Abstract
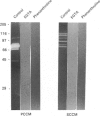
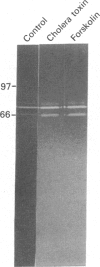
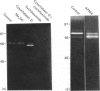
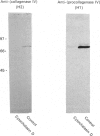
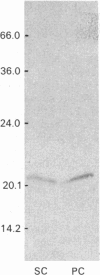
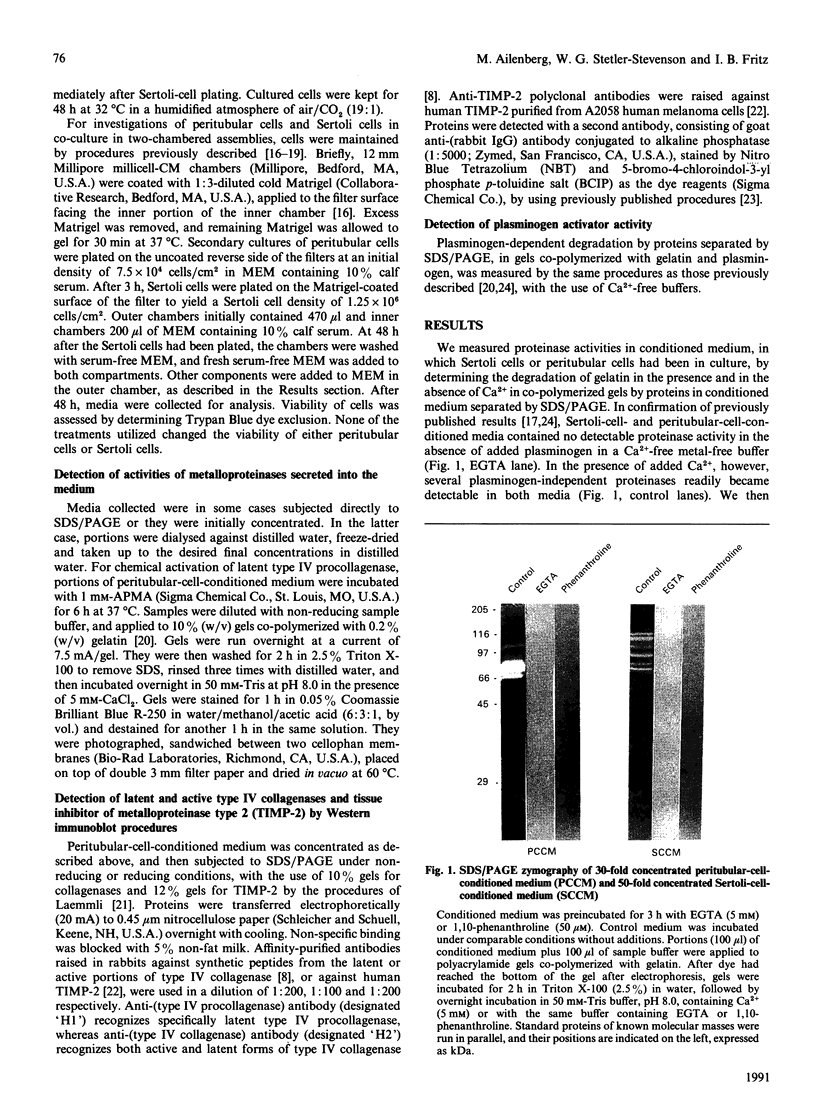
Testicular peritubular myoid cells, which have properties similar to those of vascular smooth-muscle cells, secrete a variety of metalloproteinases when maintained in culture in a chemically defined medium. The predominant metalloproteinases secreted were identified as latent type IV procollagenases having molecular masses of 72 kDa and 75 kDa, as detected in Western immunoblots with specific antibodies against type IV procollagenase. When peritubular cells were stimulated by dibutyryl cyclic AMP, forskolin or cholera toxin, they secreted increased amounts of type IV procollagenase. However, little if any of the active type IV collagenase, having a lower molecular mass of 66 kDa, could be detected under these conditions. Addition of low concentrations of cytochalasin D to peritubular cells in monoculture resulted in conversion of the latent type IV collagenase into its active form, assessed with antibody-specificity studies and by the appearance of the 66 kDa protein. In contrast, Sertoli cells in culture did not manifest an increased conversion of type IV procollagenase into type IV collagenase in the presence of cytochalasin D, even though cytochalasin D addition invariably resulted in a disruption of the microfilament assembly in each of these gonadal somatic cell populations. When peritubular cells were co-cultured with Sertoli cells, addition of cytochalasin D no longer resulted in formation of increased amounts of the active form of type IV collagenase. Sertoli cells and peritubular cells each secreted a tissue inhibitor of metalloproteinase type 2, detected with a specific antibody in a Western immunoblot to have a molecular mass of 21 kDa. We conclude that cytochalasin D acts on mesenchymal-type peritubular cells, but not on epithelial-type Sertoli cells, to enhance the conversion of latent type IV procollagenase into active type IV collagenase. This conversion of type IV procollagenase into type IV collagenase by peritubular cells was inhibited by factor(s) secreted by Sertoli cells. Interactions between Sertoli cells and peritubular cells are postulated to modulate net proteinase activities in discrete regions of the testis.
