.853, p < 0.001, sensitivity: 0.751, specificity: 0.797, Youden’s index: 0.548) and the cutoff of AFC was 10.5 (AUC = 0.836, standard error = 0.006, 95% CI: 0.822–0.847, p < 0.001, sensitivity: 0.661, specificity: 0.876, Youden’s index: 0.537). According to the AMH and AFC prediction models in the present study and the POSEIDON criteria, patients were further categorized into three groups: (1) the DOR group (AMH values ≤ 1.2 ng/mL and/or AFC ≤ 5; n = 1153); (2) the high-risk DOR group (1.2 < AMH values < 2.5 and 5 ≤ AFC ≤ 10; n = 682); and (3) the NOR group (2.5 ≤ AMH values ≤ 5.5 and/or 11 ≤ AFC ≤ 20; n = 2249).
Fig. 2.
Receiver operating characteristic (ROC) curve analyses of the young females younger than 35 years. (A) ROC analysis of the number of oocytes retrieved for predicting one viable embryo obtained (one top-quality embryo or one viable blastocyst); area under the ROC curve (AUC) 0.867 (95% CI: 0.842–0.891), sensitivity 0.875, specificity 0.733, Youden’s index 0.608, p < 0.001; (B) ROC curve of the number of oocytes retrieved for predicting the cutoff of AMH; AUC 0.847 (95%CI: 0.829–0.853), sensitivity 0.751, specificity 0.797, Youden’s index 0.548, p < 0.001; (C) ROC curve of the number of oocytes retrieved for predicting the cutoff of AFC; AUC 0.836 (95%CI: 0.822–0.847), sensitivity 0.661, specificity 0.876, Youden’s index 0.537, p < 0.001
Patient characteristics
The baseline characteristics of the patients among the three groups are shown in Table 1. The patients in the DOR group and in the high-risk DOR group were older than those in the NOR group (all p < 0.001). The patients in the high-risk DOR group had a longer duration of infertility than those did in the NOR group (p < 0.001). There were significant differences in the percentages of primary infertility between the DOR group and NOR group (50.1% vs. 44.1%, p < 0.014). As expected, there were significant differences on the number of AFC and AMH values among the three groups compared to each other (DOR group < high-risk DOR group < NOR group, all p < 0.001), and patients in the DOR group had the minimum mean number of AFC (6.47) and lowest mean value of AMH (0.952 ng/mL).
Table 1.
Characteristics of patients undergoing IVF/ICSI among the three groups
| DOR | High-risk DOR | NOR | p1 | p2 | p3 | |
|---|---|---|---|---|---|---|
| No. of oocyte aspiration cycles, n | 1153 | 682 | 2649 | |||
| Age(y), median [IQR] | 32 [29-34] | 32 [29-34] | 30 [28-33] | 0.981 | <0.001 | <0.001 |
| BMI (kg/m2), mean ± SD | 21.955±3.194 | 21.728±3.120 | 21.800±3.194 | 0.141 | 0.168 | 0.602 |
| Duration of infertility (y), median [IQR] | 3 [2-4] | 3 [2-5] | 3 [2-4] | 0.069 | 0.684 | 0.017 |
| Primary infertility, n (%) | 578 (50.1) | 301(44.1) | 1257(47.5) | 0.014 | 0.129 | 0.132 |
| AMH (ng/mL), mean ± SD | 0.952±0.892 | 1.817±0.359 | 3.819±0.840 | <0.001 | <0.001 | <0.001 |
| AFC on day 3, mean ± SD | 6.47±3.705 | 8.27±1.350 | 15.55±2.743 | <0.001 | <0.001 | <0.001 |
| Gn duration (d), mean ± SD | 10.78±4.518 | 11.32±2.369 | 10.90±1.864 | <0.001 | 0.25 | <0.001 |
p1 DOR group vs. high-risk DOR group, p2 DOR group vs. NOR group, p3 High-risk DOR group vs. NOR group, BMI Body mass index, FSH Follicle-stimulating hormone, LH Luteinizing hormone, E2 Estrogen, AMH Anti-Müllerian hormone, AFC Antral follicle count, hMG Human menopausal gonadotropin
Follicle development, and oocyte performance in oocyte aspiration cycles
The oocyte aspiration cycle characteristics of the patients among the three groups are shown in Table 2. There were significant differences in the number of retrieved oocytes (5.33 ± 3.790 vs. 8.28 ± 3.890 vs. 14.38 ± 5.765), MII oocytes (4.85 ± 3.297 vs.7.23 ± 3.559 vs. 12.47 ± 5.359), top-quality D3 embryos (1.96 ± 1.731 vs. 2.66 ± 2.036 vs. 4.43 ± 3.009), viable blastocysts in blastocyst culture cycles (1.27 ± 1.562 vs. 1.65 ± 1.870 vs. 2.98 ± 2.773), and viable blastocysts in oocyte aspiration cycles (0.64 ± 1.277 vs. 1.20 ± 1.757 vs. 2.74 ± 2.775) among the three groups (DOR group < high-risk DOR group < NOR group, p < 0.001). Compared with the high-risk DOR group and NOR group, the DOR had the highest percentages of cycles with no retrieved oocytes (3.38%, p < 0.001) and no MII oocytes (2.33%, p < 0.001), but it had the lowest percentages of cycles with viable embryos retrieved (84.39%, p < 0.001).
Table 2.
