Abstract
Purpose
Little is known about dentists’ preparedness in managing oral side effects in patients undergoing cancer therapy (CTx). The purpose of this systematic review is to identify barriers and facilitators of dentists in managing oral health of cancer patients (CPs).
Methods
The review adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and was Prospero registered (CRD42022333055). CINAHL, Embase, Medline, PsycInfo and Scopus databases were searched using keywords and MeSH terms: dentists, oral health, cancer. The outcomes were analysed descriptively and thematically.
Results
Of the 2303 articles screened 53 met eligibility criteria. Most of articles (n = 50) reported on head and neck cancer (HNC) management. Dentists’ oral cancer (OC) knowledge varied across studies (27% to 81%, n = 35). Regardless of their knowledge level, the majority of dentists expressed interest in further cancer education. Across studies, dentists perceived that their role included providing dental treatment for OC patients. However, of the few studies (n = 3) that explored dentists’ confidence in managing CPs, less than half of dentists felt confident providing advice to patients with HNC. More barriers than facilitators are identified in providing dental care provision to CPs.
Conclusion
This review demonstrates gap in dental care for patients with non-HNCs and highlights a need for methods to involve dentists in managing dental health of CPs.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12913-024-11676-8.
Keywords: Dentist, Oral health, Cancer patient, Barriers
Introduction
Patients undergoing CTx have unique oral and dental needs as cancer and its treatment often have direct and indirect impact on oral health. Attentive dental care tailored to the needs of CPs reduces oral complications [1–3], improves quality of life [4, 5], reduces mortality [6, 7] and healthcare costs [8]. To facilitate better oral care, internationally there have been a number of best practice guidelines developed (for example Elad, Cheng [9]), however the oral health of CPs receiving CTx is often overlooked and patients do not receive timely information about oral complications or oral care [10].
Traditionally, oncology patients have been managed in specialised cancer centres, however most cancer centres do not have a dental department [11], and dentists are seldom included in the oncology multidisciplinary team [10] unless treatment is focused specifically on the head and neck (H&N) region. This occurs despite patients with solid tumours outside of H&N region also experiencing chemotherapy-related mucositis, apthous ulcers and xerostomia [12]; patients receiving bone modifying agents, targeted and immunotherapies being at increased risk of osteonecrosis of the jaw [13, 14]; patients on targeted therapies experiencing oral pain, dry mouth and stomatitis [15]; and survivors of allogenic haemopoetic stem cell transplant (HSCT) patients experiencing long term oral side effects as a result of immune response to the transplantation.
With the increasing number of patients being diagnosed with cancers each year and undergoing CTx, there is an increasing need for dentists to be included in managing the oral health of these patients. Dentist’s understanding the potential oral side effects is critical as this knowledge will ensure dentists are able to discern dental disease from the transient effects of therapies and take appropriate precautions when managing oral health of these patients [16, 17]. Given the important and yet under-utilised role dentists have in the care of CPs, the aim of this review was to understand the barriers and facilitators of dentists’ management of the dental health of CPs undergoing cancer treatment. Specifically, this systematic review explored dentists’ cancer knowledge, perceptions, clinical practice and confidence of treating CPs.
Methodology
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [18] and was preregistered with the International Prospective Register for Systematic Reviews (CRD42022333055). It included both qualitative and quantitative studies capturing dentists’ knowledge, perception, clinical practice and confidence in managing the oral health of cancer patients.
Search strategy
Medline, Embase, CINAHL, PsycInfo and Scopus were searched using keyword and MeSH terms: “dentist*”, “dental specialist*”, “dental surgeon*”, “oral health professional*”, “dental practi*”; “oral health*”, “dental care”, “oral care”, “oral hygiene”, “mouth hygiene”; “neoplasm”, “cancer*”, “oncology*”, “malignan*”. Broad search terms were used as our preliminary search with narrow terms did not capture relevant studies. The search was conducted in 2022 and updated in July 2023. Reference lists of review articles were also manually searched. An example of search strategy is included in supplementary file 1.
Inclusion and exclusion criteria
Studies were included if they were in adult population and published in English between 1990 and July 2023. Review articles, conference abstracts or expert opinions were excluded.
Participants
This review included studies with general dentists (GDPs) and specialist dentists (SDs), while excluding studies involving dental students and dental auxiliaries such as hygienists, oral health therapists and dental assistants.
Study designs
We included qualitative and quantitative studies reporting dentists’ knowledge, perceptions, practice and confidence related to cancer screening, management and clinical practice.
All search results were initially uploaded into EndNote X20 (Clarivate, Philadelphia, PA, USA, 2022).
and duplicates removed. Abstracts were uploaded into Covidence (Veritas Health Innovation, Melbourne, Australia) and two reviewers (SL and JS) independently screened titles and abstracts. For studies that appeared to meet criteria, full text articles were retrieved and reviewed against the eligibility criteria. Disagreements were resolved through discussion.
Data extraction
Data extraction was conducted using a purpose-designed template (SL) and 20% of articles were reviewed by a second reviewer (JS) to assess accuracy. Data extracted included: participants’ characteristics (age, gender, experience, training background, recency of continuing education (CE), location of practice and workplace characteristics); study characteristics (country, research methods, recruitment strategies, sample size, cancer population) and outcomes of interest (dentists’ cancer and CTx knowledge, perceptions on education, role in cancer management, clinical practice, and confidence). Quality was assessed based on the Mixed Methods Assessment Tool (MMAT) [19].
Data analysis
Quantitative data was summarised descriptively, qualitative data were analysed using content analysis. Reported barriers and facilitators were categorised as: environmental/ context, dentist-related and patient factors.
Results
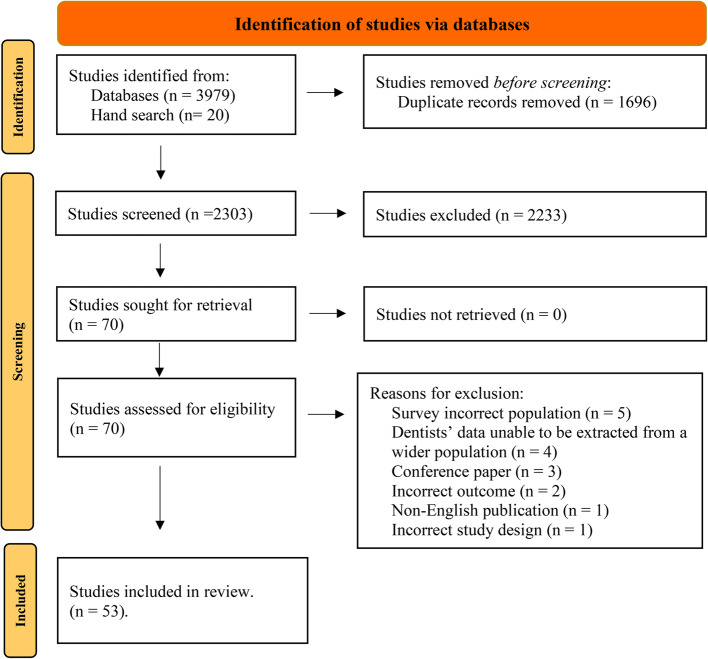
Database searches identified a total of 3,979 studies, with additional 20 abstracts found through hand searching. After removing duplicates, a total of 2,303 titles and abstracts screened. Full text review of 70 articles resulted in 53 articles identified for inclusion in the review (see Fig. 1 for PRISMA diagram).
Fig. 1.
Systematic reviews and meta-analyses (PRISMA) flow chart
Study characteristics
Of the 53 studies identified, the majority (n = 50) focused on H&N regions. Of the non-HNCs (n = 3), 1 study explored management of leukemia patients [20] and 2 studies [21, 22] explored treatment of oncology patients more broadly. The study designs were primarily surveys (n = 51), with 1 study using a qualitative focus group methodology [23] and 1 study using a mixed methods approach [24]. Seven studies focused solely on oral screening practices [25–31].
Mean sample size was 315 (range 32–3200). Studies were most commonly surveying dentists from USA (n = 16), Middle East (n = 11) or UK (n = 7). Five studies were conducted in Asia, with studies also conducted in Brazil (n = 4), Spain (n = 2), Australia (n = 2), Africa (n = 2), Canada (n = 1), and Italy (n = 1). Additionally, 2 studies conducted in combined regions: Australia/ Japan and Australia/ New Zealand. Study characteristics are summarised in Table 1.
Table 1.
