Abstract
The educational landscape of toxicology is increasingly integrating computational methodologies due to ethical concerns about animal testing and advancements in biotechnological and data analysis tools. This paper examines the evolution and significance of the Toxicology in the 21st century (Tox21) initiative and its impact on computational toxicology education. It contrasts computational toxicology with traditional methods, highlighting the limitations of conventional approaches and the new perspectives offered by computational techniques. The study emphasizes the importance of incorporating computational toxicology into curricula, including case studies that demonstrate how this integration enhances students’ problem-solving abilities, real-time data analysis skills, and innovation capabilities. Furthermore, it outlines effective teaching content and methods, including software tools, online resources, and academic literature. The paper also addresses the challenges and limitations faced in this educational shift and explores prospects for advancing computational toxicology education. By documenting these developments, the study aims to clarify the current advancements in toxicology education and the preparedness of students to address global chemical safety challenges with innovative solutions.
Keywords: Computational toxicology, Toxicology education, Risk assessment, High-throughput screening, Machine learning in toxicology
Introduction
Toxicology is a compulsory course for undergraduate and graduate students majoring in Preventive Medicine at public health schools or Pharmacology at pharmacy colleges. Additionally, some students in the fields of medicine, life sciences, biology, and molecular sciences often choose toxicology as an elective course. Toxicology is an experimental laboratory science that traditionally relies on animal experiments and laboratory testing. However, these methods are costly and time-consuming. Furthermore, due to ethical and efficacy considerations, animal experiments are facing increasing restrictions today [1, 2]. Moreover, the rapid development of biotechnology and big data analysis has provided new research methods and tools for toxicology, bringing unprecedented challenges and opportunities to toxicology education [3]. The publication of the National Research Council (NRC) report titled “Toxicity Testing in the 21st Century” ushered toxicology into a new phase by expanding the application of high-throughput in vitro screening and computer simulation methods to assess potential health risks from environmental factors [4, 5].
Computational toxicology integrates chemical information, biomolecular data, and relevant biostatistical methods to conduct computer-assisted assessments of the potential toxicity of chemical substances [6, 7]. The importance of this field lies in its ability to provide a relatively fast and cost-effective way to predict the toxicity of chemical substances. Moreover, this approach aids in the screening of potentially toxic compounds, thereby reducing the number and scope of laboratory experiments while generating more accurate risk assessment results [6]. Through computational models, researchers can assess the toxicity risks of chemical substances without conducting actual exposure experiments, which is highly important for public health and environmental protection. Moreover, with the advancement of machine learning and artificial intelligence technologies, computational toxicology is expected to achieve higher levels of automation and accuracy, providing more powerful tools for toxicological research and education [8–10]. Due to these ongoing advancements, today’s toxicology education programs must adapt and integrate various computational tools to equip students with innovative abilities and a glimpse into the future while learning the fundamentals of toxicology.
Tox21 and computational toxicology
The evolution and significance of Tox21
Tox21, which stands for Toxicology in the 21st century, signifies a pivotal shift from traditional toxicology to a modern approach based on technological and data-driven assessments. Traditional toxicology relies on animal testing and long-term biomonitoring, which are time-consuming and costly methods, to evaluate the safety of chemical substances. The emergence of Tox21 addresses the challenges posed by the rapid increase in the number of new chemicals and the accelerated demand for their assessment in modern society. High-throughput screening (HTS) technologies, bioinformatics, and computational models are utilized to predict the potential risks of chemicals to human health and the environment. The aim of Tox21 is to rapidly identify toxic chemicals and understand their mechanisms of action through these modern techniques, thus providing scientific evidence for risk assessment and chemical management. The essence of this emerging field is the integration of data and results, employing computational models to forecast the toxicity of chemical substances, which is indispensable in areas such as environmental protection, public health, and drug development.
Key concepts and applications of computational toxicology
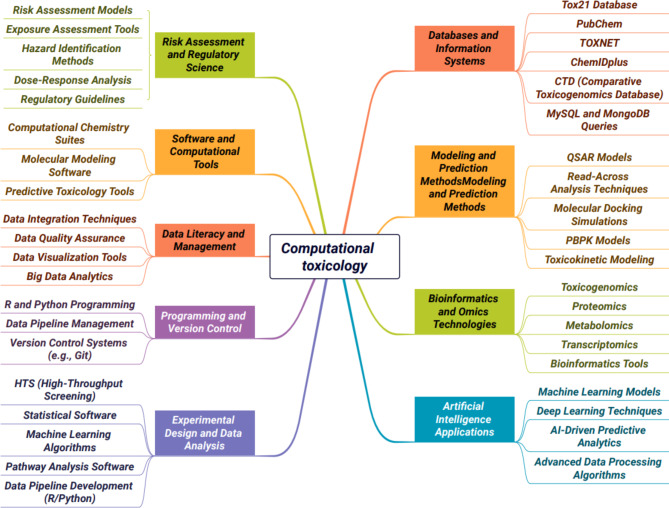
Within the domain of Tox21, computational toxicology plays a pivotal role. Computational toxicology leverages advanced technologies to predict the toxic effects of chemicals, with applications in developing databases, quantitative structure-activity relationship (QSAR) models, in vivo and in vitro models, and toxicity pathway analysis (Fig. 1). These methods enhance toxicological research by reducing animal testing and accelerating safety evaluations. The growing demand for professionals skilled in these methods underscores the importance of education in this field, equipping students with both theoretical knowledge and practical skills to address future challenges in toxicological research and chemical safety.
