Abstract
Background
Visceral Leishmaniasis (VL) is the most severe and fatal disease if left untreated. In people living with HIV/AIDS (PLHA), VL is considered an emerging opportunistic infection. The aim of this manuscript was to report a first case in Tunisia of a concomitant presentation of visceral and oral leishmaniasis in a patient LHA. A systematic review of the literature was performed according to PRISMA guidelines, as well.
Case presentation
The patient, a 43-year-old heterosexual man, treated for HIV/AIDS was referred for macrocheilitis of the upper and lower lips. A noticeable nodular and painless swelling extending to the cheeks’ mucosa was noted. The patient's poor oral hygiene was evident due to the presence of multiple dental caries. Histological analysis of the biopsied lower lip sample revealed the presence of numerous Leishmania amastigotes. The diagnosis of VL was clinically confirmed by the presence of a mild splenomegaly and pancytopenia and biologically by the identification of the parasite using PCR Lei and the species L. infantum involved using RFLP-PCR and culture. The treatment consisted of an intravenous administration of liposomal Amphotericin B (Ambisome®, 40 mg/kg/weight) for a period of 6 weeks. A favorable outcome was noted after one year with the resolution of clinical symptoms and a negative Leishmania blood PCR test. After 2 years, the patient remained asymptomatic but showed a positive Leishmania blood PCR test. Dolutegravir® was introduced in the patient’s ART regimen.
Conclusions
To the best of our knowledge, this is the first case report in Tunisia of atypical VL diagnosed through an uncommon oral location in an HIV/AIDS co-infected patient . Since VL is a severe and potentially fatal disease, it is essential for dentists to perform a thorough clinical examination and adopt a multidisciplinary approach in order to ensure an early diagnosis and an effective treatment outcome.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12981-024-00660-1.
Introduction
Leishmaniasis remains one of the world's most devastating neglected tropical diseases. The Americas, East Africa, North Africa, West Asia, and Southeast Asia are the main four regions in the world where Leishmaniasis is a major health problem. In 2018, 92 countries were considered endemic, and more than 1 billion people are at risk of being infected [1]. The disease is caused by protozoan parasites that are transmitted to humans through the bite of an infected female phlebotomine sandfly. In fact, more than 20 species of Leishmania are involved in the disease, and more than 90 species of sandfly are known to transmit the parasite [2, 3]. Over 70 species of wild and domestic animals have been identified as confirmed or potential reservoirs of Leishmania parasites, including bats, dogs, rodents, opossums, anteaters, sloths, hyraxes, and marsupials [4]. Three major forms of the disease are described: cutaneous Leishmaniasis (CL), the most prevalent form; visceral Leishmaniasis (VL), or Kala-Azar, the most severe and fatal disease if left untreated; and mucocutaneous Leishmaniasis, the most disfiguring form [1, 3]. Isolated oral mucosal forms of Leishmaniasis are rare and may occur in immune-competent patients [5–7]. According to the World Health Organization (WHO), an estimated 30,000 and 1 million new cases of VL and CL, respectively, are reported each year [1]. However, this number could be underestimated. In the Middle East and North Africa (MENA) region, CL and VL are the most important infections and are caused by L. infantum, L. donovani, and occasionally L. tropica [8]. In Tunisia, four clinical conditions are described: VL, sporadic CL, chronic CL, and zoonotic CL. VL is caused by Leishmania infantum, and the dog is the main reservoir [4, 9]. Recent epidemiological data are lacking. In 2009, an annual incidence of VL varying between 50 and 100 cases has been reported. Children under 5 years old remain most commonly affected by the disease (80%). Annually 10 VL cases per 100.000 children were reported. Although rare in adults, VL cases are on the rise [10].
In people living with HIV/AIDS (PLHA), VL is considered an emerging opportunistic infection. Nearly 35 countries have reported cases of VL/HIV co-infection, with higher rates recorded in Brazil, India (Bihar), and Ethiopia. The risk of developing active VL is 100 to 2320 times higher in HIV patients [11]. In Tunisia, HIV/VL co-infection is rare. Data are lucking and are reported as case series [12].
Co-infected patients may harbor a significant load of the parasite in their blood, making them a reservoir and source of infection for the vector. The parasite can also accelerate immune suppression through virus replication stimulation [11, 13]. As a result, the diagnosis may be challenging due to the absence of common leishmaniasis symptoms such as fever and splenomegaly [11, 14, 15]. Atypical clinical presentations have been reported in unusual sites such as the oral and gastrointestinal mucosa, lymph nodes, skin, pleura, and respiratory tract [16, 17]. The treatment of VL in HIV co-infected individuals is based on the use of lipid formulations of Amphotericin B (LAB), which can be infused at a dose of 3–5 mg daily to a total dose of 40 mg/kg or given in 10 intermittent doses (on days 1–5, 10, 17, 24, 31, and 38) [18]. As reported, VL is more severe in HIV-infected individuals, with an outcome marked by relapse and death [11, 19]. The aim of this manuscript was to report the first condition, in Tunisia, of atypical VL diagnosed through an unusual oral involvement and discuss clinical, biological features and treatment outcomes, based on a review of the literature.
