Abstract
Background
Postpartum hemorrhage is one of the leading causes of maternal mortality and morbidity. The etiology of postpartum hemorrhage exhibits variations in relation to the mode of birth; consequently, risk factors for massive transfusion in elective cesarean section might diverge from those encountered in vaginal birth or emergency cesarean section. The main purpose of this study was to investigate antepartum risk factors of massive transfusion for elective cesarean section.
Methods
We conducted a retrospective cohort study based on data from a nationwide system that collected inpatient medical records from tertiary hospitals in mainland China. We included women who had undergone elective cesarean section from January 2013 to August 2018. Primary outcome was massive transfusion defined as transfusion of more than eight units of red blood cells on the day of childbirth. Candidate risk factors were identified by the 10th revision of International Classification of Diseases Codes of admission diagnoses. The relationship between each factor and massive transfusion was assessed using multivariable logistic regression.
Results
A total of 294,695 women were included and 572 of them received massive transfusion (incidence: 194 per 100,000 elective cesarean sections). Maternal age [adjusted odds ratio (aOR) 1.22; 95% confidence interval (CI) 1.10–1.48], anemia (aOR 1.66; 95% CI 1.34–2.05), thrombocytopenia (aOR 3.54; 95% CI 2.39–5.05), coagulopathy (aOR 25.92; 95% CI 8.59–69.50), hypoalbuminemia (aOR 2.97; 95% CI 1.86–4.53), hepatic dysfunction (aOR 1.65; 95% CI 1.04–2.47), uterine scar (aOR 1.39; 95% CI 1.15–1.67), multiple pregnancy (aOR 2.84; 95% CI 1.74–4.38), polyhydramnios (aOR 2.52; 95% CI 1.19–4.68) and placenta previa (aOR 25.03; 95% CI 21.04–29.77) were associated with massive blood transfusion for elective cesarean section. Among the women receiving massive blood transfusion, 7 (1.2%) died during hospitalization, 126 (22.0%) needed hysterectomy, 25 (4.4%) uterine packing and 57 (10.0%) uterine artery ligation.
Conclusions
Ten risk factors of massive transfusion were identified in women undergoing elective cesarean section. Our findings may facilitate blood products preparation and provide opportunities for applying prophylactic strategies prior to cesarean section for women at high risk of massive transfusion.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12884-024-06875-4.
Keywords: Massive blood transfusion, Elective cesarean section, Risk factor, Real-world, Nationwide, Adverse outcomes
Introduction
Postpartum hemorrhage (PPH) is one of the leading causes of maternal mortality and morbidity worldwide [1]. Rates of PPH have been escalating in high-income countries [2–4]. Blood transfusion is frequently necessary in women with severe PPH [5, 6]. There is no agreed definition of obstetric massive blood transfusion. In previous studies, one of the definitions was transfusion of ≥ 8 units (U) of red blood cells (RBCs) within 24 h of giving birth or during hospitalization [7–10]. In severe PPH, timely availability of blood products, in combination with other interventions, is life-saving [11, 12]. However, storage of blood products might be limited and crossmatching of RBC units could be time-consuming [13].
Therefore, identifying women who are likely to require blood transfusion prior to childbirth is warranted. Previously established risk factors associated with PPH or peripartum blood transfusion included maternal age, obesity, hypertensive disorders, previous history of PPH, labor induction, placental abnormalities, multiple pregnancy, general anesthesia, emergency and elective cesarean section (CS) [7, 14–23].
In spite of these accumulating evidence in the general birthing population, little focus has been put on elective CS. Etiology of PPH exhibits variations in relation to the mode of birth; [7, 8, 24] thus, consequently, risk factors for massive transfusion in elective CS might, in addition to overlap, also show differences with those encountered in vaginal birth or emergency CS [14, 25]. Furthermore, unlike spontaneous or induced labor, which involves a series of unpredictable intrapartum factors, most risk factors in elective CS can be identified and evaluated in the antepartum period [14, 25]. Therefore, it is possible to optimize preoperative preparation and prevent avoidable adverse outcomes.
Aim of this nationwide cohort study was to investigate antepartum risk factors of massive transfusion in women undergoing elective CS in China, focusing on identifying antepartum factors that could be recognized prior to the initiation of elective CS. In the exploratory analysis, we also described maternal outcomes related to massive blood transfusion.
Methods
Data source
We performed a nationwide retrospective cohort study using data from the Hospital Quality Monitoring System (HQMS) managed by the National Health Commission of China. Launched in 2011, the system was designed to monitor and improve healthcare quality. Data were automatically uploaded from hospitals to HQMS through a secure data transmission path. This path was implemented in approximately 50% of tertiary hospitals across all 31 provincial-level regions in mainland China, allowing HQMS to collect anonymized inpatient medical records from these hospitals. The medical records contained demographic information, diagnoses on admission and discharge, details of the procedures performed during hospitalization, operation timing (elective or emergency), anesthesia methods, discharge status (alive or dead) and transfusion records (if any). Diagnoses and procedures were coded according to the 10th revision of International Classification of Diseases (ICD) and 9th revision of International Classification of Diseases, Clinical Modification. Uploading data on these variables has been a mandatory task since 2013 for the tertiary hospitals included in HQMS. The manuscript adhered to the applicable STROBE guidelines.
