Abstract
Ovarian cancer is a prevalent gynecologic malignancy with the second-highest mortality rate among gynecologic malignancies. Platinum-based chemotherapy is the first-line treatment for ovarian cancer; however, a majority of patients with ovarian cancer experience relapse and develop platinum resistance following initial treatment. Despite extensive research on the mechanisms of platinum resistance at the nuclear level, the issue of platinum resistance in ovarian cancer remains largely unresolved. It is noteworthy that mitochondrial DNA (mtDNA) exhibits higher affinity for platinum compared to nuclear DNA (nDNA). Mutations in mtDNA can modulate tumor chemosensitivity through various mechanisms, including DNA damage responses, shifts in energy metabolism, maintenance of Reactive Oxygen Species (ROS) homeostasis, and alterations in mitochondrial dynamics. Concurrently, retrograde signals produced by mtDNA mutations and their subsequent cascades establish communication with the nucleus, leading to the reorganization of the nuclear transcriptome and governing the transcription of genes and signaling pathways associated with chemoresistance. Furthermore, mitochondrial translocation among cells emerges as a crucial factor influencing the effectiveness of chemotherapy in ovarian cancer. This review aims to explore the role and mechanism of mitochondria in platinum resistance, with a specific focus on mtDNA mutations and the resulting metabolic reprogramming, ROS regulation, changes in mitochondrial dynamics, mitochondria-nucleus communication, and mitochondrial transfer.
Graphical Abstract
Keywords: Mitochondria, Platinum resistance, Mitochondria DNA (mtDNA), Metabolic reprogramming, Mitochondrial dynamics, Mitochondria-nucleus communication, Mitochondria transfer
Highlights
Directly targeting mitochondria is one of the main mechanisms by which platinum induces apoptosis in tumor cells.
mtDNA mutations occur frequently in ovarian cancer and significantly contribute to platinum resistance.
The metabolic heterogeneity induced by mtDNA mutations can directly drive platinum resistance in ovarian cancer cells.
Targeting mitochondria could be a novel approach for platinum-resistant ovarian cancer.
Introduction
As the sole double-membrane organelle harboring its independent genome within eukaryotic cells, mitochondria are not only the energy source of the organism, but also play a vital role in enabling the organism to respond to a variety of stress factors, including external drugs, nutritional changes, and environmental shifts.
Platinum-based chemotherapy regimens, including cisplatin, carboplatin, and oxaliplatin, remain a first-line treatment for ovarian cancer. These agents are globally approved as standard DNA damage inducers for treating malignancies. It is noteworthy that, beyond nuclear DNA(nDNA), mitochondria DNA(mtDNA) is also a target of these drugs, and several studies have indicated that mtDNA exhibits a significantly higher affinity for platinum than nDNA [1–3].
Cytoplasmic heterozygous cells with different mtDNA haplotypes have indicated a potential link between cisplatin sensitivity and mtDNA background [4]. The denucleation assays have demonstrated that platinum-induced apoptotic signaling pathways can occur independently of DNA damage [5–10]. Sabelle Gourdier et al. discovered that oxaliplatin-induced apoptosis primarily occurs by triggering the Bax/Bak-dependent apoptotic pathway in mitochondria [6]. The presence or absence of nDNA had no effect on the cisplatin sensitivity of head and neck squamous cell carcinoma cells, whereas mtDNA-free cells exhibited 4–5 times more resistance to cisplatin compared to their parental cells. Cisplatin shows a preference for binding to mitochondrial membrane proteins, particularly voltage-dependent anion channel proteins, leading to mitochondria damage rather than the nuclear damage [1]. Taken together, direct effect on mitochondria may be an essential mechanism through which platinum induces apoptosis in tumor cells.
This review aims to obtain crucial insights for precision medicine and establish a framework for development of cancer therapy strategies that target both the nucleus and the mitochondria by comprehending the roles and modes of mitochondria and mtDNA, as well as the communication between mitochondria and the nucleus.
mtDNA mutations occur frequently in ovarian cancer and significantly contribute to platinum resistance
The nucleotide excision repair pathway is the primary mechanism for repairing DNA damage induced by platinum-nDNA adduct [7]. The higher initial binding capacity of platinum to mtDNA, the absence of the nucleotide excision repair pathway in mtDNA, and the proximity of mtDNA to ROS generated in the organelle contribute to a mutation rate in mtDNA nearly 10-times higher than that in nuclear DNA [8].
The Cancer Genome Atlas (TCGA) sequence analysis of 226 pairs of tumors and normal tissues from five types of cancers reveals a median frequency of 36% for mtDNA mutations in ovarian adenocarcinomas [9]. Additionally, Liu and Van et al. found that somatic mtDNA mutations were present in 60% of the ovarian cancer samples [10].
Compared to individuals without mtDNA mutations, patients with high-grade serous ovarian cancer (HGSOC) harbor modest heterogeneous pathogenic somatic mutations were found to be more prone to developing platinum resistance and experiencing recurrence [11]. Some theories suggest that the progression of cancer may align with the time needed for mutant mtDNA or cellular homogeneity to become predominant [12]. In addition, tumor growth and drug resistance require the structural and functional integrity of mitochondrial electron transport chain (ETC) complex [13].
Research by Flora et al., revealed that ovarian cancers treated with carboplatin-paclitaxel chemotherapy developed a MTND4 m.10875T > C missense mutation, which results in mild energy defects and triggers drug resistance. These tumors exhibited an eosinophilic phenotype, displaying a senescence-like phenotype with a low proliferative index and hypoxia adaptation incapacity, while the missense mutation was absent in pre-chemotherapy tumors [14].
Additionally, mtDNA mutations can affect ovarian cancer drug resistance via metabolism, ROS generation, and communication with cell nucleus.
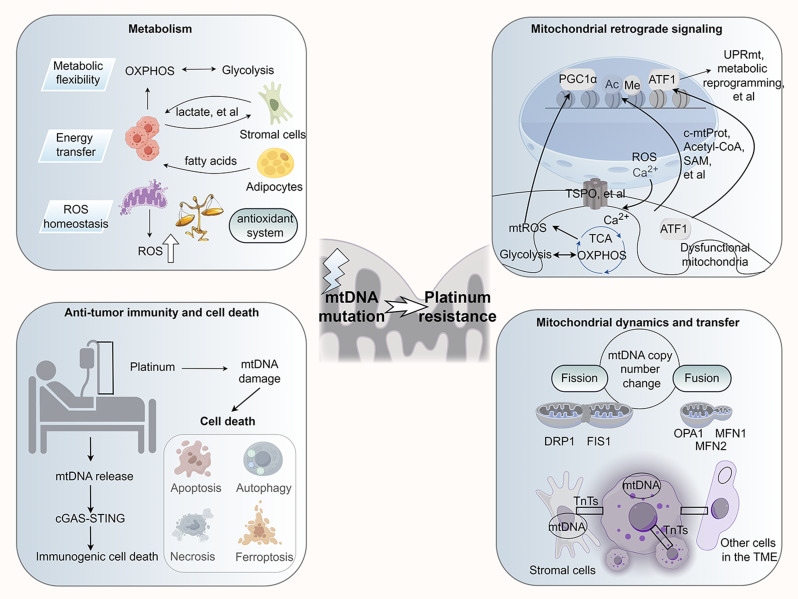
mtDNA mutations confer resistance to platinum-based treatments through regulating cancer cell metabolism (Fig. 1)
Fig. 1.