Study characteristics
| ID | Authors, publican year | Aims | Country | Research methods | Recruitment strategies | Actual sample size | Cancer population | Data analysis |
|---|---|---|---|---|---|---|---|---|
| 1 | Ahmed & Naidoo (2019) [32] | To determine dentists’ knowledge, attitudes, and practices in the prevention and early detection of OCs. To evaluate CE needs | Khartoum, Sudan | Quantitative (survey) | 130 GDPs working in public dental clinics | n = 113 (87% RR) | OC | T t-test, Mann–Whitney U test & Chi-square tests |
| 2 | Akbari et al. (2015) [33] | To assess the GDPs’ and dental SDs’ knowledge about OC in South Khorasan, Iran | Iran | Quantitative (survey) | 80 practicing GDPs & SDs taking part in CE | N = 73 (91% RR) | OC | Descriptive analysis. Chi square, t-test |
| 3 | Alhazzazi (2021) [34] | To assess the knowledge & behaviour of dentists toward screening & managing patients with HNC | Saudi Arabia | Quantitative (survey) | n = 723 GDPs & SDs | N = 206 (28.5% RR) | HNC | Descriptive analysis. Chi-square test |
| 4 | Alonge et al. (2004) [35] | To determine dentists’ OC knowledge & OC screening practices, & preferred methods for OC CE | Texas, USA | Quantitative (survey) | 398 Texas dentists practicing along the Texas-Mexico border | n = 158 (40% RR) | OC | Bivariate analysis (Chi-square test) |
| 5 | Alqahtani et al. (2021) [36] | To investigate the knowledge & awareness among dentists in Saudi Arabia towards oral & dental assessment and management of HNC patients pre and post-RT | Saudi Arabia | Quantitative (survey) | Google form via an online link though WhatsApp or Social Media Platforms | 370 responded | HNC | Descriptive analysis |
| 6 | Alqutaibi et al. (2021) [25] | To assess prosthodontis’ knowledge of & screening practices for OC and potentially malignant oral lesions | Saudi Arabia | Quantitative (survey) | n = 250 eligible prosthodontists | n = 143 (57% RR) | OC | Descriptive analysis using Chi-square test |
| 7 | Borhan-Mojabi (2012) [37] | To evaluate the degree of knowledge of physicians and GDPs on OC within the context of developing an appropriate under- & post-graduate education programme to optimize early detection & prevention of OC | Qavzin, Iran | Quantitative (survey) | Dentists: n = 100, Physicians: n = 100 | Dentists n = 86 (86% RR); Physicians n = 66 (66% RR) | OC | Descriptive analysis using t-test, chi-squared, ANOVA, Pearson correlation |
| 8 | Calvert et al. (2014) [38] | To record the current practice of restorative dentistry consultants in immediate, initial, & long-term management of patients diagnosed HNC | UK | Quantitative (survey) | 315 restorative consultants from General Dental Council website | n = 132 (43% RR), 60 of the 132 treated H&N patients | HNC | Not reported. Data presented as charts and histograms |
| 9 | Canto et al. (2001) [39] | To assess dentists’ knowledge of risk factors and diagnostic procedures for OC | Maryland, USA | Quantitative (survey) | 1000 GDPs selected from ADA Maryland mailing list | n = 508 usable questionnaires (54% RR) | OC | Descriptive analysis & logistical analysis |
| 10 | Clovis et al. (2002) [40] | To assess and describe Canadian dentists’ understanding of risk & diagnostic factors related to OC and to determine their opinions about their professional preparation to prevent & control OC | British Columbia & Nova Scotia | Quantitative (survey) | Systematic random sample of 817 licensed dentists from British Columbia n = ?? from Nova Scotia | n = 670 (55.2% RR) [n = 401 (50.4%RR) British Columbia; n = 269 (64.4%) Nova Scotia] | OC | Descriptive analysis using ANOVA |
| 11 | Colella et al. (2008) [41] | To investigate dentists and physicians’ level of knowledge, attitudes, & behaviours towards OC | Campania region, Italy | Quantitative (survey) | 1000 professionals attending 22 randomly selected association meetings | n = 457 (45.7% RR) [Dental: n = 225 Medical: n = 232] | OC | Descriptive analysis |
| 12 | Cruz et al. (2005) [26] | To examine OC prevention and early detection practice patterns in OHPs. To examine if there were any variables that were associated with lower adherence to recommended health behaviour counselling | NY, USA | Quantitative (survey) | Stratified random sample of licensed dentists (n = 904) and DHs (n = 963) | Dentists: n = 496, DHs: n = 630 | OC | Descriptive Bivariate analysis |
| 13 | Daley et al. (2011) [23] | To assess awareness among OHP regarding the HPV-OC link. To elicit OHP attitudes & perceived role to screen for HPV-related oral lesions, & to discuss HPV as OC risk factors & HPV vaccine with patients | Florida, USA | Qualitative (focus group) | Dentists or DHs recruited from local dental & dental hygiene professional associations | dentists: 3 focus groups (total n = 17) dental hygienists: 2 focus groups (total n = 21) | OC | Qualitative analysis. Coding of data |
| 14 | Dang et al. (2022) [21] | To assess dental practice patterns in oral care of medical oncology patients & to identify potential barriers to recommended care in the state of Massachusetts | Massachusetts, USA | Quantitative (survey) | Registered dentists at Massachusetts Dental Society. n = 3394 | n = 363 (10.7%RR) | All cancers | Descriptive analysis. Qualitative coding for free text responses |
| 15 | Dewan et al. (2014) [42] | To investigate the approach of restorative dentists in the treatment & dental rehabilitation of OC patients in the UK | UK | Quantitative (survey) | Delegates at the conference (n = 94) | n = 65 (69.1% RR) | OC | Descriptive analysis |
ADA Australian Dental Association, AmDA American Dental Association, CE Continuing education, DH Dental hygienist, DT Dental therapist, GDP General dental practitioner, H & N Head and neck, HNC Head and neck cancer, OC Oral cancer, OCE Oral cancer examination, OHP Oral health practitioner, OPC Oropharyngeal cancer, RR Response rate, RT Radiation therapy, SD Specialist dentist
Participants characteristics
Studies included participants who were GDPs (n = 34), SDs (prosthodontists, restorative dentists and maxillofacial surgeons) (n = 5); or a combination of GDPs and SDs (n = 14). Studies reporting gender participants (n = 36), 38% were female (range 11—79.9%). Forty studies reported participant’s clinical practice experience. Among 24 studies reporting range of years, 10 studies reported 32.6% of participants had < 5 years dental experience, 20.9% had 6–10 years and 46.3% had ≥ 10 years’ experience. Additionally, 10 studies reported a mean clinical practice duration of 11.9 ± 5.1 years. Of the studies reporting workplace characteristics (n = 30), 10 studies recruited participants from solo, partner, salaried, employee and community practices; 21 studies classified workplaces based on funding models (public vs private), of which 54.1% of dentists worked in public sector, 41.3% worked in private sector and 4.4% worked in both public and private settings.
Fourteen studies reported on the recency of OC CE, with approximately half of dentists reporting undertaking CE within the last 5 years [27, 28, 33, 40, 41, 43–51].
Participant characteristics are summarised in Table 2.
Table 2.
