Abstract
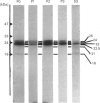
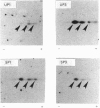
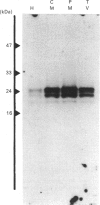
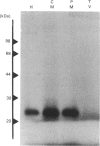
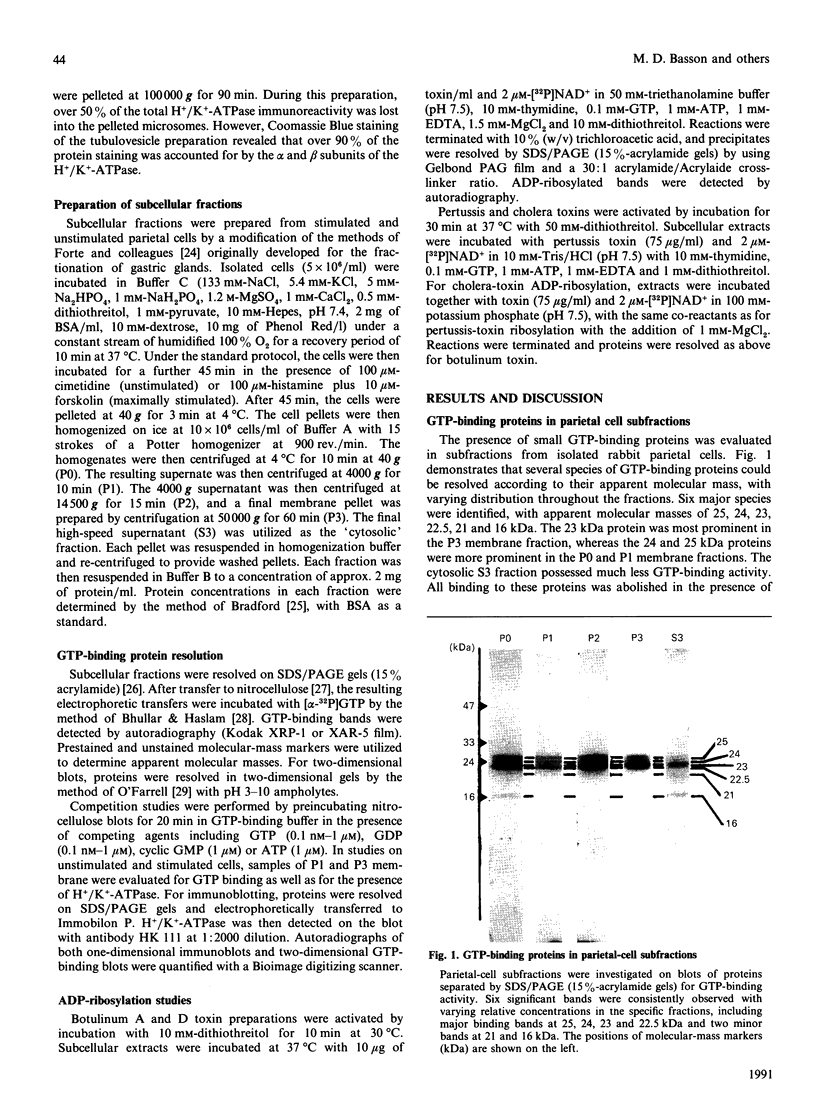
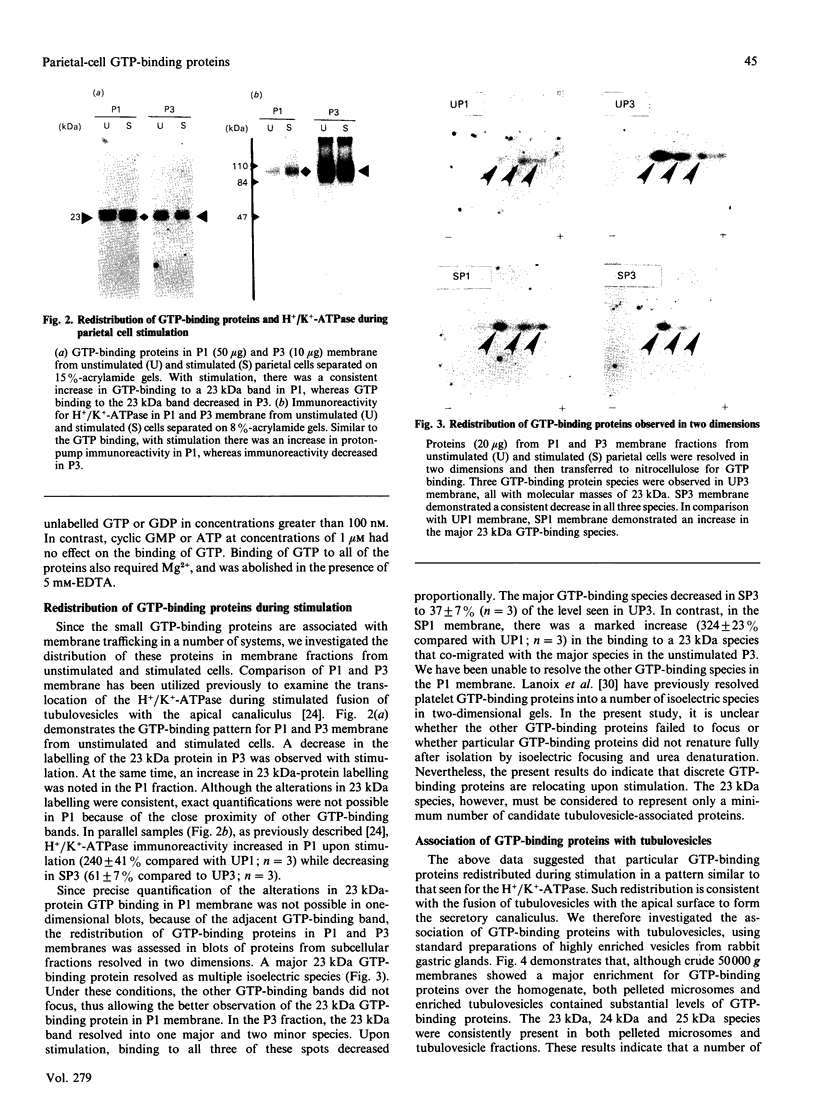
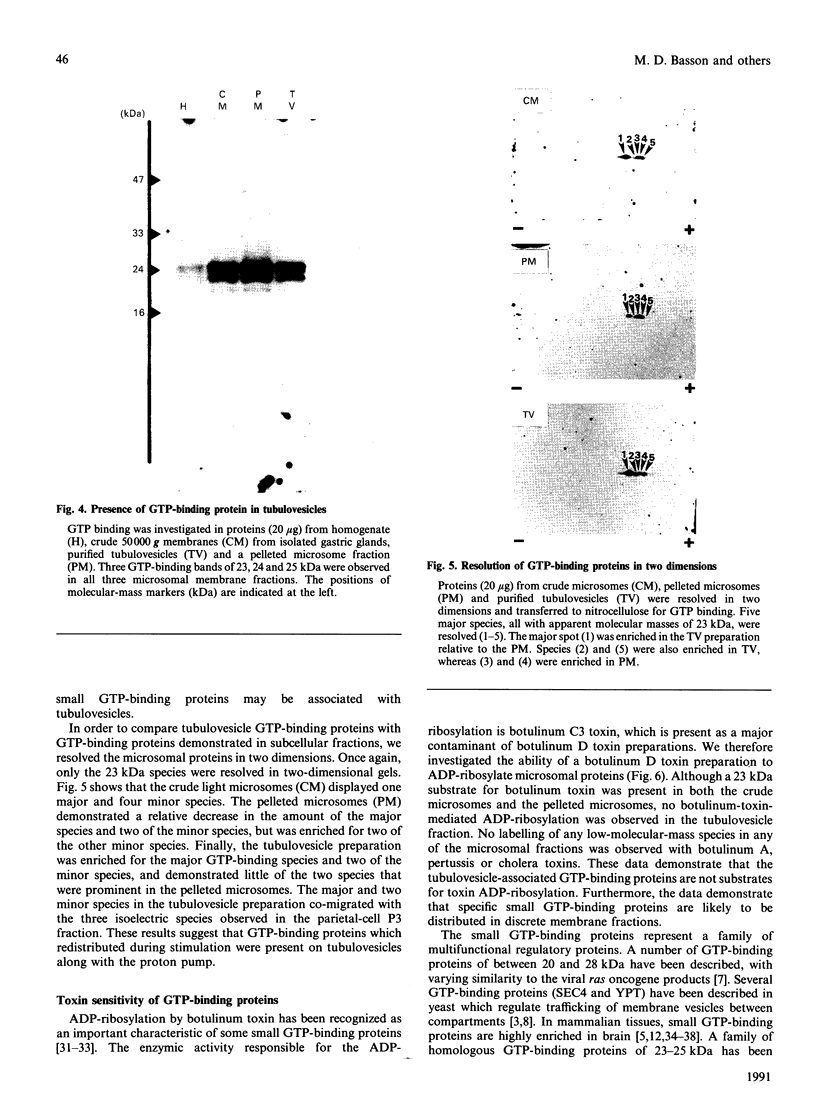
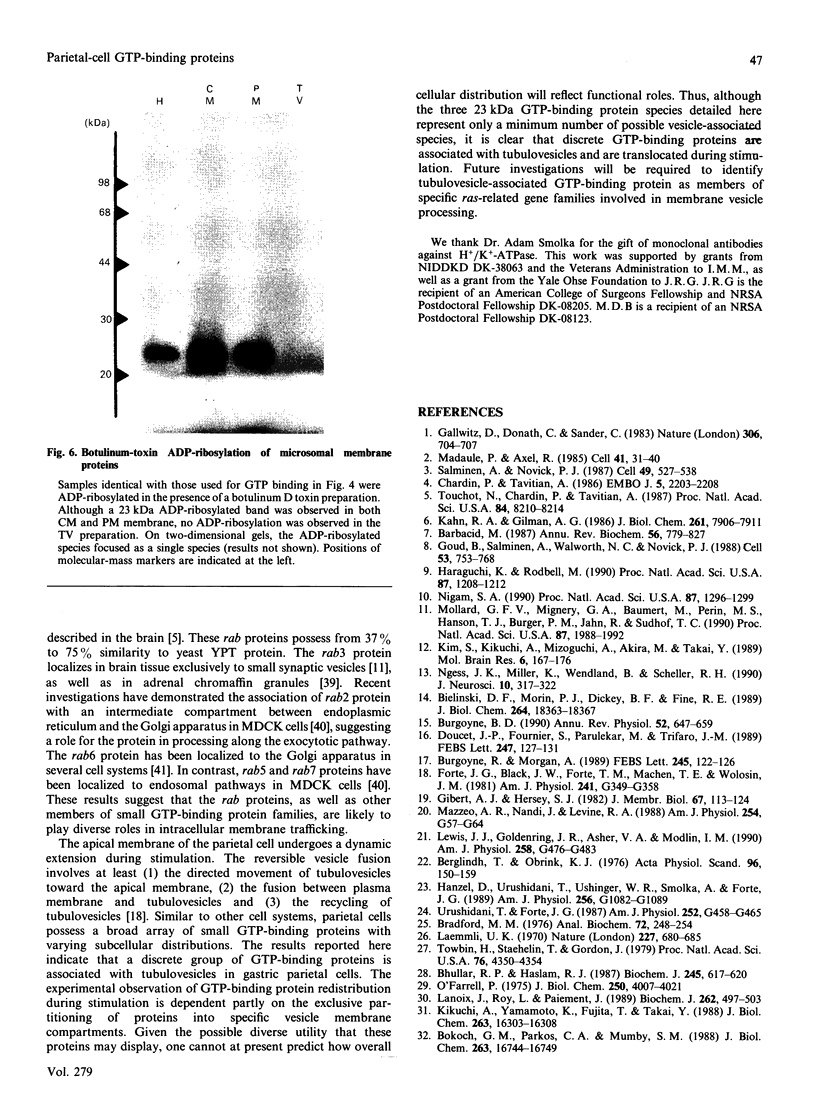
Small GTP-binding proteins are important regulators of intracellular traffic. The presence of several small GTP-binding proteins was documented in subfractions of rabbit parietal cells. Upon maximal stimulation of the cells with a combination of histamine and forskolin, one 23 kDa GTP-binding band was observed to decrease in a 50,000 g membrane fraction while increasing in 4000 g membranes. The 23 kDa band resolved into one major and two minor species on two-dimensional gels. GTP-binding species of 23 kDa, 24 kDa and 25 kDa were present in purified preparations of tubulovesicles. The three isoelectric species of the 23 kDa proteins observed in parietal cell 50,000 g microsomes were enriched in tubulovesicle preparations. None of the tubulovesicle-associated GTP-binding proteins were substrates for ADP-ribosylation by a preparation of botulinum D toxin. These results indicate that tubulovesicles contain discrete small GTP-binding proteins which redistribute during parietal cell stimulation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aktories K., Hall A. Botulinum ADP-ribosyltransferase C3: a new tool to study low molecular weight GTP-binding proteins. Trends Pharmacol Sci. 1989 Oct;10(10):415–418. doi: 10.1016/0165-6147(89)90191-0. [DOI] [PubMed] [Google Scholar]