Mitochondrial DNA (mtDNA) mutations affect the responsiveness of ovarian cancer cells to platinum-based drugs by modulating their interaction with the tumor microenvironment and the nucleus. Initially, mtDNA mutations in ovarian cancer cells disrupt their metabolism, resulting in increased metabolic flexibility and reliance on glutamate, and altered production of epigenetic modifiers. This metabolic imbalance alters the metabolic signaling to the nucleus and modifies the epigenetic profile of nuclear DNA, leading to chemoresistance. Additionally, the altered microenvironment can enhance the oncogenic effect of cells within the microenvironment, such as fibroblasts, and guide these cells to provide essential metabolites to cancer cells, thereby promoting the metabolic adaptation of ovarian cancer cells to platinum-based drugs. OXPHOS: Oxidative Phosphorylation; TCA: Tricarboxylic acid cycle; FFA: Free fatty acid; α-KG: α-ketoglutarate. Gln: Glutamine
Metabolic flexibility
Metabolic flexibility associated with heterogeneous mtDNA mutations contributed significantly to chemotherapy resistance of ovarian cancer cells. In patients with HGSOC, the presence of heterogeneous pathogenic somatic mtDNA mutations is associated with higher rates of platinum resistance and recurrence. Tumor tissues with these mutations exhibit a notably elevated lactate/pyruvate ratio compared to tissues without the mutation, indicating a shift from oxidative phosphorylation (OXPHOS) to glycolysis [11]. Cell lines carrying the MTND5 mutation also undergo metabolic switch from OXPHOS to glycolysis, and increased mutation heterogeneity promote early stage tumor development, possibly through alteration of ROS generation and apoptosis [15]. Furthermore, it has been observed that tumor cells with mutant mtDNA may possess a replication advantage, resist drug induced apoptosis, and finally retain cells with homogenous mutant mitochondria [16, 17].
Current evidence indicates that the OXPHOS pathway remains active in tumor cells. Both established ovarian cancer cell lines and patient-derived ovarian cancer cells displaying drug resistance exhibit elevated levels of OXPHOS, glycolysis, glutathione (GSH) and lipid metabolism. This metabolic flexibility allows cells to switch between OXPHOS and glycolysis, enhancing their resistance to platinum-based treatments [18–20]. Chemotherapy-resistant ovarian cancer cells demonstrated an increased capability for mitochondrial respiration, and ATP production through OXPHOS [21, 22]. Excessive reliance on OXPHOS can generate harmful ROS that hinder cell proliferation. This transition may slow cell growth but enable persistence and spread in smaller numbers.
Ovarian cancer cells in the tumor microenvironment undergo aerobic glycolysis (Warburg effect) and the lactate produced is translocated to adjacent endothelial cells and cancer-associated fibroblasts to support angiogenesis and tumour invasion [23]. However, the classical Warburg effect cannot explain the efficient ATP synthesis and intact mitochondrial function of tumors. There is accumulating evidence that ‘the reverse Warburg effect’ occurs in fibroblasts in the tumor microenvironment, where lactate created by aerobic glycolysis is transported back into the cancer cells to stimulate anabolic activities [24, 25]. In advanced epithelial ovarian cancer, the tumor-stroma proportion has been shown to be prognostic in chemotherapy-resistant patients [26], which is twice as high as in chemo-sensitive patients and correlates with significantly shorter progression-free survival and overall survival [27].
Energy transfer between cells in the tumor microenvironment also drives chemoresistance. Over 80% of ovarian cancers metastasize to the adipose of the greater omentum. Through co-cultures of human ovarian cancer cells with adipocytes, it was observed that the adipocytes supply cancer cells with high-energy mitochondrial fuels, mainly free fatty acids, through catabolism. This process enhances cancer cells OXPHOS capacity, promoting chemoresistance and distant metastasis [28]. Proteomic analysis also revealed substantial lipidomic changes and up-regulation of lipid metabolism proteins in co-cultured ovarian cancer cells. Among these, FABP4, a lipid chaperone protein, serves a key regulator of lipid responses [29]. However, the precise role of mtDNA mutations in regulating metabolic heterogeneity remains unclear.
Imbalance in reactive oxygen species (ROS) homeostasis
Mutations in mtDNA serve as both a trigger and a consequence of increased ROS within the mitochondria [15]. MtDNA is susceptible to ROS damage from the stroma due to its lack of histone protection. Mutations in mtDNA lead to diminished mitochondrial function by modifying the mitochondrial membrane potential or the redox state of electron carriers upstream and downstream, both processes resulting in ROS generation.
The regulation of mitochondrial ROS levels significantly impacts chemosensitivity of tumor cells [30]. When the mitochondria-mediated antioxidant system successfully balances the ROS levels, it triggers gene expression changes in cancer cells. These alterations involve activation of pro-oncogenic transcription factors, suppressing tumor suppressor genes, and increased levels of genes exhibiting pro-oncogenic metabolic traits, promoting cancer initiation, progression, and drug resistance. Conversely, if ROS scavenging falls short of ROS production, oxidation-induced apoptosis occures [31].
mtDNA mutations also play a direct role in mediating ROS-regulated resistance [32]. Exposure to ROS induced by anticancer drugs and hypoxia helps cancer cells developing adaptive mechanisms that ultimately favor the growth of drug-resistant clones [33–35]. The transcription factor hypoxia-inducible factor HIF-1α is a critical molecule in this process [36]. Cisplatin-resistant ovarian cancer cells maintain elevated HIF-1α levels, and knocking down of HIF-1α is able to reprogram aerobic glycolysis to OXPHOS and induce cell death [37]. To avoid excessive generation of ROS, cancer cells undergo metabolic reprogramming towards an aerobic glycolytic, generating NADPH through pathways like the pentose phosphate pathway (PPP) and the adenosine monophosphate-activated protein kinase. Additionally, cancer cells upregulate the production of antioxidants such as nuclear factor red lineage 2-related factor 2 (NRF2). Consequently, the heightened expression of these metabolic factors and antioxidants in several cancer types, including ovarian cancer, contribute to chemotherapy resistance [38, 39]. Despite the complexity of the ROS regulation pathway, it is possible to discover the determinants of ROS signaling mediated by chemotherapy. Groundbreaking research by Zhang et al. revealed that the CHK1-SSBP1 pathway mediates nuclear H2O2 accumulation and resistance to platinum drugs in ovarian cancer cells [40].
The retrograde signals caused by altered metabolites
The majority of mitochondrial proteins are nucleus-encoded. Precise coordination between the two genomes is essential for the expression of the mitochondrial proteome in both tumor cells and healthy cells. Retrograde signals from dysfunctional mitochondria are relayed to the nucleus, promoting a reorganization of the nuclear transcriptome and activation of genes and signaling pathways associated with cancer.
Mitochondrial retrograde signals are facilitated by the contact sites which comprising a multiprotein complex (including ACBD3, PKA, AKAP95 and TSPO) that tethers mitochondria to the nucleus, to regulates the cellular response to stress [41]. The translocator protein TSPO can also restrict PARK2-mediated ubiquitination of mitochondrial proteins, resulting in an accumulation of dysfunctional mitochondria [42].
PPAR-coactivator 1 (PGC1), a crucial mitochondrial biogenesis regulator [43], also mediates retrograde signaling. Cisplatin-resistant ovarian cancer cells exhibit high levels of PGC-1α, boosting mitochondrial biogenesis and reducing apoptosis [44]. In SKOV3/DDP cells, mitochondria ROS production stimulates the nucleus, increasing PGC1α expression and contributing to drug resistance [45, 46]. Deng et al. propose a feedback loop between PGC1α and NRF2 that regulates antioxidant and proteasomal activity, thereby contributes to cisplatin resistance in ovarian cancer cells [47]. Furthermore, PGC-1α boosts apoptotic resistance in ovarian cancer cells by facilitating HK2-VDAC1 binding and upregulating HSP70 gene transcription [48].
High expression of PGC1α and β can identify ovarian cancer patients who may benefit from OXPHOS inhibitors, thereby potentially delaying disease progression [49]. Unfortunately, PGC1α expression varies across different tissue sources and tumor cells, and excessive expression may not always result in treatment resistance in all individuals. Ovarian clear-cell carcinoma lacking PGC1α/TFAM and with low ERα/Ki-67 are resistant to chemotherapeutic drugs, while HGSOC with high OXPHOS showed high expression of PGC1α, TFAM, and ERα, and high Ki-67 expression, is sensitive to platinum-based chemotherapy [50, 51].
Signaling molecules involved in the process include Ca2+, ROS and misfolded proteins [52].
Mitochondrial unfolded protein response (UPRmt)
The increased demand for mitochondrial activity in tumors leads to the generation of mitochondrial ROS, mtDNA mutations, and misfolded proteins, causing detrimental effects. To counteract this, tumor cells activate the UPRmt to maintain protein homeostasis, prevent mitochondrial apoptosis, and enhance drug resistance.