Fig. 1.
Summary of keywords used in computational toxicology. Tox21: Toxicology in the 21st Century. QSAR: Quantitative Structure-Activity Relationship. AI: Artificial Intelligence
Comparison of computational toxicology and traditional toxicology education
Limitations of traditional toxicology education
Traditional toxicology education is primarily based on in vivo experiments and biochemical analyses conducted in the laboratory. This teaching method has produced generations of toxicologists who can assess the toxicity of compounds through experience and direct experimentation. However, this education model has several limitations that cannot be overlooked (Table 1). First, in public health schools, undergraduate students tend to choose disciplines that do not involve complex or cumbersome experimental procedures, such as epidemiology, statistics, health economics and management, and social medicine [11]. Second, toxicology experiments often involve high costs and time-consuming processes, greatly limiting the scale and speed of research. The biological differences between animal models and humans introduce uncertainties when extrapolating the results directly to humans [2]. Furthermore, ethical issues restrict the use of laboratory animals, and the tolerance of public and regulatory agencies for animal experiments is decreasing [1]. Therefore, alternative research and teaching methods that can replace traditional animal experiments are urgently needed to cultivate better toxicologists in the future.
Table 1.
Comparison of computational toxicology and traditional toxicology education
| Terms | Traditional Toxicology Education Limitations | Computational Toxicology Opportunities |
|---|---|---|
| Educational Resources and Methods |
a) Limited Hands-On Exposure b) Textbook-Dependent Learning c) Static Learning Materials d) One-Size-Fits-All Curriculum |
a) Interactive Simulation Tools b) Personalized Learning Platforms c) Dynamic Course Content |
| Experimental Constraints |
a) Animal Use Ethical Concerns b) High Cost of Lab Experiments c) Low Throughput Experimentation d) Limited Real-World Scenarios |
a) In Silico Model Systems b) Reduced Animal Testing c) High-Throughput Data Generation d) Diverse Exposure Scenarios Simulation |
| Data Analysis and Interpretation |
a) Simplistic Data Analysis b) Limited Statistical Training c) Overlooked Molecular Mechanisms |
a) Advanced Data Analytics b) Integrated ‘Omics’ Analysis c) Systems Biology Approaches |
| Regulatory and Risk Assessment |
a) Outdated Regulatory Frameworks b) Limited Risk Assessment Case Studies |
a) Innovative Regulatory Science Education b) Computational Risk Assessment Models |
| Global and Interdisciplinary Integration |
a) Cultural Context Neglect b) Narrow Interdisciplinary Links |
a) Global Toxicology Perspectives b) Cross-Disciplinary Collaborations |
| Research and Innovation |
a) Constrained Research Budgets b) Limited Industry Collaboration |
a) Expanded Research Opportunities b) Public‒Private Partnerships |
New perspectives brought by computational toxicology
As an interdisciplinary field, computational toxicology integrates chemistry, biology, computer science, and data analysis [12]. This requires education to go beyond traditional skills, incorporating computational modeling and bioinformatics. New research perspectives, such as systems biology and big data, provide innovative ways to explore toxicity mechanisms and predict risks, helping shape the future of toxicological assessments. By adopting these new approaches, scientists can more effectively assess untested chemicals and advance the field [13].
Importance of computational toxicology in education
Case studies in computational toxicology education
The University of California, Berkeley, and the University of Michigan have been at the forefront of integrating computational toxicology into their curricula, thereby preparing students to meet the challenges of modern toxicology. At Berkeley, foundational toxicology courses have been infused with computational toxicology content since 2001, and in 2006, a dedicated undergraduate course was launched to deepen students’ expertise in this area. The University of Michigan has similarly incorporated computational toxicology into its toxicology courses from the early 2000s and inaugurated a specialized graduate course in 2012 [14]. These educational initiatives aim to equip students with a solid foundation in the principles of computational toxicology and to acquaint them with cutting-edge predictive toxicology techniques that leverage chemical structures, toxicity-related databases, and biological systems and pathway tools.
In these courses, case studies serve as a critical pedagogical tool, enabling students to apply computational techniques and mathematical models to analyze, model, and predict potential toxic effects. Specifically, the learning objectives of these modules include:
Understanding the role of computational methods in toxicological research and risk assessment.
Developing proficiency in using computational tools to analyze chemical structures and their biological interactions.
Learning to navigate and extract data from toxicity-related databases for predictive modeling.
Applying computational approaches to investigate the mechanistic basis of toxic effects and disease pathways.
Designing and evaluating in silico experiments to test hypotheses in toxicology.
Enhancing students’ ability to solve complex problems
The integration of computational toxicology into education serves as a crucial complement to traditional toxicology education models. With the increasing variety and quantity of chemicals and growing concerns about the environment and human health, toxicology issues have become more complex. By introducing computational methods, students can utilize quantitative models, big data, and machine learning techniques to analyze and predict the toxicity of compounds. This approach greatly enhances their ability to handle and solve complex and ever-changing problems [10, 15].
Providing training in real-time data analysis and model development
Computational toxicology education teaches students how to perform real-time data analysis and construct and validate predictive models. These skills are vital in contemporary toxicology research, as are their applications in drug development, public health, and environmental protection. Students learn how to handle complex datasets, employ statistical and computational tools for data analysis, and transform data into reliable toxicity predictions [16].