Participants characteristics
| ID | Authors (publican year) | Age | Gender (female n/%) | Experience | Training background | Recency of oncology CE | Location of practice (n/%) | Workplace characteristics (n/%) |
|---|---|---|---|---|---|---|---|---|
| 1 | Ahmed & Naidoo (2019) [32] | NR | n = 77 (68.1%) | 3–5 years (35.4%), 6–10 years (42.5%), 11–15 years (11.5%) > 15 years (10.6%) | GDPs | NR | NR | Public: n = 113 (100%) |
| 2 | Akbari et al. (2015) [33] | NR | n = 36 (49%) | 1–4 years: n = 31 (42.5%), 5–9 years: n = 11 (15.1%), 10–14 years: n = 10 (13.7%), 15–19 years: n = 5 (6.8%) > 20 years: n = 16 (21.9%) | GDPs: (n = 55, 75%), SDs: (n = 18, 25%) | 24.7% attended OC CE courses | NR | NR |
| 3 | Alhazzazi (2021) [34] | NR | n = 81 (39%) | 0–2 years: 96 (47%), > 2–5 years: 31 (15%), > 5–10 years: 18 (9%), > 10 years: 61 (30%) | GDPs: n = 119 (58%), SDs: n = 14 (7%), dental consultants: n = 49 (24%), dental residents: n = 14 (7%), others: n = 3 (5%) | NR | NR | Public: n = 50 (24%), Private: n = 61 (30%), University hospital: n = 69 (33%), Mixed public & private: n = 8 (4%), mixed university & private: n = 16 (8%) |
| 4 | Alonge & Narendran (2004) [35] | 20–29 years (n = 5, 3%), 30–39 years (n = 35, 22%), 40–49 years (n = 48, 31%), 50–59 years (n = 44, 28%), 60–69 years (n = 17, 11%), 70–79 years (n = 7, 5%), missing data (n = 2) | n = 21, 14% missing data (n = 2, 1%) | 0–8 years (n = 25, 16%), 9–18 years (n = 46, 30%), 19–28 years (n = 48, 31%), 29–38 years (n = 35, 23%) | GDPs | NR | NR | Solo: n = 114 (72%) Others: n = 44 (28%) |
| 5 | Alqahtani et al. (2021) [36] | NR | n = 113 (31%) | < 5 years: n = 185 (49.5%), 5–10 years: n = 120 (32.4%), > 10 years: n = 67 (18.1%) | GDPs: n = 144 (39%), oral surgeons/ oral meds/ oral pathologists: n = 57 (15%), endodontists: n = 34 (9%), periodontists: n = 21 (9%), other specialists: n = 87 (23%) | NR | NR | Public: n = 352 (95.1%); Private: n = 18 (4.9%) |
| 6 | Alqutaibi et al. (2021) [25] | NR | n = 36 (25%) | < 10 years: n = 79 (55%); ≥ 10 years: n = 64 (45%) | Prosthodontists: 100%; master degree: n = 49 (34.3%); board certified: n = 42 (29.4%); PhD: n = 52 (36.4%) | NR | NR | Public: n = 120 (84%); Private: n = 23 (16%) |
| 7 | Borhan-Mojabi (2012) [37] | GDPs: 37.93 ± 9.22 years | Data not separated for GDPs | GDPs: 9.67 ± 9.05 years | GDPs | NR | NR | NR |
| 8 | Calvert et al. (2014) [38] | NR | NR | NR | Restorative dentists | NR | NR | NR |
| 9 | Canto et al. (2001) [39] | NR | 19% | > 25years: 23%; 16-25years: 28%; 6-15years: 34%; < = 5 years: 15% | GDPs | NR | NR | Solo: 60%, partner: 17%, salaried/ contractor: 19%, all other: 4% |
| 10 | Clovis et al. (2002) [40] | NR | n = 117 (17.9%) | > 27 years): n = 88 (13.3%); 18–27 years: n = 196 (29.6%); 8–17 years: n = 229 (34.5%); ≤ 7 years: n = 150 (22.6%) | GDPs | ~ 60% attended OC CE in the last 5 years, | NR | Solo: n = 370 (55.4%), partner: n = 177 (26.5%), salaried or contractor: n = 93 (13.9%), others: n = 28 (4.2%) |
| 11 | Colella et al. (2008) [41] | NR | n = 80 (17.5%) | ≤ 15 years: n = 186 (40.7%); 16–20 years: n = 99 (21.7%); 21–25 years: n = 80 (17.5%); 26–30 years: n = 67 (14.7%); > 30 years: n = 25 (5.4%). Mean years of graduation is 18 years | Dentists (graduates from medical school: n = 232, 50.8%; graduates from dental school: n = 225, 49.2%). GDPs: 53%, oral surgeons: 17.9%, restorative dentists/ endodontists: 9.9%, orthodontists: 7.4%, periodontics: 5.9%, oral pathologists: 3.5%, prosthetists: 2.4% |
96.1% received OC information & 20.6% attended CE course on OC in the last 12 months Main sources of info were educational (72.4%), scientific journals (22.8%) |
NR | Solo: n = 155 (33.9%) non-solo: n = 302 (66.1%) |
| 12 | Cruz et al. (2005) [26] | 20-39years: 19%; 40–59 years: 62%; 60 & older: 98% | 13% | Median years since graduation: 24 years | Dentists and DHs | NR | NR | Solo practice: 60%; partner/ employee: 26%; independent contractor: 4%; specialty practice: 4%; public health/ government/ other: 5% |
| 13 | Daley et al. (2011) [23] | 28–66 years (mean age 45 years) | n = 8 (47%) | 3–43 years (mean 19 years) | Accredited US program Dentists, DHs | NR | NR | NR |
| 14 | Dang et al. (2022) [21] | NR | NR | NR | Dentists | NR | NR | Private practice: 89%, community health centre: 4%, Hospital-based: 4%, dental school-based practice: 3% |
| 15 | Dewan et al. (2014) [42] | NR | NR | NR | Consultants or senior lecturers: n = 30 (46.1%); specialist registrars: n = 27 (41.5%). SDs in restorative dentistry: n = 8 (12.3%) | NR | NR | NHS posts (public): 73%; Academic posts: 27% |
| 16 | Dixon et al. (2021) [24] | 46.8/13.7 | n = 76 (49.35%) | < 10years: n = 37 (24%); 10-19years: n = 29 (18.8%); 20–29 years: n = 31 (20.1%); 30–39 years: n = 35 (22.7%); 40–49 years: n = 19 (12.3%); > 50 years: n = 3 (1.9%) | NZ trained dentists: n = 103 (66.9%). Interviews: 4 dentists in Sydney West Cancer Network | NR | Urban: n = 131 (85.1%); Rural: n = 23 (14.9%) | Public: n = 7 (4.5%); Private: n = 129 (83.8%); Working in both public & private: n = 14 (9.1%); Not practicing: n = 4 (2.6%); Public experience: n = 21 (14%) |
| 17 | Ekici (2020) [43] | 25–34 years: n = 67 (22.8%); 35–44 years: n = 104 (35.4%); 45–54 years: n = 108 (36.7%); ≥ 55: n = 15 (5.1%) | n = 199 (60.2%) | 1–5 years: n = 33 (11.2%); 6–10 years: n = 35 (11.9%); 11–15 years: n = 69 (23.5%); 16–20 years: n = 48 (16.3%); > 20 years: n = 109 (37.1%) | GDPs | 10% had OC training in the past 5 years | NR | Public (n = 294/ 100%) |
| 18 | Fidele et al. (2022) [52] | Mean: 33.2 ± 4.3 years. 23–29 years: 3.7%; 30–39 years: 54.3%; 40–49 years: 25.9%; 50–59 years: 11.1%; > 60 years: 4.9% | n = 56 (34.6%) | < 5 years: 30.9%; 5–10 years: 50.6%; 11–15 years: 9.9%; > 15 years: 8.6% | General practice: 81.5%; Specialty practice: 18.5% | NR | NR | NR |
| 19 | Frydrych et al. (2012) [53] | NR | NR | 0–5 years: 27.1%; 6–10 years): 12.6%; 11–15 years: 12.6%; 16–20 years: 5.3%; 21–25 years: 10%; > 25 years: 31.1%; Unknown: 1.3% | GDPs, SDs were excluded for analysis (n = 5) | NR | Urban (n = 140/ 76%); Rural (n = 44/ 23.90%) | Public: n = 19 (10.30%); Private: n = 166 (89.70%) |
| 20 | Gajendra et al. (2006) [44] | 20–39 years: 19%; 40–59 years: 61%; 60 or older: 20% | 13% | Median years of experience 24 years | Dentists, DHs | 80% of dentists attended OC prevention CE courses in past 5 years | NR | Solo: 59.8%; specialty practice: 4.1%; public health/ government: 2.5%; partner: 17%; employee: 9.4%; independent contractor: 4.3%; other: 2.9% |
| 21 | Guneri et al. (2008) [54] | 32.76 years (this includes students) | n = 92 (45%) | 1–35 years (mean 11.29 years) | GDPs: n = 113 (55.35%); final year dental students: n = 37 (18.13%); SDs: n = 54 (26.47%) | NR | NR | NR |
| 22 | Haresaku et al. (2018) [55] | Most dentists in Japan & Australia were > 46 years of age (23.8–40.2%) | Japanese: 7.3%; Australia: 45.8% | NR (data cannot be separated from hygienists) | Not specified. The study excluded Australian specialists who did not see OC patients | NR | NR | NR |
| 23 | Hashim et al. (2018) [45] | < 30 years: n = 204 (68.2%); > 30 years: n = 81 (27.1%) | n = 169 (57%) | < 15 years: n = 275 (92.3%); > 15 years: n = 23 (7.7%) | Bachelor degree: n = 256 (85.6%); MSc/ PhD: n = 41 (13.7%). GDPs & SDs | 48% attended an OC CE within the past 5 years | NR | Public: n = 31 (10.50%); private: n = 267 (89.50%) |
| 24 | Horowitz et al. (2000) [56] | NR | 14% | 16–20 years: 22%; 11–15 years: 28%; 6–10 years: 33%; 0-5years: 17% | GDPs | NR | NR | Solo: 68%; partnership: 12%; others: 6% |
| 25 | Husein et al. (2011) [57] | NR | NR | > 10 years: n = 161 (81%); 5–10 years: n = 19 (10%); < 5 years: n = 18 (9%) | UK graduates GDPs: n = 177 (89%) | NR | NR | GDPs working in mixed, mainly NHS practice: 55%; GDPs working in solely private practice: 5% |
| 26 | Joseph et al. (2012) [46] | < 40 years: 60.6%; > 40 years: 39.4% | n = 35 (22.9%) | > 15 years: 44.4%; = < 15 years: 55.6% | Dentists | 30% attended OC CE within the last 5 years | NR | Public: n = 153 (100%) |