Study population
We included women who had undergone elective CS in tertiary hospitals in mainland China from January 2013 to August 2018. Elective CS was defined as CS that was planned at least one day before surgery. We first identified patients who had at least one record of surgical procedure in HQMS. Next, we identified all parturient women who received CS using ICD code 740, 741, 742, 744, 7491 and 7499 (Supplemental Table 1). Finally, women after elective CS were identified based on the codes of operation timing.
Definition of variables
Primary outcome was the incidence of massive blood transfusion, which was defined as transfusion of ≥ 8 units of RBCs administered either intraoperatively or postoperatively on the day of CS [7–10]. Total units of RBC transfused were obtained from the transfusion records in the dataset.
Candidate correlated factors were chosen based on the results of previous similar studies and clinical experiences, with a focus on factors that could be identified prior to CS [7, 14–23]. We selected maternal age, along with a wide range of maternal morbidities and obstetric conditions, as candidate correlated factors. Maternal morbidities included anemia (hemoglobin level < 110 g/L[26]), thrombocytopenia (platelet count < 150 × 109/L[27]), coagulopathy (hereditary or acquired coagulation factor deficiencies or dysfunction [28]), hypoalbuminemia (albumin level < 35 g/L[29], not due to hepatic dysfunction), hepatic dysfunction (hepatic failure/ fibrosis/ cirrhosis, chronic active hepatitis or acute hepatitis [30]), uterine adenomyosis or leiomyoma, uterine scar (a previous history of uterine myomectomy or CS) and hypertensive disorders of pregnancy (including chronic hypertension, gestational hypertension, preeclampsia and eclampsia). Obstetric conditions included in vitro fertilization, multiple pregnancy, fetal macrosomia, polyhydramnios, placenta previa and preterm birth. We relied on the nationwide standard obstetric practice of obtaining a complete blood count, blood biochemistry and coagulation tests before conducting elective CS. These preoperative evaluations are essential for diagnosing anemia, thrombocytopenia, hypoalbuminemia, hepatic dysfunction and coagulopathy. We established an ICD code set for each candidate factor (Supplemental Table 2). The presence of each candidate factor was ascertained if there was at least one diagnosis matching to the corresponding ICD code set at the time of admission.
Maternal outcomes in relation to severe hemorrhage were compared between women receiving or not receiving massive transfusion as an exploratory analysis, including in-hospital mortality, hysterectomy, uterine packing (with gauze) and uterine artery ligation. Surgical interventions were identified by ICD codes of procedures (Supplemental Table 3).
Statistical analysis
The distribution of the continuous variables was examined using visual inspection of the histogram. Continuous normally distributed data, non-normally distributed data and categorical data were described as mean ± standard deviation, median [interquartile range] and number (percentage). 95% Confidence intervals (CI) of the incidence of massive transfusion were estimated by Wilson’s method. We first assessed the association between baseline variables and massive transfusion using univariable logistic regression and then built a multivariable logistic regression model, in which massive transfusion was the outcome variable while candidate correlated factors were explanatory variables. We did not use any techniques for variable selection in the logistic regression model, and all the explanatory variables were put into the model using the enter method. Hosmer-Lemeshow’s test was used to assess the goodness of fit for the logistic regression model. Data cleaning and analysis were completed in R (R Foundation for Statistical Computing, Vienna, Austria, version 3.5.2) and a two-sided p value < 0.05 was considered as statistically significant.
In the power analysis, the smallest significant effect measured by odds ratio (OR) was calculated with the given sample size. We used 0.05 as the two-sided probability of the type I error considering the multiplicity adjustment, 0.9 as the statistical power, 294,695 as the fixed sample size and 572 as the number of women with peripartum massive blood transfusion in the analysis. For a common exposure with a prevalence larger than 1.0%, our sample has enough statistical power to detect an OR less than 0.94 or larger than 1.12.
Results
There were 48,926,568 patients with at least one surgical procedure record from tertiary hospitals from Jan 2013 to Aug 2018 in the dataset. We identified 572,180 CSs among them. In our cohort, 277,505 women underwent emergency CS, among whom 1,854 experienced massive transfusions. This reflected an incidence of 668 (95% CI 639–699) per 100,000 CSs. After excluding these emergency CSs, we finally included 294,695 women who underwent elective CSs into the study cohort (Fig. 1). In this cohort, 572 women received massive transfusion with an incidence of 194 (95% CI 178–210) per 100,000 CSs. Of those who received massive transfusion, 484 (84.6%) received fresh frozen plasma (FFP) transfusion, 91 (15.9%) received platelet transfusion. Women who did not receive massive transfusion had significantly lower rates of FFP and platelet transfusion compared to those who received massive transfusions [FFP: 0.6% (1896/ 294123) vs. 84.6%, PLT 0.2% (613/ 294123) vs. 15.9%, p < 0.001 for both comparisons in the Chi-square test]. The number of RBC-units transfused was 10 [8, 13] U and the amount of FFP 800 ml [400, 1250].