In the C. elegans model, ATFS-1/ATF5 triggers UPRmt. Under normal conditions, ATFS-1, the homologue of ATF1 in humans, containing mitochondrial targeting sequences, is transported into mitochondria for degradation [53]. However, in dysfunctional mitochondria, it relocates to the nucleus, accumulating and activating UPRmt. ATFS-1 also interacts with the promoters of OXPHOS genes in both mitochondria and the nucleus, fine-tuning the expression of OXPHOS genes to optimize mitochondrial respiration [54]. The mammalian UPRmt involves more intricate mechanisms, with mitochondria ROS and cytoplasmic mitochondrial protein precursors (c-mtProt) as crucial signals initiating the UPRmt and promoting a nuclear transcriptional response [55]. Phosphorylation of eIF2α induces the expression of genes containing open reading frames in the 5’UTR region, such as ATF5, ATF4, and CHOP.
Drug-resistance cancers often exhibit considerable activation of the UPRmt. In ovarian cancer cells, upregulated ATF5 inhibits apoptosis by transcribing BCL2 and MCL1 [56]. Ovarian tumors upregulate HSP60 [57], an UPRmt effector protein that regulates metabolism by modulating the AMPK-mTORC1 pathway. Chemotherapy-resistant ovarian cancer cells are notably sensitized by an HSP60 antibody combined with cisplatin [58]. Resistant cells also elevate levels of Caseinolytic protease P (ClpP), which facilitates cellular exocytosis of cisplatin, maintains DNA stability by reducing the production of cisplatin-DNA adducts, and decreases sensitivity to chemotherapy [59].
Mitochondrial calcium homeostasis
Mitochondria, known for Ca2 + storage, regulate Ca2 + uptake and efflux through nucleus-encoded genes. Alterations in mitochondrial Ca2 + signaling can influence malignant transformation, tumor growth, and chemotherapy response [60, 61].
Chemotherapeutic drugs prompt Ca2 + transfer from the endoplasmic reticulum to mitochondria, triggering the opening of the mitochondrial permeability transition pore and release of various mitochondrial proteins, such as cytochrome C, into the cytoplasm, leading to apoptosis [62]. Certain proteins in mitochondria-associated endoplasmic reticulum membranes (BCL2, RAS, AKT, PTEN) regulate apoptosis by modulating the transfer of Ca2+, thereby leading to the effectiveness of chemotherapy drugs. Disruption of Ca2 + homeostasis either through interfering of Ca2 + channel or the endoplasmic reticulum stress axis could change the sensitivity of ovarian cancer to platinum [63, 64]. High Mitochondrial Calcium Uptake 1 (MICU1) expression, low mitochondrial calcium, and cisplatin resistance is seen in glycolytic ovarian cancer, with MICU1 silencing enhancing cisplatin efficacy [65].
A study by Lee et al. demonstrated calcium channel blockers’ synergistic effects in reducing ovarian cancer stem cell-like traits, inhibiting viability, proliferation, and enhancing cisplatin efficacy [66].
Epigenetic effects
The nuclear genome is intricately organized, with DNA and histone epigenetic modifications regulating gene expression by modulating chromatin accessibility and providing binding sites for regulatory proteins. Mitochondrial metabolites like acetyl coenzyme A, S-Adenosyl Methionine, and 2-Hydroxyglutarate contribute to epigenetic regulation of DNA and histone. For example, histone and DNA methyltransferases in the nucleus use S-Adenosyl Methionine as a methyl group donor. Researchers analyzing metabolomics data and post-transcriptional histone modifications in cytoplasmic hybrid cell lines with varying levels of the tRNALeu (UUR) m.3243G mutation discovered that increased levels of this specific mtDNA mutation resulted in alterations in mitochondrial intermediates and redox status, leading to significant changes in histone modification. At high heterozygosity mutations (90-100%), levels of glucose-derived acetyl coenzyme A were reduced, resulting in decreased histone H4 acetylation. In contrast, moderate heterozygosity mutations (69-70%) led to elevated α-ketoglutarate levels, which were inversely correlated with histone H3 methylation [34].
In ovarian cancer, while direct evidence linking mitochondrial metabolites to epigenetic modifications and resistance is limited, chemotherapy-induced epigenetic changes contribute to resistance. Post-chemotherapy, ovarian cancer stem cells remaining in HGSOC display characteristics such as aldehyde dehydrogenase positive (ALDH+), rendering them highly resistant to treatment. These cells exhibit increased DNA methylation in gene promoter regions associated with differentiation pathways, leading to chromatin compaction and gene expression repression. The DNA methyltransferase inhibitor SGI-110 can induce differentiation in ALDH + cells, making them more sensitive to platinum-based therapies [67].
Histone acetylation governs the activity of distal enhancers, super enhancers and their associated genes, influencing the transcriptional program that leads to platinum resistance in ovarian cancer [68]. The relationship between super enhancer-driven mechanisms and chemoresistance in ovarian cancer involves the modulation of cancer stem cell formation, cellular plasticity, the tumor microenvironment, gene linked to chemoresistance, ncRNAs, and tumor immunity [69]. Methylation and acetylation modifications of various sites on histone H3, as well as DNA methylation changes in specific genes like H3K79 methylation, H3K27 methylation, FANCF methylation, and Fanconi anemia pathway, have been linked to cisplatin sensitivity in ovarian cancers [70–72].
Targeting epigenetic modifications can enhance chemosensitivity and reverse acquired resistance in ovarian cancer, potentially through combining chemotherapy with immunotherapy [73]. This suggests that mtDNA mutations may play a role in tumor resistance by influencing metabolites that impact epigenetic alterations.
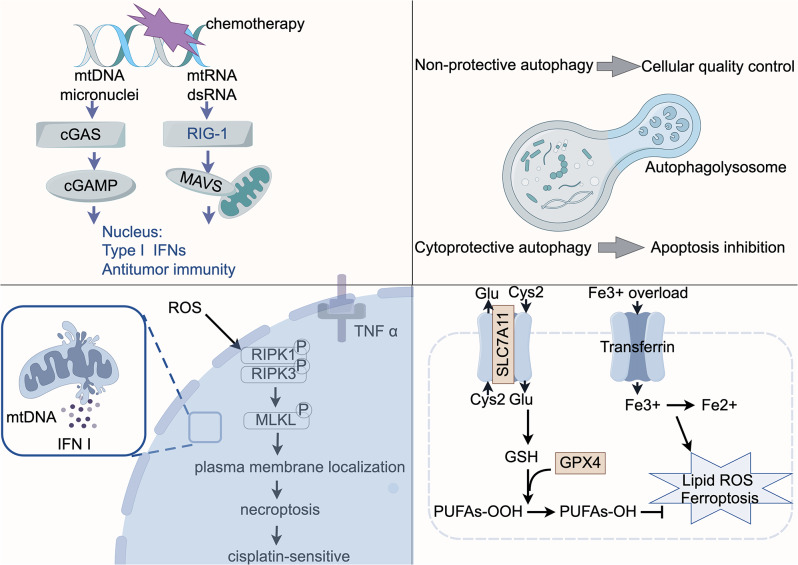
mtDNA damage signaling regulates platinum resistance via the cGAS/STING signaling pathway and multiple modes of cell death (Fig. 2)
Fig. 2.
mtDNA damage signals can regulate the sensitivity of ovarian cancer cells to platinum-based drug by modulating the cGAS/STING pathway and cell death signaling pathways. In addition to the apoptotic signaling pathway, signals derived from damaged mtDNA could potentially impact the sensitivity of ovarian cancer cells to platinum-based drugs by influencing antitumor immunity and the cell’s responsiveness to other cell death signals. GSH: glutathione; PUFAs: Polyunsaturated Fatty Acids
The cGAS/STING signaling pathway
The efficacy of cancer chemotherapy is significantly affected by anti-tumor immunity. Mitochondria serve as crucial components in innate immune recognition [74]. It can affect the immunogenicity of cancer cells and T cell activity through two pathways: (1) cGAS/STING pathway: mtDNA initially activates cGAS, which then transforms ATP/GTP into cGAMP, activates the downstream protein STING. This activation leads to the expression of ISGs genes. (2) MAVS/RIG-I/MDA5 pathway: Cytoplasmic dsRNA activates RIG-I and MDA5, which interact with the mitochondrial protein MAVS, triggering downstream effector TRAF proteins. This interaction forms the MAVS signaling complex, inducing the expression of interferon-stimulated genes.