Oocyte aspiration cycle characteristics of the patients among the DOR group, high-risk DOR group and NOR group
| DOR | High-risk DOR | NOR | p1 | p2 | p3 | |
|---|---|---|---|---|---|---|
| Oocytes aspiration | ||||||
| No. of oocyte aspiration cycles, n | 1153 | 682 | 2649 | |||
| No. of retrieved oocytes, n, mean ± SD | 5.33±3.790 | 8.28±3.890 | 14.38±5.765 | <0.001 | <0.001 | <0.001 |
| No. of MII oocytes, n, mean ± SD | 4.85±3.297 | 7.23±3.559 | 12.47±5.359 | <0.001 | <0.001 | <0.001 |
| Mature oocyte rates, % | 88.5 | 87.4 | 86.5 | 0.146 | <0.001 | 0.189 |
| No. of cycles with no oocyte retrieved, n (%) | 39 (3.38) | 3 (0.44) | 5 (0.19) | <0.001 | <0.001 | 0.213 |
| No. of cycles with no MII oocyte retrieved, n (%) | 26 (2.33) | 3 (0.44) | 3 (0.11) | 0.003 | <0.001 | 0.104 |
| Insemination | ||||||
| Methods of Insemination | 1088 | 676 | 2641 | |||
| IVF, n (%) | 748 (68.8) | 435 (64.4) | 1658 (62.8) | 0.061 | <0.001 | 0.475 |
| ICSI, n (%) | 281 (25.8) | 165 (24.4) | 549 (20.8) | 0.536 | <0.001 | 0.046 |
| Half ICSI, n (%) | 59 (5.4) | 76 (11.2) | 434 (16.4) | <0.001 | <0.001 | 0.001 |
| Normal fertilization rates in MII oocyte cycles, %, mean ± SD | ||||||
| IVF (%) | 70.8±27.2 | 69.8±22.0 | 70.8±21.1 | 0.448 | 0.969 | 0.414 |
| ICSI (%) | 75.3±29.8 | 79.6±21.1 | 76.1±18.5 | 0.052 | 0.617 | 0.083 |
| Half ICSI (%) | 69.5±22.6 | 75.1±19.3 | 72.6±16.6 | 0.070 | 0.210 | 0.258 |
| Embryos culture | ||||||
| No. of cycles with viable embryos retrieved, n (%) | 973(84.39) | 654(95.89) | 2618(98.83) | <0.001 | <0.001 | <0.001 |
| No. of top-quality D3 embryos, n, mean ± SD | 1.96±1.731 | 2.66±2.036 | 4.43±3.009 | <0.001 | <0.001 | <0.001 |
| Top-quality D3 embryo rates, %, mean ± SD | 52.1±36.0 | 50.6±30.7 | 49.6±25.9 | 0.318 | 0.023 | 0.432 |
| No. of blastocyst culture cycles, n (%) | 572 (49.6) | 491 (72.0) | 2426 (91.6) | <0.001 | <0.001 | <0.001 |
| No. of viable blastocysts in blastocyst culture cycles, n, mean ± SD | 1.27±1.562 | 1.65±1.870 | 2.98±2.773 | 0.015 | <0.001 | <0.001 |
| Blastocyst rates per blastocyst culture cycles, %, mean ± SD | 38.4±38.4 | 37.0±34.4 | 40.0±28.9 | 0.665 | 0.271 | 0.117 |
| No. of viable D3 embryos, n, mean ± SD | 1.84±1.328 | 2.37±1.245 | 2.62±1.378 | <0.001 | <0.001 | <0.001 |
| No. of viable blastocysts in oocyte aspiration cycles, n, mean ± SD | 0.64±1.277 | 1.20±1.757 | 2.74±2.775 | <0.001 | <0.001 | <0.001 |
p1 DOR group vs. high-risk DOR group, p2 DOR group vs. NOR group, p3 High-risk DOR group vs. NOR group, IVF In-vitro fertilization, ICSI Intracytoplasmic sperm injection, Half ICSI Half number of MII oocytes fertilized by in-vitro fertilization and half number of MII oocytes fertilized by intracytoplasmic sperm injection
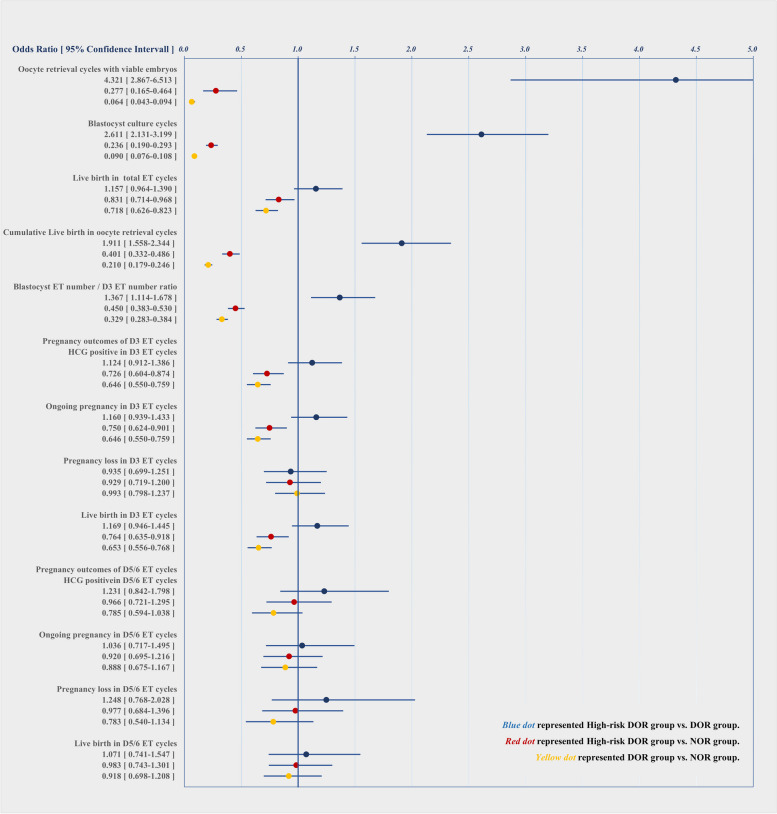
To evaluate the relative predictive value of group allocation for the number of cycles with viable embryos obtained and cycles with blastocyst culture, logistic regression analyses were performed for the respective populations of each comparison (Fig. 3). High-risk DOR was associated with greater odds of the number of cycles with viable embryos obtained (OR 4.321, 95%CI: 2.867–6.513, p < 0.001) and blastocyst culture cycles (OR 2.611, 95%CI: 2.131–3.199, p < 0.001) than the DOR group, and with lower odds of the number of cycles with viable embryos obtained (OR 0.277, 95%CI: 0.165–0.464, p < 0.001) and cycles with blastocyst culture (OR 0.236, 95%CI: 0.190–0.293, p < 0.001) than the NOR group.
Fig. 3.
Univariate logistic regression analyses of the associations between group allocation and the outcomes of IVF/ICSI cycles among the three groups
Outcomes of fresh embryo transfer and frozen-thawed embryo transfer cycles
The outcomes of fresh ET and FET are presented in Table 3. Compared with those in the NOR group, the live birth rates per total ET, including fresh ET and FET, were significantly lower in the DOR group (39.7% vs. 47.8%, p < 0.001) and high-risk DOR group (43.2% vs. 47.8%, p = 0.019). There were significant differences in the percentages of CLBRs per oocyte aspiration cycle among the three groups (43.0% vs. 59.0% vs. 78.2%, p < 0.001), and the DOR group had the lowest percentage of CLBRs per oocyte aspiration cycle. To investigate the effects of embryo development stage and the effects of whether embryos were frozen, the outcomes of D3 embryos and blastocysts which were frozen-thawed or not, were analyzed separately. Compared with those in the NOR group, the live birth rates per frozen D3 ET were significantly lower in the DOR group (35.9% vs. 47.8%, p < 0.001) and high-risk DOR group (39.2% vs. 47.8%, p = 0.004), compared to that of NOR group.
Table 3.