Fig. 1.
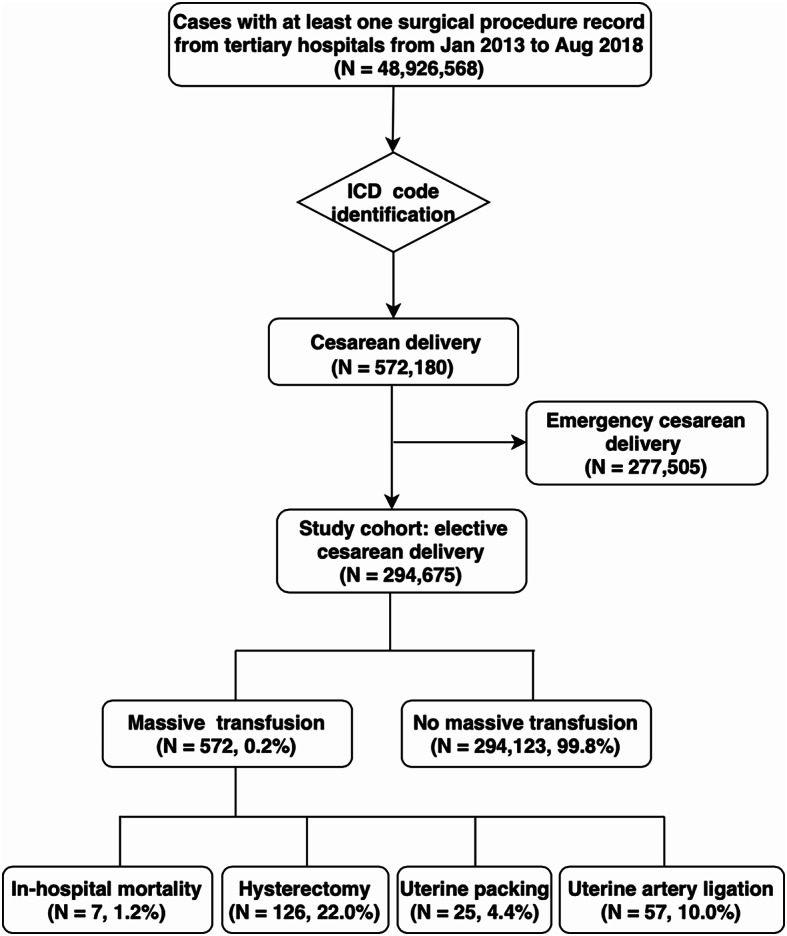
Flow diagram of the selection and identification of the women included in the cohort
The comparison of the candidate correlated factors between the women receiving and not receiving massive blood transfusion was presented in Table 1. Women receiving massive blood transfusion were more likely to have an advanced maternal age, anemia, thrombocytopenia, coagulopathy, hypoalbuminemia, hepatic dysfunction, uterine scar, multiple pregnancy, fetal macrosomia, polyhydramnios, placenta previa or preterm birth.
Table 1.
