Abstract
Background
Cryptococcus is an opportunistic infection acquired through inhalation from the environment, primarily affecting individuals with compromised immune systems. It typically leads to pneumonia upon passing through lung tissue. The infection can disseminate to various organs via the bloodstream, resulting in meningitis or encephalitis in the central nervous system. Disseminated Cryptococcus has been reported to involve the skin, liver, eyes, lymph nodes, bone marrow, spleen, kidneys, and intestines, significantly increasing morbidity and mortality. However, pancreatic involvement in Cryptococcus is relatively rare, and a few case reports have highlighted severe organ damage and high mortality rates.
Case presentation
In this case report, we present the case of a 36-year-old Asian man who presented with a 2-week history of headaches and blurred vision in his right eye. Brain magnetic resonance imaging revealed multiple brain masses, along with a mass in the lower left lung field and a tumor in the pancreatic tail, as detected by chest computed tomography. Endoscopic ultrasound-guided fine needle biopsy and computed tomography-guided lung biopsy confirmed the diagnosis of disseminated cryptococcal infection involving the pancreas, lung, and brain. The patient’s clinical condition improved following antifungal therapy. Additionally, we identified anti-granulocyte–macrophage colony-stimulating factor antibody as a risk factor for disseminated cryptococcal infection in this patient.
Conclusion
Disseminated cryptococcosis can be a potentially lethal condition, as highlighted by previous literature. However, early diagnosis using contrast-enhanced harmonic endoscopic ultrasound and endoscopic ultrasound-guided biopsies, as well as prompt treatment as demonstrated in our case, can improve outcomes and prevent mortality.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13256-024-04836-1.
Keywords: Disseminated cryptococcosis, Case report, Pancreatic involvement with Cryptococcus, Endoscopic ultrasound, Pancreatic malignancy
Background
Cryptococcus is a potentially lethal fungal infection that may be categorized into either the C. neoformans or C. gattii species. The former is globally distributed and abundant in avian excreta [1, 2], whereas C. gattii has classically been viewed as a tropical or subtropical fungus. In nature, Cryptococcus is inhaled from the environment and passes through lung tissue, subsequently causing pneumonia in an immune-suppressed status. Otherwise, these fungal cells are also able to establish asymptomatic latent infection in immunocompetent cases. Upon becoming flared up once the immune system has been suppressed, the cells may disseminate to other tissue via the bloodstream. This most commonly occurs in the central nervous system, causing meningitis or encephalitis with increased intracranial pressure and, in turn, posing a lethal threat to individuals, as witnessed in our case.
Although cryptococcosis is typically rare in immunocompetent individuals, a recent increase in cases has been noted in healthy populations in North America and Canada [3]. Within a cohort of 300 human immunodeficiency virus (HIV)-negative patients diagnosed with cryptococcal infection, 25% had received steroid therapy, 24% had chronic liver, kidney, or lung diseases, 16% had malignancies, and 15% had undergone solid organ transplants [4]. Notably, 30% of these patients had no discernible underlying medical conditions. Generally, Cryptococcus meningitis seldom occurs in previously healthy individuals, with an annual incidence of approximately 3000 reported cases in the USA, corresponding to an estimated occurrence of one in 100,000 individuals per year. Among the identified risk factors, pulmonary alveolar proteinosis, characterized by autoantibodies against granulocyte–macrophage colony-stimulating factor (GM-CSF), has been linked to intracellular infections, including cryptococcosis. In our case, this association potentially inhibited macrophage signaling by blocking STAT5 phosphorylation [5].
Additionally, other mentioned organs in previously published literature have included the skin, liver, eyes, lymph nodes, bone marrow, spleen, kidneys, and intestines. Cryptococcal involvement in pancreas tissue is relatively rare, while disseminated Cryptococcus may deteriorate causing severe organ damage and a high mortality rate. This case report highlights the awareness of pancreatic cryptococcal infection as opposed to pancreatic malignancy. After contrast-enhanced harmonic endoscopic ultrasound (CEH-EUS) and EUS-guided fine needle biopsy (EUS-FNB), pancreatic Cryptococcus was diagnosed in our patient. Nontraditional risk factors regarding autoantibodies against GM-CSFs were also featured, with the patient finally presenting with a favorable outcome after undergoing antifungal therapy.