| 27 | Kogi et al. (2019) [58] | NR | 28.2% | < 1 year: 35.5%; 2–5 years: 20.9%; 6–15 years: 20.9%; > 16 years: 26.4% | Restorative dentists (operative, endodontics, periodontics, prosthodontics): 60.9%; non-restorative dentists (dental anaesthesiology, dental public health, dental radiology, orthodontics, paediatric): 39.1% | NR | NR | NR |
| 28 | Kujan et al. (2006) [27] | NR | NR | 43–52 years: n = 1 (3%); 33–42 years: n = 50 (14.8%); 23–32 years: n = 104 (30.8%); 13–22 years: n = 113 (33.5%); 0–12 years: n = 66 (19.5%) | GDPs and SDs | 52.3% SDs & 26.3% GDPs attended OC CE in the last 12 months | NR | NR |
| 29 | Leão et al. (2005) [59] | 40.4 years/ 12.9 | 52% | Mean: 16 years | GDPs | NR | Urban: n = 129 (100%) | Public: 38%; Private or public/private: 62% |
| 30 | LeHew et al. (2010) [28] | NR | n = 28 (27.5%) | Median: 13 years (range 0–50 years) | GDPs: 90%; SDs (orthodontics, oral surgery, endodontics, peadiatrics, prosthodontics): 10% | 37.3% never attended OC CE | NR | NR |
| 31 | Lopez-Jornet et al. (2010) [60] | NR | 40.3% | Mean: 13.3 years (1- 42 years) | GDPs | NR | NR | NR |
| 32 | Marino et al. (2017) [29] | ≤ 25 years: 7.4%; 26–35 years: 19%; 36–45 years: 23.2%; 46–55 years: 25.2%; > 55 years: 25.2% | 44.2% | ≤ 5 years: 15.7%; 6–10 years: 12.4%; 11–15 years: 11.6%; 16–20 years: 9.9%; 21–25 years: 9.9%; > 25 years: 40.5% | GDPs: 63.6%; SDs: 8.4%; DHs: 13.7%; Oral health therapists: 12.2%; Dental therapists: 2.1% | NR | Urban: 76.60%; Rural: 23.40% | NR |
| 33 | Martins et al. (2021) [61] | 20–30 years: 41.07%; 31–40 years: 34.64%; 41–50 years: 14.28%; 51–60 years: 6.7%; > 60 years: 3.21%. Most were 20–40 years | n = 195 (69.64%) | Time in specialty: < 5 years = 43.92%; 5–10 years = 12.85%; 10–20 years = 18.21%; > 20 years = 25% |
Group A: n = 160 (57.14%) working in oral oncology Group B: n = 120 (42.86%) OMFS (n = 25), orthodontics (n = 21), oral rehabilitation/ prosthodontics (n = 20), paediatric dentistry (n = 14), endodontics (n = 13), dentistry specialists (n = 11), periodontics (n = 10), forensic/social legal dentistry (n = 6) |
NR | NR | NR |
| 34 | Maybury et al. (2012) [47] | NR | n = 107 (24%) | < 10 years: 14%; 10–19 years: 15%; 20–29 years: 35%; 30–39 years: 34%; ≥ 40 years: 2% | GPDs | OC CE course: Within the last 12 months: 29%; 2–5 years: 54%; ≥ 5 years: 15%. Never taken a course: < 1% | NR | Solo practice: 62%; group private practice: 36%; community health centre: 1%; other: 1% |
| 35 | McCann et al. (2000) [48] | NR | NR | NR | GDPs | GDPs: 44% had OC training in the last 2 years, 25% had OC training in 3–5 years, 17% had no OC training for > 10 years | NR | Public: n = 73 (32%); private: n = 152 (68%) |
| 36 | Nazar et al. (2022) [62] | 25.8 ± 2.4 years | n = 139 (44.8%) | Mean: 1.5 ± 1.7 years | Bachelor degree: 94.5%; Master degree: 2.6%; MFDS: 1.9%; MEGD: 0.3%; PhD: 0.6% | NR | NR | 100% of participants worked at polyclinics, specialty dental centres and School of Oral Health Program clinics as part of their rotation |
| 37 | Nazar et al. (2019) [63] | 35.2/ 10.9 years | n = 109 (37.7%) | Mean: 11.7/ 11.3 years | Bachelor degree: 75%; Master degree, MEGD or PhD: 25% | NR | NR | Public: n = 289 (100%) |
| 38 | Nicholls & Ilankovan (1998) [64] | NR | NR | NR | Oral maxillofacial surgeons | NR | NR | NR |
| 39 | Patel et al. (2012) [65] | NR | NR | < 10 year: n = 65 (15.9%); 10–19 years: n = 62 (15.1%); 20–29 years: n = 121 (29.5%); 30–39 years: n = 109 (26.6%); ≥ 40 years: n = 53 (12.9%) | Dentists & Radiation oncologists | NR | NR | NR |
| 40 | Patton et al. (2006) [66] | NR | NR | NR | Dentists, DHs, physicians, nurse practitioners | NR | NR | NR |
| 41 | Pavão Spaulonci et al. (2018) [49] | NR | NR | NR | Junior dentists: 55.9% GDPs, 38.1% specialists; Senior dentists: 56.2% specialists, 21% GDPs, 15.2% Master degree, 7.6% PhD | Attended OC CE: 15.9% in the last year, 23.3% in the last 2 years, 37.6% > 2 years ago | NR | NR |
| 42 | Reed et al. (2010) [67] | Not separated for dentists | NR | NR | GDPs & SDs, physicians & medical specialists | NR | NR | NR |
| 43 | Saleh et al. (2014) [50] | ≤ 30 years: 35.1%; 31–40 years: 26.2%; 41–50 years: 19.9%; 51–60 years: 15.2%; 61–70 years: 2.8%; 71–80 years: 0.8% | n = 247 (68.2%) | 50.8% graduated > 10 years ago | Place of graduation: Malaysia (72.4%), Asia (18%), Oceanic (4.1%), UK (2.8%), Others (2.8%). Postgraduate training 21.5% | Number of OC CE attended: 0: 26.5%; 1–5: 67.4%; > 5: 6.1% | NR | Public: 50.3%; private: 48.6%; both public & private: 1.1% |
| 44 | Seals (1990) [68] | NR | NR | NR | Recent graduate dentists | NR | NR | NR |
| 45 | Seoane et al. (2006) [30] | NR | NR | Mean: 9.1/ 5.9 years | GDPs | NR | NR | NR |
| 46 | Shadid & Habash (2023) [69] | > 30 years: 65.7% | 43.7% | ≤ 5 years: 33.5%; 6–15 years: 42.5%; > 15 years: 24% | GDPs: 79.9%; SDs: 20.1% | NR | NR | Public: n = 8 (3.20%); private: n = 205 (80.70%); both public & private: n = 41 (16.1%) |
| 47 | Strey et al. (2022) [31] | 37.6 ± 10.4 years (range 22–66 years) | 79.7% | 14.2 ± 10.4 years (1–42 years). Public system experience: 9.2 ± 8.2 years | Dentists | NR | NR | Public: 100% |
| 48 | Taheri et al. (2018) [70] | 36.8 years (range 25–60 years) | n = 80 (52%) | Mean: 9.88 (1–35 years) | GDPs | NR | NR | Private: 100% |
| 49 | Tami-Maury et al. (2016) [71] | 51–65 years: 62% | 32% | NR | Dentists | NR | NR | NR |
| 50 | Vijay Kumar & Suresan (2012) [72] | 20–39 years: 30%; 40–59 years: 62%; 60 & above: 8% | 45% | NR | Private dentists. Post-graduate qualification: 24% | NR | NR | Private: 100%. Solo: 44%; partnership: 25%; employee/ contractor: 24%; others: 7% |
| 51 | Wong & Toljanic (2009) [20] | NR | NR | NR | Maxillofacial dentists | NR | NR | NR |
| 52 | Wright et al. (2011) [22] | NR | NR | NR | Managers of dentists | NR | NR | Public: n = 83 (100%). 100% Salaried dentists |
| 53 | Yellowitz et al. (1998) [51] | NR | 11% | Range from 1–30 years | GDPs | 53% attended OC CE within the past 5 years | NR | Solo practitioners: 68% |
CE Continuing education, DH Dental hygienist, GDP General dental practitioner, NR Not reported, OC Oral cancer, SD Specialist dentist
Study outcomes
Knowledge
Thirty-five studies explored dentists’ cancer knowledge, with no studies exploring cancers outside of H&N regions. Twenty-eight evaluated OC knowledge, 24 surveyed OC identification skills, 25 assessed OC risk factors and 2 studied CTx side effects (see supplementary file 2 in the Appendix).
Cancer knowledge
There was significant variability in dentists’ overall OC knowledge across studies, with correct responses ranging from 27% [35] to 81.3% [62]. In 4 of 5 studies, > 90% of dentists recognised that early detection of OC improves patient survival rates [40, 51, 60, 66]. Several factors were identified to be positively associated with OC knowledge. Recent CE (n = 7) [28, 37, 39, 41, 46, 47, 59], recent dental graduates (n = 7) [32, 33, 39, 49, 50, 69, 70], SDs in oral surgery/ pathology (n = 2) [36, 41] and dentists with experience in public settings (n = 4) [36, 49, 50, 59] reportedly had significantly better OC knowledge. Dentists who rated their undergraduate OC training favourably were more likely to agree that their OC knowledge was current [60], and 2.2 times more likely to score higher on knowledge of OC [49]. In terms of gender, 3 studies found female dentists faired significantly better in OC knowledge [47, 50, 62], while others found no significant influence of gender on OC knowledge [54, 60, 69].
Cancer identification skills
To evaluate OC identification skills, the domains assessed included knowledge of sites, signs and symptoms of OC. There was a considerable degree of variability in survey responses. Notably, recent CE (n = 2) were found to positively correlated with skills in OC identification [40, 41]. Nevertheless, our findings showed divergent relationship between clinical experience and cancer identification skills amongst dentists. Two studies found recent graduates had better skills [40, 69] while 2 studies indicated that dentists with more clinical experience [47, 52] were better at OC identification. Additionally, Maybury et al. [47] found that dentists working in a group private practice were more likely to have better OC identification skills than dentists working in solo private practice.