Case presentation
The patient, a 43-year-old heterosexual man, from the region of Moknine (Monastir-Tunisia), living with HIV/AIDS and treated for a chronic schizophrenia (Haldol® and Largactil®), was referred in March 2016 by the psychiatric department, for a swelling of the right lower mandibular cheek. The diagnosis of cellulitis of dental origin was made, at that time, on the basis of a poor oral hygiene and the presence of multiple dental infectious foci. Systemic antibiotics (β-Lactams 2 g/day and Metronidazole 750 mg/day for 7 days) and pain killers were prescribed as well as a panoramic radiography. The patient didn’t show up for teeth extractions. In June 2017, he was referred by the infectious diseases’ department for a macrocheilitis of both upper and lower lips. As for his medical history, the patient was treated for hypothyroidism (l-thyroxine), iron-deficiency anemia, and HIV/AIDS co-infected with HBV since 1994. In Germany, he was put on Zidovudine® and Nevirapine®, with poor compliance with treatment. On his first consultation in the infectious diseases’ department in 2004, his CD4 count was 625/mm3 and viral load count was 2625 copies/mm3. He was treated with Zidovudine®/Lamivudine® and Efavirenz® changed to Zidovudine®/Lamivudine® and Indinavir® because of Efavirenz® intolerance. In 2008, he was put on Zidovudine®/ Lamivudine and Lopinavir® with a poor compliance to treatment. In 2010, virological and immunological failure was diagnosed with a CD4 count of 255/mm3 and a viral load count of 87.932 copies/mm3. Because of the occurrence of pulmonary tuberculosis, the treatment was shifted to Tenofovir®/Emtricitabine® and Reyataz®. The viral load was undetectable and the CD4 count was 81/mm3.
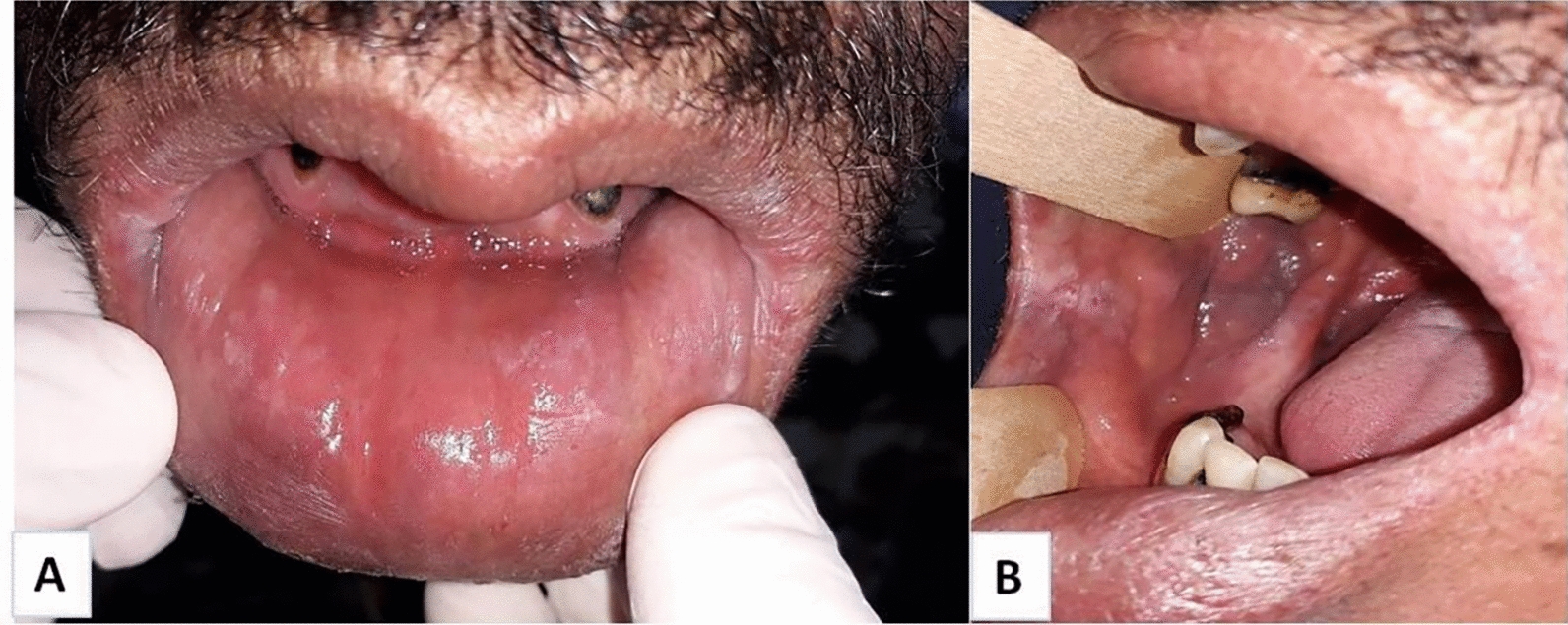
During the patient’s oral examination, a history of a swelling of the lower mandibular cheek that appeared 12 months ago, spontaneously resolved, and reappeared on the upper lip was reported. No history of dental pain was recorded. A noticeable nodular swelling with multiple fissures of both the upper and lower lips was noted. The swelling was extending to the cheeks’ mucosa. Upon palpation, the swelling was painless and appeared inflammatory. Additionally, small eruptions (2 to 3) were observed at the corner of the mouth (Fig. 1A–D). Facial palsy, fissured tongue, and cervical adenopathy were absent. The patient's poor oral hygiene was evident due to the presence of multiple dental caries and infected roots. However, there was no tooth mobility or periodontal pockets. Panoramic radiography confirmed the presence of dental infectious foci without any bone anomalies (Fig. 2). Since a dental cause for the swelling was ruled out; and various infectious and non-infectious diagnoses were considered, an incisional biopsy of the lower lip was performed under local anesthesia. The incision was very hemorrhagic, but homeostasis was achieved through a surgical wound suturing. The histological analysis revealed a significant lymphocyte inflammatory infiltrate with multiple round to oval bodies in the cytoplasm of macrophages consistent with Leishmania amastigotes (Fig. 3A and B). The patient returned to the infectious diseases’ department for further exploration. In fact, a mild splenomegaly was detected during the clinical examination with no associated fever. The biological results showed pancytopenia, with a total white blood cell count of 900/mm3, neutrophils of 600/mm3, hemoglobin of 7.2 g/dL, and a platelet count of 62,000/mm3. The CD4 count was 80 cells/mm3. No other opportunistic infections were noted. The diagnosis of VL with an unusual oral manifestation was made.
Fig. 1.