Case presentation
A 36-year-old Asian man with no prior medical history, considered to be relatively healthy, was admitted due to the presentation of headache, dizziness, and blurred vision in the right eye having occurred for a period of 2 weeks. He was employed as a chicken vendor. Seeking assistance, he visited our neurosurgery department with initial vital signs showing a blood pressure of 134/97 mmHg, heart rate of 89 bpm, respiratory rate of 18 breaths per minute, and a body temperature of 36.4 °C. A neurological examination was performed, assessing orientation, memory, abstract thinking, and calculation—all of which were intact. However, visual field examination revealed hemianopia. Additional tests, including assessments of pupil size, light reflex, eye movement, facial sensation, jaw opening, corneal reflex, mouth angle, uvula/palate movement, and sensory/motor function, showed no abnormalities. Subsequent physical examinations indicated symmetric chest expansion, clear breath sounds, a soft abdomen, normoactive bowel sounds, and no abdominal tenderness.
His brain magnetic resonance imaging (MRI) revealed multiple brain masses (Fig. 1). However, he had no associated symptoms, including fever, body weight loss, or abdominal discomfort. The tentative diagnosis was brain tumors with the suspicion of intracranial metastasis. Therefore, he underwent a series of examinations for tumor survey, including abdominal sonography (Fig. 