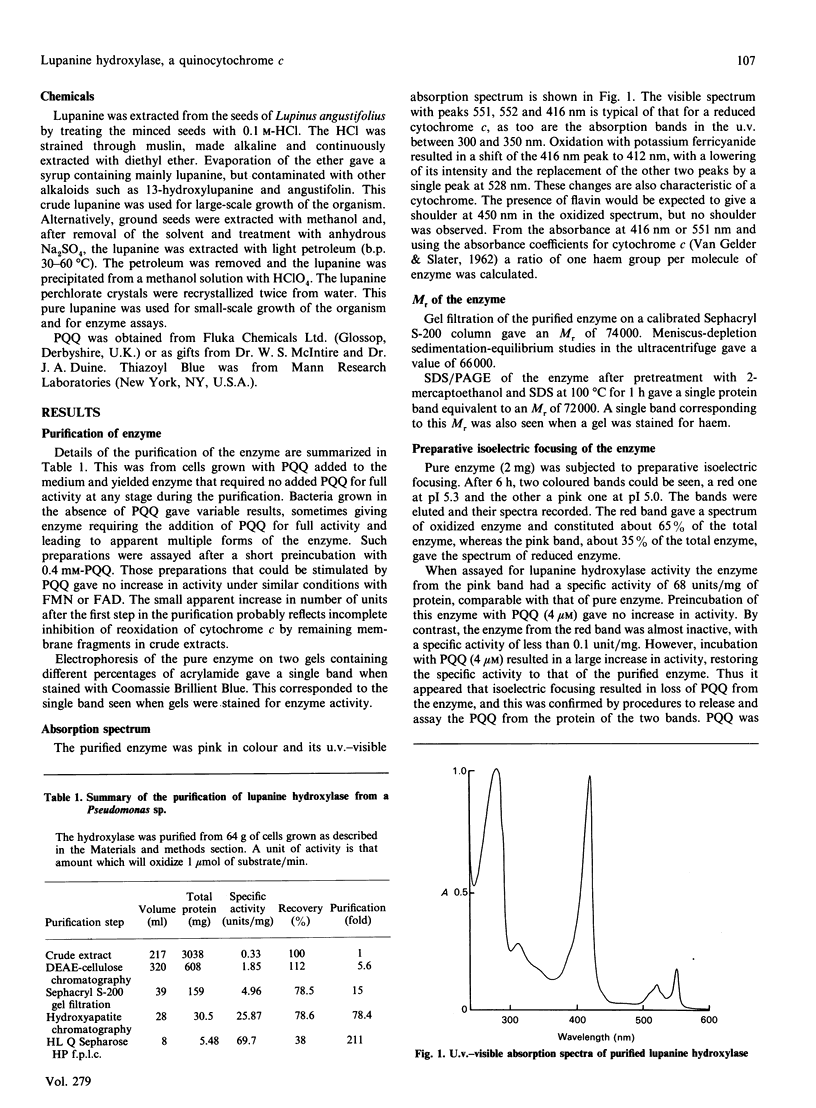
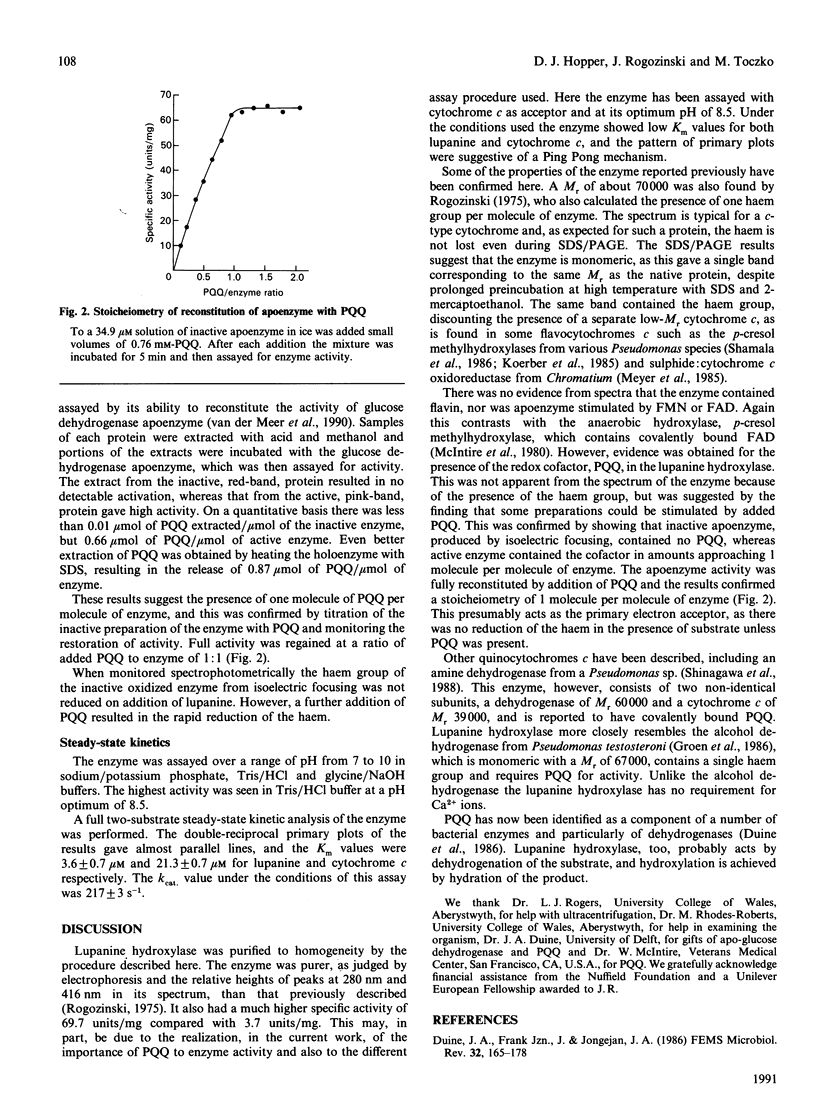
Abstract
Lupanine 17-hydroxylase, the first enzyme in the pathway for bacterial degradation of the alkaloid, lupanine, was purified from a Pseudomonas sp. The enzyme acts by initial dehydrogenation of the substrate, and cytochrome c was used as electron acceptor in assays. It had an Mr of 66,000 by ultracentrifuge studies and 74,000 by gel filtration. The visible absorption spectrum was that of a cytochrome c, and a stoicheiometry of one haem group per molecule of enzyme was calculated. SDS/PAGE gave a single band of Mr 72,000 containing the haem group. The enzyme also contained pyrroloquinoline quinone (PQQ), which could be removed by isoelectric focusing. The apoenzyme was reconstituted to full activity with addition of PQQ, and a stoicheiometry of one molecule of PQQ per molecule of enzyme was calculated. Steady-state kinetics gave values of 3.6 microM for the Km for lupanine, 21.3 microM for the Km for cytochrome c and 217 s-1 for the Kcat.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Groen B. W., van Kleef M. A., Duine J. A. Quinohaemoprotein alcohol dehydrogenase apoenzyme from Pseudomonas testosteroni. Biochem J. 1986 Mar 15;234(3):611–615. doi: 10.1042/bj2340611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerber S. C., Hopper D. J., McIntire W. S., Singer T. P. Formation and properties of flavoprotein-cytochrome hybrids by recombination of subunits from different species. Biochem J. 1985 Oct 15;231(2):383–387. doi: 10.1042/bj2310383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MOZEJKO-TOCZKO M. [Decomposition of lupanine by Pseudomonas lupanini]. Acta Microbiol Pol. 1960;9:157–171. [PubMed] [Google Scholar]
- McIntire W., Edmondson D. E., Hopper D. J., Singer T. P. 8 alpha-(O-Tyrosyl)flavin adenine dinucleotide, the prosthetic group of bacterial p-cresol methylhydroxylase. Biochemistry. 1981 May 26;20(11):3068–3075. doi: 10.1021/bi00514a013. [DOI] [PubMed] [Google Scholar]