West et al. demonstrated that mtDNA is safeguarded from degradation and cytoplasmic release by the mitochondrial transcription factor TFAM [75]. However, dysregulated TFAM expression, mitochondrial stress, and damage can lead to the release of mtDNA into the cytoplasm, activating the cGAS/STING pathway and inducing immunogenic cell death (ICD).
Cisplatin has been found to enhance the anticancer properties by stimulating type I IFN production [76]. DNA damage caused by chemotherapy leads to micronuclei formation, triggering an innate anti-tumor immune response [77]. Additionally, tumor cells undergoing necrosis and releasing mtDNA are crucial for cisplatin-induced immune activation. Moreover, tumor-derived cGAMP from cancer cells is transferred to non-cancer cells, activating STING and fostering anti-tumor immunity [77]. Additionally, it has been uncovered that tumor cells undergoing necrosis under the influence of receptor-interacting protein kinase 3 (RIPK3) and releasing mtDNA are crucial for cisplatin-induced immune activation [78, 79]. Due to chromatin interference with micronuclei promoted by cGAS DNA sensing, mtDNA may hold greater significance than micronuclear DNA.
In advanced cancer stages, impairment of the cGAS-STING pathway gene expression is common, while epigenetic modifications hinder cGAS and STING expression in ovarian cancer cells, contributing to immune evasion and drug resistance [80].
STING activation has been linked to mediating chemoresistance, with the expression of type I IFN-stimulated genes associated with resistance to chemotherapy across various tumor types [81, 82]. In ovarian cancer, heightened expression of HDAC4 following chemotherapy can deacetylate STAT1, thereby promoting resistance to platinum-based treatments [83]. Based on these findings, it seems that combining STING agonists with DNA-damaging drugs could present a novel therapeutic strategy, potentially leading to a sensitizing effect in cells respond to ICD.
Cell death in relation to platinum resistance
The main mechanism of action of chemotherapeutic drugs is to induce apoptosis in tumor cells. Recent studies have shown that tumor metabolic reprogramming can sensitize cancer cells to non-apoptotic forms of programmed cell death, including autophagy, necrosis, and ferroptosis. Mitochondria are pivotal in regulating the diverse forms of cell death that can impact the responsiveness to chemotherapy or the development of resistance to it.
Autophagy
Autophagy, a cellular process that transports cytoplasmic components to lysosomes for degradation and recycling, is regulated by autophagy-associated genes. It plays a dual role in tumor cells. On the one hand, it acts as a tumor suppressor by eliminating damaged proteins and organelles, reducing oxidative stress within cells, and averting genetic damage that could lead to cancer. On the other hand, autophagy mediates mitochondrial preservation in ovarian cancer cells with acquired resistance after cisplatin treatment. For instance, the rate-limiting enzyme of glycolysis HK2 promotes cisplatin resistance in ovarian cancer by boosting cisplatin-induced, ERK-mediated autophagy [84]. Blocking autophagy can result in fetal DNA damage and subsequent mitochondria-mediated apoptosis [85]. In hypoxic microenvironments, HIF1α induces autophagy and inhibits apoptosis, rendering OVCAR3 cells resistant to cisplatin [86].
The status of P53 has been demonstrated to impact the efficacy of autophagy inhibition in certain tumors. In ovarian cancer with p53 mutation, the cytoplasmic translocation and phosphorylation of HIF-1α and HDAC4 triggered by p53 and RAS signaling molecules govern both apoptotic and autophagic pathways, thereby influencing cisplatin resistance [87].
Given the crucial role of autophagy in cancer chemotherapy, altering autophagy has been recognized as a potentially valuable strategy to sensitize cancer cells to chemotherapy. Chloroquine (CQ) and its analogue hydroxychloroquine (HCQ) have been employed as autophagy inhibitors in various tumors, including ovarian cancer. Combining CQ with cisplatin enhances the effectiveness of chemotherapy in both cisplatin-sensitive and cisplatin-resistant cancers [88]. It is worth noting that the beneficial therapeutic strategy of autophagy inhibition in combination with cisplatin is closely related to the tumor type. Exploring how to strategically target autophagy to overcome chemoresistance while mitigating the impact of systemic autophagy inhibition on normal tissues in cancer patients is a subject for future investigation.
Necrosis
Necrosis is a form of programmed, caspase-independent cell death that is typically associated with a pro-inflammatory response [89]. The tumor necrosis factor α (TNFα) is involved in the activation of the receptor-interacting protein kinases RIPK1 and RIPK3, resulting in the formation of necrosomes. Subsequently, RIPK3 phosphorylates the mixed-spectrum kinase structural domain-like pseudokinase (MLKL), activating it, which then triggers the opening of the mitochondrial permeability transition pore and cell death. In some cell types, mitochondrial ROS facilitate the initiation of necrosis by promoting the autophosphorylation of RIPK1. This sets off a feed-forward activation mechanism where RIPK3 kinase boosts the activity of the pyruvate dehydrogenase complex, leading to increased aerobic respiration and elevated ROS Production [90].
Inducing cell necrosis is a strategy to heighten the platinum sensitivity of tumor cells. Treatment with inhibitors of “inhibitors of apoptotic proteins” (IAPs) combined with caspase inhibitors selectively triggers TNFα- and RIPK3-dependent cell death in various ovarian cancer-resistant cell lines and patient xenograft models [91]. Dey and colleagues discovered that targeting chemotherapy-resistant ovarian cancers that overexpress BMI1, where apoptotic pathways are often compromised, could inhibit the PINK1-PARK2-dependent mitochondrial pathway, leading to necroptosis [92].
Cisplatin elicits a more robust concurrent effect compared to oxaliplatin or carboplatin by activating RIPK3-dependent necrosis in tumor cells, lead to the release of mtDNA, initiation of the cyclic GMP-AMP synthase-Type I IFN genes pathway, and Type I IFN secretion [78]. The absence of RIPK3 or mtDNA in tumor cells contribute to their resistance to cisplatin.
Ferroptosis
The mechanism of ferroptosis was discovered by Dixon et al. [93]. It occurs under two major conditions, first, lipid peroxidation resulting from the suppression of the cystine/glutamate inverse transporter (SLC7A11) or glutathione peroxidase 4 (GPX4). GPX4 converts endogenous lipid peroxides from PUFAs-OOH to PUFAs-OH by using GSH as a cofactor, thus preventing the accumulation of intracellular ROS. The second is iron overload. Circulating Fe3 + binds to the transferrin and enters the cell through endocytosis transport. The Fenton reaction subsequently converts Fe3 + to Fe2+, which in turn generates lipid peroxides.
Ovarian cancer cells exhibit iron-dependency, with reduced ferroportin 1(FPN1) and transferrin receptor (TFR1) in tumor tissues from patients with HGSOC [94]. Targeting ferroptosis to combat chemoresistance in ovarian cancer could capitalized on increased lipid peroxidation and abnormal iron accumulation. In ovarian cancer patients, high co-expression levels of SLC7A11 and GPX4 correlate with a 60-fold increase in platinum resistance compared to those with low co-expression levels [95]. Gentric et al. discovered that the PML-PGC-1α axis, regulated by oxidative stress, and ferroptosis collectively enhance sensitivity to traditionally chemotherapy in HGSOCs with high OXPHOS [51]. Additionally, overexpression of SCD1 and FADS2, two crucial fatty acid desaturases, in peritoneal water-sourced ovarian cancer cells accelerates lipid metabolic activity, tumor invasiveness, and mediated cisplatin resistance [96]. Inhibiting fatty acid metabolism induces ferroptosis in ovarian cancer, synergizing with cisplatin to impede peritoneal metastasis.
Mitochondrial dynamics and mitochondria transfer contributes to platinum resistance of ovarian cancer cells
Mitochondria dynamics
In whole genome sequencing data encompassing 2658 tumor samples from 38 tumor types, mitochondrial copy numbers exhibited significant variation among different tumor types and even within the same tumor type, with ovarian cancers displaying the highest copy number at 644 copies/cell [97]. The mitochondrial content, mitochondrial ROS, and apoptosis in cisplatin resistant HGSOC cell lines are lower compared to sensitive cells [98].