Pregnancy outcomes of embryo transfer cycles among the three groups
| DOR | High-risk DOR | NOR | p1 | p2 | p3 | |
|---|---|---|---|---|---|---|
| No. of fresh and frozen ET cycles, n | 1147 | 846 | 3848 | |||
| Live birth rates per total ETs, % (n) | 39.7 (439/1107) | 43.2 (356/824) | 47.8(1781/3727) | 0.123 | <0.001 | 0.019 |
| Live birth rates per fresh D3 ET, %(n) | 45.1 (105/233) | 46.6 (110/236) | 50.6 (359/710) | 0.781 | 0.152 | 0.294 |
| Live birth rates per frozen D3 ET, %(n) | 35.9 (229/638) | 39.2 (143/365) | 47.8 (633/1324) | 0.309 | <0.001 | 0.004 |
| Live birth rates per fresh D5/6 ET, %(n) | 54.5 (6/11) | 66.7(8/12) | 47.3 (43/91) | 0.68 | 0.754 | 0.235 |
| Live birth rates per frozen D5/6 ET, %(n) | 44.0 (99/225) | 45.0 (95/211) | 46.6 (746/1602) | 0.848 | 0.476 | 0.714 |
| Cumulative Live birth rates per oocyte aspiration cycles, % (n) | 43.0 (439/1021) | 59.0 (356/603) | 78.2 (1781/2277) | <0.001 | <0.001 | <0.001 |
| No. of removed cases in ET cycles, n | 40 | 22 | 121 | |||
| No. of removed cases in oocyte aspiration cycles, n | 132 | 79 | 372 | |||
| Lost to follow-up | 5 | 2 | 14 | |||
| Gestational duration | 35 | 20 | 107 | |||
| No live birth with remained embryos | 92 | 57 | 251 | |||
| No. of total D3 ET cycles,n (%) | 894 (77.9) | 610 (72.1) | 2070 (53.8) | 0.003 | <0.001 | <0.001 |
| No. of removed cases, n (%) | 23 (2.6) | 9 (1.5) | 36 (1.7) | 0.202 | 0.152 | 0.724 |
| Endometrial thickness on ET day, mean ± SD | 10.40±2.09 | 10.46±2.06 | 10.41±1.99 | 0.583 | 0.896 | 0.605 |
| No. of transferred embryos per ET, mean ± SD | 1.77±0.429 | 1.88±0.330 | 1.93±0.265 | <0.001 | <0.001 | 0.003 |
| HCG positive rates per ET, % (n) | 53.8 (469/871) | 56.7 (341/601) | 64.4 (1309/2034) | 0.287 | <0.001 | <0.001 |
| Clinical pregnancy rates per ET, % (n) | 45.6 (397/871) | 49.8 (299/601) | 56.3 (1146/2034) | 0.124 | <0.001 | 0.004 |
| Ongoing pregnancy rates per ET, % (n) | 39.5 (344/871) | 43.1 (259/601) | 50.2 (1022/2034) | 0.178 | <0.001 | 0.002 |
| Pregnancy loss rates per ET, % (n) | 15.5 (135/871) | 14.6 (88/601) | 15.6 (317/2034) | 0.658 | 0.956 | 0.607 |
| Live birth rates per ET, % (n) | 38.3 (334/871) | 42.1 (253/601) | 48.8 (992/2034) | 0.159 | <0.001 | 0.005 |
| HCG-positive cases in total D3 ET cycles | 469 | 341 | 1309 | |||
| Biochemical pregnancy rates per HCG-positives, % (n) | 12.8 (60/469) | 9.7 (33/341) | 10.2 (133/1309) | 0.182 | 0.12 | 0.84 |
| Ectopic pregnancy rates per HCG-positives, % (n) | 2.6 (12/469) | 2.6 (9/341) | 2.3 (30/1309) | 1.000 | 0.86 | 0.842 |
| Early miscarriage rates per HCG-positives, % (n) | 11.3 (53/469) | 11.7 (40/341) | 9.5 (124/1309) | 0.911 | 0.281 | 0.223 |
| Late miscarriage rates per HCG-positives, % (n) | 2.1 (10/469) | 1.8 (6/341) | 2.3 (30/1309) | 0.802 | 0.86 | 0.679 |
| Live birth rates per HCG-positives, % (n) | 71.2 (334/469) | 74.2 (253/341) | 75.8 (992/1309) | 0.381 | 0.055 | 0.572 |
| No. of total D5/D6 ET cycles, n (%) | 253 (22.1) | 236 (27.9) | 1778 (46.2) | 0.003 | <0.001 | <0.001 |
| No. of removed cases, n (%) | 17 (6.7) | 13 (5.5) | 85 (4.8) | 0.707 | 0.216 | 0.628 |
| Endometrial thickness on ET day, mean ± SD | 10.603±1.978 | 9.939±1.808 | 10.217±1.974 | <0.001 | 0.003 | 0.041 |
| No. of transferred embryos per ET, mean ± SD | 1.11±0.315 | 1.09±0.292 | 1.13±0.340 | 0.562 | 0.311 | 0.082 |
| HCG positive rates per ET, %(n) | 60.2 (142/236) | 65.0 (145/223) | 65.8 (1114/1693) | 0.29 | 0.094 | 0.822 |
| Clinical pregnancy rates per ET, % (n) | 52.1 (123/236) | 57.0 (127/223) | 56.5 (957/1693) | 0.304 | 0.208 | 0.943 |
| Ongoing pregnancy rates per ET, % (n) | 45.8 (108/236) | 46.6 (104/223) | 48.7 (825/1693) | 0.852 | 0.405 | 0.569 |
| Pregnancy loss rates per ET, % (n) | 15.7 (37/236) | 18.8 (42/223) | 19.2 (325/1693) | 0.389 | 0.213 | 0.928 |
| Live birth rates per ET, % (n) | 44.5 (105/236) | 46.2 (103/223) | 46.6 (789/1693) | 0.778 | 0.577 | 0.943 |
| HCG-positive cases in total D5/D6 ET cycles | 142 | 145 | 1114 | |||
| Biochemical pregnancy rates per HCG-positives, % (n) | 12.7 (18/142) | 10.3 (15/145) | 13.1 (146/1114) | 0.582 | 0.897 | 0.361 |
| Ectopic pregnancy rates per HCG-positives, % (n) | 0.70 (1/142) | 2.1 (3/145) | 1.0 (11/1114) | 0.622 | 1.000 | 0.212 |
| Early miscarriage rates per HCG-positives, % (n) | 10.6 (15/142) | 15.9 (23/145) | 11.8 (132/1114) | 0.224 | 0.682 | 0.179 |
| Late miscarriage rates per HCG-positives, % (n) | 2.10 (3/142) | 0.7 (1/145) | 3.2 (36/1114) | 0.367 | 0.613 | 0.114 |
| Live birth rates per HCG-positives, %(n) | 73.9 (105/142) | 71.0 (103/145) | 70.8 (789/1114) | 0.599 | 0.491 | 1.000 |
p1 DOR group vs. high-risk DOR group, p2 DOR group vs. NOR group, p3 High-risk DOR group vs. NOR group, ET Embryo transfer, Removed cases in ET cycles include cases “lost to follow-up”, and cases “in the process of the gestation”; Removed cases in oocyte aspiration cycles include cases “lost to follow-up”, cases “in the process of the gestation” and cases “no live birth with remained embryos”
Considering that the number of fresh ET cycles was limited, we focused on the effects of embryo development stage (D3 embryo or D5/6 embryo) on IVF/ICSI outcomes. In D3 ET cycles, significant differences were detected in HCG-positive rates per ET (53.8% vs. 64.4% vs. 64.4%), clinical pregnancy rates per ET (45.6% vs. 56.3% vs. 56.3%), ongoing pregnancy rates per ET (39.5% vs. 43.1% vs. 50.2%), and live birth rates per ET (38.3% vs. 42.1% vs. 48.8%) among the three groups compared with each other (DOR group < high-risk DOR group < NOR group, p < 0.05). Interestingly, the significant difference in live birth rates disappeared in the D3 ET cycles of the positive HCG population. The pregnancy loss rates per ET or per positive HCG did not differ significantly among the three groups in the D3 ET cycles (p > 0.05). There were no significant differences in any of the pregnancy outcomes in the blastocyst ET cycles (p > 0.05).