Promoting critical thinking and innovation skills development
Computational toxicology education goes beyond skill development; it also encourages students to cultivate critical thinking and innovation skills. Faced with evolving data and emerging scientific issues, students need the ability to question the status quo, propose new hypotheses, and devise novel approaches. This mode of thinking is crucial for scientific progress, particularly in the pursuit of safer and more sustainable chemicals and drugs [17].
Teaching content and methods in computational toxicology
Teaching content
Basic principles and concepts
Students will first learn the fundamental principles and key concepts of biology, chemistry, biochemistry, and toxicology, including molecular mechanisms of toxicity, dose‒response relationships, and the bioaccumulation and persistence of chemicals. In addition, students need to understand the basic statistical principles and the foundations of the bioinformatics used in computational toxicology. This includes introductory courses in computer science and data analysis so that students can handle and interpret toxicological data effectively.
Data literacy and programming skills
With the increasing demand for informatics and data engineering in computational toxicology, data literacy and programming skills are essential core competencies [18]. This includes proficiency in databases, modeling methods, experimental design tools, and data management. To address future challenges, students must master data integration, quality assurance, and visualization techniques. Programming languages such as R and Python are crucial for developing and managing data pipelines, while version control systems like Git are important for facilitating research collaboration and ensuring reproducibility. Artificial intelligence technologies, particularly machine learning and deep learning, provide powerful support for data processing and predictive modeling, enhancing the accuracy and efficiency of research. Integrating these skills into the educational framework will ensure that students are equipped with the comprehensive capabilities needed to succeed in the evolving field of computational toxicology.
Use of computational models and software tools
The computational toxicology course will cover various predictive models and their applications, including Quantitative Structure-Activity Relationship (QSAR), molecular docking, and dynamic simulations. Students will learn how to use professional software and tools to assess the toxicity of chemicals. The following table lists recommended models and tools, incorporating not only traditional QSAR tools but also the latest tools and data literacy resources (Table 2). These tools and resources include various modern data processing packages, databases, and APIs that will help students handle complex toxicity data and perform advanced analyses. By utilizing these tools, students will enhance their computational toxicology skills and be well-prepared for future research and professional development.
Table 2.
Recommended computational models and software tools
| Category | Tool/Resource | Description |
|---|---|---|
| Predictive Models | QSAR | Classic Quantitative Structure-Activity Relationship models used for predicting the biological activity and toxicity of chemicals. [19] |
| Molecular Docking | Molecular docking simulations used to predict interactions between chemicals and biological targets. [20] | |
| Dynamic Simulations | Dynamic simulations used to study the behavior and effects of chemicals within biological systems. [21] | |
| Professional Software and Tools | OpenTox | A computational toxicology platform for predicting chemical toxicity. [22] |
| ADMET Predictor | Evaluates absorption, distribution, metabolism, excretion, and toxicity of chemicals. [23] | |
| VEGA | Virtual Environment for Genomics Applications (VEGA), used for toxicity predictions. [24] | |
| Databases and Resources | CompTox Chemicals Dashboard | EPA’s chemical information database for retrieving and analyzing toxicology data. [25] |
| httk Package | An R package for processing and analyzing high-throughput screening data. [26] | |
| tcpl Package | An R package for processing ToxCast data for toxicity predictions. [27] | |
| Various APIs | Application Programming Interfaces (APIs) for accessing and integrating toxicology data. |
Case studies and data analysis
Case-based teaching was introduced by Clyde Freeman Herreid in the late 1990s as a way to teach science by fostering content mastery and analytical skills [28]. Compared to traditional teacher-centered instruction, case studies are more effective at developing students’ noncognitive abilities, such as oral communication skills [29]. Integrating case-based teaching into computational toxicology education helps students better understand relevant concepts and enhances their critical thinking abilities [29]. For example, students may analyze the toxicological data of a drug molecule or assess the potential health impacts of environmental pollutants. Case studies not only increase the practicality of learning but also strengthen students’ skills in data processing and interpretation.
Teaching methods
Interactive lectures and flipped classroom
The teaching of computational toxicology will employ interactive lectures and a flipped classroom approach. A flipped classroom is a teaching strategy that reverses the traditional model of knowledge delivery and homework, allowing students to independently study theoretical knowledge outside the classroom. Classroom time is then primarily dedicated to interactive discussions and practical applications, thereby increasing student engagement and learning effectiveness [30].
Laboratory simulations and virtual experiments
Due to the heavy reliance on computers and software tools in computational toxicology, laboratory simulations and virtual laboratory techniques will be widely used in teaching. These techniques provide a risk-free environment for students when designing experiments and analyzing results in the absence of actual chemicals or biological samples.
Group projects and case discussions
Group projects and case discussions will be used to enhance students’ collaborative skills and communication abilities. Students will work in groups to solve real-world toxicology problems by applying the computational toxicology knowledge and skills they have learned. Group discussions and case analyses help students understand different perspectives and learn how to effectively express and synthesize information within a team.
Applications of computational toxicology in teaching
Application 1: compound toxicity prediction and screening
Computational toxicology plays an important role in the prediction and screening of compound toxicity. The teaching design will focus on developing students’ abilities to use computational methods to predict and screen potential toxic substances.