OC risk factors
Of the studies that explored common OC risk factors such as alcohol and tobacco use (n = 23), 19 studies reported > 80% of participants identified alcohol [35, 39, 40, 43–52, 60, 62, 63, 69, 70, 72] as a risk factor while 21 studies reported > 80% of participants identified tobacco as a risk factor for OC [35, 39–41, 43–52, 55, 60, 62, 63, 67, 69, 70]. In all 12 studies, [35, 39–41, 43, 45–47, 51, 52, 60, 69] exploring prior OC risk, > 80% of participants were aware of its significance. Among the 18 studies investigating older age, 11 studies reported > 60% of participants correctly identified older age as a risk factor [39, 40, 45–49, 52, 60, 69, 70]. Of the 16 studies that explored Human Papilloma Virus (HPV), 9 out of 15 reported that > 60% of participants were aware of the association between HPV and OC [32, 34, 43, 45–47, 49, 50, 69], although a focus group study revealed that dentists had limited knowledge [23]. In contrast, of the 10 studies investigating consumption of fruit/ vegetables, 8 studies reported < 50% of participants identified low consumption as a risk factor [32, 39–41, 44, 47, 49, 69].
CTx side effects and management
In two studies that assessed dentists’ knowledge of H&N radiation therapy (RT) and side effects, > 80% of dentists were able to identify radiation-related caries as an oral complication following RT [54, 61]. Dentists working in the field of H&N RT were more aware of radiation related complications than dentists not working in the clinical area [61].
Perceptions
Twenty-one studies surveyed dentists’ perception of their cancer knowledge, 5 studies assessed dentists’ perceived role in cancer management, 14 studies investigated dentists’ role in OC screening, and 33 studies examined the adequacy and interest in further cancer training (see supplementary file 3 in the Appendix).
Perceived knowledge
In studies investigating dentists’ perception of cancer knowledge, knowledge was categorised into perceived (i) currency and (ii) sufficiency. On average, 56.9% dentists (n = 13) perceived their OC knowledge was current [32, 39, 40, 44, 46, 47, 51, 52, 60, 62, 63, 66, 72]. However, 35.3% of dentists (n = 3) found their OC knowledge was sufficient [37, 50, 58]. Furthermore, an average of 60.4% of dentists (n = 3) perceived their OC prevention knowledge was sufficient [32, 43, 50]. Two studies found there was no correlation between perceived and actual knowledge [51, 59], while other studies reported a positive correlation [49, 50]. Additionally, 2 studies investigated dentists’ perceived cancer management knowledge, with one study reporting 37.1% of dentists felt their knowledge was current [53] and the other study found dentists with more experience were more likely to treat CPs [24].
Perceived role of dentists in managing CPs
Two studies reported > 75% of participants agreed that GDPs should provide dental treatment for OC patients [24, 53]. However, dentists’ willingness to provide dental treatment to H&N CPs receiving RT varied; < 50% of dentists expressed comfort in managing these patients [57], and the preference to refer these patients to dentists who specialised in the field ranged from 32.9% [53] to 77.1% [61]. A study exploring dentists’ perceived roles in treating patients with a history of cancer, found that 91% of GDPs were happy to provide dental treatment to cancer survivors [71].
Perceived role of dentists in cancer screening
On average, 88.3% of dentists (n = 7) acknowledged the role of dentists in OC screening [25, 28, 48, 50, 55, 60, 69]. However, some dentists believed that oral screening should be performed selectively, with an average of 71.6% of dentists (n = 5) perceiving dentists have a role in performing OC screening in high-risk patients [34, 36, 51, 60, 72], and in one study 78% of dentists indicated a role in screening patients with a history of HNC [34].
Perceived adequacy of training
On average, 63.6% of dentists (n = 4) perceived their OC training was sufficient [31, 35, 47, 56], 59.6% of dentists (n = 17) perceived their OC screening practice was sufficient [25, 27, 32, 41, 43, 46, 49, 51, 52, 56, 60, 62, 63, 66, 69, 72, 73], while 47% of dentists (n = 4) perceived they were adequately trained to treat CPs [21, 24, 53, 65]. With regards to further training needs, studies consistently reported a strong inclination among dentists for further training. On average, 87% of dentists (n = 2) were interested in receiving OC CE [41, 43], 81.7%% dentists (n = 11) were interested in specific training on OC detection [32, 35, 39, 40, 45, 50, 52, 62, 63, 67, 73], and 92.5% of dentists (n = 2) were interested in training on managing CPs [53, 57].
Practice
Studies that surveyed practice of dentists can be grouped in oral screening practice (n = 33), management of suspicious oral lesions (n = 13), managing CPs (n = 14) and communication with other health professionals (n = 7) (see supplementary file 4 in the Appendix).
Oral screening practice
In clinical practice, an average of 53.4% of dentists (n = 16) reportedly performed oral screening examinations on every patient [25, 28, 29, 32, 34, 37, 41, 48, 49, 51, 52, 55, 58, 63, 69, 72], while 71.8% of dentists (n = 9) selectively screened high risk patients [26, 35, 43, 44, 50–52, 56, 69]. Recent graduates (n = 2) [35, 50] or dentists who perceived to have adequate training (n = 3) [50, 60, 66] were more likely to perform OC examinations on their patients.
Management of suspicious oral lesions
Majority of dentists refer suspicious lesions to a SD to confirm diagnosis. Only 36.7% of GDPs (n = 4) performed the biopsy on patients with suspicious oral lesions [27, 43, 46, 72].
Management of CPs
Of the studies that explored SDs (n = 4), over 60% of restorative specialists [38, 42] and over 78% of oral maxillofacial surgeons [64] managed H&N CPs in their clinical practice. In one study, 55% of oral maxillofacial surgeons reportedly reviewed leukaemia patients pre-chemotherapy [20]. However, among studies of GDPs (n = 6), 4 studies found > 50% of dentists saw CPs undergoing CTx [21, 22, 53, 57, 65] although one study reported GDPs rarely see CPs [73]. Location of practice (metropolitan or urban) [53], gender, age and duration of practice [54] reportedly was not associated with dentists seeing CPs. However, a study suggests place of graduation influenced if a GDP would refer patients with HNCs to a SD for dental management [24].
Communication with other health professionals
Amongst the studies with GDPs (n = 3), majority of dentists who treated CPs communicated with the oncology team [21, 53], however they rarely received updates from the oncology team [21]. A study reported that 88.5% GDPs reported that having a referral guideline could improve the quality of referrals [32]. Amongst the studies of restorative specialists (n = 2), 52% attended multi-disciplinary meetings (MDTs) [38] and most patients seen at oncology assessment clinics were referred from MDTs [42]. A study on dentists in management roles found that 13% of dental managers believed dental service for CPs can be improved by having earlier referral for dental care [22].
Confidence
Three studies explored dentists’ beliefs in their capabilities in managing CPs. In two studies, < 50% felt confident in treating HNC patients [24, 53]. One study reported that GDPs were most comfortable with performing non-invasive or less complex procedures on CPs [57].
Barriers and facilitators
Content analysis of free text responses or qualitative data within studies reporting barriers and facilitators to providing oral care to CPs, identified professional, organisational and patients’ factors that influence dentists’ willingness to provide dental care to CPs.
Professional barriers and facilitators
Lack of training [37], knowledge and skills [21, 65] were identified in 3 studies as a barrier to providing dental treatment for patients undergoing CTx. The increased time required to manage oral health of CPs [38] and the short timelines to perform dental screening between diagnosis and commencement of CTx [21, 38, 65] were also highlighted as barriers.
The complexity and consideration of CTx needed before performing dental treatment could pose as barrier for dentists to treat CPs [42]. For example, a survey of dentists managing CPs with bone modifying agents found that 94% of dentists considered risk of extracting a tooth (osteoradionecrosis), 81% of dentists considered the prognosis of the tooth (extension of caries) and 76% of dentists considered success of conservative management (restorability of tooth) in their dental treatment planning for patients undergoing RT [42].
Organisational barriers and facilitators
Structural barriers also impact on dentists’ ability to provide dental care to patients. Studies (n = 2) highlighted that the medical team does not prioritise or refer patients for dental screening prior to treatment or provide information to patients [21, 65]. One study reported the need for inclusion of oral health and referral pathways in the overall care plan of CPs [65]. The short time frame between diagnosis and treatment commencing also restricts time available for screening [38]. Similarly, dentists lack of clear guidance on safe treatment options [65]. Having referral sources and a policy to provide long-term continuing care for patients following completion of CTx was highlighted as a potential facilitator to the continuity of care for patients [22].
Lack of communication between dentists and the cancer team was also a barrier to care. For example, in one study, 31% of dentists reported a lack of correspondence with the patient’s oncology team [21] and other studies (n = 2) reported weak links with oncology services and primary care providers impacted on timely communication [22, 65].
Funding models were also highlighted as a barrier to dental care for many. For example, in two US studies it was reported 1/5 of dental practices did not accept CPs on Medicaid [21, 65] and insurers do not provide cover for dental treatment (n = 2) as it is not viewed as necessary for cancer management [20, 65]. UK managers also highlighted need for specific funding for dental treatment of CPs [22].