Logistic regression analyses were further performed to evaluate the relative predictive value of group allocation for live birth chance in total ET cycles, cumulative live birth in oocyte aspiration cycles, the blastocyst ET number/D3 ET number ratio, and pregnancy outcomes of D3 and D5/6 ET (Fig. 3). Compared with the NOR group, the high-risk DOR group was associated with lower odds of live birth (OR 0.831, 95%CI: 0.714–0.968, p < 0.001). Compared with the NOR group, the high-risk DOR group was associated with lower odds of the blastocyst ET/D3 ET ratio (OR 0.450, 95%CI: 0.383–0.530, p < 0.05); Compared with the DOR group, the high-risk DOR group was associated with higher odds of the blastocyst ET number/D3 ET number ratio (OR 1.367, 95%CI: 1.114–1.678, p < 0.05). In oocyte aspiration cycles, compared with the NOR group, the high-risk DOR group was associated with lower odds of cumulative live birth cases (OR 0.401, 95%CI: 0.332–0.486, p < 0.001), whereas the high-risk DOR group was associated with higher odds of cumulative live birth cases compared with the DOR group (OR 1.911, 95%CI: 1.558–2.344, p < 0.001). Compared with the NOR group, high-risk DOR was associated with lower odds of HCG positive cases (OR 0.726, 95%CI: 0.604–0.874, p < 0.001), ongoing pregnancy cases (OR 0.750, 95%CI: 0.624–0.901, p = 0.002) and live birth cases (OR 0.764, 95%CI: 0.635–0.918, p = 0.005) in D3 ET cycles. In D5 ET cycles, group allocation was not associated with pregnancy outcomes in the respective population of each comparison.
Comparison between high-risk DOR and NOR after propensity score matching
As shown in Table 4, the basic parameters, including age, infertility duration, BMI, and infertility type, were not significantly different after PSM (p > 0.05). Table 4 showed that the high-risk DOR group had a significantly lower number of retrieved oocytes (8.23 ± 3.971 vs. 14.54 ± 5.822, p < 0.001), a lower number of MII oocytes (7.16 ± 3.633 vs. 12.67 ± 5.390, p < 0.001) and a lower number of top-quality D3 embryos (2.60 ± 2.03 vs. 4.26 ± 3.02, p < 0.001) than did the NOR group. A lower number of cycles with blastocyst culture was observed in the high-risk DOR group than in the NOR group (p < 0.001). In the cycles with blastocyst culture, the number of blastocysts was significantly lower in the high-risk DOR group than in the NOR group (1.55 ± 1.850 vs. 2.18 ± 2.477, p < 0.001). The number of viable embryos (3.47 ± 2.02 vs. 5.43 ± 2.61, p < 0.001), including D3 embryos (2.36 ± 1.26 vs. 3.52 ± 1.57, p < 0.001) and blastocysts (1.11 ± 1.72 vs. 1.91 ± 2.44, p < 0.001), that could be frozen and transferred in the high-risk DOR group was lower than that in the NOR group. The percentages of cycles with no MII oocyte obtained (1.3% vs. 0.2%, p = 0.038) and no embryos obtained (4.6% vs. 1.0%, p < 0.001) were significantly greater in the high-risk DOR group than in the NOR group.
Table 4.