A–C Clinical views showing diffuse swelling of both upper and lower lips extending to the mucosa of the cheeks. Note the presence of fissures in the lower lip and cheeks. D Poor oral hygiene
Fig. 2.

Panoramic radiography shows multiple dental caries, infected dental roots, and periapical lesions. related to root
Fig. 3.
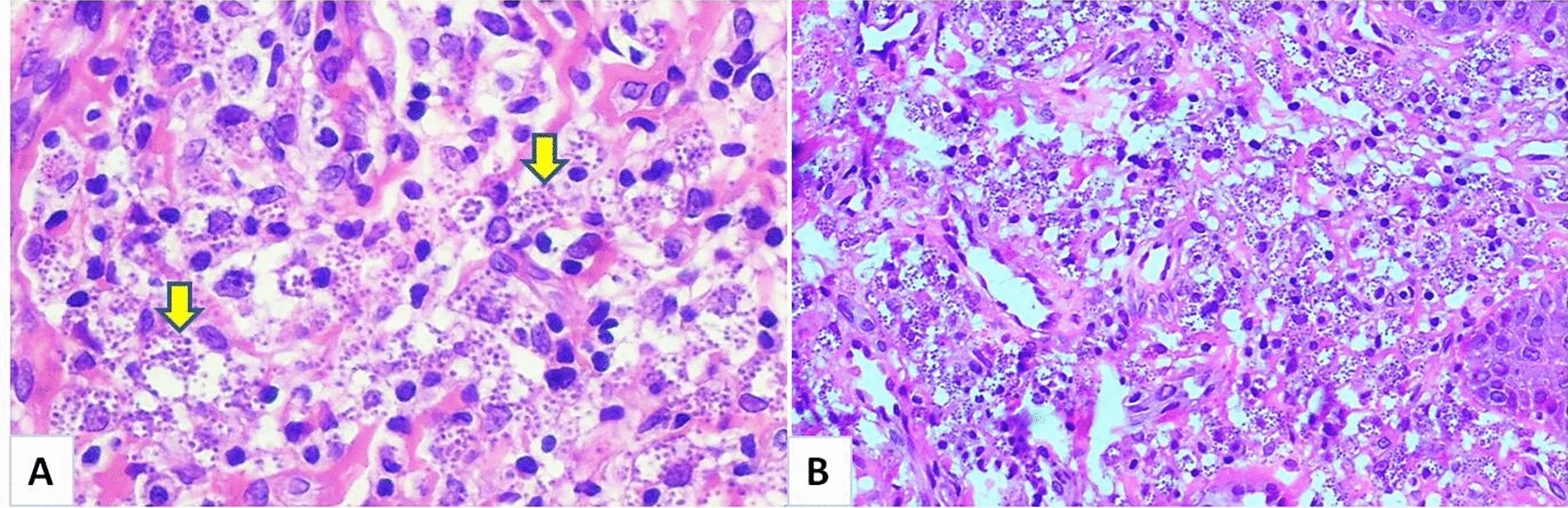
A, B Histological finding of the biopsied lower lip show a significant lymphocyte inflammatory infiltrate with multiple round to oval bodies in the cytoplasm of macrophages, which were consistent with Leishmania amastigotes (hematoxylin and eosin staining, ×400 magnification)
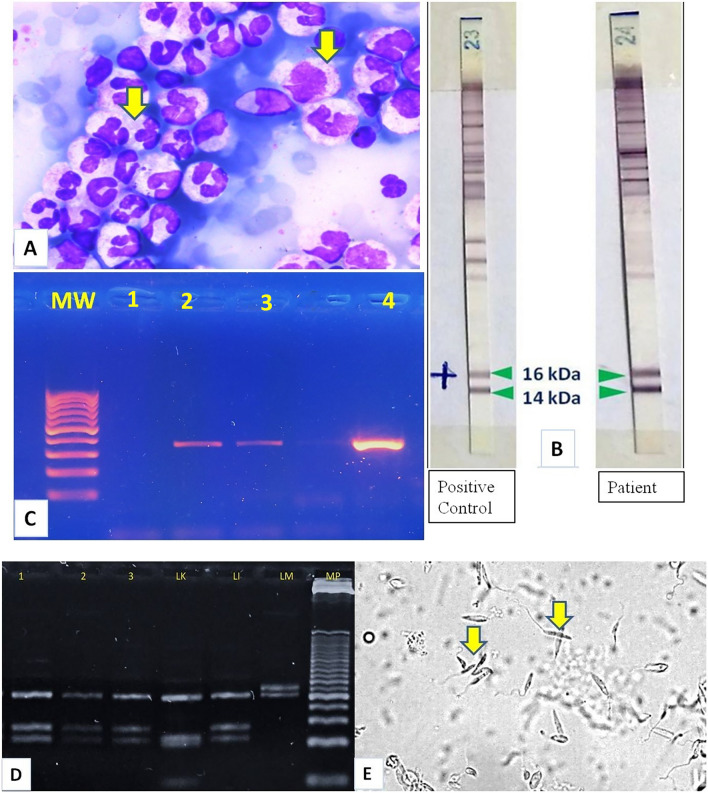
Several methods were used to detect, isolate and identify Leishmania species involved in the present case. Microscopic observation of Leishmania was positive after May Grumwald-Giemsa staining (MGG) of concentrated leukocytes isolated from the patient's blood sample (Fig. 4A). Immunoprinting using Leishmania Western Blot IgG LDBIO Diagnostics® yielded positive results and showed the presence of antibodies (IgG) to two Leishmania protein bands: p14 and p16 (Fig. 4B).The identification of the parasite's genus from the blood sample and from the histological slices of the biopsied lower lip was performed by PCR, using a single pair of primers (Lei70L and Lei70R) to amplify a conservative region of the 18S rRNA (345 base pairs or bp) [20] (Fig. 4C). The PCR–RFLP was used to differentiate between the species L. infantum, L. major and L. killicki using the ITS1 locus (Internal Transcribed Spacer 1, 300–350 bp), which is flanked by two highly conserved regions (18S rRNA et 5.8S). This locus is characterized by its polymorphism among Leishmania species [21, 22]. In fact, 3 reference species were used, based on parasites’ species involved in Leishmaniasis in Tunisia [9]: L. infantum MON-24 (MHOM/DZ/82/LIPA59), L. major MON-25 (MHOM/MA/81/LEM265) and L. killicki MON-8 (MHOM/TN/80/LEM163) (Reference National Center for Leishmaniasis, Montpellier, France) (Fig. 4D). The confirmation of L. infantum was done through culture using the appropriate medium for samples obtained from the blood and small eruptions scraped from the corner of the mouth (Fig. 4E).