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Berglindh T., Obrink K. J. A method for preparing isolated glands from the rabbit gastric mucosa. Acta Physiol Scand. 1976 Feb;96(2):150–159. doi: 10.1111/j.1748-1716.1976.tb10184.x. [DOI] [PubMed] [Google Scholar]
- Bhullar R. P., Haslam R. J. Detection of 23-27 kDa GTP-binding proteins in platelets and other cells. Biochem J. 1987 Jul 15;245(2):617–620. doi: 10.1042/bj2450617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielinski D. F., Morin P. J., Dickey B. F., Fine R. E. Low molecular weight GTP-binding proteins are associated with neuronal organelles involved in rapid axonal transport and exocytosis. J Biol Chem. 1989 Nov 5;264(31):18363–18367. [PubMed] [Google Scholar]
- Bokoch G. M., Parkos C. A., Mumby S. M. Purification and characterization of the 22,000-dalton GTP-binding protein substrate for ADP-ribosylation by botulinum toxin, G22K. J Biol Chem. 1988 Nov 15;263(32):16744–16749. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D., Morgan A. Low molecular mass GTP-binding proteins of adrenal chromaffin cells are present on the secretory granule. FEBS Lett. 1989 Mar 13;245(1-2):122–126. doi: 10.1016/0014-5793(89)80204-2. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D. Secretory vesicle-associated proteins and their role in exocytosis. Annu Rev Physiol. 1990;52:647–659. doi: 10.1146/annurev.ph.52.030190.003243. [DOI] [PubMed] [Google Scholar]
- Chardin P., Tavitian A. The ral gene: a new ras related gene isolated by the use of a synthetic probe. EMBO J. 1986 Sep;5(9):2203–2208. doi: 10.1002/j.1460-2075.1986.tb04485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavrier P., Parton R. G., Hauri H. P., Simons K., Zerial M. Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell. 1990 Jul 27;62(2):317–329. doi: 10.1016/0092-8674(90)90369-p. [DOI] [PubMed] [Google Scholar]