Comparison of the candidate risk factors between women receiving and not receiving massive blood transfusion
| Variables | Massive Transfusion (N = 572)a |
No Massive Transfusion (N = 294,123)b |
OR | 95% CI |
|---|---|---|---|---|
| Age (year) | 31 ± 6 | 30 ± 6 | 1.04 | 0.99–1.06 |
| Anemiac | 130 (22.7%) | 29,549 (10.0%) | 2.63 | 2.16–3.20 |
| Thrombocytopeniad | 34 (5.9%) | 3984 (1.4%) | 4.60 | 3.25–6.52 |
| Coagulopathye | 7 (1.2%) | 32 (0.0%) | 113.86 | 50.05-259.04 |
| Hypoalbuminemiaf | 25 (4.4%) | 2147 (0.7%) | 6.22 | 4.15–9.30 |
| Hepatic dysfunctiong | 23 (4.0%) | 6687 (2.3%) | 1.80 | 1.19–2.73 |
| Adenomyosis/ leiomyoma | 6 (1.0%) | 1996 (0.7%) | 1.55 | 0.69–3.47 |
| Uterine scar | 179 (31.3%) | 80,514 (27.4%) | 1.21 | 1.01–1.44 |
| Hypertension | 6 (1.0%) | 3152 (1.1%) | 0.98 | 0.44–2.19 |
| In vitro fertilization | 196 (34.3%) | 106,842 (36.3%) | 0.91 | 0.77–1.09 |
| Multiple pregnancy | 16 (2.8%) | 2812 (1.0%) | 2.98 | 2.62–3.38 |
| Fetal macrosomia | 18 (3.1%) | 19,156 (6.5%) | 0.47 | 0.29–0.75 |
| Polyhydramnios | 9 (1.6%) | 1756 (0.6%) | 2.66 | 1.38–5.15 |
| Placenta previa | 287 (50.2%) | 11,029 (3.8%) | 25.85 | 21.92–30.49 |
| Preterm birth | 45 (7.9%) | 7515 (2.6%) | 3.26 | 2.40–4.42 |
Abbreviations OR, odd ratio; CI, confidence interval. a, b Continuous data were expressed as mean ± standard deviation, and categorical data were described as number (percentage). cHemoglobin level < 110 g/L; dplatelet count < 150 × 109/L; ehereditary or acquired coagulation factor deficiencies or dysfunction; falbumin level < 35 g/L, not due to hepatic dysfunction; ghepatic failure/ fibrosis/ cirrhosis, chronic active hepatitis or acute hepatitis
In the multivariable logistic regression model that included all the candidate antepartum risk factors (Table 2), factors associated with massive transfusion were maternal age [adjusted odds ratio (aOR) 1.22; 95% CI 1.10–1.48], anemia (aOR 1.66; 95% CI 1.34–2.05), thrombocytopenia (aOR 3.54; 95% CI 2.39–5.05), coagulopathy (aOR 25.92; 95% CI 8.59–69.50), hypoalbuminemia (aOR 2.97; 95% CI 1.86–4.53), hepatic dysfunction (aOR 1.65; 95% CI 1.04–2.47), uterine scar (aOR 1.39; 95% CI 1.15–1.67), multiple pregnancy (aOR 2.84; 95% CI 1.74–4.38), polyhydramnios (aOR 2.52; 95% CI 1.19–4.68) and placenta previa (aOR 25.03; 95% CI 21.04–29.77). The Hosmer and Lemeshow’s tests implied acceptable goodness-of-fit (p 0.152).
Table 2.
Multiple logistic regression model included the candidate risk factors of massive blood transfusion (N = 294,695)
| Variables | aOR | 95% CI |
|---|---|---|
| Age (year) | 1.02 | 1.01–1.04 |
| Anemiaa | 1.66 | 1.34–2.05 |
| Thrombocytopeniab | 3.54 | 2.39–5.05 |
| Coagulopathyc | 25.92 | 8.59–69.50 |
| Hypoalbuminemiad | 2.97 | 1.86–4.53 |
| Hepatic dysfunctione | 1.65 | 1.04–2.47 |
| Adenomyosis/ leiomyoma | 1.67 | 0.65–3.48 |
| Uterine scar | 1.39 | 1.15–1.67 |
| Hypertension | 1.03 | 0.40–2.17 |
| In vitro fertilization | 1.14 | 0.94–1.37 |
| Multiple pregnancy | 2.84 | 1.74–4.38 |
| Fetal Macrosomia | 0.77 | 0.46–1.20 |
| Polyhydramnios | 2.52 | 1.19–4.68 |
| Placenta previa | 25.03 | 21.04–29.77 |
| Preterm birth | 0.91 | 0.65–1.25 |
Abbreviations aOR, adjusted odd ratio; CI, confidence interval; aHemoglobin level < 110 g/L; bplatelet count < 150 × 109/L; ccoagulation factor deficiencies or dysfunction; dalbumin level < 35 g/L, not due to hepatic dysfunction; ehepatic failure/ fibrosis/ cirrhosis, chronic active hepatitis or acute hepatitis
We further investigated adverse outcomes related to severe PPH (Table 3). Among the women receiving massive blood transfusion, 7 (1.2%) died during hospitalization, 126 (22.0%) needed hysterectomy, 25 (4.4%) uterine packing and 57 (10.0%) uterine artery ligation. Women requiring massive blood transfusion were more likely to suffer from these adverse outcomes.
Table 3.
Comparison of the maternal outcomes between women receiving and not receiving massive blood transfusion
| Maternal outcomes | Massive Transfusion (N = 572)a |
No Massive Transfusion (N = 294,123)b |
OR | 95% CI |
|---|---|---|---|---|
| In-hospital mortality | 7 (1.2%) | 32 (0.0%) | 113.86 | 50.05-259.04 |
| Hysterectomy | 126 (22.0%) | 1199 (0.4%) | 69.02 | 56.19–84.78 |
| Uterine packing | 25 (4.4%) | 1290 (0.4%) | 10.37 | 6.92–15.55 |
| Uterine artery ligation | 57 (10.0%) | 1695 (0.6%) | 19.09 | 14.46–25.21 |
Abbreviations OR, odd ratio; CI, confidence interval. a, b Categorical data were described as number (percentage)
Discussion
In this nationwide retrospective cohort study, the risk factors associated with peripartum massive transfusion for elective CS were maternal age, anemia, thrombocytopenia, coagulopathy, hypoalbuminemia, hepatic dysfunction, uterine scar, multiple pregnancy, polyhydramnios and placenta previa. Additionally, among women who required massive blood transfusion the rate of in-hospital mortality occurred in 7 (1.2%), hysterectomy in 126 (22.0%), uterine packing in 25 (4.4%) and uterine artery ligation in 57 (10.0%).