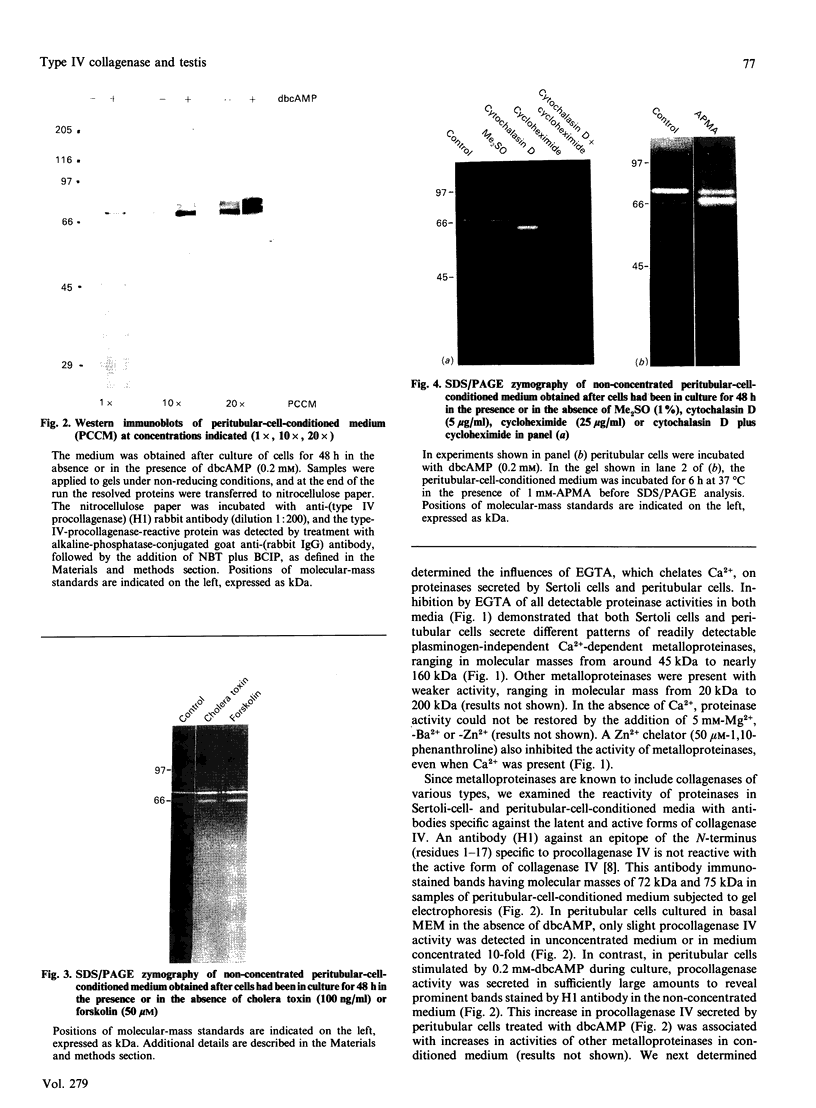
Full text
PDF
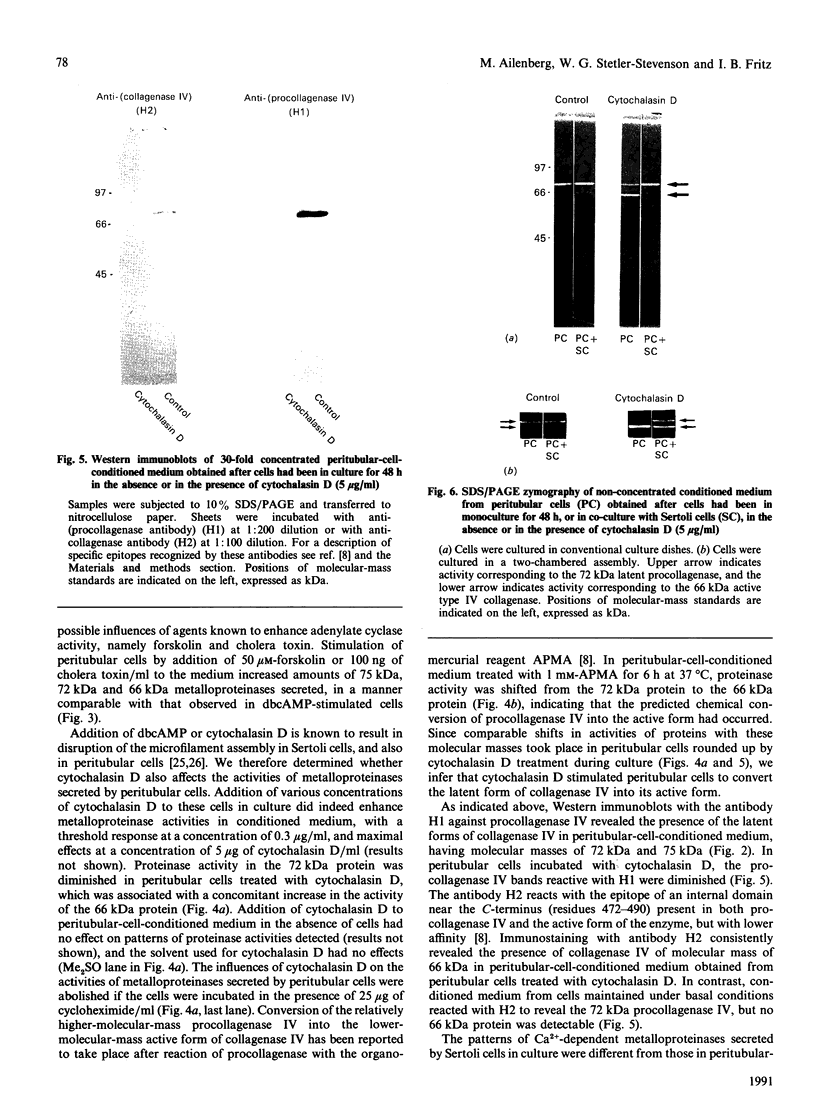


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aggeler J., Frisch S. M., Werb Z. Changes in cell shape correlate with collagenase gene expression in rabbit synovial fibroblasts. J Cell Biol. 1984 May;98(5):1662–1671. doi: 10.1083/jcb.98.5.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggeler J., Frisch S. M., Werb Z. Collagenase is a major gene product of induced rabbit synovial fibroblasts. J Cell Biol. 1984 May;98(5):1656–1661. doi: 10.1083/jcb.98.5.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ailenberg M., Fritz I. B. Control of levels of plasminogen activator activity secreted by Sertoli cells maintained in a two-chamber assembly. Endocrinology. 1988 Jun;122(6):2613–2618. doi: 10.1210/endo-122-6-2613. [DOI] [PubMed] [Google Scholar]
- Ailenberg M., Fritz I. B. Influences of follicle-stimulating hormone, proteases, and antiproteases on permeability of the barrier generated by Sertoli cells in a two-chambered assembly. Endocrinology. 1989 Mar;124(3):1399–1407. doi: 10.1210/endo-124-3-1399. [DOI] [PubMed] [Google Scholar]
- Ailenberg M., McCabe D., Fritz I. B. Androgens inhibit plasminogen activator activity secreted by Sertoli cells in culture in a two-chambered assembly. Endocrinology. 1990 Mar;126(3):1561–1568. doi: 10.1210/endo-126-3-1561. [DOI] [PubMed] [Google Scholar]
- Ailenberg M., Tung P. S., Fritz I. B. Transforming growth factor-beta elicits shape changes and increases contractility of testicular peritubular cells. Biol Reprod. 1990 Mar;42(3):499–509. doi: 10.1095/biolreprod42.3.499. [DOI] [PubMed] [Google Scholar]
- Ailenberg M., Tung P. S., Pelletier M., Fritz I. B. Modulation of Sertoli cell functions in the two-chamber assembly by peritubular cells and extracellular matrix. Endocrinology. 1988 Jun;122(6):2604–2612. doi: 10.1210/endo-122-6-2604. [DOI] [PubMed] [Google Scholar]
- Collier I. E., Wilhelm S. M., Eisen A. Z., Marmer B. L., Grant G. A., Seltzer J. L., Kronberger A., He C. S., Bauer E. A., Goldberg G. I. H-ras oncogene-transformed human bronchial epithelial cells (TBE-1) secrete a single metalloprotease capable of degrading basement membrane collagen. J Biol Chem. 1988 May 15;263(14):6579–6587. [PubMed] [Google Scholar]
- Danø K., Andreasen P. A., Grøndahl-Hansen J., Kristensen P., Nielsen L. S., Skriver L. Plasminogen activators, tissue degradation, and cancer. Adv Cancer Res. 1985;44:139–266. doi: 10.1016/s0065-230x(08)60028-7. [DOI] [PubMed] [Google Scholar]