2), chest computed tomography (CT) (Fig. 3), and serum tumor markers. One mass (4 cm) over the left lower lung field and a pancreatic tail tumor were found at the same time. Based on a series of MRI scans (Fig. 4), pancreatic cancer with brain and lung metastasis was suspected. The patient therefore underwent a EUS-guided fine needle biopsy (EUS-FNB) (Fig. 5) for the pancreatic tumor, and then a subsequent CT-guided biopsy for the lung mass. A Latex test for Cryptococcus yielded a result of 1:32, and a fungus element with a thick capsule was determined from the pathological findings (Fig. 6), with the CT-guided biopsy of the left lung cavity lesion also revealing fungal contamination due to cryptococcal infection involving both xanthomatous and necrotizing inflammation. The final diagnosis for this nonimmunodeficient patient was disseminated cryptococcal infection with pancreas, lung, and brain involvement. Tests for other risk factors for disseminated cryptococcal infection that had been previously mentioned in the literature were conducted, including malignancy, HIV status, and liver cirrhosis, all of which were determined to be negative by our panel.
Fig. 1.
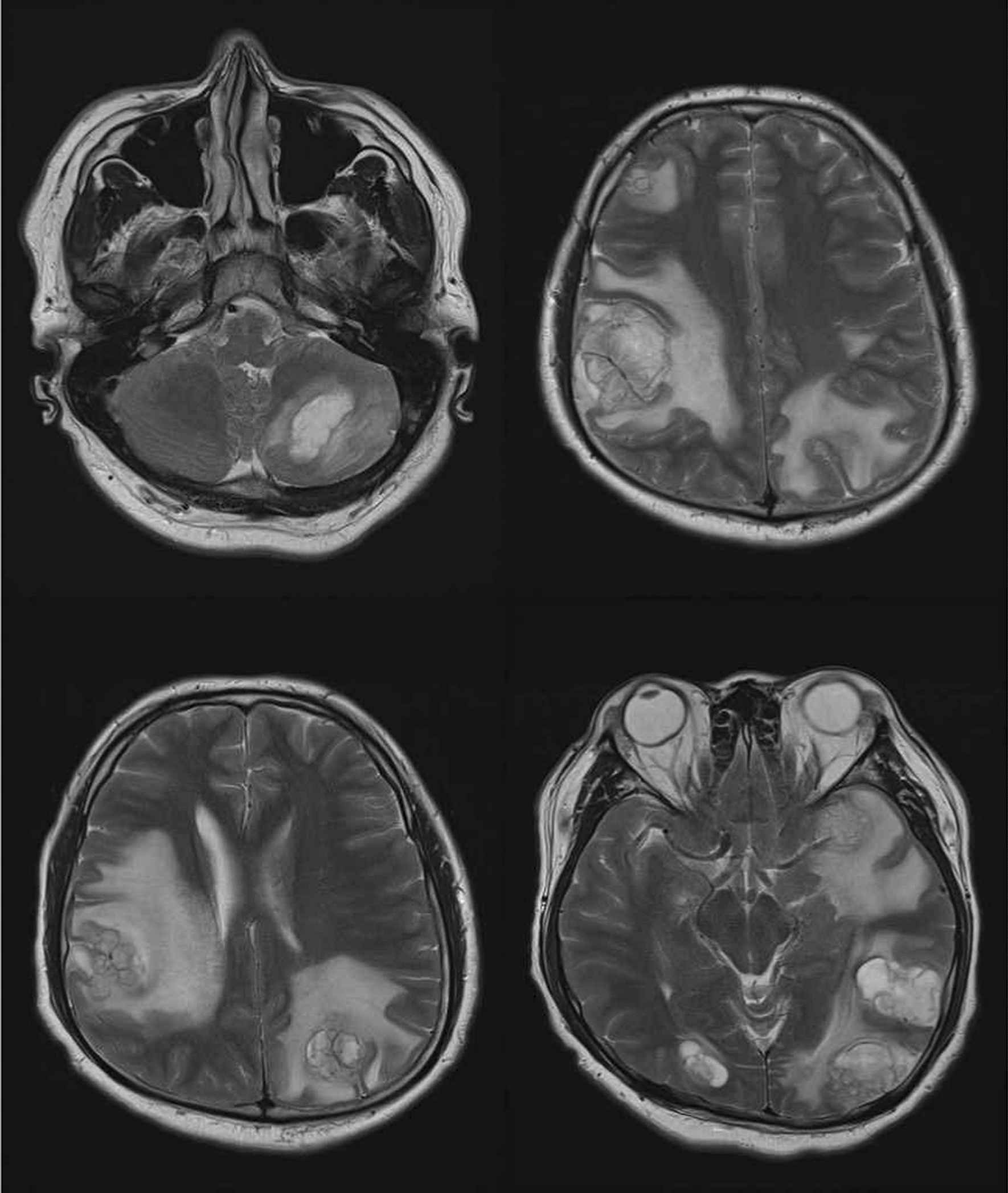
Multiple enhancing lesions over bilateral cerebellar hemisphere were noted with initial impression of brain metastasis
Fig. 2.
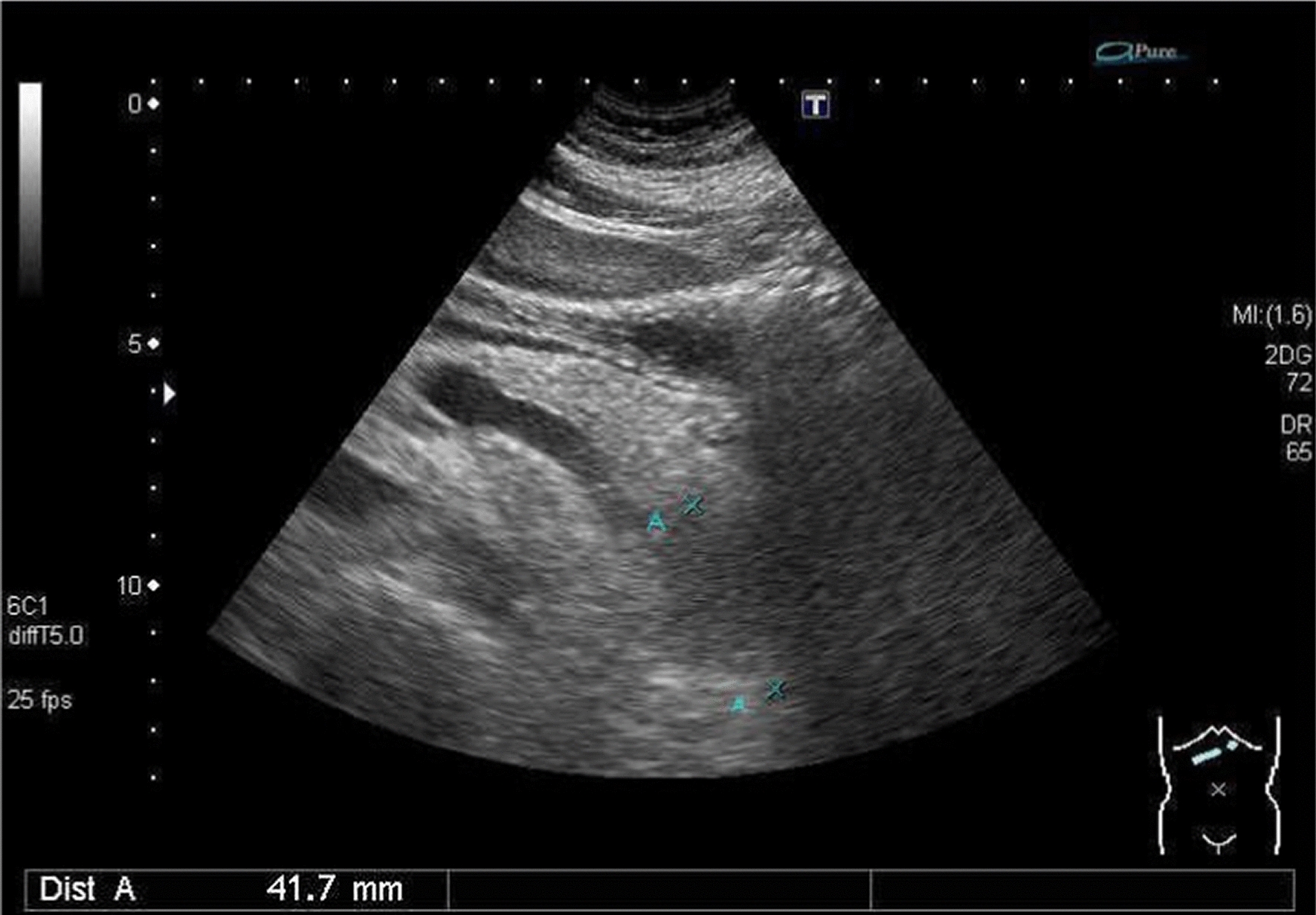
Abdominal sonography showed a 41 mm hypoechoic mass over the pancreatic tail