Baseline characteristics of patients and oocyte aspiration cycle characteristics between the high-risk DOR group and NOR group after propensity score matching
| High-risk DOR | NOR | p | |
|---|---|---|---|
| No. of patients, n | 603 | 603 | |
| Age (y), mean ± SD | 31.19±2.974 | 30.96±2.904 | 0.186 |
| BMI (kg/m2), mean ± SD | 21.687±3.140 | 21.682±2.938 | 0.978 |
| Duration of infertility (y), mean ± SD | 3.546±2.574 | 3.669±2.400 | 0.392 |
| Primary infertility, n (%) | 269(44.6) | 265(43.9) | 0.862 |
| AMH (ng/mL), mean ± SD | 1.822±0.358 | 3.817±0.842 | <0.001 |
| AFC, mean ± SD | 8.28±1.330 | 15.48±2.809 | <0.001 |
| Gn (d), mean ± SD | 11.33±2.349 | 11.37±1.843 | <0.001 |
| Oocytes retrieved | |||
| No. of oocytes retrieved, n, mean ± SD | 8.23±3.971 | 14.54±5.822 | <0.001 |
| No. of MII oocytes retrieved, n, mean ± SD | 7.16±3.633 | 12.67±5.390 | <0.001 |
| Mature oocyte rates, % | 86.9 | 86.6 | 0.698 |
| No. of cycles with no oocyte retrieved, n (%) | 3 (0.5) | 3 (0.5) | 1.000 |
| No. of cycles with no MII oocyte retrieved, n (%) | 8 (1.3) | 1 (0.2) | 0.038 |
| Insemination | |||
| Methods of insemination | |||
| IVF, n (%) | 379 (63.4) | 378 (63.2) | 1.000 |
| ICSI, n (%) | 153 (25.6) | 114 (19.1) | 0.008 |
| Half ICSI, n (%) | 66 (11.0) | 106 (17.7) | 0.001 |
| Normal fertilization rates per MII oocyte cycles, %, mean ± SD | |||
| IVF (%) | 69.5±22.6 | 71.7±16.2 | 0.115 |
| ICSI (%) | 79.2±21.2 | 74.5±19.4 | 0.063 |
| Half ICSI (%) | 75.8±19.3 | 74.5±16.5 | 0.622 |
| Embryos culture | |||
| No. of top-quality D3 embryos in oocyte aspiration cycles, n, mean ± SD | 2.60±2.03 | 4.26±3.02 | <0.001 |
| Top-quality D3 embryo rates, %, mean ± SD | 50.2±30.9 | 47.3±26.9 | 0.084 |
| No. of viable embryos in oocyte aspiration cycles, n, mean ± SD | 3.47±2.02 | 5.43±2.61 | <0.001 |
| No. of viable D3 embryos in oocyte aspiration cycles, n, mean ± SD | 2.36±1.26 | 3.52±1.57 | <0.001 |
| No. of blastocyst culture cycles, n (%) | 430 (71.3) | 525 (87.1) | <0.001 |
| No. of viable blastocyst in blastocyst culture cycles, n, mean ± SD | 1.55±1.850 | 2.18±2.477 | <0.001 |
| Blastocyst rates per blastocyst culture cycles, %, mean ± SD | 35.0±33.3 | 31.0±28.5 | 0.082 |
| No. of viable blastocyst in oocyte aspiration cycles, n, mean ± SD | 1.11±1.72 | 1.91±2.44 | <0.001 |
| No. of cycles with blastocysts retrieved, n (%) | 277 (45.9) | 366 (60.7) | <0.001 |
| No. of cycles with no viable embryos retrieved, n (%) | 28 (4.6) | 6 (1.0) | <0.001 |
IVF In-vitro fertilization, ICSI Intracytoplasmic sperm injection, Half ICSI Half number of MII oocytes fertilized by in-vitro fertilization and half number of MII oocytes fertilized by intracytoplasmic sperm injection; viable embryos include transferred and frozen embryos
Logistic regression analyses further revealed (Fig. 4) that the high-risk DOR group was associated with lower odds of the number of cycles with viable embryos obtained (OR 0.206, 95%CI: 0.085–0.502, p < 0.001) and with lower odds of the number of cycles with blastocysts retrieved (OR 0.550, 95%CI: 0.438–0.692, p < 0.001) than the NOR group was. The difference in the normal fertilization rates between the groups was not significant, although the methods of insemination differed between the groups.
Fig. 4.
Univariate logistic regression analyses of the associations between group allocation and the outcomes of IVF/ICSI cycles in the high-risk DOR group and NOR group
The pregnancy outcomes of fresh ET and FET between the high-risk DOR group and NOR group after PSM are presented in Table 5. Compared with the NOR group, the high-risk DOR group presented a lower number of mean ET cycles(1.285 ± 0.677 vs. 1.519 ± 0.854, p < 0.002), lower live birth rates per total number of ET cycles (45.9% vs. 51.1%, p = 0.035), and lower CLBRs per number of oocyte aspiration cycles (59.0% vs. 77.6%, p < 0.001). Owing to the lower number of blastocysts obtained in the high-risk DOR group, the percentages of D3 ET cycles (74.3% vs. 69.4, p = 0.027) differed between the two groups. In the D3 ET cycles, compared with the NOR group, the high-risk DOR group had a significantly lower number of transferred embryos (1.88 ± 0.334 vs. 1.95 ± 0.219, p < 0.001), lower HCG-positive rates per ET (57.5% vs. 65.1%, p = 0.007), lower clinical pregnancy rates per ET (50.5% vs. 58.2%, p = 0.008), lower ongoing pregnancy rates per ET (44.8% vs. 52.7%, p = 0.007), and lower live birth rates per ET (43.9% vs. 51.3%, p = 0.011). No significant difference was found in the pregnancy loss rates between the two groups in the D3 ET cycles. In the blastocyst ET cycles, the number of transferred embryos was significantly lower in the high-risk DOR group than in the NOR group (1.10 ± 0.295 vs. 1.28 ± 0.451, p < 0.001). The HCG-positive rates per ET, clinical pregnancy rates per ET, ongoing pregnancy rates per ET, live birth rates per ET and pregnancy loss rates per ET did not differ between the two groups in terms of the number of blastocyst ET cycles. Interestingly, in the HCG positive cycles, the pregnancy outcomes, including HCG-positive rates per ET, clinical pregnancy rates per ET, ongoing pregnancy rates per ET and live birth rates per ET, did not differ between the two groups in the D3 ET cycles and blastocyst ET cycles.
Table 5.
Pregnancy outcomes of embryo transfer cycles between the high-risk DOR group and NOR group after propensity score matching
| High-risk DOR | NOR | p | |
|---|---|---|---|
| No. of fresh and frozen cycles, n | 775 | 916 | |
| No. of patients, n | 603 | 603 | |
| No. of mean ETs in oocytes aspiration cycles, n, mean ± SD | 1.285±0.677 | 1.519±0.854 | <0.002 |
| Live birth rates per total ET cycles, %(n) | 45.9 (356/775) | 51.1 (468/916) | 0.035 |
| Cumulative live birth rates per oocytes aspiration cycles, % (n) | 59.0 (356/603) | 77.6 (468/603) | <0.001 |
| No. of D3 ET cycles,n (%) | 576 (74.3) | 636(69.4) | 0.027 |
| Endometrial thickness on ET day, mean ± SD | 10.50±2.00 | 10.45±1.99 | 0.700 |
| No. of transferred embryos in ET, mean ± SD | 1.88±0.33 | 1.95±0.22 | <0.001 |
| HCG positive rates per ET, % (n) | 57.5 (331/576) | 65.1 (414/636) | 0.007 |
| Clinical pregnancy rates per ET, % (n) | 50.5 (291/576) | 58.2 (370/636) | 0.008 |
| Ongoing pregnancy rates per ET, % (n) | 44.8 (258/576) | 52.7 (335/636) | 0.007 |
| Pregnancy loss rates per ET, % (n) | 13.5 (78/576) | 13.8 (88/636) | 0.933 |
| Live birth rates per ET, % (n) | 43.9 (253/576) | 51.3 (326/636) | 0.011 |
| HCG positive cases in D3 ET cycles, n | 331 | 414 | |
| Biochemical pregnancy rates per HCG-positives, % (n) | 9.4 (31/331) | 9.2 (38/414) | 0.998 |
| Ectopic pregnancy rates per HCG-positives, % (n) | 2.7 (9/331) | 1.4 (6/414) | 0.295 |
| Early miscarriage rates per HCG-positives, % (n) | 10.0 (33/331) | 8.5 (35/414) | 0.302 |