- Darchen F., Zahraoui A., Hammel F., Monteils M. P., Tavitian A., Scherman D. Association of the GTP-binding protein Rab3A with bovine adrenal chromaffin granules. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5692–5696. doi: 10.1073/pnas.87.15.5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet J. P., Fournier S., Parulekar M., Trifaró J. M. Detection of low molecular mass GTP-binding proteins in chromaffin granules and other subcellular fractions of chromaffin cells. FEBS Lett. 1989 Apr 10;247(1):127–131. doi: 10.1016/0014-5793(89)81254-2. [DOI] [PubMed] [Google Scholar]
- Fischer von Mollard G., Mignery G. A., Baumert M., Perin M. S., Hanson T. J., Burger P. M., Jahn R., Südhof T. C. rab3 is a small GTP-binding protein exclusively localized to synaptic vesicles. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1988–1992. doi: 10.1073/pnas.87.5.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forte J. G., Black J. A., Forte T. M., Machen T. E., Wolosin J. M. Ultrastructural changes related to functional activity in gastric oxyntic cells. Am J Physiol. 1981 Nov;241(5):G349–G358. doi: 10.1152/ajpgi.1981.241.5.G349. [DOI] [PubMed] [Google Scholar]
- Gallwitz D., Donath C., Sander C. A yeast gene encoding a protein homologous to the human c-has/bas proto-oncogene product. Nature. 1983 Dec 15;306(5944):704–707. doi: 10.1038/306704a0. [DOI] [PubMed] [Google Scholar]
- Gibert A. J., Hersey S. J. Morphometric analysis of parietal cell membrane transformations in isolated gastric glands. J Membr Biol. 1982;67(2):113–124. doi: 10.1007/BF01868654. [DOI] [PubMed] [Google Scholar]
- Goud B., Salminen A., Walworth N. C., Novick P. J. A GTP-binding protein required for secretion rapidly associates with secretory vesicles and the plasma membrane in yeast. Cell. 1988 Jun 3;53(5):753–768. doi: 10.1016/0092-8674(88)90093-1. [DOI] [PubMed] [Google Scholar]