- McIntire W., Hopper D. J., Singer T. P. p-Cresol methylhydroxylase. Assay and general properties. Biochem J. 1985 Jun 1;228(2):325–335. doi: 10.1042/bj2280325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T. E., Vorkink W. P., Tollin G., Cusanovich M. A. Chromatium flavocytochrome c: kinetics of reduction of the heme subunit, and the flavocytochrome c-mitochondrial cytochrome c complex. Arch Biochem Biophys. 1985 Jan;236(1):52–58. doi: 10.1016/0003-9861(85)90605-8. [DOI] [PubMed] [Google Scholar]
- ROSENBERGER R. F., ELSDEN S. R. The yields of Streptococcus faecalis grown in continuous culture. J Gen Microbiol. 1960 Jun;22:726–739. doi: 10.1099/00221287-22-3-726. [DOI] [PubMed] [Google Scholar]
- Reeve C. D., Carver M. A., Hopper D. J. The purification and characterization of 4-ethylphenol methylenehydroxylase, a flavocytochrome from Pseudomonas putida JD1. Biochem J. 1989 Oct 15;263(2):431–437. doi: 10.1042/bj2630431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogoziński J. Molecular properties of the inducible lupanine hydroxylase from growing cultures of Pseudomonas lupanini. Acta Biochim Pol. 1975;22(1):57–66. [PubMed] [Google Scholar]
- Shamala N., Lim L. W., Mathews F. S., McIntire W., Singer T. P., Hopper D. J. Structure of an intermolecular electron-transfer complex: p-cresol methylhydroxylase at 6.0-A resolution. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4626–4630. doi: 10.1073/pnas.83.13.4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]
- van GELDER B., SLATER E. C. The extinction coefficient of cytochrome c. Biochim Biophys Acta. 1962 Apr 23;58:593–595. doi: 10.1016/0006-3002(62)90073-2. [DOI] [PubMed] [Google Scholar]
- van der Meer R. A., Groen B. W., van Kleef M. A., Frank J., Jongejan J. A., Duine J. A. Isolation, preparation, and assay of pyrroloquinoline quinone. Methods Enzymol. 1990;188:260–283. doi: 10.1016/0076-6879(90)88043-a. [DOI] [PubMed] [Google Scholar]