First, students will learn basic toxicology knowledge and use computational toxicology tools. These authors explored different computational toxicology methods, including compound descriptor calculations and training and prediction of machine learning models. Subsequently, students apply the acquired knowledge to design a virtual toxic screening project. A given compound library was used, and computational toxicology methods were combined for toxicity prediction and screening. Students need to explain their screening results and discuss the potential and limitations of computational toxicology in toxic screening. Throughout this project, students will cultivate practical application skills through the use of computational toxicology tools and methods. The authors will understand the importance of computational toxicology in toxicity screening and how to assess the accuracy and reliability of models.
Applications 2: computational prediction of toxicity mechanisms
Computational toxicology plays a key role in uncovering toxicity mechanisms. The teaching design will guide students in using computational methods to study the mechanism of action of toxins and predict potential targets and toxicity pathways.
Students learn about the chemical properties, biological activities, and toxic effects of toxins and use computational tools to analyze and model relevant data. The authors explored network analysis tools and bioinformatics databases to predict the interactions between toxins and biomolecules and infer toxicity pathways and mechanisms. In this project, students will design a computational toxicology research plan and write a research report. The authors need to describe the research design, analyze the results, draw conclusions, and discuss the importance and application potential of computational toxicology in toxicity mechanism research. Through this project, students will develop the ability to apply computational toxicology methods in studying toxicity mechanisms. These authors will gain an in-depth understanding of the potential and limitations of computational toxicology and learn to integrate computational predictions with experimental results to provide comprehensive explanations of toxicological mechanisms.
Applications 3: drug safety assessment
Computational toxicology plays a significant role in drug safety assessment. The teaching design will help students understand and apply computational toxicology methods to evaluate the potential toxicity and safety of drugs.
Students learn about the absorption, distribution, metabolism, and excretion (ADME) characteristics of drugs and master the use of computational tools for ADME prediction and toxicity assessment. The authors analyzed the interactions between drugs and targets, predicted drug toxicity and pharmacokinetic properties, and evaluated drug safety in different populations. In this project, students will conduct drug safety assessments and write risk assessment reports. The authors need to explain the assessment methods, results, and recommendations, as well as discuss the practical application capabilities of computational toxicology in drug safety assessment. They will learn to collect and analyze relevant data, predict drug toxicity and safety, and provide corresponding risk assessment reports.
The design of these projects will help students gain an in-depth understanding of the importance of computational toxicology in toxicological research and risk assessment. Students will learn to integrate computational methods with experimental results to provide comprehensive toxicity assessment and risk management recommendations. By designing specific toxicology projects, students can apply computational toxicology methods to real-world problems and gain a deeper understanding of the importance and application potential of computational toxicology. Additionally, through teamwork and scientific communication, students develop comprehensive skills and contribute to future toxicological research and risk assessment.
Teaching resources in computational toxicology
Software tools and databases
Software tools and databases are essential resources in computational toxicology education. Software tools such as ADMET Predictor, Gaussian, and Schrödinger enable students to perform molecular modeling, dynamic simulations, and toxicity predictions (Table 3). Databases such as PubChem, Comparative Toxicogenomics Database (CTD), and ChemSpider provide a wide range of chemical property, biological activity, and toxicology data. These resources not only provide basic training with experimental data but also facilitate students’ understanding and research on real-world issues. Additionally, there are knowledge databases that students need to explore further, such as the AOP database. The adverse outcome pathway (AOP) is a conceptual framework that links the molecular, cellular, and organ-level responses of chemical substances to adverse outcomes at the organism level. Mechanism-based detection methods can be used in AOP pathways to systematically assess whether a compound is likely to induce the target adverse reaction [31]. Human transmembrane proteome (HTP) is a knowledge database, which focuses on identifying and understanding mechanisms of human toxicity, offering valuable data for assessing chemical safety [32].
Table 3.
Some publicly available data resource libraries related to computational toxicology
| Database | Data Type | Reference |
|---|---|---|
| PubChem | Structure and properties of chemical substances | [38] |
| ChemSpider | Structure and properties of chemical substances | [39] |
| ToxBase (EU-ToxRisk) | A large integrated European in vitro toxicology project | [40] |
| EURL ECVAM DB-ALM | Database on Alternative Methods | [41] |
| Toxicity ForeCaster (ToxCast) | In vitro medium- and high-throughput screening assay data | [42] |
| Aggregated Computational Toxicology Resource (ACToR) | Toxicological properties of chemicals | [43] |
| OECD eChemPortal | Chemical safety data and regulatory information | [44] |
| OECD QSAR Toolbox | Data for QSAR modeling and read-across | [45] |
| ChEMBL | Bioactivity data for drug-like compounds | [46] |
| US EPA Chemistry Dashboard | Data, models, and information on chemical use and safety | [47] |
|
Toxicity Forecastern (ToxCast) |
Toxicity related In Vitro testing | [48] |
|
Comparative Toxicogenomics Database (CTD) |
Chemical gene interaction | [49] |
|
Integrated Risk Information System (IRIS) |
Health risk assessment report | [50] |
|
Hazardous Substances Data Bank (HSDB) |
Hazardous substances and their impact on human health | [51] |
| International Toxicity Estimates for Risk (ITER) | Toxicity assessment and risk standards | [52] |
|
Toxicology in the 21st Century (Tox21) |
High throughput screening test results | [53] |
|
Integrated Chemical Environment (ICE) |
In Vivo and In Vitro toxicity data | [54] |
|
Gene Expression Omnibus (GEO) |
Gene expression data | [55] |
In addition to these U.S.-based resources, several significant European Union (EU) research initiatives contribute to the global landscape of computational toxicology. For instance, the Animal-free Safety assessment of chemicals: Project cluster for Implementation of novel Strategies (ASPIS) cluster, which includes projects like ONTOX, RISK-HUNT3R, and PrecisionTOX, focuses on advancing alternative methods for chemical safety assessment without relying on animal testing [33, 34]. These initiatives aim to refine and promote computational and in vitro methods, thereby enhancing the predictive capacity of toxicology studies. Another major EU initiative is the PARC (Partnership for the Assessment of Risks from Chemicals) program, which emphasizes the development of next-generation risk assessment tools, integrating exposure data, and leveraging computational models to assess the safety of chemicals more accurately [35]. Additionally, the InSilicoWorld and VPH4Safety projects are part of the EU’s Horizon 2020 and Horizon Europe frameworks, respectively. InSilicoWorld focuses on creating a unified platform for in silico medicine, including tools for toxicological assessments [36], while VPH4Safety is dedicated to developing Virtual Physiological Human models to improve drug safety evaluations through computational simulations [37].