Fig. 4.
A–E Identification of Leishmania. A MGG from peripheral blood leucocytoconcentration shows intracellular Leishmania amastigotes. B Immunoprinting using Leishmania Western Blot IgG LDBIO Diagnostics® yielded positive results and showed the presence of two Leishmania protein bands: p14 and p16 (band 23: positive control and band 24: patients’ results). C Agarose gel electrophoresis of 18S rRNA PCR product. Lane 1: negative control; Lane 2 patient blood, Lane 3: patient lip biopsy, Lane 4: positive control 343 bp; Lane MW: Molecular Weight marker (100 bp DNA ladder). D Restriction pattern of the amplified ribosomal Internal Transcribed Spacer 1 using HaeIII. Reference strains used were: Leishmania infantum MHOM/DZ/82/LIPA59 (LI) (three fragments of 187 bp, 72 bp and 55 bp), Leishmania killicki MHOM/TN/LEM163 (LK) (three fragments of 188 bp, 57 bp and 26 bp) and L. major MHOM/MA/81/LEM265 (LM) (two fragments of 206 bp and 132 bp). Lanes 1, 2, and 3: L. infantum identified from blood, lip biopsy and lip scraping product. MP: Molecular Weight marker (25-bp DNA ladder). E Culture using biphasic medium (blood horse based) from the peripheral blood leukocyte layer shows the promastigote form of Leishmania
The treatment consisted of intravenous administration of LAB (Ambisome®, 40 mg/kg/weight) for 6 weeks. Favorable clinical and biological outcomes were recorded. The swelling of the lips and cheeks has resolved after 6 months with a follow-up period of five years (Fig. 5A and B). The blood PCR test for Leishmaniasis was negative. In June 2020, the PCR test turned positive and the serology test was negative. The patient was asymptomatic. In October 2023, a new ART regimen including Dolutegravir® was initiated with Tenofovir®, Lamivudine®. Currently, the patient is still monitored; he remains asymptomatic with a positive blood PCR test.
Fig. 5.
A, B Clinical views after treatment showing resolution of the swelling of the lips and cheeks
Review and discussion
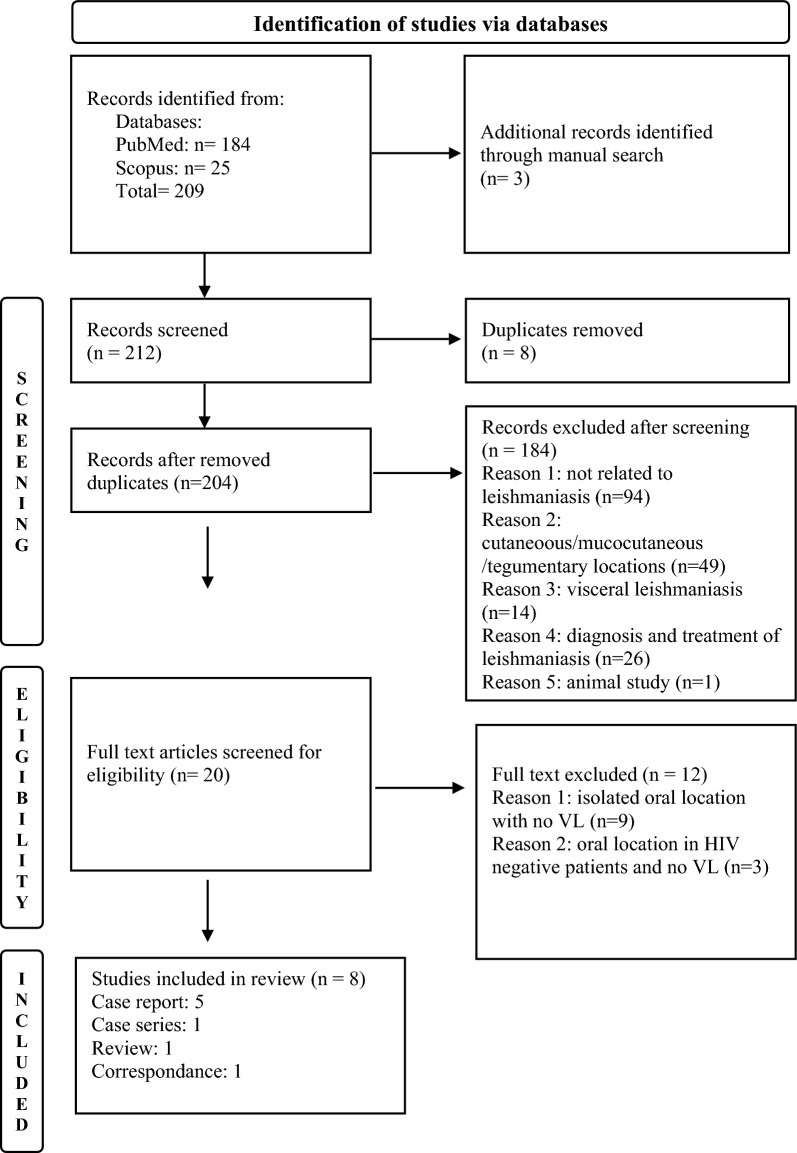
The review was independently conducted by two authors, LB and I.K. Only adult PLHA presenting concomitant mucosal oral and visceral leishmaniasis were included. HIV positive and negative adults and children with isolated VL, or isolated oral, nasal, laryngeal and pharyngeal locations were excluded from the review. No restrictions regarding the year or the language were considered. The literature review was conducted using MEDLINE via its interface PubMed and Scopus databases with the following MeSh/Key words “HIV”, “AIDS”, “Visceral Leishmaniasis”, “Atypical” “Oral Leishmaniasis”, “Lip Leishmaniasis”, “Ulcer”, “gingival Leishmaniasis” and their combinations using the Boolean equations “AND” “OR”. The PRISMA flow chart shows the selection process of studies by titles, abstracts and full texts. Other references were searched from the reference lists of full text-publications (Fig. 6). References obtained from the database search were managed using Mendeley Desktop 1.19.5. Data from the eligible 8 full texts, retrieved from 1994 to 2023, were extracted using a Microsoft Excel customized data sheet. Table 1 provides information on patients' characteristics, medical history, oral lesions, biological, clinical features, and treatment outcomes.