There has been scarce data regarding the incidence of massive transfusion in relation to elective CS before. Population-based cohort studies using a similar definition of massive transfusion indicated that the incidence was 23 to 91 per 10,000 births in high-income countries [7–10]. Notably, these studies included women undergoing various modes of birth. Since CS is associated with an increased risk of PPH [14, 31], the relatively high incidence in our study may be explainable.
We found ten antepartum risk factors of massive transfusion for elective CS. The mechanisms of their association with PPH lie in the following three aspects. The first one is abnormal placentation, encompassing placenta previa and placenta accreta spectrum. In line with previous studies, placenta previa is the strongest factor of massive transfusion in our study [15, 21, 32]. Uterine scarring contributes to the development of placenta accreta, increta or percreta, which may lead to uncontrolled PPH [33]. Second, some factors are associated with coagulation function. Despite the low prevalences of thrombocytopenia and coagulopathy in our cohort, both conditions were found to be significantly associated with the risk of massive transfusion. Hepatitis B is indeed a significant concern among pregnant women in Mainland China with an approximate prevalence of 4.5% and this may explain the high prevalence of hepatic dysfunction in our study [30]. Severe hepatic dysfunction may cause impaired synthesis of coagulation factors [34]. The third mechanism is uterine atony. Anemia and hypoalbuminemia during pregnancy are indicators of malnutritional status, which may cause diminished oxygen transportation and myometrial weakness [35, 36]. Multiple pregnancy and polyhydramnios can lead to overdistention of the uterus, which may weaken myometrial contractility [37].
We did not find statistically significant associations between adenomyosis/ leiomyoma, fetal macrosomia or in vitro fertilization with massive transfusion. Some studies showed that adenomyosis/ leiomyoma could impair uterine myometrium both structurally and functionally and further attenuate uterine contraction [19, 38, 39]. In contrast, it was also found that the effects of leiomyoma on pregnancy outcomes depended on its size and location [38]. Fetal macrosomia has been believed to be associated with obstetric transfusion [18, 39]. It is worth noting that the majority of women involved in previous studies underwent vaginal birth, during which women are at a high risk of shoulder dystocia, uterine atony and episiotomy [40–42]. Conversely, a reduced risk of PPH has been observed in planned CS with predicted fetal macrosomia [43]. Several studies showed assisted reproductive technology to be associated with increased risks of preeclampsia, abnormal placentation, multiple pregnancy and polyhydramnios and thus may contribute to PPH [14, 44]. However, it is unknown whether assisted reproductive technology is an independent risk factor of PPH.
Rates of maternal outcomes after massive transfusion in our study are comparable with previous data [7–9]. Those women who required massive transfusion were more likely to have adverse outcomes, which punctuated the necessity of identifying women with high-risk pregnancies in advance of CS. For women with multiple strong risk factors, prophylactic attempts and proactive preparations may be instrumental in reducing the risks of these adverse outcomes.
Strengths and limitations
Our study has the following strengths. First, Our study specially identified risk factors for massive transfusion among women undergoing elective CS. First, our study was focused on antepartum risk factors. We found that pre-existing anemia, thrombocytopenia, coagulopathy, hypoalbuminemia, and hepatic dysfunction are associated with massive transfusion. Identifying these potentially amendable factors in advance can provide opportunities to reduce the risk of massive transfusion. Furthermore, since the date of elective CS is usually predefined, physicians are able to make full preparations for massive transfusion for women with multiple risk factors, including adequate blood products, venous access, prohemostatic agents (tranexamic acid, fibrinogen concentrate and prothrombin complex concentrate [45]), point-of-care thromboelastometry monitoring and active warming devices (electric blankets, warm-water mattresses, intravenous fluids warming and anesthetic air warming [46]). Another main strength of the present study was the nationwide sample of elective CSs, which was representative of the entire Chinese population after elective CS. This allowed us to assess candidate correlated factors for the exceptionally low-incidence event, massive blood transfusion. The large sample size also gave sufficient statistical power to include multiple variables in the regression model, including the ones that have been seldom investigated, such as hypoalbuminemia.