Online courses and open educational resources
With the development of educational technology, a series of online courses and open educational resources are available for computational toxicology. These resources are provided through Massive Open Online Course (MOOC) platforms such as Coursera, edX, and Udemy, as well as the Society of Toxicology (SOT), providing students with flexible learning pathways. These platforms often include courses designed by domain experts, covering content from the beginner to advanced levels, allowing students at all levels to find suitable learning materials (Table 4). In addition, there are also third-party organizations that offer valuable training activities to enhance knowledge and skills in computational methods. For example, the Physicians Committee for Responsible Medicine (PCRM) provides resources and training to promote the use of non-animal testing methods and alternatives.
Table 4.
Some online educational resources related to computational toxicology
| Course Name | Platform | Link |
|---|---|---|
| Toxicology 21: Scientific Applications | Coursera | https://www.coursera.org/learn/toxicology-21 |
| Chemicals and Health | Coursera | https://www.coursera.org/learn/chemicals-health |
| Chemical biology, Pharmacology & Computational Toxicology | Udemy | https://www.udemy.com/course/chemical-biology-pharmacology-computational-toxicology/ |
| Adverse Outcome Pathway (AOP) Development and Evaluation | CEd-Tox | https://www.toxicology.org/education/ce/onlineCourses.asp#courses |
| Alternative In Vitro Toxicology Testing for the 21st Century | ||
| Applications of In Vitro and In Silico New Approach Methodologies for Predictive and Mechanistic Thyroid Toxicity Testing | ||
| Applications of Computational Systems Biology for Toxicology | ||
| The NAM Use for Regulatory Application (NURA) program’s recorded content | PCRM | https://www.pcrm.org/ethical-science/animal-testing-and-alternatives/nura |
Academic journals and latest research
Keeping up with academic journals and the latest research is essential for education in computational toxicology. Journals such as Toxicological Sciences, Environmental Health Perspectives, Chemical Research in Toxicology, and Computational Toxicology regularly publish the latest research findings, enabling teachers and students to stay at the forefront of science. This not only enriches teaching materials but also encourages students to engage in independent research and develop critical thinking skills.
In addition to academic journals, participating in webinars organized by institutions like PCRM, EPA, or NICEATM can provide valuable insights into emerging trends and methodologies. Furthermore, attending scientific conferences, such as those hosted by the SOT and the American Society for Cellular and Computational Toxicology (ASSCT), offers opportunities to engage with the latest research and network with professionals in the field. These additional resources complement journal readings by providing interactive learning experiences and direct exposure to current scientific advancements.
Challenges and limitations
Despite the significant advancements in computational toxicology, there remain substantial barriers to the widespread acceptance of these models by regulatory agencies, particularly as replacements for traditional animal testing [55]. While computational models offer promising prospects for improving the efficiency and ethical aspects of toxicological assessments, their integration into regulatory frameworks faces notable challenges. Key issues include the lack of comprehensive validation and standardization, which limits the confidence in the predictions made by these models. Additionally, there are socio-technical barriers, such as resistance to change within established regulatory processes and the need for extensive data to support the reliability of alternative methods [10, 56]. Students and researchers should be aware that while computational models represent a cutting-edge approach, they are still in a developmental phase and face significant hurdles before becoming universally accepted. For instance, the PrecisionTox report highlights the socio-technical barriers to the uptake of New Approach Methodologies (NAMs), underscoring the gap between the technological potential of these models and their current regulatory acceptance. Addressing these challenges is crucial for advancing the field and ensuring that emerging technologies can effectively contribute to the evolution of toxicological practices.