Mitochondria exhibit morphologically flexibility and dynamics, creating a dynamic mitochondrial network through processes like cristae remodeling, fusion, and fission [99], which are involved in the regulation of cell apoptosis. The cisplatin-resistant ovarian cancer cells typically exhibit elevated levels of mitochondrial fusion proteins like MFN2, OPA1, prohibitin 1 (PHB1), and serine 637 phosphorylated DRP1 [100], [101]. An E3 ubiquitin ligase complex, CRL4, which inhibits mitochondrial fission by regulating the phosphorylation of DRP1 at Ser637, and inhibits mitophagy, was significantly upregulated in cisplatin-resistant ovarian cancer cells [102].
However, hypoxia-induced ROS can trigger resistance to cisplatin by promoting mitochondrial fission and downregulating mitochondrial fusion proteins [103], indicating that dynamics of mitochondria may also serve as a strategy for cells to adapt to the tumor microenvironment and resist therapy.
Mitochondria transfer
Tumors are now regarded as intricate systems where tumor and stromal components engage in extensive interactions. Beyond the transfer of energy between cells, the transfer of mitochondria and mtDNA also contributes to platinum resistance. Several studies have underscored the importance of mitochondrial exchange in maintaining tissue homeostasis, with implications for enhancing chemosensitivity in tumor cells.
Give the substantial mitochondrial damage and high energy demands in cancer cells, the transfer of mitochondria from donor cells-often originating from mesenchymal origin and fibroblasts-to damaged tumor cells (those with defective or deleted mtDNA), can restore respiration, boost proliferative and migratory capabilities, reduce ROS levels, and heighten drug resistance [104, 105]. Pioneering work by Lou and Pasquier et al. has highlighted tunneling nanotubes (TNTs) as conduits facilitating direct communication between stromal cells and cancer cells, or between cancer cells themselves, enabling mitochondrial transfer—an important mechanism observed in various tumors [106]. TNT formation occurs more frequently between platinum-resistant ovarian cancer cells cultures under hypoxic conditions compared to chemotherapy-sensitive malignant or benign epithelial cells, and TNT promotes angiogenesis in platinum-resistant cells [107, 108]. Moreover, exosome fractions and increased exocellular secretion of mitochondrial derived vesicles, which contains mitochondria with mutated mtDNA, are directed from resistant cells to sensitive cells’ mitochondria, contributing to acquired and extended chemoresistance in ovarian cancer cells [109, 110].
Conversely, studies have revealed that mitochondria originating from mesenchymal stem cells do not transfer to cells carrying human pathogenic mtDNA mutations (such as A3243G mutation or 4,977 bp deletion), but only to cells with nearly complete mitochondrial dysfunction [111].
Summary and outlook
mtDNA mutations, especially those induced by chemotherapeutic agents like platinum, hold significant clinical relevance. Delving deeply into these mutations is expected to offer vital insights for identifying high-risk individuals, screening pre-cancerous lesions, monitoring cancer progression, and predicting the prognosis of ovarian cancer.
Unfortunately, functional investigations into mutations at specific mitochondrial loci are still scarce. The question of whether the known mitochondrial mutations are pathogenic or confer drug-resistance remains unanswered, primarily due to limitations in mitochondrial base editing technology. There is a pressing need to develop enhanced tools, including more accessible mitochondrial base editors, to delve into mtDNA mutations. With the advent of single-cell sequencing technology, research on mitochondrial mutations in both drug-sensitive and drug-resistant ovarian cancers can progress by sequencing mtDNA at the single-cell level.
Under specific clinical circumstances, studies have revealed that proteins typically localized in the nucleus or cytoplasm, such Estrogen receptor-β [112], can translocate into mitochondria and influence cellular activities. The integration of spatial transcriptomics and proteomics at the mitochondrial level is anticipated to yield valuable insights into the identification of mitochondria-localized proteins and their functions in physiological and pathological contexts.
Targeting mitochondria have shown great potential for the treatment of platinum resistant ovarian cancer cells. Drugs targeting mitochondrial metabolism, such as metformin and 2-Deoxy-D-Glucose (2-DG), have demonstrated promising results in preclinical studies across various cancers, including ovarian cancer [113–115]. Furthermore, enhancing platinum sensitivity can be accomplished by specifically targeting mitochondria through delivering agents specifically to mitochondria to affect mtDNA [116, 117], utilizing drugs that disrupt the Tricarboxylic acid cycle (TCA) to impede ATP production, and employing agents that target proteins in the ETC [118]. As our understanding of mitochondrial biology deepens, the development and utilization of mitochondria-targeted therapeutics are expected to rise. These tailored treatments hold great potential for the effective management of ovarian cancer, particularly in drug-resistant cases, in the near future.
Conclusion
Mutations in mtDNA contribute to platinum resistance of ovarian cancer by affecting mitochondrial function and communication with the cell nucleus. Targeting mitochondria could provide a new approach to tackle platinum-resistant ovarian cancer.
Acknowledgements
We are grateful to Figdraw (www.figdraw.com) for their invaluable expertise in figure drawing.
Author contributions
Xin Cui: Writing-original draft, Investigation, visualization and funding acquisition. Juan Xu: Writing-review and editing, conceptualization, investigation, supervision and funding acquisition. Xuemei Jia: Writing-review and editing, conceptualization and funding acquisition.
Funding
This study was financially supported by the National Natural Science Foundation of China (81872126), Jiangsu Province Capability Improvement Project through Science, Technology and Education Jiangsu Provincial Medical Key Discipline (ZDXK202211), 333 project of Jiangsu Province (Xuemei Jia & Juan Xu) and Research Innovation Program for Graduates of Jiangsu Province (SJCX22_0670).
Data availability
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
All authors declared that there are no conflicts of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xin Cui and Juan Xu contributed equally to this work.
Contributor Information
Juan Xu, Email: xujuannj@njmu.edu.cn.
Xuemei Jia, Email: xmjia@njmu.edu.cn.
References
- 1.Yang Z, et al. Cisplatin preferentially binds mitochondrial DNA and voltage-dependent anion channel protein in the mitochondrial membrane of head and neck squamous cell carcinoma: possible role in apoptosis. Clin Cancer Res. 2006;12:5817–25. [DOI] [PubMed] [Google Scholar]
- 2.Olivero OA, Chang PK, Lopez-Larraza DM, Semino-Mora MC, Poirier MC. Preferential formation and decreased removal of cisplatin-DNA adducts in Chinese hamster ovary cell mitochondrial DNA as compared to nuclear DNA. Mutat Res. 1997;391:79–86. [DOI] [PubMed] [Google Scholar]
- 3.Kohno K, et al. Mitochondrial transcription factor A and mitochondrial genome as molecular targets for cisplatin-based Cancer Chemotherapy. Int J Mol Sci. 2015;16:19836–50. 10.3390/ijms160819836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abedi S, et al. Differential effects of cisplatin on cybrid cells with varying mitochondrial DNA haplogroups. PeerJ. 2020;8:e9908. 10.7717/peerj.9908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mandic A, Hansson J, Linder S, Shoshan MC. Cisplatin induces endoplasmic reticulum stress and nucleus-independent apoptotic signaling. J Biol Chem. 2003;278:9100–6. [DOI] [PubMed] [Google Scholar]
- 6.Gourdier I, Crabbe L, Andreau K, Pau B, Kroemer G. Oxaliplatin-induced mitochondrial apoptotic response of colon carcinoma cells does not require nuclear DNA. Oncogene. 2004;23:7449–57. [DOI] [PubMed] [Google Scholar]