- Goud B., Zahraoui A., Tavitian A., Saraste J. Small GTP-binding protein associated with Golgi cisternae. Nature. 1990 Jun 7;345(6275):553–556. doi: 10.1038/345553a0. [DOI] [PubMed] [Google Scholar]
- Hanzel D. K., Urushidani T., Usinger W. R., Smolka A., Forte J. G. Immunological localization of an 80-kDa phosphoprotein to the apical membrane of gastric parietal cells. Am J Physiol. 1989 Jun;256(6 Pt 1):G1082–G1089. doi: 10.1152/ajpgi.1989.256.6.G1082. [DOI] [PubMed] [Google Scholar]
- Haraguchi K., Rodbell M. Isoproterenol stimulates shift of G proteins from plasma membrane to pinocytotic vesicles in rat adipocytes: a possible means of signal dissemination. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1208–1212. doi: 10.1073/pnas.87.3.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshijima M., Kondo J., Kikuchi A., Yamamoto K., Takai Y. Purification and characterization from bovine brain membranes of a GTP-binding protein with a Mr of 21,000, ADP-ribosylated by an ADP-ribosyltransferase contaminated in botulinum toxin type C1--identification as the rhoA gene product. Brain Res Mol Brain Res. 1990 Jan;7(1):9–16. doi: 10.1016/0169-328x(90)90067-n. [DOI] [PubMed] [Google Scholar]
- Kahn R. A., Gilman A. G. The protein cofactor necessary for ADP-ribosylation of Gs by cholera toxin is itself a GTP binding protein. J Biol Chem. 1986 Jun 15;261(17):7906–7911. [PubMed] [Google Scholar]
- Kikuchi A., Yamamoto K., Fujita T., Takai Y. ADP-ribosylation of the bovine brain rho protein by botulinum toxin type C1. J Biol Chem. 1988 Nov 5;263(31):16303–16308. [PubMed] [Google Scholar]