Prospects
Computational toxicology is a rapidly developing field in which mathematical models, computational algorithms, and big data analysis are used to predict and evaluate the toxicity of chemical substances. With advancements in computational power and algorithms, computational toxicology will be able to simulate human reactions to chemical substances more accurately and understand the mechanisms of toxicity at the molecular level. In the future, computational toxicology may further improve the accuracy of toxicity prediction by integrating omics technologies such as genomics, proteomics, and metabolomics, as well as bioinformatics [57]. Additionally, with the rise of personalized medicine and precision therapeutics, the demand for computational toxicology experts will continue to grow. Pharmaceutical companies need these experts to predict the toxicity of drug molecules and reduce the risk of clinical trial failure. Environmental protection agencies and chemical regulatory authorities also need computational toxicology to assess the potential risks of environmental pollutants and emerging chemicals. The demand for developing new algorithms and computational models in academia will drive further research in computational toxicology.
Computational toxicology education provides students with a range of important professional skills, including data analysis, programming, statistics, and understanding of complex biological systems. These skills not only are useful in the field of toxicology but are also applicable to a wide range of biomedical, environmental science, and data science disciplines. Therefore, students who receive computational toxicology education will have greater employability and broader career development prospects.
Conclusion
Computational toxicology education is a critical innovation in the public health education system that cultivates students’ ability to solve real-world toxicology problems through interdisciplinary courses and practices. This type of education not only includes foundational knowledge of machine learning and artificial intelligence but also emphasizes the enhancement of students’ practical skills through hands-on projects and case analyses. Its core lies in interdisciplinary collaboration and networking knowledge from fields such as biology, chemistry, and computer science to enhance students’ comprehensive analysis and problem-solving abilities. Through participation in research projects, students’ innovative spirit and ability to independently tackle complex problems are further stimulated. This education model is not only about knowledge transfer but also about comprehensive training in thinking and professional skills, aiming to cultivate a new generation of toxicologists capable of effectively addressing chemical safety challenges globally.
Acknowledgements
We express our gratitude to the OpenAI team for providing access to ChatGPT, which greatly assisted in the translation and linguistic enhancement of portions of this manuscript.
Author contributions
J.W. and J.L. proposed article ideas, participated in project implementation, data collection and analysis, and wrote and revised the paper. All authors reviewed the manuscript.
Funding
The study was supported by Jiangsu Key Laboratory of Preventive and Translational Medicine for Geriatric Diseases as well as the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). The study was also supported by a Joint-Project of Four Government Departments (MX13901123).
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Selter F, Hetzel T, Kahrass H, Mertz M. Animal Research Ethics as Interaction of Research Ethics, Animal Ethics, and (Animal Protection) Law. Altex-Altern Anim Ex. 2023;40(3):541–4. [DOI] [PubMed] [Google Scholar]
- 2.Robinson NB, Krieger K, Khan FM, Huffman W, Chang M, Naik A, Yongle R, Hameed I, Krieger K, Girardi LN, et al. The current state of animal models in research: a review. Int J Surg. 2019;72:9–13. [DOI] [PubMed] [Google Scholar]
- 3.Hartung T. Making big sense from big data in toxicology by read-across. Altex. 2016;33(2):83–93. [DOI] [PubMed] [Google Scholar]
- 4.Krewski D, Andersen ME, Tyshenko MG, Krishnan K, Hartung T, Boekelheide K, Wambaugh JF, Jones D, Whelan M, Thomas R, et al. Toxicity testing in the 21st century: progress in the past decade and future perspectives. Arch Toxicol. 2020;94(1):1–58. [DOI] [PubMed] [Google Scholar]
- 5.Predictive NRCCoAoTTt. The National Academies Collection: reports funded by National Institutes of Health. Applications of Toxicogenomic Technologies to Predictive Toxicology and Risk Assessment. edn. Washington (DC): National Academies Press (US); 2007. [PubMed] [Google Scholar]
- 6.Bolt HM, Hengstler JG. The rapid development of computational toxicology. Arch Toxicol. 2020;94(5):1371–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rusyn I, Daston GP. Computational toxicology: realizing the promise of the toxicity testing in the 21st century. Environ Health Perspect. 2010;118(8):1047–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin Z, Chou WC. Machine learning and Artificial Intelligence in Toxicological sciences. Toxicol Sci. 2022;189(1):7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tetko IV, Klambauer G, Clevert DA, Shah I, Benfenati E. Artificial Intelligence meets Toxicology. Chem Res Toxicol. 2022;35(8):1289–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jia X, Wang T, Zhu H. Advancing computational toxicology by interpretable machine learning. Environ Sci Technol. 2023;57(46):17690–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qu W, Zheng W, Zheng Y. The developments and Challenges of Toxicology Education, Research, and funding in China. Chem Res Toxicol. 2008;21(9):1643–6. [DOI] [PubMed] [Google Scholar]
- 12.Wang Z, Chen J. Background, Tasks, Modeling Methods, and Challenges for Computational Toxicology. In: Advances in Computational Toxicology: Methodologies and Applications in Regulatory Science. edn. Edited by Hong H. Cham: Springer International Publishing; 2019: 15–36.
- 13.Kostal J. Chapter Four - Computational Chemistry in Predictive Toxicology: status quo et quo vadis? In: Advances in Molecular Toxicology. Volume 10, edn. Edited by Fishbein JC, Heilman JM: Elsevier; 2016: 139–186.
- 14.Johnson DE, Richardson RJ. CHAPTER 13:Educational Programs for Computational Toxicology and Pharmacology. In: 2017; 2017.