- 7.Ju YS, et al. Frequent somatic transfer of mitochondrial DNA into the nuclear genome of human cancer cells. Genome Res. 2015;25:814–24. 10.1101/gr.190470.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown WM, George M, Wilson AC. Rapid evolution of animal mitochondrial DNA. Proc Natl Acad Sci U S A. 1979;76:1967–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larman TC, et al. Spectrum of somatic mitochondrial mutations in five cancers. Proc Natl Acad Sci U S A. 2012;109:14087–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu VW, et al. High incidence of somatic mitochondrial DNA mutations in human ovarian carcinomas. Cancer Res. 2001;61:5998–6001. [PubMed] [Google Scholar]
- 11.Ni J, et al. Pathogenic heteroplasmic somatic mitochondrial DNA mutation confers platinum-resistance and recurrence of high-Grade Serous Ovarian Cancer. Cancer Manag Res. 2020;12:11085–93. 10.2147/CMAR.S277724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kozakiewicz P, Grzybowska-Szatkowska L, Ciesielka M, Rzymowska J. The role of Mitochondria in Carcinogenesis. Int J Mol Sci. 2021;22. 10.3390/ijms22105100. [DOI] [PMC free article] [PubMed]
- 13.Iommarini L, et al. Different mtDNA mutations modify tumor progression in dependence of the degree of respiratory complex I impairment. Hum Mol Genet. 2014;23:1453–66. 10.1093/hmg/ddt533. [DOI] [PubMed] [Google Scholar]
- 14.Guerra F, et al. Mitochondrial DNA mutation in serous ovarian cancer: implications for mitochondria-coded genes in chemoresistance. J Clin Oncol. 2012;30:e373–8. 10.1200/JCO.2012.43.5933. [DOI] [PubMed] [Google Scholar]
- 15.Park JS, et al. A heteroplasmic, not homoplasmic, mitochondrial DNA mutation promotes tumorigenesis via alteration in reactive oxygen species generation and apoptosis. Hum Mol Genet. 2009;18:1578–89. 10.1093/hmg/ddp069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shidara Y, et al. Positive contribution of pathogenic mutations in the mitochondrial genome to the promotion of cancer by prevention from apoptosis. Cancer Res. 2005;65:1655–63. [DOI] [PubMed] [Google Scholar]
- 17.Ohta S. Contribution of somatic mutations in the mitochondrial genome to the development of cancer and tolerance against anticancer drugs. Oncogene. 2006;25:4768–76. [DOI] [PubMed] [Google Scholar]
- 18.Dar S, et al. Bioenergetic adaptations in Chemoresistant Ovarian Cancer cells. Sci Rep. 2017;7:8760. 10.1038/s41598-017-09206-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dier U, Shin D-H, Hemachandra LPMP, Uusitalo LM, Hempel N. Bioenergetic analysis of ovarian cancer cell lines: profiling of histological subtypes and identification of a mitochondria-defective cell line. PLoS ONE. 2014;9:e98479. 10.1371/journal.pone.0098479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montopoli M, et al. Metabolic reprogramming in ovarian cancer cells resistant to cisplatin. Curr Cancer Drug Targets. 2011;11:226–35. [DOI] [PubMed] [Google Scholar]
- 21.Giddings EL, et al. Mitochondrial ATP fuels ABC transporter-mediated drug efflux in cancer chemoresistance. Nat Commun. 2021;12:2804. 10.1038/s41467-021-23071-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matassa DS, et al. Oxidative metabolism drives inflammation-induced platinum resistance in human ovarian cancer. Cell Death Differ. 2016;23:1542–54. 10.1038/cdd.2016.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tyagi K, Mandal S, Roy A. Recent advancements in therapeutic targeting of the Warburg effect in refractory ovarian cancer: a promise towards disease remission. Biochim Biophys Acta Rev Cancer. 2021;1876:188563. 10.1016/j.bbcan.2021.188563. [DOI] [PubMed] [Google Scholar]
- 24.Bonuccelli G, et al. Ketones and lactate fuel tumor growth and metastasis: evidence that epithelial cancer cells use oxidative mitochondrial metabolism. Cell Cycle. 2010;9:3506–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pavlides S, et al. The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle. 2009;8:3984–4001. [DOI] [PubMed] [Google Scholar]
- 26.Labiche A, et al. Stromal compartment as a survival prognostic factor in advanced ovarian carcinoma. Int J Gynecol Cancer. 2010;20:28–33. 10.1111/IGC.0b013e3181bda1cb. [DOI] [PubMed] [Google Scholar]
- 27.Lou E, et al. Tumor-stroma proportion to Predict Chemoresistance in patients with ovarian Cancer. JAMA Netw Open. 2024;7:e240407. 10.1001/jamanetworkopen.2024.0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nieman KM, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17:1498–503. 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mukherjee A, et al. Adipocyte-Induced FABP4 expression in ovarian Cancer cells promotes metastasis and mediates Carboplatin Resistance. Cancer Res. 2020;80:1748–61. 10.1158/0008-5472.CAN-19-1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sabharwal SS, Schumacker PT. Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles’ heel? Nat Rev Cancer. 2014;14:709–21. 10.1038/nrc3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Idelchik MDPS, Begley U, Begley TJ, Melendez JA. Mitochondrial ROS control of cancer. Semin Cancer Biol. 2017;47:57–66. 10.1016/j.semcancer.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh RK, et al. mtDNA germ line variation mediated ROS generates retrograde signaling and induces pro-cancerous metabolic features. Sci Rep. 2014;4:6571. 10.1038/srep06571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marullo R, et al. Cisplatin induces a mitochondrial-ROS response that contributes to cytotoxicity depending on mitochondrial redox status and bioenergetic functions. PLoS ONE. 2013;8:e81162. 10.1371/journal.pone.0081162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kopinski PK, et al. Regulation of nuclear epigenome by mitochondrial DNA heteroplasmy. Proc Natl Acad Sci U S A. 2019;116:16028–35. 10.1073/pnas.1906896116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okon IS, Zou M-H. Mitochondrial ROS and cancer drug resistance: implications for therapy. Pharmacol Res. 2015;100:170–4. 10.1016/j.phrs.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li H-S, et al. HIF-1α protects against oxidative stress by directly targeting mitochondria. Redox Biol. 2019;25:101109. 10.1016/j.redox.2019.101109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ai Z, Lu Y, Qiu S, Fan Z. Overcoming cisplatin resistance of ovarian cancer cells by targeting HIF-1-regulated cancer metabolism. Cancer Lett. 2016;373:36–44. 10.1016/j.canlet.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kobayashi H, Imanaka S, Shigetomi H. Revisiting therapeutic strategies for ovarian cancer by focusing on redox homeostasis. Oncol Lett. 2022;23:80. 10.3892/ol.2022.13200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tossetta G, Fantone S, Montanari E, Marzioni D, Goteri G. Role of NRF2 in Ovarian Cancer. Antioxid (Basel). 2022;11. 10.3390/antiox11040663. [DOI] [PMC free article] [PubMed]
- 40.Zhang J, et al. Systematic identification of anticancer drug targets reveals a nucleus-to-mitochondria ROS-sensing pathway. Cell. 2023;186. 10.1016/j.cell.2023.04.026. [DOI] [PMC free article] [PubMed]
- 41.Desai R, et al. Mitochondria form contact sites with the nucleus to couple prosurvival retrograde response. Sci Adv. 2020;6. 10.1126/sciadv.abc9955. [DOI] [PMC free article] [PubMed]
- 42.Gatliff J, et al. TSPO interacts with VDAC1 and triggers a ROS-mediated inhibition of mitochondrial quality control. Autophagy. 2014;10:2279–96. 10.4161/15548627.2014.991665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scarpulla RC. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol Rev. 2008;88:611–38. 10.1152/physrev.00025.2007. [DOI] [PubMed] [Google Scholar]
- 44.Shen L, et al. PGC1α promotes cisplatin resistance in human ovarian carcinoma cells through upregulation of mitochondrial biogenesis. Int J Oncol. 2018;53:404–16. 10.3892/ijo.2018.4401. [DOI] [PubMed] [Google Scholar]