Fig. 6.
PRISMA flowchart
Table 1.
Summary of clinical, biological features and therapeutic outcome of oral leishmaniasis in HIV-VL co-infected patients
| Authors | Age/Gender/ Origin |
History | Location and Type of oral lesions | Hepatomegaly/Splenomegaly | Other | Fever / Weigh loss | CD4/mm3 /Viral Load cp/ mm3 |
Blood count | Oral biopsy/ Other |
BMB/ Serology/ Other |
Species | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Michiels JF et al.1994 [16] |
28, M/MSM France |
NM |
Tonsil/Palate Ulcer/polypoid |
No No |
Yes | Yes | 50/ NM |
Anemia Pancytopenia |
+ | + /No | ND |
LAB/ ATB |
D |
| Pineiro TV et al. 1998 [23] |
34, M/heterosexual Spain |
IVDU, HCV, Genital herpes LLei/Gluc |
Tongue Nodule Ulcer |
Yes No |
No |
Yes Yes |
140/ NM | Leucopenia |
+ / KS |
Neg/+ | NM | NM | D |
|
Nigro L et al 2003 [24] |
46, M, bisexual Italy |
VL /AIDS Pulmonary Lei HAART Oral Lei |
Oral mucosa Ulcer |
Yes Yes |
Lung | Yes, asthenia | 10/ NM |
Anemia Pancytopenia hyperγGlobulinemia |
+ | +/+ |
L. infantum MON1 |
Gluc /60mg/kg) |
Sympt |
| Montineri A. et al.2007 [25] |
48 M Italy |
Gingival ulcers/ Candidiasis |
Gingiva Ulcer |
No No |
No | Yes | 334 |
54.800/MA /hyperγGlobulinemia |
+ | Neg/+ | NM | LAB | F/6 m |
|
Kumar et al 2007 [26] |
35 F India |
Ulcer Tongue/ TTT for TB |
Tongue Ulcer Bleeding |
Yes No |
No | Yes |
12/CD4 18/CD8 |
Anemia |
+FNA Lymph node |
ND/+ | NM | NM | D |
| Rodrigo L.M. et al.2007 [27] |
65, M Spain |
AIDS/HCV |
Palate/Uvula Ulcer |
Yes Yes |
No |
Yes No |
NM |
Anemia /Thrombocytopenia |
+ | NM | NM | Gluc | F |
|
Presmanes M.M. et al 2008 [28] |
31, M Spain |
IVDU, HCV,AIDS HAART, |
Lower lip: Swelling Ulcer |
No No |
No |
No Yes |
160 NM |
Anemia | Neg | +/Neg | ND | LAB | F |
|
Diro E et al 2015 [17] |
29, F Ethiopia |
Systemic symptoms Oral lesion |
Palate/Mass Bleeding |
Yes No |
No |
Yes No |
71 NM |
Anemia Pancytopenia |
+ | + | ND |
LAB ATB |
D |
|
Present case 2017 |
43, M, heterosexual Moknine-Tunisia |
HIV/HBV/TB/Anemia/Ht/S Oral swelling/ ATB |
Lips/Cheeks/ Nodules |
No Yes |
No |
No Yes |
81 Undetected |
Anemia Pancytopenia |
+ |
No/+/ Culture+ PCR+/ PCR RFLP+ |
L infantum |
LAB |
F 2020: PCR+ Asympt |
M: Man; MSM: Men who have sex with men; F: Female; IVDU: Intravenous drug user; HCV: hepatitis C virus HIV: LLei: Laryngeal Leishmaniasis; Gluc: Glucantime; VL: Visceral leishmaniasis; AIDS: Acquired immune deficiency syndrome; HAART: Highly Active Antiretroviral Treatment;TTT: Treatment; TB: Tunerculosis; BMB; Bone marrow biopsy; NM: Not mentioned;KS: Kaposi Sarcoma; +: Positive; Neg.: Negative; FNA: Fine Needle Aspiration; ND: Not done; LAB: Liposomal amphotericin B; PCR FRLP: Polymerase chain reaction-Restriction fragment length polymorphism; D: Dead; Sympt: Symptomatic; F: Favorable; Asympt: Asymptomatic
Demographic data and medical history
The age of the patients ranged from 28 to 65 years, 6 patients were males and 2 were females [17, 26]. Among them, one was a MSM [16] and 1 man was bisexual [24]. Most patients were from Europe (France, Belgium, Italy, Spain, n = 6), while the other two were from India [26] and Africa (Ethiopia) [17], respectively. The present patient is a heterosexual adult man from an endemic area in the Sahel of Tunisia (Moknine). Medical history was available for 7 patients and not mentioned for one patient [16]. Six of the individuals were known to be HIV positive, 3 patients were co-infected with HCV [23, 27, 28], and 2 patients were intravenous drug users (IVDU) [23, 28]. Three patients each received treatments for laryngeal leishmaniasis [23], pulmonary leishmaniasis [24], and suspected abdominal tuberculosis [26]. One patient received prophylaxis against pneumocystis jiroveccii [27]. Oral lesions, such as candidiasis, and leishmaniasis were observed in 5 patients and were accompanied by symptoms such as fever, diarrhea, and vomiting [17, 24–26]. In one case, a patient with multiple gingival ulcers did not show any improvement after being treated with fluconazole and acyclovir [25]. The patient under study has been diagnosed with HIV since 1994 and was also co-infected with HBV. He has received treatment for iron-deficiency anemia, hypothyroidism and pulmonary tuberculosis. The patient experienced episodes of swelling in the right mandibular cheek. The diagnosis of cellulitis of dental origin was based on poor oral hygiene and the presence of multiple infected dental foci. Antibiotics were prescribed to treat the "cellulitis."