Patients’ barriers and facilitators
Patients’ lack of awareness can also be a barrier to accessing care. For example, in one study 56% of dentists reported a lack of patient education on oral complications [21] and a second study reported inadequate patient education of oral risks associated with RT [65].
Quality of studies
Overall, most studies (n = 43) scored over 71% on MMAT. The quality assessment can be found in supplementary file 5. No studies were excluded from the review based on their quality assessment. The review did identify a high risk potential bias in one paper included in our analysis [64]. This risk is related to lack of details about how recruitment processes were conducted. Given we used a narrative synthesis to summarise study results in this review, it is unlikely inclusion of this study resulted in mis-representation or inflation of the review findings. Fifty-one of the 53 studies were surveys, it is worth noting that these survey-based studies have their limitations including self-selecting samples, small sample size and low response rate which increased the risk of selection bias. Further, most studies adopted study specific questionnaires due to a lack of standardization in outcome measures. Hence, this review is classified as level V primarily relying on descriptive and qualitative research.
Discussion
This is the first systematic review that synthesizes the perspective of dentists in managing CPs. In our review, 94% (n = 50) of studies limited their focus to HNC. This is despite all CPs potentially experiencing short and long-term treatment related oral complications.
It is well known that having clinical guidelines alone are not sufficient to change practice; rather multi-level factors are required to implement evidence-based research into clinical practice [74]. In this review we sought to map the current literature to the Theoretical Domains Framework [75], to provide a conceptually robust exploration of factors that may influence clinical practice change among dentists. We specifically focused on the domains of knowledge, skills, professional role and identity, beliefs about capabilities, and environmental context and resources.
The review found that there is great variability in dentists’ OC knowledge. This is likely to due to a lack of standardised measure and variation in how knowledge was assessed. Across studies, while there was a high percentage of respondents who were able to identify alcohol and tobacco are associated with OC, they showed less awareness of other risk factors and myths. Not surprisingly, higher cancer knowledge was linked to clinical exposure, prior cancer education and positive perception of cancer training. Regardless of their current knowledge or perception, dentists expressed desire in further CE in deepening their cancer knowledge, OC detection and CTx.
Our review found that while dentists view their responsibilities as including OC screening and management, however, fewer conduct screening in their clinical practice. This pattern is also observed in managing patients with OC, possibly due to insufficient oncology training in dentistry programs. There is limited information on dentists’ perceptions of their role in non-HNC management. With evolving CTx and side effects such as opportunistic infection [76], dental caries, gingivitis and febrile episodes from odontogenic origin [77], the need for managing patients with non-HNCs is becoming crucial.
In our review, GDPs reported lower proficiency in giving advice to CPs [24, 53] and were reportedly less comfortable performing complex dental procedures for such patients [57], in comparison to SDs [38, 42, 64]. However, after implementation of education programs in Texas [78, 79], there was a notable change, with majority (91%) of GDPs in recent local survey providing dental treatment to patients undergoing CTx or with a history of cancer [71]. This highlights the benefits of cancer-specific training.
Our review found where dental treatment is dictated by third parties (such as insurance companies or government agencies), dental care in patients with non-HNCs was not deemed as a necessity. Previous research also found 56% of cancer centres did not have a dental department [11]. This is despite research demonstrating a 26% reduction of oral complications following implementation of dental services to patients with non-HNCs [3]. Furthermore, research demonstrated that dental intervention reduced blood stream infection in patients following allogenic HSCT [80], decreased incidence of osteonecrosis in patients with bone metastases treated with bisphosphonates [81], and lowered risk of mucositis in breast CPs undergoing chemotherapy [82].
Dentists working in the community have an important role in cancer care. A hospital-based dental intervention demonstrated positive outcomes in reducing adverse oral side effects in patients with HSCT, however there was a 86% drop-out rate at the 3-month follow-up due to the distance patients were required to travel to the hospital [2]. This highlights the need for accessible dental care closer to patients’ home, ensuring continuity of care and leveraging existing rapport with their dentists during cancer treatment. Our review found that 92.5% of dentists were willing to receive further training on managing CPs. This affirms Fantozzi et al. [16] view that adequately trained dentists working in the community are critical to providing safe and effective oral care for CPs.
A limitation of this study is that most literature focused on HNCs, resulting in over-representation of HNCs. Given this study is on patients with all forms of cancer, it might not reflect dentists’ perspective on managing CPs more broadly. Secondly, a lack of standardised questionnaires in assessing outcomes across studies could leading to challenges in drawing consensus results. Furthermore, utilising study-specific and non-validated questionnaires can lead to misinterpretation of the results. For example, Martins et al. [61] assumed “radiation-related caries leads to osteonecrosis” while in fact, it is the extraction of carious teeth in the irradiated bone rather than having caries that leads to osteonecrosis of the jaw. In another study [59], the authors introduced uncommon terminologies “initiating” and “promoting” factors for OC and pointed out participants could not differentiate between the two. This can result in potentially inaccurate data being collected. Lastly, no studies was excluded based on their quality or biases. Results were based on data reported by the studies.
Conclusion
This review highlights the paucity of research related to dentists’ knowledge, perception, practice and confidence in treating CPs outside of H&N regions. There is a need for future studies to understand barriers that hinder dental involvement with oncology patients and to identify strategies to facilitate clinical practice amongst dentists to be in alignment with advancement in cancer treatments.
Supplementary Information
Acknowledgments
Code availability
Not applicable
Authors’ contributions
All authors (SL, AH and JS) were involved in conceptualising the review. SL carried out initial database searches. SL and JS screened initial results and articles that met inclusion criteria. Any disagreements were resolved with AH. All authors contributed to quality review of a subset of included reviews. SL wrote the first draft of the manuscript. JS and AH made contributions to subsequent drafts. All authors read and approved the final manuscript.
Funding
The authors did not receive any funding for this systematic review.
Availability of data and materials
These are submitted as supplementary files.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Borowski B, Benhamou E, Pico JL, Laplanche A, Margainaud JP, Hayat M. Prevention of oral mucositis in patients treated with high-dose chemotherapy and bone marrow transplantation: a randomised controlled trial comparing two protocols of dental care. Eur J Cancer B Oral Oncol. 1994;30B(2):93–7. [DOI] [PubMed] [Google Scholar]
- 2.Gurgan CA, Ozcan M, Karakus O, Zincircioglu G, Arat M, Soydan E, et al. Periodontal status and post-transplantation complications following intensive periodontal treatment in patients underwent allogenic hematopoietic stem cell transplantation conditioned with myeloablative regimen. Int J Dent Hyg. 2013;11(2):84–90. [DOI] [PubMed] [Google Scholar]
- 3.Sonis S, Kunz A. Impact of improved dental services on the frequency of oral complications of cancer therapy for patients with non-head-and-neck malignancies. Oral Surg Oral Med Oral Pathol. 1988;65(1):19–22. [DOI] [PubMed] [Google Scholar]
- 4.Nunez-Aguilar J, Oliveros-Lopez LG, Fernandez-Olavarria A, Torres-Lagares D, Serrera-Figallo MA, Gutierrez-Corrales A, et al. Influence of dental treatment in place on quality of life in oral cancer patients undergoing chemoradiotherapy. Med Oral Patol Oral Cir Bucal. 2018;23(4):e498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sadler GR, Stoudt A, Fullerton JT, Oberle-Edwards LK, Nguyen Q, Epstein JB. Managing the oral sequelae of cancer therapy. Medsurg Nurs. 2003;12(1):28–36. [PubMed] [Google Scholar]
- 6.Elad S, Thierer T, Bitan M, Shapira MY, Meyerowitz C. A decision analysis: the dental management of patients prior to hematology cytotoxic therapy or hematopoietic stem cell transplantation. Oral Oncol. 2008;44(1):37–42. [DOI] [PubMed] [Google Scholar]
- 7.Smith DK, Castellanos EH, Murphy BA. Financial and socio-economic factors influencing pre- and post-cancer therapy oral care. Support Care Cancer. 2018;26(7):2143–8. [DOI] [PubMed] [Google Scholar]
- 8.Epstein JB, Guneri P, Barasch A. Appropriate and necessary oral care for people with cancer: guidance to obtain the right oral and dental care at the right time. Support Care Cancer. 2014;22(7):1981–8. [DOI] [PubMed] [Google Scholar]
- 9.Elad S, Cheng KKF, Lalla RV, Yarom N, Hong C, Logan RM, et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer. 2020;126(19):4423–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilberg P, Hjermstad MJ, Ottesen S, Herlofson BB. Chemotherapy-associated oral sequelae in patients with cancers outside the head and neck region. J Pain Symptom Manage. 2014;48(6):1060–9. [DOI] [PubMed] [Google Scholar]
- 11.Epstein JB, Parker IR, Epstein MS, Gupta A, Kutis S, Witkowski DM. A survey of National Cancer Institute-designated comprehensive cancer centers’ oral health supportive care practices and resources in the USA. Support Care Cancer. 2007;15(4):357–62. [DOI] [PubMed] [Google Scholar]
- 12.de Paula Novaes C, e Silva RT, Coelho ÂM, Fabri GMC, Chaves MdGAM. Orofacial complaints and complications of chemotherapy. Arch Oncol. 2017;23(1):9–14.