- Kikuchi A., Yamashita T., Kawata M., Yamamoto K., Ikeda K., Tanimoto T., Takai Y. Purification and characterization of a novel GTP-binding protein with a molecular weight of 24,000 from bovine brain membranes. J Biol Chem. 1988 Feb 25;263(6):2897–2904. [PubMed] [Google Scholar]
- Kim S., Kikuchi A., Mizoguchi A., Takai Y. Intrasynaptosomal distribution of the ras, rho and smg-25A GTP-binding proteins in bovine brain. Brain Res Mol Brain Res. 1989 Nov;6(2-3):167–176. doi: 10.1016/0169-328x(89)90051-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lanoix J., Roy L., Paiement J. Detection of GTP-binding proteins in purified derivatives of rough endoplasmic reticulum. Biochem J. 1989 Sep 1;262(2):497–503. doi: 10.1042/bj2620497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J. J., Goldenring J. R., Asher V. A., Modlin I. M. Effects of epidermal growth factor on signal transduction in rabbit parietal cells. Am J Physiol. 1990 Mar;258(3 Pt 1):G476–G483. doi: 10.1152/ajpgi.1990.258.3.G476. [DOI] [PubMed] [Google Scholar]
- Madaule P., Axel R. A novel ras-related gene family. Cell. 1985 May;41(1):31–40. doi: 10.1016/0092-8674(85)90058-3. [DOI] [PubMed] [Google Scholar]
- Matsui Y., Kikuchi A., Kondo J., Hishida T., Teranishi Y., Takai Y. Nucleotide and deduced amino acid sequences of a GTP-binding protein family with molecular weights of 25,000 from bovine brain. J Biol Chem. 1988 Aug 15;263(23):11071–11074. [PubMed] [Google Scholar]
- Mazzeo A. R., Nandi J., Levine R. A. Effects of ethanol on parietal cell membrane phospholipids and proton pump function. Am J Physiol. 1988 Jan;254(1 Pt 1):G57–G64. doi: 10.1152/ajpgi.1988.254.1.G57. [DOI] [PubMed] [Google Scholar]