Oral lesions and systemic involvement
Regarding oral lesions’ features, the palate [16, 17, 27] and the tongue [23, 26] were primarily affected, followed by the gingiva [25] and the lower lip [28]. In one case, the location was not specified (oral mucosa) [24]. All lesions were ulcerated and painful. Two cases reported bleeding on touch [17, 26]. The lesions were described as polypoid [16], nodular and exophytic [23], vegetative [27], and fungating [17]. Dysphagia and hoarseness were also reported [17, 23]. One patient had bilateral enlargement of submental and submandibular lymph nodes [26]. Fever was present in 7 cases, accompanied by weight loss [23, 25, 26, 28]. hepatomegaly [17, 23, 26], or hepatosplenomegaly [24, 27] were also observed. Other mucosal involvements were noted in 2 cases, including the esophageal, gastric, and duodenal mucosa in one case [16] and the lung in another case) [24]. In the present case, the patient had mild splenomegaly with no associated fever. He presented with macrocheilitis of both upper and lower lips. The lesion was nodular and extended to the mucosa of the cheeks. According to Rosenthal et al. [29], the clinical triad of fever, splenomegaly, and hepatomegaly was less frequently seen in patients with low CD4 counts. Considering the diverse clinical manifestations of oral leishmaniasis, it is important to rule out the following differential diagnoses for both granulomatous (such as tuberculosis, actinomycosis, sarcoidosis, deep fungal infections, syphilis, Melkersson-Rosenthal syndrome, and lymphogranuloma venereum) and non- granulomatous diseases (including allergy, chronic cellulitis, herpes infections, lymphoma, carcinoma, and Kaposi sarcoma) [30, 31].
Biological investigations
All patients were positive for HIV, with CD4 counts reported for 7 patients: < 100 cells/mm3 for 4 patients (ranging from 10 to 71/mm3) [16, 17, 24, 26], > 100/mm3 for 2 patients [23, 28] and > 200/mm3 for one patient (334/mm3) with a mentioned viral load of 54,800 copies/ml [25]. Anemia was recorded in all cases. Pancytopenia [16, 17, 24], thrombocytopenia [27], and hyperγ-globulinemia were also noted [24, 25]. Oral biopsy, performed in all patients was positive in 7 cases and negative in one case [28]. In addition, oral Kaposi sarcoma was diagnosed in a patient with CD4 T cell counts of 140/mm3 and a CD4/CD8 ratio of 0.338 [23]. Bone marrow aspiration was positive in 4 cases [16, 17, 24, 28], negative in 2 cases [23, 25], not mentioned in one case [27], and not done in another case [26]. Serology testing was positive in 4 cases [23–26], negative in one case [28], not performed in one case [16], and not mentioned in 2 cases [17, 27]. Regarding the identification of the parasitic species, L. infantum zimodeme MON1 was characterized in one case report using the NNN medium (Novy-MacNeil-Nicole) [24]. The identification was not done in 3 cases [16, 17, 28] and not mentioned in 4 cases [23, 25–27]. For the present patient, the diagnosis of leishmaniasis was initially based on histopathology findings from the lip biopsy. Serology and leishmaniasis culture were positive. Indeed, the genus of Leishmania parasite and L. infantum species were identified using molecular methods (Lei PCR and RFLP-PCR) [31].
Treatments and outcomes
Different treatment regimens were prescribed. LAB was used in 5 cases [16, 17, 25, 27, 28], and antimonials were used in one case [24]. The drug was not specified in 2 cases [23, 26]. In one case, authors used intermittent doses of LAB on days 1–5, 10, 17, 31, and 38 [25], according to WHO guidelines [18]. LAB was associated with antibiotics (Ceftriaxone and Metronidazole) to prevent sepsis from the oral cavity in 2 cases [16, 17]. N-methyl-glucamine antimoniate (60 mg/kg) was prescribed in one case [24], and antimonials were substituted by LAB in one case [27]. The outcome was favorable or satisfactory in 3 cases [25, 27, 28], unfavorable with complications leading to death in 3 cases [16, 17, 26], and not specified in 2 cases [23, 24]. In the present case, the patient was treated with LAB at a total dose of 40 mg/kg for 6 weeks [18]. The outcome was favorable with resolution of biological and clinical symptoms, including swelling of the lips and cheeks. However, in 2020, as the patient discontinued his ART, the blood PCR test for Leishmaniasis was positive and the serology test was negative. Indeed, clinical symptoms were absent. In fact, to better control the parasite and prevent the recurrence of VL, a new ART regimen incorporating dolutegravir has recently been reintroduced. Some authors propose including dolutegravir to help the body eliminate the parasite, and suggest improving adherence and counseling services. Additionally, they recommend prolonging the duration of leishmaniasis treatment; implementing molecular monitoring and enhancing follow-up [32, 33]. In 2023, the patient remains asymptomatic with a positive PCR, despite ART treatment including dolutegravir. As reported by Molina R et al., there is no consensus to define “asymptomatic case’, HIV/VL coinfected patients are easily infectious for sand flies, even those receiving prophylactic treatment for leishmaniasis. They represent an important source of VL transmission and spread of the disease, making coinfection prevention and control challenging. Therefore, the authors recommend to screen for VL in HIV infected patients in endemic and non-endemic areas [34].