- 45.Kim B, et al. PGC1α induced by reactive oxygen species contributes to chemoresistance of ovarian cancer cells. Oncotarget. 2017;8:60299–311. 10.18632/oncotarget.19140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shen L, et al. PGC1α regulates mitochondrial oxidative phosphorylation involved in cisplatin resistance in ovarian cancer cells via nucleo-mitochondrial transcriptional feedback. Exp Cell Res. 2021;398:112369. 10.1016/j.yexcr.2020.112369. [DOI] [PubMed] [Google Scholar]
- 47.Deng X et al. The Nrf2/PGC1α Pathway Regulates Antioxidant and Proteasomal Activity to Alter Cisplatin Sensitivity in Ovarian Cancer. Oxid Med Cell Longev 2020, 4830418, 10.1155/2020/4830418 (2020). [DOI] [PMC free article] [PubMed]
- 48.Li Y, et al. PGC1α promotes Cisplatin Resistance in Ovarian Cancer by regulating the HSP70/HK2/VDAC1 signaling pathway. Int J Mol Sci. 2021;22. 10.3390/ijms22052537. [DOI] [PMC free article] [PubMed]
- 49.Ghilardi C, et al. PGC1α/β expression predicts therapeutic response to oxidative phosphorylation inhibition in Ovarian Cancer. Cancer Res. 2022;82:1423–34. 10.1158/0008-5472.CAN-21-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gabrielson M, Björklund M, Carlson J, Shoshan M. Expression of mitochondrial regulators PGC1α and TFAM as putative markers of subtype and chemoresistance in epithelial ovarian carcinoma. PLoS ONE. 2014;9:e107109. 10.1371/journal.pone.0107109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gentric G, et al. PML-Regulated mitochondrial metabolism enhances Chemosensitivity in Human ovarian cancers. Cell Metab. 2019;29. 10.1016/j.cmet.2018.09.002. [DOI] [PMC free article] [PubMed]
- 52.Walker BR, Moraes CT. Nuclear-Mitochondrial Interactions. Biomolecules 12, 10.3390/biom12030427 (2022). [DOI] [PMC free article] [PubMed]
- 53.Nargund AM, Pellegrino MW, Fiorese CJ, Baker BM, Haynes CM. Mitochondrial import efficiency of ATFS-1 regulates mitochondrial UPR activation. Science. 2012;337:587–90. 10.1126/science.1223560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nargund AM, Fiorese CJ, Pellegrino MW, Deng P, Haynes CM. Mitochondrial and nuclear accumulation of the transcription factor ATFS-1 promotes OXPHOS recovery during the UPR(mt). Mol Cell. 2015;58:123–33. 10.1016/j.molcel.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sutandy FXR, Gößner I, Tascher G, Münch C. A cytosolic surveillance mechanism activates the mitochondrial UPR. Nature. 2023;618:849–54. 10.1038/s41586-023-06142-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen A, et al. ATF5 is overexpressed in epithelial ovarian carcinomas and interference with its function increases apoptosis through the downregulation of Bcl-2 in SKOV-3 cells. Int J Gynecol Pathol. 2012;31:532–7. 10.1097/PGP.0b013e31824df26b. [DOI] [PubMed] [Google Scholar]
- 57.Guo J, et al. HSP60-regulated mitochondrial proteostasis and protein translation promote Tumor Growth of Ovarian Cancer. Sci Rep. 2019;9:12628. 10.1038/s41598-019-48992-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harper AK, Fletcher NM, Fan R, Morris RT, Saed GM. Heat shock protein 60 (HSP60) serves as a potential target for the sensitization of Chemoresistant Ovarian Cancer cells. Reprod Sci. 2020;27:1030–6. 10.1007/s43032-019-00089-2. [DOI] [PubMed] [Google Scholar]
- 59.Zhang Y, Maurizi MR. Mitochondrial ClpP activity is required for cisplatin resistance in human cells. Biochim Biophys Acta. 2016;1862:252–64. 10.1016/j.bbadis.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marchi S, Giorgi C, Galluzzi L, Pinton P. Ca2 + fluxes and Cancer. Mol Cell. 2020;78:1055–69. 10.1016/j.molcel.2020.04.017. [DOI] [PubMed] [Google Scholar]
- 61.Kerkhofs M, et al. Emerging molecular mechanisms in chemotherapy: Ca2 + signaling at the mitochondria-associated endoplasmic reticulum membranes. Cell Death Dis. 2018;9:334. 10.1038/s41419-017-0179-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Patergnani S, et al. Various aspects of Calcium Signaling in the regulation of apoptosis, Autophagy, Cell Proliferation, and Cancer. Int J Mol Sci. 2020;21. 10.3390/ijms21218323. [DOI] [PMC free article] [PubMed]
- 63.Yan T, et al. A novel CSN5/CRT O-GlcNAc/ER stress regulatory axis in platinum resistance of epithelial ovarian cancer. Int J Biol Sci. 2024;20:1279–96. 10.7150/ijbs.89700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dziegielewska B, et al. T-Type Ca2 + Channel Inhibition sensitizes ovarian Cancer to Carboplatin. Mol Cancer Ther. 2016;15:460–70. 10.1158/1535-7163.MCT-15-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chakraborty PK, et al. MICU1 drives glycolysis and chemoresistance in ovarian cancer. Nat Commun. 2017;8:14634. 10.1038/ncomms14634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee H, et al. Calcium channels as Novel therapeutic targets for ovarian Cancer stem cells. Int J Mol Sci. 2020;21. 10.3390/ijms21072327. [DOI] [PMC free article] [PubMed]
- 67.Wang Y, et al. Epigenetic targeting of ovarian cancer stem cells. Cancer Res. 2014;74:4922–36. 10.1158/0008-5472.CAN-14-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shang S, et al. Chemotherapy-Induced Distal Enhancers Drive Transcriptional Programs to maintain the Chemoresistant State in Ovarian Cancer. Cancer Res. 2019;79:4599–611. 10.1158/0008-5472.CAN-19-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li G-H, et al. Super-enhancers: a new frontier for epigenetic modifiers in cancer chemoresistance. J Exp Clin Cancer Res. 2021;40:174. 10.1186/s13046-021-01974-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zeller C, et al. Candidate DNA methylation drivers of acquired cisplatin resistance in ovarian cancer identified by methylome and expression profiling. Oncogene. 2012;31:4567–76. 10.1038/onc.2011.611. [DOI] [PubMed] [Google Scholar]
- 71.Curry E, et al. Genes predisposed to DNA hypermethylation during Acquired Resistance to Chemotherapy are identified in ovarian tumors by bivalent chromatin domains at initial diagnosis. Cancer Res. 2018;78:1383–91. 10.1158/0008-5472.CAN-17-1650. [DOI] [PubMed] [Google Scholar]
- 72.Liu D, et al. C/EBPβ enhances platinum resistance of ovarian cancer cells by reprogramming H3K79 methylation. Nat Commun. 2018;9:1739. 10.1038/s41467-018-03590-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhao L, et al. Tackling drug resistance in ovarian cancer with epigenetic targeted drugs. Eur J Pharmacol. 2022;927. 10.1016/j.ejphar.2022.175071. [DOI] [PubMed]
- 74.Kanneganti T-D, Kundu M, Green DR. Innate immune recognition of mtDNA–an undercover signal? Cell Metab. 2015;21:793–4. 10.1016/j.cmet.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 75.West AP, et al. Mitochondrial DNA stress primes the antiviral innate immune response. Nature. 2015;520:553–7. 10.1038/nature14156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Salvagno C, et al. Therapeutic targeting of macrophages enhances chemotherapy efficacy by unleashing type I interferon response. Nat Cell Biol. 2019;21:511–21. 10.1038/s41556-019-0298-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yum S, Li M, Chen ZJ. Old dogs, new trick: classic cancer therapies activate cGAS. Cell Res. 2020;30:639–48. 10.1038/s41422-020-0346-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Luo R, et al. Necroptosis-dependent immunogenicity of cisplatin: implications for enhancing the Radiation-induced Abscopal Effect. Clin Cancer Res. 2023;29:667–83. 10.1158/1078-0432.CCR-22-1591. [DOI] [PubMed] [Google Scholar]
- 79.Yang H, et al. Contribution of RIP3 and MLKL to immunogenic cell death signaling in cancer chemotherapy. Oncoimmunology. 2016;5:e1149673. 10.1080/2162402X.2016.1149673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.de Queiroz NMGP, Xia T, Konno H, Barber GN. Ovarian Cancer cells commonly exhibit defective STING Signaling which affects sensitivity to viral oncolysis. Mol Cancer Res. 2019;17:974–86. 10.1158/1541-7786.MCR-18-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weichselbaum RR, et al. An interferon-related gene signature for DNA damage resistance is a predictive marker for chemotherapy and radiation for breast cancer. Proc Natl Acad Sci U S A. 2008;105:18490–5. 10.1073/pnas.0809242105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Erdal E, Haider S, Rehwinkel J, Harris AL, McHugh PJ. A prosurvival DNA damage-induced cytoplasmic interferon response is mediated by end resection factors and is limited by Trex1. Genes Dev. 2017;31:353–69. 10.1101/gad.289769.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stronach EA, et al. HDAC4-regulated STAT1 activation mediates platinum resistance in ovarian cancer. Cancer Res. 2011;71:4412–22. 10.1158/0008-5472.CAN-10-4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang X-Y, et al. Hexokinase 2 confers resistance to cisplatin in ovarian cancer cells by enhancing cisplatin-induced autophagy. Int J Biochem Cell Biol. 2018;95. 10.1016/j.biocel.2017.12.010. [DOI] [PubMed]