- 13.Soutome S, Otsuru M, Hayashida S, Murata M, Yanamoto S, Sawada S, et al. Relationship between tooth extraction and development of medication-related osteonecrosis of the jaw in cancer patients. Sci Rep. 2021;11(1):17226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nifosi AF, Zuccarello M, Nifosi L, HervasSaus V, Nifosi G. Osteonecrosis of the jaw in the era of targeted therapy and immunotherapy in oncology. J Korean Assoc Oral Maxillofac Surg. 2019;45(1):3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Georgakopoulou E, Stebbing J, Scully C. Targeted cancer therapies: Oral health care implications. J Am Dent Assoc. 2018;149(2):100–11. [DOI] [PubMed] [Google Scholar]
- 16.Fantozzi PJ, Monopoli M, Villa A. Oral Oncology and Oral Medicine Fellowship for the General Dentist. Multidisciplinary Digital Publishing Institute Proceedings. 2019. 10.3390/proceedings2019035023.
- 17.Haverman TM, Raber-Durlacher JE, Raghoebar II, Rademacher WMH, Rozema FR, Hazenberg MD, et al. Oral chronic graft-versus-host disease: What the general dental practitioner needs to know. J Am Dent Assoc. 2020;151(11):846–56. [DOI] [PubMed] [Google Scholar]
- 18.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hong QN, Gonzalez-Reyes A, Pluye P. Improving the usefulness of a tool for appraising the quality of qualitative, quantitative and mixed methods studies, the Mixed Methods Appraisal Tool (MMAT). J Eval Clin Pract. 2018;24(3):459–67. [DOI] [PubMed] [Google Scholar]
- 20.Wong F, Toljanic JA. A survey of clinicians: prioritization of dental treatment in leukemia patients prior to chemotherapy. Int J Prosthodont. 2009;22(3):303–6. [PubMed] [Google Scholar]
- 21.Dang RR, Brar B, Pasco JM, Rebhun C, Sohn W, Salama A. Dental practice patterns for oral care in medical oncology patients-a survey-based assessment of massachusetts dentists. J Cancer Educ. 2022;37(3):555–60. [DOI] [PubMed] [Google Scholar]
- 22.Wright DA, Porter SA, Kumar N, Moles DR. An overview of the provision of oral health care for patients with oncological disease attending salaried primary dental care services in England. J Disabil Oral Health. 2011;12(1):10–6. [Google Scholar]
- 23.Daley E, DeBate R, Dodd V, Dyer K, Fuhrmann H, Helmy H, et al. Exploring awareness, attitudes, and perceived role among oral health providers regarding HPV-related oral cancers. J Public Health Dent. 2011;71(2):136–42. [DOI] [PubMed] [Google Scholar]
- 24.Dixon H, Thomson W, Ting G. Dentists’ knowledge and experiences of treating patients with Head and Neck Cancer. New Zealand Dent J. 2021;117(1):15-21.
- 25.Alqutaibi AY, Borzangy S, Al-Maweri SA, Aboalrejal A. Early detection of oral cancer and potentially malignant disorders: Experiences, practices, and beliefs of prosthodontists practicing in Saudi Arabia. J Prosthet Dent. 2021;126(4):569–74. [DOI] [PubMed] [Google Scholar]
- 26.Cruz GD, Ostroff JS, Kumar JV, Gajendra S. Preventing and detecting oral cancer. Oral health care providers’ readiness to provide health behavior counseling and oral cancer examinations. J Am Dent Assoc. 2005;136(5):594–601; quiz 81–2. [DOI] [PMC free article] [PubMed]
- 27.Kujan O, Duxbury AJ, Glenny AM, Thakker NS, Sloan P. Opinions and attitudes of the UK’s GDPs and specialists in oral surgery, oral medicine and surgical dentistry on oral cancer screening. Oral Dis. 2006;12(2):194–9. [DOI] [PubMed] [Google Scholar]
- 28.LeHew CW, Epstein JB, Kaste LM, Choi YK. Assessing oral cancer early detection: clarifying dentists’ practices. J Public Health Dent. 2010;70(2):93–100. [DOI] [PubMed] [Google Scholar]
- 29.Marino R, Haresaku S, McGrath R, Bailey D, McCullough M, Musolino R, et al. Oral cancer screening practices of oral health professionals in Australia. BMC Oral Health. 2017;17(1):151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seoane J, Warnakulasuriya S, Varela-Centelles P, Esparza G, Dios PD. Oral cancer: experiences and diagnostic abilities elicited by dentists in North-western Spain. Oral Dis. 2006;12(5):487–92. [DOI] [PubMed] [Google Scholar]
- 31.Strey JR, Roxo-Goncalves M, Guzenski BD, Martins MAT, Romanini J, de Figueiredo MAZ, et al. Oral medicine experience and attitudes toward oral cancer: an evaluation of dentists working in primary health care. J Cancer Educ. 2022;37(6):1621–8. [DOI] [PubMed] [Google Scholar]
- 32.Ahmed NHM, Naidoo S. Oral cancer knowledge, attitudes, and practices among dentists in Khartoum State. Sudan J Cancer Educ. 2019;34(2):291–6. [DOI] [PubMed] [Google Scholar]
- 33.Akbari N, Raeesi V, Khazaei T, Ramezanzadeh K, Ebrahimipour S. Evaluation of general dentists’ and dental specialists’ knowledge about oral cancer in South Khorasan-Iran 2014. Asian Pac J Cancer Prev. 2015;16(16):6987–90. [DOI] [PubMed] [Google Scholar]
- 34.Alhazzazi TY. Knowledge and behavioral assessment of dentists toward screening and managing patients with head and neck cancer in Saudi Arabia. Niger J Clin Pract. 2021;24(5):735–46. [DOI] [PubMed] [Google Scholar]
- 35.Alonge OK, Narendran S. Oral cancer knowledge and practices of dentists along the Texas-Mexico border. J Cancer Educ. 2004;19(1):6–11. [DOI] [PubMed] [Google Scholar]
- 36.Alqahtani AS, Alshamrani Y, Alhazmi Y, Halboub E. Oral and dental complications of radiotherapy for head and neck cancer: knowledge of dental practitioners in Saudi Arabia. Asian Pac J Cancer Prev. 2021;22(7):2033–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borhan-Mojabi K, Moradi A, Yazdabadi A. Evaluating the degree of knowledge on oral cancer among general practitioners and dentists in Qazvin. J Eval Clin Pract. 2012;18(2):498–501. [DOI] [PubMed] [Google Scholar]
- 38.Calvert G, Barclay SC, Owens JS, Alani A. A national survey of restorative consultants’ treatment provision for head and neck oncology patients. Br Dent J. 2014;217(10):E21. [DOI] [PubMed] [Google Scholar]
- 39.Canto MT, Drury TF, Horowitz AM. Maryland dentists’ knowledge of oral cancer risk factors and diagnostic procedures. Health Promotion Practice. 2001;2(3):255–62. [Google Scholar]
- 40.Clovis JB, Horowitz AM, Poel DH. Oral and pharyngeal cancer: knowledge and opinions of dentists in British Columbia and Nova Scotia. J Can Dent Assoc. 2002;68(7):415–20. [PubMed] [Google Scholar]
- 41.Colella G, Gaeta GM, Moscariello A, Angelillo IF. Oral cancer and dentists: knowledge, attitudes, and practices in Italy. Oral Oncol. 2008;44(4):393–9. [DOI] [PubMed] [Google Scholar]
- 42.Dewan K, Kelly RD, Bardsley P. A national survey of consultants, specialists and specialist registrars in restorative dentistry for the assessment and treatment planning of oral cancer patients. Br Dent J. 2014;216(12):E27. [DOI] [PubMed] [Google Scholar]
- 43.Ekici ö. Knowledge and attitudes of the dentists regarding oral cancer in Ankara, Turkey. Turk J Oncol. 2020;35(4):373–9.