- Frisch S. M., Clark E. J., Werb Z. Coordinate regulation of stromelysin and collagenase genes determined with cDNA probes. Proc Natl Acad Sci U S A. 1987 May;84(9):2600–2604. doi: 10.1073/pnas.84.9.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg G. I., Marmer B. L., Grant G. A., Eisen A. Z., Wilhelm S., He C. S. Human 72-kilodalton type IV collagenase forms a complex with a tissue inhibitor of metalloproteases designated TIMP-2. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8207–8211. doi: 10.1073/pnas.86.21.8207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley M. A., Dym M. Immunocytochemistry of extracellular matrix in the lamina propria of the rat testis: electron microscopic localization. Biol Reprod. 1987 Dec;37(5):1283–1289. doi: 10.1095/biolreprod37.5.1283. [DOI] [PubMed] [Google Scholar]
- Harris E. D., Jr, Reynolds J. J., Werb Z. Cytochalasin B increases collagenase production by cells in vitro. Nature. 1975 Sep 18;257(5523):243–244. doi: 10.1038/257243a0. [DOI] [PubMed] [Google Scholar]
- Hettle J. A., Balekjian E., Tung P. S., Fritz I. B. Rat testicular peritubular cells in culture secrete an inhibitor of plasminogen activator activity. Biol Reprod. 1988 Mar;38(2):359–371. doi: 10.1095/biolreprod38.2.359. [DOI] [PubMed] [Google Scholar]
- Hettle J. A., Waller E. K., Fritz I. B. Hormonal stimulation alters the type of plasminogen activator produced by Sertoli cells. Biol Reprod. 1986 Jun;34(5):895–904. doi: 10.1095/biolreprod34.5.895. [DOI] [PubMed] [Google Scholar]
- Heussen C., Dowdle E. B. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal Biochem. 1980 Feb;102(1):196–202. doi: 10.1016/0003-2697(80)90338-3. [DOI] [PubMed] [Google Scholar]
- Lacroix M., Smith F. E., Fritz I. B. Secretion of plasminogen activator by Sertoli cell enriched cultures. Mol Cell Endocrinol. 1977 Dec;9(2):227–236. doi: 10.1016/0303-7207(77)90124-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Tryggvason K., Garbisa S., Hart I., Foltz C. M., Shafie S. Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature. 1980 Mar 6;284(5751):67–68. doi: 10.1038/284067a0. [DOI] [PubMed] [Google Scholar]
- Saksela O., Rifkin D. B. Cell-associated plasminogen activation: regulation and physiological functions. Annu Rev Cell Biol. 1988;4:93–126. doi: 10.1146/annurev.cb.04.110188.000521. [DOI] [PubMed] [Google Scholar]