Conclusions
To the best of our knowledge, this is the first case report in Tunisia of unusual presentation of VL and it could be a concomitant VL/ML infection in a patient LHA. However, the nodular presentation of the oral condition is uncommon, compared to reports in the literature. Indeed this is the first report that investigates Leishmaniasis using both molecular and non-molecular methods. The scarcity of these atypical locations may explain a lack of clinical suspicion. Therefore, as the oral cavity could be the first site of VL presentation, dentists play a crucial role in conducting a proper clinical examination and ruling out major infectious and non-infectious diseases that can mimic the oral condition. Finally, a multidisciplinary approach is mandatory to better treat VL-HIV co-infected patients and ensure a favorable outcome.
Supplementary Information
Acknowledgements
The authors gratefully acknowledge Dr. Nour Mellouli who performed the lip biopsy with Dr. Latifa Berrezouga. They also acknowledge the patient and the patient’s tutor for their contribution.
Abbreviations
- PLHA
People living with HIV/AIDS
- VL
Visceral Leishmaniasis
- LAB
Liposomal Amphotericin B
- HBV
Hepatitis B virus
Author contributions
LB performed oral and radiographic examinations, ruled out the dental origin of the lesion, indicated the lip biopsy and took pre and postoperative photos; AZ and SBH made the histological diagnosis from the lip biopsy; SB and HB performed biological analysis for the identification of Leishmania genus and species; IK, WM, AT and MC are the treating physicians who contributed to the treatment and monitoring of the patient. LB and IK made the review and wrote the manuscript. All authors took part in drafting and revising the manuscript and approved the publication of the final version of the article.
Funding
The International Association for Dental, Oral and Craniofacial Research (IADR, www.iadr.org) funds this study in the context of the Regional Development Program Award ($18,290) in 2021.
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The present case report is part of Latifa Berrezouga research project on PLHIV that was approved by the committee of ethics of the faculty of pharmacy, University of Monastir, Tunisia, under the reference CER-SVS/ISBM 013/2020. Professor Mohamed Chakroun is the Head of the Infectious Diseases’ Department and Director of the Project. Informed consent from the patient’s tutor was obtained with respect to the Helsinki declaration.
Consent for publication
Informed consent for publication related to clinical management data that are compliant with the infectious diseases’ department was obtained from the patient’s tutor.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO. Leishmaniasis. https://www.who.int/health-topics/leishmaniasis#tab=tab_1
- 2.WHO. Leishmaniasis: Key factors-2 March 2020. https://www.who.int/news-room/fact-sheets/detail/leishmaniasis
- 3.WHO. Leishmaniasis: Key factors- 12 January 2023. https://www.who.int/news-room/fact-sheets/detail/leishmaniasis
- 4.Derghal M, Tebai A, Balti G, et al. High-resolution melting analysis identifies reservoir hosts of zoonotic Leishmania parasites in Tunisia. Parasites Vectors. 2022;15:12. 10.1186/s13071-021-05138-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Passi D, Sharma S, Dutta S, Gupta C. Localised leishmaniasis of oral mucosa: report of an unusual clinicopathological entity. Case Rep Dent. 2014;2014: 753149. 10.1155/2014/753149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Celentano A, Ruoppo E, Mansueto G, Mignogna MD. Primary oral leishmaniasis mimicking oral cancer: a case report. Br J Oral Maxillofac Surg. 2019;53(4):396–8. 10.1016/j.bjoms.2015.01.021. [DOI] [PubMed] [Google Scholar]
- 7.Fleissig Y, Dan-Gur M, Michael-Gayego A, et al. A trespasser from a foreign land? A case report of primary mucosal leishmaniasis. BMC Infect Dis. 2022;22:212. 10.1186/s12879-022-07169-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tabbabi A. Review of Leishmaniasis in the Middle East and North Africa. Afr Health Sci. 2019;19(1):1329–37. 10.4314/ahs.v19i1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haouas N, Babba H. Leishmaniasis in Tunisia: History and New Insights into the Epidemiology of a Neglected Disease, The Epidemiology and Ecology of Leishmaniasis, David Claborn, IntechOpen, 2017. 10.5772/65000. https://www.intechopen.com/books/the-epidemiology-and-ecology-of-leishmaniasis/leishmaniasis-in-tunisia-history-and-new-insights-into-the-epidemiology-of-a-neglected-disease
- 10.Aoun K, Jeddi F, Amri F, Ghrab J, Bouratbine A. Current epidemiological data on visceral leishmaniasis in Tunisia. Med Mal Infect. 2009;39(10):775–9. [DOI] [PubMed] [Google Scholar]
- 11.WHO. Leishmaniasis and HIV co-infection. https://www.who.int/leishmaniasis/burden/hiv_coinfection/burden_hiv/coinfection/en/
- 12.Kallel K, Ammari L, Kaouech E, Belhadj S, Anane S, Kilani B, Chaker E. Asymptomatic bearing of Leishmania infantum among Tunisian HIV infected patients. Pathol Biol. 2007;55(10):521–4. 10.1016/j.patbio.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 13.Alvar J, Aparicio P, Aseffa A, Den Boer M, Canavate C, Dedet JP, et al. The relationship between leishmaniasis and AIDS: the second 10 years. Clin Microbiol Rev. 2008;21:334–59. 10.1128/CMR.00061-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindoso JAL, Moreira CHV, Mirella Alves Cunha MA, Queiroz IT. Visceral leishmaniasis and HIV coinfection: current perspectives. HIV/AIDS Res Palliat Care. 2018;10:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pagliano P, Esposito S. Visceral leishamiosis in immunocompromised host: an update and literature review. J Chemother. 2017. 10.1080/1120009X.2017.1323150. [DOI] [PubMed] [Google Scholar]