Our study also has limitations. First, we recognize the absence of certain known risk factors, such as previous PPH and obesity, in our analysis due to data constraints, which may limit interpretability of our results [18, 39]. We believe that a prospective study with comprehensive data collection, including these variables, would be beneficial to further our understanding of the risk factors for massive transfusion in elective CS. Second, we recognize a distinct gap between the analysis of risk factors and the implementation of risk stratification in clinical practice. Future endeavors should focus on developing and validating a prediction model based on the risk factors identified in this study. Third, since all the data were collected from tertiary hospitals in urban areas, our findings may not be applicable to poorly resourced medical settings or rural regions. Third, certain risk factors were treated as binary variables, defined by established laboratory thresholds, such as anemia, thrombocytopenia and hypoalbuminemia. Consequently, we were unable to explore the potential association between the severity of these comorbidities and the occurrence of massive transfusion. Nonetheless, our results are consistent with previous studies that indicated associations between even mild or moderate thrombocytopenia or anemia and PPH [36, 47–49]. Fourth, we included only women undergoing elective CS, which limited the generalizability of our findings to vaginal births or emergency CS. A future risk factor analysis across different modes of birth could provide more comprehensive evidence. Finally, although more than 294,000 women were included in our analysis, massive transfusion was a rare event and the prevalences of some candidate risk factors, such as adenomyosis/ leiomyoma (0.7%) and hypertension (1.1%), were very low in our particular cohort of women with elective CS. As a result, our analysis did not have enough statistical power to analyze these rare exposures and non-significant results of these factors cannot totally exclude the potential association.
Conclusions
We identified ten antepartum risk factors of massive transfusion in women who had undergone elective cesarean section. Our findings could facilitate the identification of women who may require preparations and prophylactic strategies in the antenatal period or before birth in women who got massive blood transfusion after cesarean section.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank National Health Commission of China, for allowing us to get access to Hospital Quality Monitoring System.
Abbreviations
- PPH
Postpartum hemorrhage
- U
Unit
- RBC
Red blood cell
- FFP
Fresh frozen plasma
- CS
Cesarean section
- HQMS
Hospital Quality Monitoring System
- ICD
International Classification of Diseases
- CI
Confidence interval
- OR
Odds ratio
- aOR
Adjusted odds ratio
Author contributions
XX and YX conceived and designed this study. XX and ZY implemented the study, collected the data, and interpreted the data. XX drafted the manuscript. YX and HY revised it critically. All authors have approved the manuscript for publication and there is no conflict of interest in this submission.
Funding
This work was supported by National High Level Hospital Clinical Research Funding (2022-PUMCH-B-119), Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (2021-1-I2M-060), and National Natural Science Foundation of China (72304281). The funding body did not participate in the design of the study or collection, analysis, and interpretation of data or in writing the manuscript.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the institutional review board of Peking Union Medical College Hospital (reference number: S-K 1491). All methods were carried out in accordance with relevant guidelines and regulations. The requirement of written informed consent was waived by the institutional review board of Peking Union Medical College Hospital, since all the data was recorded anonymously without any information that may help to identify certain participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiaohan Xu and Yuelun Zhang contribute equally to this article.
Contributor Information
Xuerong Yu, Email: yxr313@aliyun.com.
Yuguang Huang, Email: garypumch@163.com.
References
- 1.Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2(6):e323–333. [DOI] [PubMed] [Google Scholar]
- 2.Ford JB, Patterson JA, Seeho SK, Roberts CL. Trends and outcomes of postpartum haemorrhage, 2003–2011. BMC Pregnancy Childbirth. 2015;15:334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thurn L, Wikman A, Westgren M, Lindqvist PG. Massive blood transfusion in relation to delivery: incidence, trends and risk factors: a population-based cohort study. BJOG. 2019;126(13):1577–86. [DOI] [PubMed] [Google Scholar]