- Sang Q. X., Dym M., Byers S. W. Secreted metalloproteinases in testicular cell culture. Biol Reprod. 1990 Dec;43(6):946–955. doi: 10.1095/biolreprod43.6.946. [DOI] [PubMed] [Google Scholar]
- Sang Q. X., Stetler-Stevenson W. G., Liotta L. A., Byers S. W. Identification of type IV collagenase in rat testicular cell culture: influence of peritubular-Sertoli cell interactions. Biol Reprod. 1990 Dec;43(6):956–964. doi: 10.1095/biolreprod43.6.956. [DOI] [PubMed] [Google Scholar]
- Springman E. B., Angleton E. L., Birkedal-Hansen H., Van Wart H. E. Multiple modes of activation of latent human fibroblast collagenase: evidence for the role of a Cys73 active-site zinc complex in latency and a "cysteine switch" mechanism for activation. Proc Natl Acad Sci U S A. 1990 Jan;87(1):364–368. doi: 10.1073/pnas.87.1.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetler-Stevenson W. G., Brown P. D., Onisto M., Levy A. T., Liotta L. A. Tissue inhibitor of metalloproteinases-2 (TIMP-2) mRNA expression in tumor cell lines and human tumor tissues. J Biol Chem. 1990 Aug 15;265(23):13933–13938. [PubMed] [Google Scholar]
- Stetler-Stevenson W. G., Krutzsch H. C., Liotta L. A. Tissue inhibitor of metalloproteinase (TIMP-2). A new member of the metalloproteinase inhibitor family. J Biol Chem. 1989 Oct 15;264(29):17374–17378. [PubMed] [Google Scholar]
- Stetler-Stevenson W. G., Krutzsch H. C., Wacher M. P., Margulies I. M., Liotta L. A. The activation of human type IV collagenase proenzyme. Sequence identification of the major conversion product following organomercurial activation. J Biol Chem. 1989 Jan 25;264(3):1353–1356. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung P. S., Dorrington J. H., Fritz I. B. Structural changes inducted by follicle-stimulating hormone or dibutyryl cyclic AMP on presumptive Sertoli cells in culture. Proc Natl Acad Sci U S A. 1975 May;72(5):1838–1842. doi: 10.1073/pnas.72.5.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung P. S., Fritz I. B. Cell-substratum and cell-cell interactions promote testicular peritubular myoid cell histotypic expression in vitro. Dev Biol. 1986 May;115(1):155–170. doi: 10.1016/0012-1606(86)90237-x. [DOI] [PubMed] [Google Scholar]
- Tung P. S., Fritz I. B. Characterization of rat testicular peritubular myoid cells in culture: alpha-smooth muscle isoactin is a specific differentiation marker. Biol Reprod. 1990 Feb;42(2):351–365. doi: 10.1095/biolreprod42.2.351. [DOI] [PubMed] [Google Scholar]