| Late miscarriage rates per HCG-positives, % (n) | 1.5 (5/331) | 2.2 (9/414) | 0.594 |
| Live birth rates per HCG-positives, % (n) | 76.4 (253/331) | 78.7 (326/414) | 0.479 |
| No. of D5/D6 ET cycles, n (%) | 199 (25.7) | 280 (30.6) | 0.027 |
| Endometrial thickness on ET day, mean ± SD | 9.94±1.76 | 10.16±1.91 | 0.205 |
| No. of transferred embryos in ETs, mean ± SD | 1.10±0.30 | 1.28±0.45 | <0.001 |
| HCG positive rates per ET, % (n) | 64.8 (129/199) | 68.6 (192/280) | 0.43 |
| Clinical pregnancy rates per ET, % (n) | 58.8 (117/199) | 59.6 (167/280) | 0.925 |
| Ongoing pregnancy rates per ET, % (n) | 52.3 (104/199) | 51.8 (145/280) | 0.926 |
| Pregnancy loss rates per ET, % (n) | 13.1 (26/199) | 17.9 (50/280) | 0.165 |
| Live birth rates per ET, % (n) | 51.8 (103/199) | 50.7 (142/280) | 0.853 |
| HCG-positive cases in D5/6 ET cycles, n | 129 | 192 | |
| Biochemical pregnancy rates per HCG-positives, % (n) | 7.00 (9/129) | 12.5 (24/192) | 0.134 |
| Ectopic pregnancy rates per HCG-positives, % (n) | 2.30 (3/129) | 0.50 (1/192) | 0.306 |
| Early miscarriage rates per HCG-positives, % (n) | 10.1 (13/129) | 11.5 (22/192) | 0.72 |
| Late miscarriage rates per HCG-positives, % (n) | 0.80 (1/129) | 1.60 (3/192) | 0.651 |
| Live birth rates per HCG-positives, % (n) | 79.8 (103/129) | 74.0 (142/192) | 0.232 |
Logistic regression analyses were performed between the high-risk DOR group and NOR group after PSM (Fig. 4). Compared with NOR group, the high-risk DOR group was associated with lower odds of HCG-positive cases (OR 0.725, 95%CI: 0.574–0.913, p = 0.007), clinical pregnancy cases (OR 0.734, 95%CI: 0.585–0.920, p = 0.008), ongoing pregnancy cases (OR 0.729, 95% CI: 0.581–0.913, p = 0.007), and live birth cases (OR 0.745, 95%CI: 0.594–0.933, p = 0.011) in D3 ET cycles. In D5 ET cycles, group allocation was not associated with pregnancy outcomes between the high-risk DOR group and NOR group. Compared with the NOR group, the high-risk DOR was associated with lower odds of live birth cases in all ET cycles (OR 0.813, 95%CI: 0.671–0.985, p = 0.035). Compared with the NOR group, the high-risk DOR had lower odds of cumulative live births in oocyte aspiration cycles (OR 0.416, 95%CI: 0.323–0.534, p < 0.001).
Discussion
For the first time, the present study identified a population of high-risk DOR patients (1.20 ng/mL < AMH values < 2.50 ng/mL, with 6 ≤ AFC ≤ 10; n = 682) on the basis of ROC analyses and the POSEIDON criteria. Their outcomes were further compared to those of DOR patients (positive control, AMH values ≤ 1.2 ng/mL, and/or AFC ≤ 5; n = 1153) and NOR patients (negative control, 2.5 ng/mL ≤ AMH values ≤ 5.5 ng/mL, and 11 ≤ AFC ≤ 20; n = 2649). Additionally, patients with high-risk DOR could achieve relatively higher CLBRs than those with DOR, and there were fewer with no oocytes retrieved, no MII oocytes retrieved, and no embryos obtained versus patients with DOR, all of which ultimately reduce the heavy burden on young infertile patients. The results also revealed that AMH and AFC could serve as predictors for oocyte quantity and the ovarian response to stimulation, but could not effectively predict oocyte quality in young females (≤ 35 years old). All the above comparisons between the high-risk DOR and NOR results were further confirmed by PSM.
The ovarian response was clinically evaluated according to the number of retrieved oocytes, MII oocytes, top-quality D3 embryos, viable D3 embryos and viable blastocysts and the percentages of cycles with no oocytes retrieved, no MII oocytes retrieved, and nonviable embryos obtained. DOR usually exhibited POR. As expected, the number of retrieved oocytes, MII oocytes, top-quality D3 embryos, viable D3 embryos, and viable blastocysts significantly decreased, and the percentages of cycles with no oocyte retrieval, and no MII oocyte retrieval significantly increased in DOR and high-risk DOR females (Table 3), which was associated with the degree of depletion of the ovarian oocyte pool. These results indicated that DOR, even mildly decreased ovarian reserve, could predict a poor ovarian response in young females. Comparisons between the high-risk DOR and NOR groups after PSM were conducted, and the results were consistent with those observed between the two groups before PSM (Table 4). Recently, some studies have shown similar results in young DOR females [11, 12]. A study by Morin et al. revealed that the numbers of retrieved oocytes, 2PNs embryos, and usable blastocysts were significantly lower in young DOR patients (< 38 years old), as determined by the values of AMH and by the number of obtained oocytes, than in the control group [11]. Chang et al. reported that the number of retrieved eggs was significantly lower in the young DOR group (< 37 years old) than that in the control group [12]. The patients included in the above studies were older than the females included in the present study. Owing to a depleted ovarian oocyte pool, early-onset DOR in young females also results in a decrease in the number of obtained oocyte or embryos, which is consistent with the findings in DOR aged patients [13, 16].
Recently, a large-scale retrospective study revealed that AMH was strongly associated with CLBR in women with a reduced ovarian reserve independent of age [6]. The greater the number of oocytes retrieved was, the greater the CLBR; however, the trend was evident up to 41 years of age, and no clear benefit was observed when the females were older than 44 years of age [16]. There was a positive correlation between the CLBR and the number of frozen embryos [17, 18]. Fewer cryopreserved embryos reduced the chance of ET [17]. In the present study, high-risk DOR patients had higher CLBR than DOR patients did but had lower CLBR than NOR patients did, which might be due to the differences in the number of available embryos among the three groups. Additionally, our logistic regression analysis revealed that the cumulative live birth chances of DOR patients decreased to 20.1% of those of NOR patients; meanwhile, the cumulative live birth chances of high-risk DOR patients declined to 40.0% of those of NOR patients (Fig. 3), indicating that patients with mildly decreased ovarian reserves, such as patients in the high-risk DOR group, should begin to consider receiving fertility assistance and not wait until being diagnosed with DOR before ART interventions. The comparison between the high-risk DOR group and the DOR group was further confirmed by PSM. Thus, a decreased ovarian reserve could be a negative marker of the quantity of the oocytes and embryos, which is associated with a decrease in the CLBR, and the cutoff values of AMH and AFC for predicting high-risk DOR could lead to more valuable information for clinicians and patients in making proper decisions.