- 44.Gajendra S, Cruz GD, Kumar JV. Oral cancer prevention and early detection: knowledge, practices, and opinions of oral health care providers in New York State. J Cancer Educ. 2006;21(3):157–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hashim R, Abo-Fanas A, Al-Tak A, Al-Kadri A, Abu Ebaid Y. Early detection of oral cancer- dentists’ knowledge and practices in the United Arab Emirates. Asian Pac J Cancer Prev. 2018;19(8):2351–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Joseph BK, Sundaram DB, Sharma P. Oral cancer awareness among dentists in Kuwait. Med Princ Pract. 2012;21(2):164–70. [DOI] [PubMed] [Google Scholar]
- 47.Maybury C, Horowitz AM, Yan AF, Green KM, Wang MQ. Maryland dentists’ knowledge of oral cancer prevention and early detection. J Calif Dent Assoc. 2012;40(4):341–50. [PubMed] [Google Scholar]
- 48.McCann MF, Macpherson LM, Binnie VI, Stephen KW. A survey of Scottish primary care dental practitioners’ oral cancer-related practices and training requirements. Community Dent Health. 2000;17(1):24–30. [PubMed] [Google Scholar]
- 49.Pavao Spaulonci G, Salgado de Souza R, Gallego Arias Pecorari V, Lauria Dib L. Oral cancer knowledge assessment: Newly graduated versus senior dental clinicians. Int J Dent. 2018;2018:9368918. [DOI] [PMC free article] [PubMed]
- 50.Saleh A, Kong YH, Vengu N, Badrudeen H, Zain RB, Cheong SC. Dentists’ perception of the role they play in early detection of oral cancer. Asian Pac J Cancer Prev. 2014;15(1):229–37. [DOI] [PubMed] [Google Scholar]
- 51.Yellowitz J, Horowitz AM, Goodman HS, Canto MT, Farooq NS. Knowledge, opinions and practices of general dentists regarding oral cancer: a pilot survey. J Am Dent Assoc. 1998;129(5):579–83. [DOI] [PubMed] [Google Scholar]
- 52.Fidele NB, Patrick SMN, Okonji OC, Kazadi EK. Oral cancer awareness and knowledge: Survey of dentists in Democratic Republic of the Congo. J Cancer Policy. 2022;32:100332. [DOI] [PubMed] [Google Scholar]
- 53.Frydrych A, Slack-Smith L, Park J, Smith A. Expertise regarding dental management of oral cancer patients receiving radiation therapy among Western Australian dentists. Open Dent J. 2012;6:197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guneri P, Cankaya H, Kaya A, Boyacioglu H. Turkish dentists’ knowledge of head and neck cancer therapy-related complications: implications for the future. Eur J Cancer Care (Engl). 2008;17(1):84–92. [DOI] [PubMed] [Google Scholar]
- 55.Haresaku S, Makino M, Sugiyama S, Naito T, Marino RJ. Comparison of practices, knowledge, confidence, and attitude toward oral cancer among oral health professionals between Japan and Australia. J Cancer Educ. 2018;33(2):429–35. [DOI] [PubMed] [Google Scholar]
- 56.Horowitz AM, Drury TF, Goodman HS, Yellowitz JA. Oral pharyngeal cancer prevention and early detection. Dentists’ opinions and practices. J Am Dent Assoc. 2000;131(4):453–62. [DOI] [PubMed]
- 57.Husein AB, Butterworth CJ, Ranka MS, Kwasnicki A, Rogers SN. A survey of general dental practitioners in the North West of England concerning the dental care of patients following head and neck radiotherapy. Prim Dent Care. 2011;18(2):59–65. [DOI] [PubMed] [Google Scholar]
- 58.Kogi S, DaSilva J, Mikasa Y, Lee C, Ishikawa-Nagai S, Yang Q, et al. Knowledge and practice of oral cancer screening in teaching faculty-comparison of specialty and year of clinical experience. J Cancer Educ. 2019;34(3):455–62. [DOI] [PubMed] [Google Scholar]
- 59.Leao JC, Goes P, Sobrinho CB, Porter S. Knowledge and clinical expertise regarding oral cancer among Brazilian dentists. Int J Oral Maxillofac Surg. 2005;34(4):436–9. [DOI] [PubMed] [Google Scholar]
- 60.Lopez-Jornet P, Camacho-Alonso F, Molina-Minano F. Knowledge and attitudes about oral cancer among dentists in Spain. J Eval Clin Pract. 2010;16(1):129–33. [DOI] [PubMed] [Google Scholar]
- 61.Martins B, Palmier NR, Prado-Ribeiro AC, de Goes MF, Lopes MA, Brandao TB, et al. Awareness of the risk of radiation-related caries in patients with head and neck cancer: A survey of physicians, dentists, and patients. Oral Surg Oral Med Oral Pathol Oral Radiol. 2021;132(4):398–408. [DOI] [PubMed] [Google Scholar]
- 62.Nazar HS, Ariga J, Shyama M. Oral Cancer Knowledge, Attitudes, and Practices among Newly Graduated Dentists in Kuwait. Asian Pac J Cancer Prev. 2022;23(2):459–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nazar H, Shyama M, Ariga J, El-Salhy M, Soparkar P, Alsumait A. Oral cancer knowledge, attitudes and practices among primary oral health care dentists in Kuwait. Asian Pac J Cancer Prev. 2019;20(5):1531–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nicholls C, Ilankovan V. An audit of oral and dental health regimens practised in the management of oropharyngeal cancer. Br J Oral Maxillofac Surg. 1998;36(1):63–6. [DOI] [PubMed] [Google Scholar]
- 65.Patel Y, Bahlhorn H, Zafar S, Zwetchkenbaum S, Eisbruch A, Murdoch-Kinch CA. Survey of Michigan dentists and radiation oncologists on oral care of patients undergoing head and neck radiation therapy. J Mich Dent Assoc. 2012;94(7):34–45. [PubMed] [Google Scholar]
- 66.Patton LL, Ashe TE, Elter JR, Southerland JH, Strauss RP. Adequacy of training in oral cancer prevention and screening as self-assessed by physicians, nurse practitioners, and dental health professionals. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102(6):758–64. [DOI] [PubMed] [Google Scholar]
- 67.Reed SG, Cartmell KB, Duffy NG, Wahlquist AE, Sinha D, Hollinger A, et al. Oral cancer preventive practices of South Carolina dentists and physicians. J Cancer Educ. 2010;25(2):166–73. [DOI] [PubMed] [Google Scholar]
- 68.Seals R. Dental care for cancer patients: a survey of Texas dental school graduates. Texas Dental Journal. 1990;107(6):17-20. [PubMed]
- 69.Shadid RM, Habash G. Knowledge, Opinions, and Practices of Oral Cancer Prevention among Palestinian Practicing Dentists: An Online Cross-Sectional Questionnaire. Healthcare (Basel). 2023;11(7):1005. [DOI] [PMC free article] [PubMed]
- 70.Taheri JB, Namazi Z, Azimi S, Mehdipour M, Behrovan R, Rezaei Far K. Knowledge of oral precancerous lesions considering years since graduation among dentists in the capital city of Iran: a pathway to early oral cancer diagnosis and referral? Asian Pac J Cancer Prev. 2018;19(8):2103–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tami-Maury I, Wagh AJ, Abou Khalil NE, Gritz ER, Chambers MS. Dental care in Texas: An opportunity for implementing a comprehensive and patient-centric approach with special emphasis on cancer patients and survivors. Tex Dent J. 2016;133(6):364–73. [PubMed] [Google Scholar]
- 72.Vijay Kumar KV, Suresan V. Knowledge, attitude and screening practices of general dentists concerning oral cancer in Bangalore city. Indian J Cancer. 2012;49(1):33–8. [DOI] [PubMed] [Google Scholar]
- 73.Lotlikar V, Kedar U, Shidhaye S, Kadam V. pH-responsive dual pulse multiparticulate dosage form for treatment of rheumatoid arthritis. Drug Dev Ind Pharm. 2010;36(11):1295–302. [DOI] [PubMed] [Google Scholar]
- 74.Frantsve-Hawley J, Rindal DB. Translational research: bringing science to the provider through guideline implementation. Dent Clin North Am. 2019;63(1):129–44. [DOI] [PubMed] [Google Scholar]
- 75.Cane J, O’Connor D, Michie S. Validation of the theoretical domains framework for use in behaviour change and implementation research. Implement Sci. 2012;7:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Souza ESVC, Oliveira VC, Sousa AFL, Bim FL, Macedo AP, Andrade D, et al. Prevalence and susceptibility profile of Candida spp. isolated from patients in cancer therapy. Arch Oral Biol. 2020;119:104906. [DOI] [PubMed]
- 77.Hong CH, Napenas JJ, Hodgson BD, Stokman MA, Mathers-Stauffer V, Elting LS, et al. A systematic review of dental disease in patients undergoing cancer therapy. Support Care Cancer. 2010;18(8):1007–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rankin KV. Preserving oral health in cancer therapy. Tex Dent J. 2004;121(6):468–9. [PubMed] [Google Scholar]
- 79.https://www.texascancer.info/tcplan/goal3/goal3_objb.html. [Web page]. Texas Cancer Council [Available from: https://www.texascancer.info/tcplan/goal3/goal3_objb.html.
- 80.Suwabe T, Fuse K, Katsura K, Soga M, Katagiri T, Shibasaki Y, et al. Intensive oral care can reduce bloodstream infection with coagulase-negative staphylococci after neutrophil engraftment in allogeneic hematopoietic stem-cell transplantation. Support Care Cancer. 2022;30(1):475–85. [DOI] [PubMed] [Google Scholar]
- 81.Ripamonti CI, Maniezzo M, Campa T, Fagnoni E, Brunelli C, Saibene G, et al. Decreased occurrence of osteonecrosis of the jaw after implementation of dental preventive measures in solid tumour patients with bone metastases treated with bisphosphonates. The experience of the National Cancer Institute of Milan. Ann Oncol. 2009;20(1):137–45. [DOI] [PubMed]
- 82.Saito H, Watanabe Y, Sato K, Ikawa H, Yoshida Y, Katakura A, et al. Effects of professional oral health care on reducing the risk of chemotherapy-induced oral mucositis. Support Care Cancer. 2014;22(11):2935–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
These are submitted as supplementary files.