- Tung P. S., Fritz I. B. Morphogenetic restructuring and formation of basement membranes by Sertoli cells and testis peritubular cells in co-culture: inhibition of the morphogenetic cascade by cyclic AMP derivatives and by blocking direct cell contact. Dev Biol. 1987 Mar;120(1):139–153. doi: 10.1016/0012-1606(87)90112-6. [DOI] [PubMed] [Google Scholar]
- Tung P. S., Skinner M. K., Fritz I. B. Cooperativity between Sertoli cells and peritubular myoid cells in the formation of the basal lamina in the seminiferous tubule. Ann N Y Acad Sci. 1984;438:435–446. doi: 10.1111/j.1749-6632.1984.tb38304.x. [DOI] [PubMed] [Google Scholar]
- Tung P. S., Skinner M. K., Fritz I. B. Fibronectin synthesis is a marker for peritubular cell contaminants in Sertoli cell-enriched cultures. Biol Reprod. 1984 Feb;30(1):199–211. doi: 10.1095/biolreprod30.1.199. [DOI] [PubMed] [Google Scholar]
- Unemori E. N., Werb Z. Collagenase expression and endogenous activation in rabbit synovial fibroblasts stimulated by the calcium ionophore A23187. J Biol Chem. 1988 Nov 5;263(31):16252–16259. [PubMed] [Google Scholar]
- Unemori E. N., Werb Z. Reorganization of polymerized actin: a possible trigger for induction of procollagenase in fibroblasts cultured in and on collagen gels. J Cell Biol. 1986 Sep;103(3):1021–1031. doi: 10.1083/jcb.103.3.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wart H. E., Birkedal-Hansen H. The cysteine switch: a principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5578–5582. doi: 10.1073/pnas.87.14.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Aggeler J. Proteases induce secretion of collagenase and plasminogen activator by fibroblasts. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1839–1843. doi: 10.1073/pnas.75.4.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Hembry R. M., Murphy G., Aggeler J. Commitment to expression of the metalloendopeptidases, collagenase and stromelysin: relationship of inducing events to changes in cytoskeletal architecture. J Cell Biol. 1986 Mar;102(3):697–702. doi: 10.1083/jcb.102.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Tremble P. M., Behrendtsen O., Crowley E., Damsky C. H. Signal transduction through the fibronectin receptor induces collagenase and stromelysin gene expression. J Cell Biol. 1989 Aug;109(2):877–889. doi: 10.1083/jcb.109.2.877. [DOI] [PMC free article] [PubMed] [Google Scholar]