Embryo quality was clinically assessed on the basis of the fertilization condition, high-quality D3 embryo rates and blastocyst rates, together with pregnancy outcomes (HCG-positive rates, pregnancy rates, ongoing pregnancy rates, live birth rates, and pregnancy loss rates). No significant differences were found in the fertilization conditions, blastocyst rates, or pregnancy loss rates between D3 ET cycles and blastocyst ET cycles among the three groups. The top-quality D3 embryo rates were not lower in the DOR/high-risk DOR than those in the NOR. These findings suggested that young females with DOR/high-risk DOR had a decreased ovarian response to stimulation, but little corresponding effect on the quality of embryos. Interestingly, our results revealed that only DOR and high-risk DOR were negatively correlated with the pregnancy outcomes, including HCG-positive rates, clinical pregnancy rates, ongoing pregnancy rates, and live birth rates, in D3 ET cycles; moreover, the correlation disappeared in blastocyst ET cycles (Table 3). Additionally, the live birth rates per positive HCG did not differ among the three groups in either D3 ET cycles or blastocyst cycles, suggesting that once embryonic implantation occurred, DOR/high-risk DOR did not disturb pregnancy development. These results were consistent with those of previous publications [3, 9, 11, 12]. However, some of these studies did not analyze the pregnancy outcomes of D3 ET cycles and blastocyst ET cycles separately [3, 12]. The study by Morin et al. focused on the pregnancy outcomes of blastocyst transfer and revealed that young women with evidence of accelerated follicular depletion presented equivalent blastocyst rates, aneuploidy rates and live birth rates per euploid ET as age-matched controls with a normal precycle (AMH ranging from 1.1 ng/mL to 4.5 ng/mL) and postcycle parameters (the number of retrieved oocytes ranged from 10 to 21) [11]. Thus, the quality of the embryo was not associated with DOR or high-risk DOR. On the basis of the findings of the present study and previous studies, the cultivation of all D3 embryos to blastocysts is recommended, even if DOR/high-risk DOR females (≤ 35 years old) have fewer D3 embryos.
Decreased fertility is related to advanced age, which is accompanied by a reduction in the quantity of oocytes and the quality of oocytes, and with an increased incidence of embryonic aneuploidy [19, 20]. However, recently, previous evidence has demonstrated that women older than 34 years of age face a steep increase in the aneuploidy rates [21, 22], and the CLBR gradually decreases with increasing female age, especially in females older than 35 years [23, 24]. However, the majority of studies have reported the pregnancy outcomes of females with a diminished ovarian reserve who were less than 37 or 38 years of age [11, 12, 25]. Thus, to separate age-related decreases in oocyte quality and to differentiate age-related increases in embryonic aneuploidy, the patients included in the present study were younger than 35 years old. Previous studies have demonstrated that aneuploidy is an important factor in the spontaneous miscarriage [26]. The present study revealed that DOR and high-risk DOR were not associated with pregnancy loss rates (biochemical pregnancy rates, ectopic pregnancy rates, early miscarriage rates and late miscarriage rates) (Table 3), further indirectly demonstrating that aneuploidy might not play a key role in the young population (≤ 35 years of age) with DOR/high-risk DOR.
There were strengths and weaknesses in the present study. To our knowledge, this first large-scale study is the first to determine the thresholds of AMH and AFC in the females younger than 35 years with mildly diminished ovarian reserve. However, this study was retrospective; thus, prospective or interventional studies should be conducted to better address these problems. Another limitation of this study was that the transferred embryos were not tested by preimplantation genetic testing for aneuploidy (PGT-A), although previous studies have demonstrated that the incidence of embryonic aneuploidy dose not increase in the women younger than 35 years [21, 22]. The conditions of the ovarian stimulation protocols and cancellation of ovarian stimulation cycles were not checked in the manuscript.
Conclusion
In summary, our study is the first to identify young infertile women with mildly reduced ovarian reserve, through the combined measurements of AMH and AFC. The values of AMH ranging from 1.2 to 2.5 and AFC ranging from 6 to 10 appeared to constitute meaningful thresholds to target the above young women. The results could guide clinicians to better provide proper counseling services for young infertile couples with DOR/high-risk DOR, which are different from aged patients with DOR. Since the present study was retrospective, these findings need to be confirmed in future prospective studies or randomized controlled trials.
Acknowledgements
We thank Wenzhou Key Laboratory of Reproduction and Genetics for consultation that supported this work.
Abbreviations
- AFC
Antral follicle count
- AMH
Anti-Mullerian hormone
- ART
Assisted reproductive technology
- CLBR
Cumulative live birth rate
- DOR
Diminished ovarian reserve
- FSH
Follicular stimulating hormone
- FET
Frozen-thawed embryo transfer
- Half-ICSI
Half number of MII oocytes fertilized by in-vitro fertilization and half number of MII oocytes fertilized by intracytoplasmic sperm injection
- IVF
In-vitro fertilization
- ICSI
Intracytoplasmic sperm injection
- MII oocyte
Metaphase II oocyte
- POR
Poor ovarian response
Authors’ contributions
Y.T.: Data analysis and manuscript writing. P.P.: Data analysis and management. C.L.: Data collection and management. Y.L.: Data collection and manuscript editing. X.Z.: Table preparation. S.W.: Data collection. H.Y.: Figure preparation. X.H.: manuscript editing. F.L.: study design and manuscript revising.All authors reviewed the manuscript.