- Ngsee J. K., Miller K., Wendland B., Scheller R. H. Multiple GTP-binding proteins from cholinergic synaptic vesicles. J Neurosci. 1990 Jan;10(1):317–322. doi: 10.1523/JNEUROSCI.10-01-00317.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigam S. K. Subcellular distribution of small GTP binding proteins in pancreas: identification of small GTP binding proteins in the rough endoplasmic reticulum. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1296–1299. doi: 10.1073/pnas.87.4.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Salminen A., Novick P. J. A ras-like protein is required for a post-Golgi event in yeast secretion. Cell. 1987 May 22;49(4):527–538. doi: 10.1016/0092-8674(87)90455-7. [DOI] [PubMed] [Google Scholar]
- Touchot N., Chardin P., Tavitian A. Four additional members of the ras gene superfamily isolated by an oligonucleotide strategy: molecular cloning of YPT-related cDNAs from a rat brain library. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8210–8214. doi: 10.1073/pnas.84.23.8210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urushidani T., Forte J. G. Stimulation-associated redistribution of H+-K+-ATPase activity in isolated gastric glands. Am J Physiol. 1987 Apr;252(4 Pt 1):G458–G465. doi: 10.1152/ajpgi.1987.252.4.G458. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Kondo J., Hishida T., Teranishi Y., Takai Y. Purification and characterization of a GTP-binding protein with a molecular weight of 20,000 in bovine brain membranes. Identification as the rho gene product. J Biol Chem. 1988 Jul 15;263(20):9926–9932. [PubMed] [Google Scholar]
- Zahraoui A., Touchot N., Chardin P., Tavitian A. Complete coding sequences of the ras related rab 3 and 4 cDNAs. Nucleic Acids Res. 1988 Feb 11;16(3):1204–1204. doi: 10.1093/nar/16.3.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]