- 16.Michiels JF, Monteil RA, Hofman P, Perrin C, Fuzibet JG, Lefichoux J, Loubière R. Oral leishmaniasis and Kaposi’s sarcoma in an AIDS patient. J Oral Pathol & Med. 1994;23:45–6. [DOI] [PubMed] [Google Scholar]
- 17.Diro E, Van Griensven J, Mohammed R, Colebunders R, Asefa M, Hailu A, Lynen L. Atypical manifestations of visceral leishmaniasis in patients with HIV in north Ethiopia: a gap in guidelines for the management of opportunistic infections in resource poor settings. Lancet Infect Dis. 2015;15:122–9. [DOI] [PubMed] [Google Scholar]
- 18.WHO guideline for the treatment of visceral leishmaniasis in HIV co-infected patients in East Africa and South-East Asia, 7 June 2022. (https://www.who.int/publications/i/item/9789240048294 [PubMed]
- 19.Costa LDLN, Lima US, Rodrigues V, Lima MIS, Silva LA, Ithamar J, Azevedo CMPS. Factors associated with relapse and hospital death in patients coinfected with visceral leishmaniasis and HIV: a longitudinal study. BMC Infect Dis. 2023;23(1):141. 10.1186/s12879-023-08009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spanakos G, Patsoula E, Kremastinou T, Saroglou G, Vakalis N. Development of a PCR-based method for diagnosis of Leishmania in blood samples. Mol Cell Probes. 2002;16(6):415–20. [DOI] [PubMed] [Google Scholar]
- 21.Schönian G, Nasereddin A, Dinse N, Schweynoch C, Schallig HDFH, Presber W, et al. PCR diagnosis and characterization of Leishmania in local and imported clinical samples. Diag Microbiol Infect Dis. 2003;47(1):349–58. [DOI] [PubMed] [Google Scholar]
- 22.Chargui N, Haouas N, Jaouadi K, Gorcii M, Pratlong F, Dedet JP, et al. Usefulness of a PCR-based method in the detection and species identification of Leishmania from clinical samples. Pathol Biol. 2012;60(6):e75-79. [DOI] [PubMed] [Google Scholar]
- 23.Vázquez-Piñeiro T, Fernández Alvarez JM, Gonzalo Lafuente JC, Cano J, Gimeno M, Berenguer J. Visceral leishmaniasis: a lingual presentation in a patient with HIV infection. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;86(2):179–82. [DOI] [PubMed] [Google Scholar]
- 24.Nigro L, Montineri A, La Rosa R, Zuccarello M, Iacobello C, Iacobello C, Vinci C, Pulizia R, Fatuzzo F. Visceral leishmaniasis and HIV co-infection: a rare case of pulmonary and oral localization. Infez Med. 2003;11(2):93–6. [PubMed] [Google Scholar]
- 25.Montineria A, La Rosaa R, Laroccaa L, Brisolesea V, Brancatia G, Fatuzzoa F, Nigrob L. Gingival ulcers as first manifestation of leishmaniasis and HIV infection. AIDS. 2008;22(1):160–1. [DOI] [PubMed] [Google Scholar]
- 26.Kumar P, Sharma PK, Jain RK, Gautam RK, Bhardwaj M, Kar HK. Oral ulcer as an unusual feature of visceral leishmaniasis in an AIDS patient. Indian J Med Sci. 2007;61(2):97–101. [PubMed] [Google Scholar]
- 27.Rodrigo LM, Sierra JO, Jiménez ADA, Fernández IR. Oro-pharyngeal mass in a patient with HIV infection. Acta Otorrinolaringol Esp. 2008;59(4):202–3. [PubMed] [Google Scholar]
- 28.Presmanes MM, Cabedo GT, Cavero FV, Pastor GP, Santander RL. Lip ulceration due to visceral leishmaniasis in a HIV-positive patient. Med Cutan Iber Lat Am. 2008;36(4):195–8. [Google Scholar]
- 29.Rosenthal E, Marty P, del Giudice P, Pradier C, Ceppi C, Gastaut JA, et al. HIV and Leishmania coinfection: a review of 91 cases with focus on atypical locations of Leishmania. Clin Infect Dis. 2000;31:1093–5. [DOI] [PubMed] [Google Scholar]
- 30.Gabriele G, Cascino F, Funaioli F, Gennaro P. Granulomatous cheilitis: differential diagnosis in patients with macrocheilia. BMJ Case Rep. 2023;16(5): e251829. 10.1136/bcr-2022-251829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohammadpour I, Motazedian MH, Handjani F, Hatam GR. Lip leishmaniasis: a case series with molecular identification and literature review. BMC Infect Dis. 2017;17(1):96. 10.1186/s12879-016-2178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takele Y, Mulaw T, Adem E, Shaw CJ, Franssen SU, Womersley R, Kaforou M, Taylor GP, Levin M, Müller I, Cotton JA, Kropf P. Immunological factors, but not clinical features, predict visceral leishmaniasis relapse in patients co-infected with HIV. Cell Rep Med. 2021;3(1): 100487. 10.1016/j.xcrm.2021.100487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guedes DL, Justo AM, Barbosa Júnior WL, Silva EDD, Aquino SR, Lima Junior MSDC, Montarroyos U, Bezerra GSN, Vieira AVB, Pereira VRA, Medeiros ZM. Asymptomatic Leishmania infection in HIV-positive outpatients on antiretroviral therapy in Pernambuco, Brazil. PLoS Negl Trop Dis. 2021;15(1): e0009067. 10.1371/journal.pntd.0009067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Molina R, Jiménez M, García-Martínez J, San Martín JV, Carrillo E, Sánchez C, Moreno J, Alves F, Alvar J. Role of asymptomatic and symptomatic humans as reservoirs of visceral leishmaniasis in a Mediterranean context. PLoS Negl Trop Dis. 2020;14(4): e0008253. 10.1371/journal.pntd.0008253. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.