- 15.Ciallella HL, Zhu H. Advancing computational toxicology in the Big Data era by Artificial Intelligence: data-driven and mechanism-driven modeling for Chemical toxicity. Chem Res Toxicol. 2019;32(4):536–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paganelli AI, Mondéjar AG, da Silva AC, Silva-Calpa G, Teixeira MF, Carvalho F, Raposo A, Endler M. Real-time data analysis in health monitoring systems: a comprehensive systematic literature review. J Biomed Inf. 2022;127:104009. [DOI] [PubMed] [Google Scholar]
- 17.García-Carmona A. Scientific thinking and critical thinking in science education · two distinct but symbiotically related intellectual processes. Sci Educ 2023, Online first.
- 18.Patlewicz G. Navigating the Minefield of Computational Toxicology and Informatics: looking back and charting a New Horizon. Front Toxicol 2020, 2. [DOI] [PMC free article] [PubMed]
- 19.Golbraikh A, Wang XS, Zhu H, Tropsha A. Predictive QSAR Modeling: Methods and Applications in Drug Discovery and Chemical Risk Assessment. In: Handbook of Computational Chemistry. edn. Edited by Leszczynski J, Kaczmarek-Kedziera A, Puzyn T, G. Papadopoulos M, Reis H, K. Shukla M. Cham: Springer International Publishing; 2017: 2303–2340.
- 20.Trisciuzzi D, Alberga D, Leonetti F, Novellino E, Nicolotti O, Mangiatordi GF. Molecular Docking for Predictive Toxicology. Methods Mol Biol. 2018;1800:181–97. [DOI] [PubMed] [Google Scholar]
- 21.Sakkiah S, Kusko R, Tong W, Hong H. Applications of Molecular Dynamics Simulations in Computational Toxicology. In: Advances in Computational Toxicology: Methodologies and Applications in Regulatory Science. edn. Edited by Hong H. Cham: Springer International Publishing; 2019: 181–212.
- 22.Willighagen EL, Jeliazkova N, Hardy B, Grafström RC, Spjuth O. Computational toxicology using the OpenTox application programming interface and Bioclipse. BMC Res Notes. 2011;4(1):487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chandrasekaran B, Abed SN, Al-Attraqchi O, Kuche K, Tekade RK. Chap. 21 - Computer-Aided Prediction of Pharmacokinetic (ADMET) Properties. In: Dosage Form Design Parameters. edn. Edited by Tekade RK: Academic Press; 2018: 731–755.
- 24.Pedretti A, Mazzolari A, Gervasoni S, Fumagalli L, Vistoli G. The VEGA suite of programs: an versatile platform for cheminformatics and drug design projects. Bioinformatics. 2021;37(8):1174–5. [DOI] [PubMed] [Google Scholar]
- 25.Williams AJ, Grulke CM, Edwards J, McEachran AD, Mansouri K, Baker NC, Patlewicz G, Shah I, Wambaugh JF, Judson RS, et al. The CompTox Chemistry Dashboard: a community data resource for environmental chemistry. J Cheminform. 2017;9(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pearce RG, Setzer RW, Strope CL, Wambaugh JF, Sipes NS. Httk: R Package for High-Throughput Toxicokinetics. J Stat Softw. 2017;79(4):1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Filer DL, Kothiya P, Setzer RW, Judson RS. Martin MT: tcpl: the ToxCast pipeline for high-throughput screening data. Bioinformatics. 2017;33(4):618–20. [DOI] [PubMed] [Google Scholar]
- 28.Herreid CF. Case studies in Science–A Novel Method of Science Education. J Coll Sci Teach 1994, 23.
- 29.Noblitt L, Vance D, Smith M. A comparison of Case Study and traditional teaching methods for improvement of oral communication and critical-thinking skills. J Coll Sci Teach 2010, 39.
- 30.Akçayır G, Akçayır M. The flipped classroom: a review of its advantages and challenges. Comput Educ. 2018;126:334–45. [Google Scholar]
- 31.Ankley GT, Bennett RS, Erickson RJ, Hoff DJ, Hornung MW, Johnson RD, Mount DR, Nichols JW, Russom CL, Schmieder PK, et al. Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ Toxicol Chem. 2010;29(3):730–41. [DOI] [PubMed] [Google Scholar]
- 32.Dobson L, Reményi I, Tusnády GE. The human transmembrane proteome. Biol Direct. 2015;10:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.The ASPIS cluster is a joint collaboration of the H2020 funded projects ONTOX, PrecisionTox. RISK-HUNT3R and represents Europe’s effort towards the sustainable, animal-free and reliable chemical risk assessment of tomorrow [https://aspis-cluster.eu/].