- 4.Kramer MS, Berg C, Abenhaim H, et al. Incidence, risk factors, and temporal trends in severe postpartum hemorrhage. Am J Obstet Gynecol. 2013;209(5):e449441–447. [DOI] [PubMed] [Google Scholar]
- 5.Dahlke JD, Mendez-Figueroa H, Maggio L, et al. Prevention and management of postpartum hemorrhage: a comparison of 4 national guidelines. Am J Obstet Gynecol. 2015;213(1):e7671–7610. [DOI] [PubMed]
- 6.Abdul-Kadir R, McLintock C, Ducloy AS, et al. Evaluation and management of postpartum hemorrhage: consensus from an international expert panel. Transfusion. 2014;54(7):1756–68. [DOI] [PubMed] [Google Scholar]
- 7.Green L, Knight M, Seeney FM, et al. The epidemiology and outcomes of women with postpartum haemorrhage requiring massive transfusion with eight or more units of red cells: a national cross-sectional study. BJOG. 2016;123(13):2164–70. [DOI] [PubMed] [Google Scholar]
- 8.Ramler PI, van den Akker T, Henriquez D, Zwart JJ, van Roosmalen J. Incidence, management and outcome of women requiring massive transfusion after childbirth in the Netherlands: secondary analysis of a nationwide cohort study between 2004 and 2006. BMC Pregnancy Childbirth. 2017;17(1):197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramler PI, van den Akker T, Henriquez D, et al. Women receiving massive transfusion due to postpartum hemorrhage: a comparison over time between two nationwide cohort studies. Acta Obstet Gynecol Scand. 2019;98(6):795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mhyre JM, Shilkrut A, Kuklina EV, et al. Massive blood transfusion during hospitalization for delivery in New York State, 1998–2007. Obstet Gynecol. 2013;122(6):1288–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hendriks J, Zwart JJ, Briet E, Brand A, van Roosmalen J. The clinical benefit of blood transfusion: a hypothetical experiment based on a nationwide survey of severe maternal morbidity. Vox Sang. 2013;104(3):234–9. [DOI] [PubMed] [Google Scholar]
- 12.Pasquier P, Gayat E, Rackelboom T, et al. An observational study of the fresh frozen plasma: red blood cell ratio in postpartum hemorrhage. Anesth Analg. 2013;116(1):155–61. [DOI] [PubMed] [Google Scholar]
- 13.Yazer MH, Waters JH, Spinella PC, Aabb /Trauma HORNWP. Use of Uncrossmatched erythrocytes in emergency bleeding situations. Anesthesiology. 2018;128(3):650–6. [DOI] [PubMed] [Google Scholar]
- 14.Butwick AJ, Ramachandran B, Hegde P, Riley ET, El-Sayed YY, Nelson LM. Risk factors for severe Postpartum Hemorrhage after Cesarean Delivery: case-control studies. Anesth Analg. 2017;125(2):523–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bao Y, Xu C, Qu X, Quan S, Dong Y, Ying H. Risk factors for transfusion in cesarean section deliveries at a tertiary hospital. Transfusion. 2016;56(8):2062–8. [DOI] [PubMed] [Google Scholar]
- 16.Akinlusi FM, Rabiu KA, Durojaiye IA, Adewunmi AA, Ottun TA, Oshodi YA. Caesarean delivery-related blood transfusion: correlates in a tertiary hospital in Southwest Nigeria. BMC Pregnancy Childbirth. 2018;18(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu J, Yu ZP, Wang P, Shi CY, Yang HX. Clinical Analysis of Postpartum Hemorrhage Requiring Massive Transfusions at a Tertiary Center. Chin Med J. 2017;130(5):581–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ononge S, Mirembe F, Wandabwa J, Campbell OM. Incidence and risk factors for postpartum hemorrhage in Uganda. Reproductive Health. 2016;13:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jegasothy E, Patterson J, Randall D, et al. Assessing the effect of risk factors on rates of obstetric transfusion over time using two methodological approaches. BMC Med Res Methodol. 2018;18(1):139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmadzia HK, Phillips JM, James AH, Rice MM, Amdur RL. Predicting peripartum blood transfusion in women undergoing cesarean delivery: a risk prediction model. PLoS ONE. 2018;13(12):e0208417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ekin A, Gezer C, Solmaz U, Taner CE, Dogan A, Ozeren M. Predictors of severity in primary postpartum hemorrhage. Arch Gynecol Obstet. 2015;292(6):1247–54. [DOI] [PubMed] [Google Scholar]
- 22.Bloch EM, Ingram C, Hull J, et al. Risk factors for peripartum blood transfusion in South Africa: a case-control study. Transfusion. 2018;58(9):2149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Zirqi I, Vangen S, Forsen L, Stray-Pedersen B. Prevalence and risk factors of severe obstetric haemorrhage. BJOG: Int J Obstet Gynecol. 2008;115(10):1265–72. [DOI] [PubMed] [Google Scholar]
- 24.Herstad L, Klungsoyr K, Skjaerven R, et al. Elective cesarean section or not? Maternal age and risk of adverse outcomes at term: a population-based registry study of low-risk primiparous women. BMC Pregnancy Childbirth. 2016;16:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magann EF, Evans S, Hutchinson M, Collins R, Lanneau G, Morrison JC. Postpartum hemorrhage after cesarean delivery: an analysis of risk factors. South Med J. 2005;98(7):681–5. [DOI] [PubMed] [Google Scholar]