- 85.Liu Y, et al. TRPML1-induced autophagy inhibition triggers mitochondrial mediated apoptosis. Cancer Lett. 2022;541:215752. 10.1016/j.canlet.2022.215752. [DOI] [PubMed] [Google Scholar]
- 86.Long F, et al. HIF-1α-induced autophagy contributes to cisplatin resistance in ovarian cancer cells. Pharmazie. 2018;73:533–6. 10.1691/ph.2018.8514. [DOI] [PubMed] [Google Scholar]
- 87.Zhang X, Qi Z, Yin H, Yang G. Interaction between p53 and Ras signaling controls cisplatin resistance via HDAC4- and HIF-1α-mediated regulation of apoptosis and autophagy. Theranostics. 2019;9:1096–114. 10.7150/thno.29673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hwang JR, et al. Chloroquine reverses chemoresistance via upregulation of p21WAF1/CIP1 and autophagy inhibition in ovarian cancer. Cell Death Dis. 2020;11:1034. 10.1038/s41419-020-03242-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Newton K, et al. Activity of protein kinase RIPK3 determines whether cells die by necroptosis or apoptosis. Science. 2014;343:1357–60. 10.1126/science.1249361. [DOI] [PubMed] [Google Scholar]
- 90.Yang Z, et al. RIP3 targets pyruvate dehydrogenase complex to increase aerobic respiration in TNF-induced necroptosis. Nat Cell Biol. 2018;20:186–97. 10.1038/s41556-017-0022-y. [DOI] [PubMed] [Google Scholar]
- 91.McCabe KE, et al. Triggering necroptosis in cisplatin and IAP antagonist-resistant ovarian carcinoma. Cell Death Dis. 2014;5:e1496. 10.1038/cddis.2014.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dey A, et al. Inhibition of BMI1 induces autophagy-mediated necroptosis. Autophagy. 2016;12:659–70. 10.1080/15548627.2016.1147670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dixon SJ, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–72. 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Basuli D, et al. Iron addiction: a novel therapeutic target in ovarian cancer. Oncogene. 2017;36:4089–99. 10.1038/onc.2017.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wu X, et al. High co-expression of SLC7A11 and GPX4 as a predictor of platinum resistance and poor prognosis in patients with epithelial ovarian cancer. BJOG. 2022;129(2):40–9. 10.1111/1471-0528.17327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xuan Y, et al. SCD1/FADS2 fatty acid desaturases equipoise lipid metabolic activity and redox-driven ferroptosis in ascites-derived ovarian cancer cells. Theranostics. 2022;12:3534–52. 10.7150/thno.70194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yuan Y, et al. Comprehensive molecular characterization of mitochondrial genomes in human cancers. Nat Genet. 2020;52:342–52. 10.1038/s41588-019-0557-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kleih M, et al. Direct impact of cisplatin on mitochondria induces ROS production that dictates cell fate of ovarian cancer cells. Cell Death Dis. 2019;10:851. 10.1038/s41419-019-2081-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pernas L, Scorrano L, Mito-Morphosis. Mitochondrial Fusion, Fission, and Cristae Remodeling as Key mediators of Cellular function. Annu Rev Physiol. 2016;78:505–31. 10.1146/annurev-physiol-021115-105011. [DOI] [PubMed] [Google Scholar]
- 100.De Rasmo D, Cormio A, Cormio G, Signorile A. Ovarian Cancer: a Landscape of Mitochondria with emphasis on mitochondrial dynamics. Int J Mol Sci. 2023;24. 10.3390/ijms24021224. [DOI] [PMC free article] [PubMed]
- 101.Zou G-P, et al. Mitochondrial dynamics mediated by DRP1 and MFN2 contributes to Cisplatin Chemoresistance in Human Ovarian Cancer SKOV3 cells. J Cancer. 2021;12:7358–73. 10.7150/jca.61379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Meng Y, et al. Targeting CRL4 suppresses chemoresistant ovarian cancer growth by inducing mitophagy. Signal Transduct Target Ther. 2022;7:388. 10.1038/s41392-022-01253-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Han Y, et al. Mitochondrial fission causes cisplatin resistance under hypoxic conditions via ROS in ovarian cancer cells. Oncogene. 2019;38:7089–105. 10.1038/s41388-019-0949-5. [DOI] [PubMed] [Google Scholar]
- 104.Spees JL, Olson SD, Whitney MJ, Prockop DJ. Mitochondrial transfer between cells can rescue aerobic respiration. Proc Natl Acad Sci U S A. 2006;103:1283–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tan AS, et al. Mitochondrial genome acquisition restores respiratory function and tumorigenic potential of cancer cells without mitochondrial DNA. Cell Metab. 2015;21:81–94. 10.1016/j.cmet.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 106.Pasquier J, et al. Preferential transfer of mitochondria from endothelial to cancer cells through tunneling nanotubes modulates chemoresistance. J Transl Med. 2013;11:94. 10.1186/1479-5876-11-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Desir S, et al. Tunneling nanotube formation is stimulated by hypoxia in ovarian cancer cells. Oncotarget. 2016;7:43150–61. 10.18632/oncotarget.9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lou E, et al. Cellular and Molecular networking within the ecosystem of Cancer Cell Communication via Tunneling nanotubes. Front Cell Dev Biol. 2018;6:95. 10.3389/fcell.2018.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Abad E, Lyakhovich A. Movement of Mitochondria with Mutant DNA through Extracellular vesicles helps Cancer cells acquire Chemoresistance. ChemMedChem. 2022;17(e202100642). 10.1002/cmdc.202100642. [DOI] [PubMed]
- 110.Gagliardi S, et al. Defects of mitochondria-lysosomes communication induce secretion of mitochondria-derived vesicles and drive chemoresistance in ovarian cancer cells. Cell Commun Signal. 2024;22:165. 10.1186/s12964-024-01507-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cho YM, et al. Mesenchymal stem cells transfer mitochondria to the cells with virtually no mitochondrial function but not with pathogenic mtDNA mutations. PLoS ONE. 2012;7:e32778. 10.1371/journal.pone.0032778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liao TL, Tzeng CR, Yu CL, Wang YP, Kao SH. Estrogen receptor-β in mitochondria: implications for mitochondrial bioenergetics and tumorigenesis. Ann NY Acad Sci. 2015;1350:52–60. 10.1111/nyas.12872. [DOI] [PubMed] [Google Scholar]
- 113.Brown JR, et al. Phase II clinical trial of metformin as a cancer stem cell-targeting agent in ovarian cancer. JCI Insight. 2020;5. 10.1172/jci.insight.133247. [DOI] [PMC free article] [PubMed]
- 114.Tossetta G. Metformin improves ovarian Cancer sensitivity to Paclitaxel and Platinum-based drugs: a review of in Vitro findings. Int J Mol Sci. 2022;23. 10.3390/ijms232112893. [DOI] [PMC free article] [PubMed]
- 115.Loar P, et al. Inhibition of glycolysis enhances cisplatin-induced apoptosis in ovarian cancer cells. Am J Obstet Gynecol. 2010;202:e371371–378. 10.1016/j.ajog.2009.10.883. [DOI] [PubMed] [Google Scholar]
- 116.Marrache S, Pathak RK, Dhar S. Detouring of cisplatin to access mitochondrial genome for overcoming resistance. Proc Natl Acad Sci U S A. 2014;111:10444–9. 10.1073/pnas.1405244111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yang G-G, et al. Precisely assembled nanoparticles against Cisplatin Resistance via Cancer-Specific Targeting of Mitochondria and Imaging-guided chemo-photothermal therapy. ACS Appl Mater Interfaces. 2020;12:43444–55. 10.1021/acsami.0c12814. [DOI] [PubMed] [Google Scholar]
- 118.Lee C, Park S-H, Yoon SK. Genetic mutations affecting mitochondrial function in cancer drug resistance. Genes Genomics. 2023;45:261–70. 10.1007/s13258-022-01359-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.