Funding
This work was supported by National Natural Science Foundation of China (82174429 and 82274573 to Fang Lian; 82201856 to Peipei Pan). We thank Wenzhou Key Laboratory of Reproduction and Genetics for consultation that supported this work.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The present study was reviewed and preapproved by the Review of Ethics Committee in Clinical Research of the First Affiliated Hospital of Wenzhou Medical University (protocol number, KY2023-R224). All procedures were in accordance with the guidelines of use of Human Subjects as per the Helsinki Declaration (2013) and Ethical Reviews for Biomedical Research Comments Involving Human Subjects (2016) the National Health Commission of People’s Republic of China. An informed consent form was signed by all participants at the beginning of the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Penzias A, Azziz R, Bendikson K, Falcone T, Hansen K, Hill M, Hurd W, Jindal S, Kalra S, Mersereau J, Racowsky C. Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril. 2020;114:1151–7. [DOI] [PubMed] [Google Scholar]
- 2.Cedars MI. Managing poor ovarian response in the patient with diminished ovarian reserve. Fertility Steril. 2022;117:655–6 United States. [DOI] [PubMed] [Google Scholar]
- 3.Hu S, Xu B, Jin L. Perinatal outcome in young patients with diminished ovarian reserve undergoing assisted reproductive technology. Fertility Steril. 2020;114:118-124.e1. United States. [DOI] [PubMed] [Google Scholar]
- 4.Annalisa R, Dominic S, Nikolaos PP. Editorial: diminished ovarian reserve and poor ovarian response: diagnostic and therapeutic management. Front Physiol. 2022;13:827678. Switzerland. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lew R. Natural history of ovarian function including assessment of ovarian reserve and premature ovarian failure. Best Pract Res Clin Obstet Gynaecol. 2019;55:2–13. Netherlands. [DOI] [PubMed] [Google Scholar]
- 6.Tal R, Seifer DB, Tal R, Granger E, Wantman E, Tal O. AMH highly correlates with cumulative live birth rate in women with diminished ovarian reserve independent of age. J Clin Endocrinol Metab. 2021;106:2754–66. United States. [DOI] [PubMed] [Google Scholar]
- 7.Ata B, Seyhan A, Seli E. Diminished ovarian reserve versus ovarian aging: overlaps and differences. Curr Opin Obstet Gynecol. 2019;31:139–47. [DOI] [PubMed] [Google Scholar]
- 8.Alviggi C, Andersen CY, Buehler K, Conforti A, De Placido G, Esteves SC, et al. A new more detailed stratification of low responders to ovarian stimulation: from a poor ovarian response to a low prognosis concept. Fertil Steril. 2016;105:1452–3. United States. [DOI] [PubMed] [Google Scholar]
- 9.Bishop LA, Richter KS, Patounakis G, Andriani L, Moon K, Devine K. Diminished ovarian reserve as measured by means of baseline follicle-stimulating hormone and antral follicle count is not associated with pregnancy loss in younger in vitro fertilization patients. Fertility Steril United States. 2017;108:980–7. United States. [DOI] [PubMed] [Google Scholar]
- 10.Busnelli A, Somigliana E, Cirillo F, Levi-Setti PE. Is diminished ovarian reserve a risk factor for miscarriage? Results of a systematic review and meta-analysis. Hum Reprod Update. 2021;27:973–88. England. [DOI] [PubMed] [Google Scholar]
- 11.Morin SJ, Patounakis G, Juneau CR, Neal SA, Scott RT, Seli E. Diminished ovarian reserve and poor response to stimulation in patients < 38 years old: a quantitative but not qualitative reduction in performance. Human Reprod (Oxford, England). 2018;33:1489–98. England. [DOI] [PubMed] [Google Scholar]
- 12.Chang Y, Li J, Li X, Liu H, Liang X. Egg quality and pregnancy outcome in young infertile women with diminished ovarian reserve. Med Sci Monit. 2018;24:7279–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi W, Zhou H, Tian L, Zhao Z, Zhang W, Shi J. Cumulative live birth rates of good and low prognosis patients according to POSEIDON criteria: a single center analysis of 18,455 treatment cycles. Front Endocrinol (Lausanne). 2019;10:409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maheshwari A, McLernon D, Bhattacharya S. Cumulative live birth rate: time for a consensus? Hum Reprod (Oxford, England). 2015;30:2703–7. England. [DOI] [PubMed] [Google Scholar]
- 15.Simon L, Brunborg G, Stevenson M, Lutton D, McManus J, Lewis SEM. Clinical significance of sperm DNA damage in assisted reproduction outcome. Human Reprod (Oxford, England). 2010;25:1594–608. England. [DOI] [PubMed] [Google Scholar]
- 16.Devesa M, Tur R, Rodríguez I, Coroleu B, Martínez F, Polyzos NP. Cumulative live birth rates and number of oocytes retrieved in women of advanced age. A single centre analysis including 4500 women ≥ 38 years old. Human Reprod (Oxford, England). 2018;33:2010–7. England. [DOI] [PubMed] [Google Scholar]
- 17.Wang Q, Qi D, Zhang L, Wang J, Du Y, Lv H, et al. Association of the cumulative live birth rate with the factors in assisted reproductive technology: a retrospective study of 16,583 women. J Clin Med. 2023;12(2):493.Switzerland. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ji J, Liu Y, Tong XH, Luo L, Ma J, Chen Z. The optimum number of oocytes in IVF treatment: an analysis of 2455 cycles in China. Hum Reprod (Oxford, England). 2013;28:2728–34. England. [DOI] [PubMed] [Google Scholar]
- 19.Park SU, Walsh L, Berkowitz KM. Mechanisms of ovarian aging. Reproduction (Cambridge, England). 2021;162:R19-33. England. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaswa EG, McCulloch CE, Simbulan R, Cedars MI, Rosen MP. Diminished ovarian reserve is associated with reduced euploid rates via preimplantation genetic testing for aneuploidy independently from age: evidence for concomitant reduction in oocyte quality with quantity. Fertility Steril. 2021;115:966–73. United States. [DOI] [PubMed] [Google Scholar]
- 21.Franasiak JM, Forman EJ, Hong KH, Werner MD, Upham KM, Treff NR, et al. The nature of aneuploidy with increasing age of the female partner: a review of 15,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertility Steril. 2014;101:656-e6631.United States. [DOI] [PubMed] [Google Scholar]
- 22.Irani M, Canon C, Robles A, Maddy B, Gunnala V, Qin X, et al. No effect of ovarian stimulation and oocyte yield on euploidy and live birth rates: an analysis of 12 298 trophectoderm biopsies. Hum Reprod (Oxford, England). 2020;35:1082–9. England. [DOI] [PubMed] [Google Scholar]
- 23.Hu KL, Liu FT, Xu H, Li R, Qiao J. Association of serum anti-Müllerian hormone and other factors with cumulative live birth rate following IVF. Reprod Biomed Online. 2020;40:675–83. Netherlands. [DOI] [PubMed] [Google Scholar]
- 24.Khalife D, Nassar A, Khalil A, Awwad J, Abu Musa A, Hannoun A, et al. Cumulative live-birth rates by maternal age after one or multiple in vitro fertilization cycles: an institutional experience. Int J Fertility Steril. 2020;14:34–40. Iran. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu X, Lv X, Dong X, Li Y, Turathum B, Liu S, et al. Increased serine synthesis in cumulus cells of young infertile women with diminished ovarian reserve. Human Reprod (Oxford, England). 2023;38:1723–32. England. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yi Y, Fu J, Xie S, Zhang Q, Xu B, Wang Y, et al. Association between ovarian reserve and spontaneous miscarriage and their shared genetic architecture. Hum Reprod (Oxford, England). 2023;38:2247–58. England. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.