- 26.Butwick AJ, McDonnell N. Antepartum and postpartum anemia: a narrative review. Int J Obstet Anesth. 2021;47:102985. [DOI] [PubMed] [Google Scholar]
- 27.Pishko AM, Levine LD, Cines DB. Thrombocytopenia in pregnancy: diagnosis and approach to management. Blood Rev. 2020;40:100638. [DOI] [PubMed] [Google Scholar]
- 28.Gernsheimer TB. Congenital and acquired bleeding disorders in pregnancy. Hematol Am Soc Hematol Educ Program. 2016;2016(1):232–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franch-Arcas G. The meaning of hypoalbuminaemia in clinical practice. Clin Nutr. 2001;20(3):265–9. [DOI] [PubMed] [Google Scholar]
- 30.Kumar M, Abbas Z, Azami M, et al. Asian Pacific association for the study of liver (APASL) guidelines: hepatitis B virus in pregnancy. Hepatol Int. 2022;16(2):211–53. [DOI] [PubMed] [Google Scholar]
- 31.Williams O, Fisher N, Bayya J, et al. 601: preparing for blood transfusion in the peripartum period: usefulness of admission risk factors. Am J Obstet Gynecol. 2014;210(1, Supplement):S296. [Google Scholar]
- 32.Holm C, Langhoff-Roos J, Petersen KB, Norgaard A, Diness BR. Severe postpartum haemorrhage and mode of delivery: a retrospective cohort study. BJOG: Int J Obstet Gynecol. 2012;119(5):596–604. [DOI] [PubMed] [Google Scholar]
- 33.Eshkoli T, Weintraub AY, Sergienko R, Sheiner E. Placenta accreta: risk factors, perinatal outcomes, and consequences for subsequent births. Am J Obstet Gynecol. 2013;208(3):e219211–217. [DOI] [PubMed] [Google Scholar]
- 34.Mufti AR, Reau N. Liver disease in pregnancy. Clin Liver Dis. 2012;16(2):247–69. [DOI] [PubMed] [Google Scholar]
- 35.Gomez-Cantarino S, Agullo-Ortuno MT, de Dios-Aguado M, Ugarte-Gurrutxaga MI, Bouzas-Mosquera C. Prevalence of Hypoproteinemia and Hypoalbuminemia in pregnant women from three different socioeconomic populations. Int J Environ Res Public Health. 2020;17(17):6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glenzer MM, Correia M, Nhantumbo V, et al. Postpartum hemorrhage in Sub-saharan Africa-a prospective study in metropolitan Mozambique. J Thromb Haemost. 2023;21(12):3463–76. [DOI] [PubMed] [Google Scholar]
- 37.Blitz MJ, Yukhayev A, Pachtman SL, et al. Twin pregnancy and risk of postpartum hemorrhage. J Matern Fetal Neonatal Med. 2020;33(22):3740–5. [DOI] [PubMed] [Google Scholar]
- 38.Zhao R, Wang X, Zou L, et al. Adverse obstetric outcomes in pregnant women with uterine fibroids in China: a multicenter survey involving 112,403 deliveries. PLoS ONE. 2017;12(11):e0187821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nyflot LT, Sandven I, Stray-Pedersen B, et al. Risk factors for severe postpartum hemorrhage: a case-control study. BMC Pregnancy Childbirth. 2017;17(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weissmann-Brenner A, Simchen MJ, Zilberberg E, et al. Maternal and neonatal outcomes of macrosomic pregnancies. Med Sci Monitor: Int Med J Experimental Clin Res. 2012;18(9):Ph77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Turkmen S, Johansson S, Dahmoun M. Foetal macrosomia and foetal-maternal outcomes at Birth. J Pregnancy. 2018;2018:4790136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Said AS, Manji KP. Risk factors and outcomes of fetal macrosomia in a tertiary centre in Tanzania: a case-control study. BMC Pregnancy Childbirth. 2016;16(1):243–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vitner D, Bleicher I, Kadour-Peero E, Lipworth H, Sagi S, Gonen R. Does prenatal identification of fetal macrosomia change management and outcome? Arch Gynecol Obstet. 2019;299(3):635–44. [DOI] [PubMed] [Google Scholar]
- 44.Qin J, Liu X, Sheng X, Wang H, Gao S. Assisted reproductive technology and the risk of pregnancy-related complications and adverse pregnancy outcomes in singleton pregnancies: a meta-analysis of cohort studies. Fertil Steril. 2016;105(1):73–85. e71-76. [DOI] [PubMed] [Google Scholar]
- 45.Sentilhes L, Merlot B, Madar H, Sztark F, Brun S, Deneux-Tharaux C. Postpartum haemorrhage: prevention and treatment. Expert Rev Hematol. 2016;9(11):1043–61. [DOI] [PubMed] [Google Scholar]
- 46.Sessler DI. Perioperative Thermoregulation and heat balance. Lancet. 2016;387(10038):2655–64. [DOI] [PubMed] [Google Scholar]
- 47.Attali E, Epstein D, Lavie M, et al. Mild thrombocytopenia and the risk for postpartum hemorrhage in twin pregnancies. Int J Gynaecol Obstet. 2022;159(3):790–5. [DOI] [PubMed] [Google Scholar]
- 48.Arcudi S, Ronchi A, Capecchi M, et al. Assessment of post-partum haemorrhage risk among women with moderate thrombocytopenia. Br J Haematol. 2022;197(4):482–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nair M, Choudhury MK, Choudhury SS, et al. Association between maternal anaemia and pregnancy outcomes: a cohort study in Assam, India. BMJ Glob Health. 2016;1(1):e000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