- 34.Hardy B, Mohoric T, Exner T, Dokler J, Brajnik M, Bachler D, Mbegbu O, Kleisli N, Farcal L, Maciejczuk K, et al. Knowledge infrastructure for integrated data management and analysis supporting new approach methods in predictive toxicology and risk assessment. Toxicol in Vitro. 2024;100:105903. [DOI] [PubMed] [Google Scholar]
- 35.Ramhøj L, Svingen T, Vanhaecke T. Editorial: European partnership on the assessment of risks from chemicals (PARC): focus on new approach methodologies (NAMs) in risk assessment. Front Toxicol. 2024;6:1461967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Viceconti M, Emili L, Afshari P, Courcelles E, Curreli C, Famaey N, Geris L, Horner M, Jori MC, Kulesza A, et al. Possible contexts of Use for in Silico trials methodologies: a Consensus-based review. IEEE J Biomed Health Inf. 2021;25(10):3977–82. [DOI] [PubMed] [Google Scholar]
- 37.Virtual Human Platform for Safety Assessment. (VPH4Safety) [https://docs.vhp4safety.nl/en/latest/
- 38.Wang Y, Bryant SH, Cheng T, Wang J, Gindulyte A, Shoemaker BA, Thiessen PA, He S, Zhang J. PubChem BioAssay: 2017 update. Nucleic Acids Res. 2017;45(D1):D955–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Editorial. ChemSpider–a tool for Natural products research. Nat Prod Rep. 2015;32(8):1163–4. [DOI] [PubMed] [Google Scholar]
- 40.Daneshian M, Kamp H, Hengstler J, Leist M, van de Water B. Highlight report: launch of a large integrated European in vitro toxicology project: EU-ToxRisk. Arch Toxicol. 2016;90(5):1021–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.European Commission JRC. EURL ECVAM dataset on alternative methods to animal experimentation (DB-ALM). In.: European Commission. Joint Research Centre (JRC); 2019.
- 42.Feshuk M, Kolaczkowski L, Dunham K, Davidson-Fritz SE, Carstens KE, Brown J, Judson RS, Paul Friedman K. The ToxCast pipeline: updates to curve-fitting approaches and database structure. Front Toxicol. 2023;5:1275980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Judson R, Richard A, Dix D, Houck K, Elloumi F, Martin M, Cathey T, Transue TR, Spencer R, Wolf M. ACToR — aggregated computational Toxicology Resource. Toxicol Appl Pharmcol. 2008;233(1):7–13. [DOI] [PubMed] [Google Scholar]
- 44.Kim D, Cho S, Jeon JJ, Choi J. Inhalation toxicity screening of Consumer Products Chemicals using OECD Test Guideline Data-based machine learning models. J Hazard Mater. 2024;478:135446. [DOI] [PubMed] [Google Scholar]
- 45.Diderich R. Tools for category formation and read-across: overview of the OECD (Q)SAR application toolbox. Silico Toxicology: Principles Appl 2010:385–407.
- 46.Gaulton A, Hersey A, Nowotka M, Bento AP, Chambers J, Mendez D, Mutowo P, Atkinson F, Bellis LJ, Cibrián-Uhalte E, et al. The ChEMBL database in 2017. Nucleic Acids Res. 2017;45(D1):D945–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Judson RS, Houck KA, Kavlock RJ, Knudsen TB, Martin MT, Mortensen HM, Reif DM, Rotroff DM, Shah I, Richard AM, et al. In vitro screening of environmental chemicals for targeted testing prioritization: the ToxCast project. Environ Health Perspect. 2010;118(4):485–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Davis AP, Grondin CJ, Johnson RJ, Sciaky D, Wiegers J, Wiegers TC, Mattingly CJ. Comparative toxicogenomics database (CTD): update 2021. Nucleic Acids Res. 2021;49(D1):D1138–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Committee to Review the IP, Board on, Environmental S, Toxicology, Division on E, Life S, National Research C. Review of EPA’s Integrated Risk Information System (IRIS) Process. In., edn. Washington (DC): National Academies Press (US) Copyright 2014 by the National Academy of Sciences. All rights reserved.; 2014. [PubMed]
- 50.Fonger GC, Hakkinen P, Jordan S, Publicker S. The National Library of Medicine’s (NLM) Hazardous Substances Data Bank (HSDB): background, recent enhancements and future plans. Toxicology. 2014;325:209–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wullenweber A, Kroner O, Kohrman M, Maier A, Dourson M, Rak A, Wexler P, Tomljanovic C. Resources for global risk assessment: the International Toxicity estimates for risk (ITER) and risk Information Exchange (RiskIE) databases. Toxicol Appl Pharmacol. 2008;233(1):45–53. [DOI] [PubMed] [Google Scholar]
- 52.Attene-Ramos MS, Miller N, Huang R, Michael S, Itkin M, Kavlock RJ, Austin CP, Shinn P, Simeonov A, Tice RR, et al. The Tox21 robotic platform for the assessment of environmental chemicals–from vision to reality. Drug Discov Today. 2013;18(15–16):716–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bell SM, Phillips J, Sedykh A, Tandon A, Sprankle C, Morefield SQ, Shapiro A, Allen D, Shah R, Maull EA, et al. An Integrated Chemical Environment to Support 21st-Century Toxicology. Environ Health Perspect. 2017;125(5):054501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Edgar R, Domrachev M, Lash AE. Gene expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30(1):207–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Report on Socio-Technical. Barriers to the Uptake of NAMs [https://precisiontox.org/read-the-report-on-socio-technical-barriers-to-the-uptake-of-nams-here/
- 56.Sydow D, Burggraaff L, Szengel A, van Vlijmen HWT, AP IJ, van Westen GJP, Volkamer A. Advances and challenges in Computational Target Prediction. J Chem Inf Model. 2019;59(5):1728–42. [DOI] [PubMed] [Google Scholar]
- 57.Canzler S, Schor J, Busch W, Schubert K, Rolle-Kampczyk UE, Seitz H, Kamp H, von Bergen M, Buesen R, Hackermüller J. Prospects and challenges of multi-omics data integration in toxicology. Arch Toxicol. 2020;94(2):371–88. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.