Fig. 3.
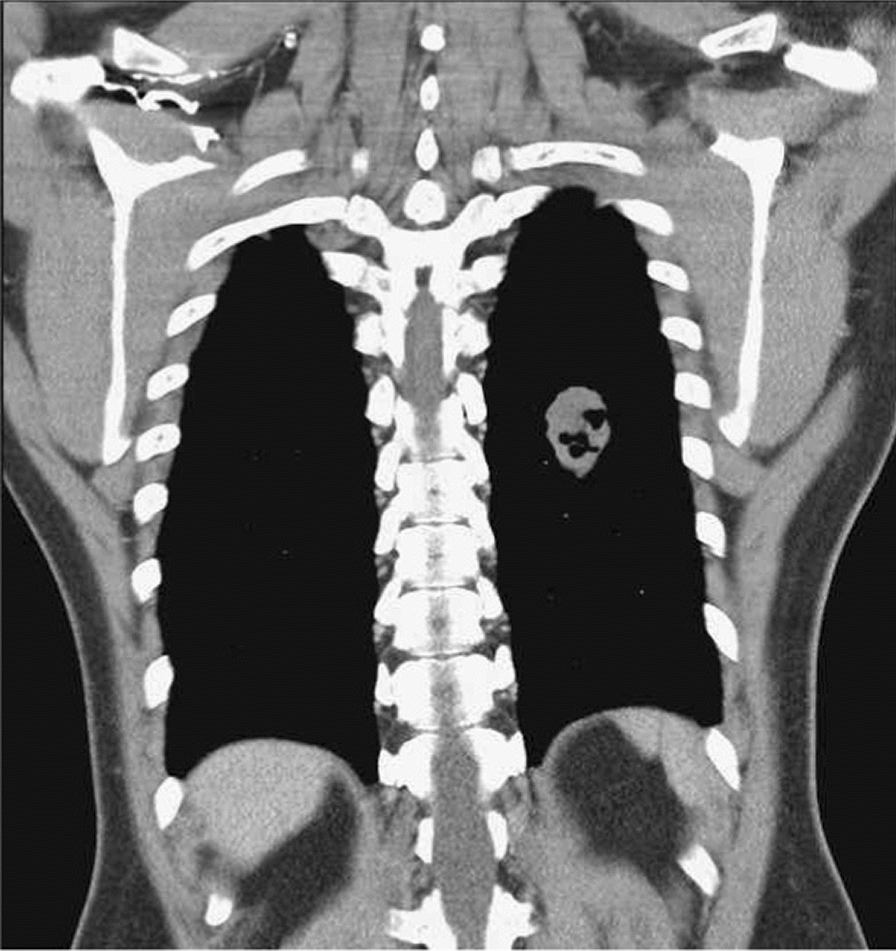
Chest computed tomography showed an oval cavity mass (4 cm) over the left lower lung field
Fig. 4.
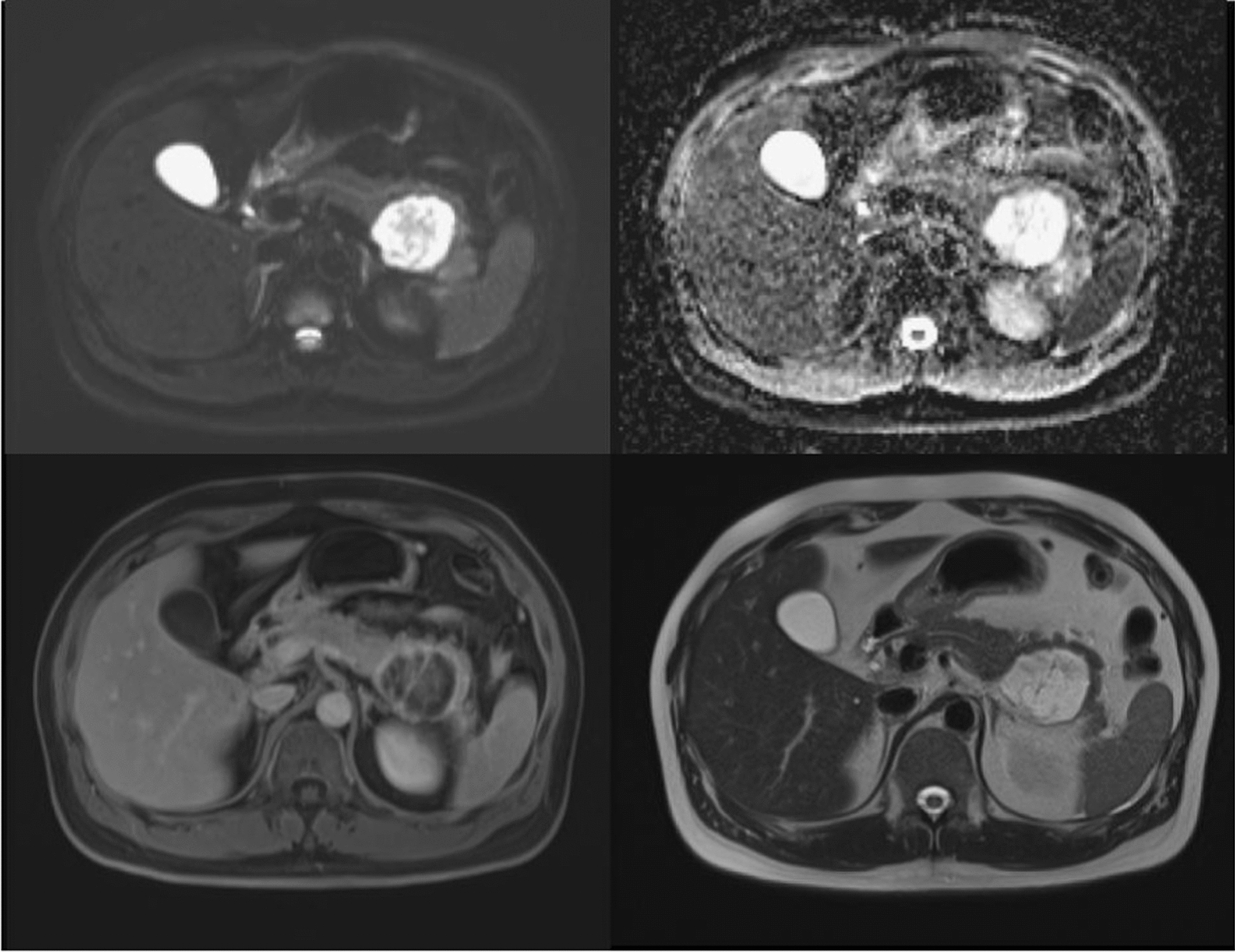
Magnetic resonance cholangiopancreatography (MRCP) made raising suspicion of pseudocyst or cystic neoplasm, suspected mucinous cystadenoma
Fig. 5.
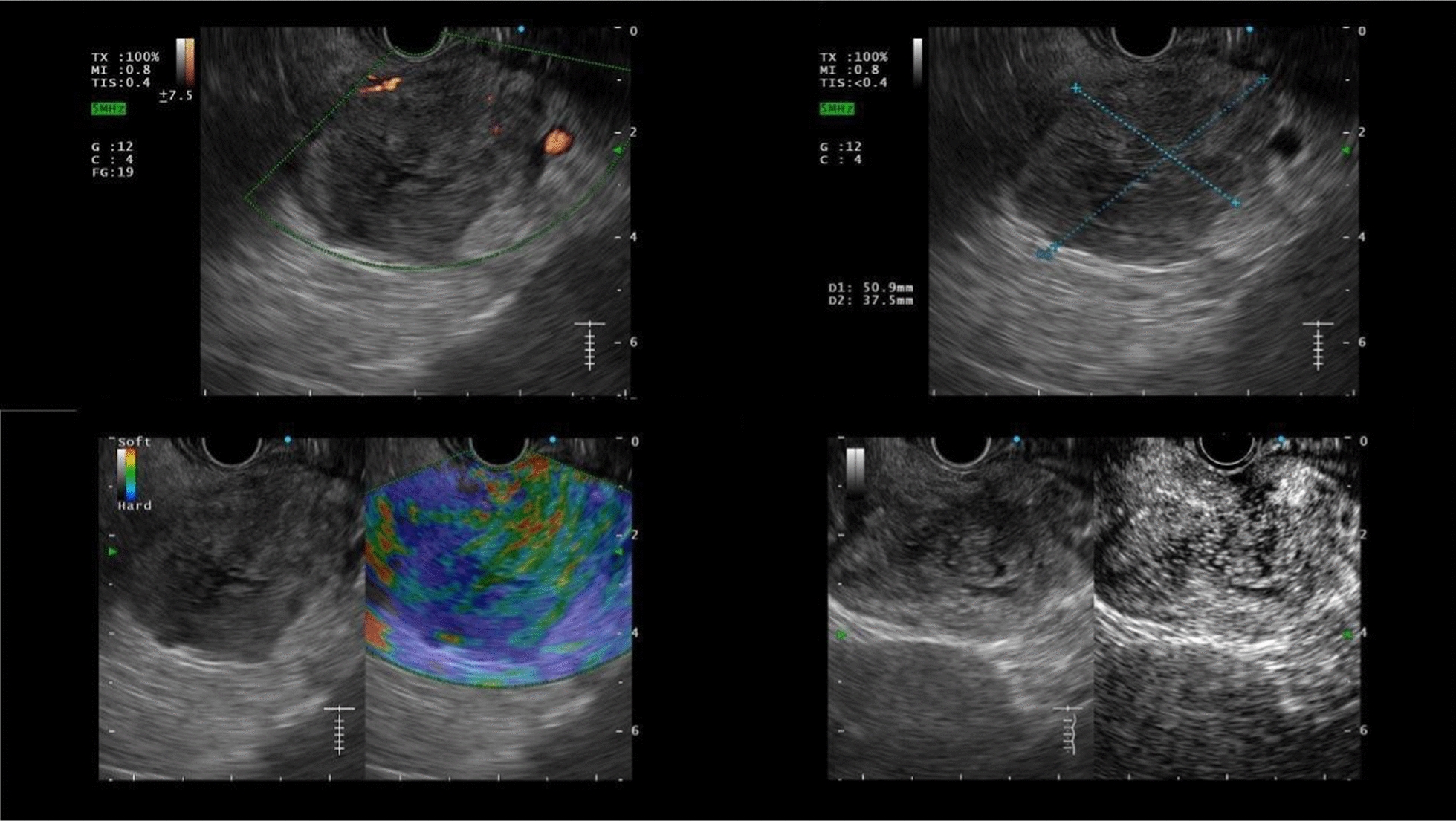
Contrast-enhanced harmonic endoscopic ultrasound (CEH-EUS) showed hypovascularity, iso-enhanced lesion with soft echotexture under elastography
Fig. 6.
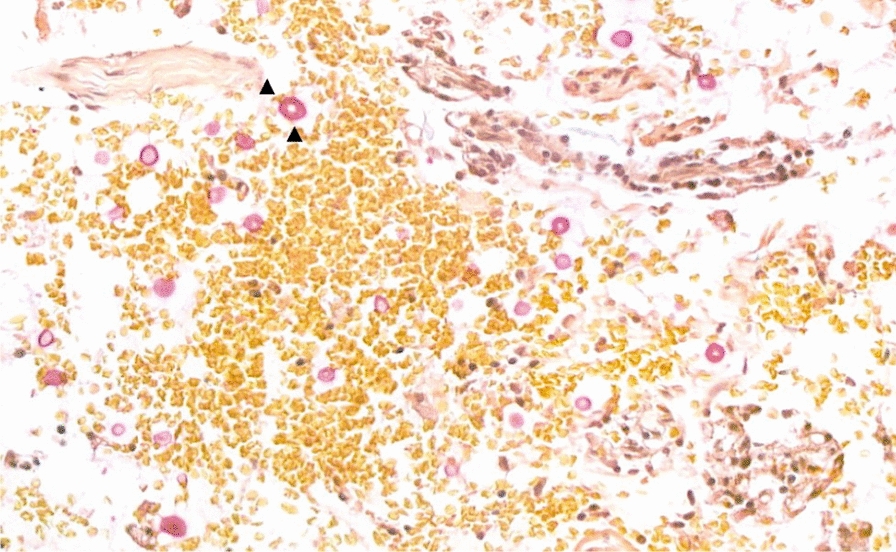
Endoscopic ultrasound fine needle aspiration (FNA) pathology cytologic finding showed fungal yeasts with thickened capsule highlighted with mucicarmine stain (black arrow)
Afterwards, a serum sample of anti-GM CSF antibody was tested revealing a positive result, which is one of the predisposing factors for non-HIV cryptococcal meningitis. Treatment commenced with flucytosine 1500 mg 4 times a day/quater in die (QID) from 19 April 2022 to 14 June 2022, alongside ambisome 150 mg from 19 April to 28 April, titrated to 350 mg from 29 April to 14 June. Subsequently, the regimen shifted to fluconazole 600 mg QD from 14 June to 15 August, tapering to 450 mg QD from 16 August to 30 November, and finally down to 300 mg QD from 16 August 2022 to 24 November 2023. Throughout follow-up appointments with the infectious disease department, there were no reports of double vision. The cryptococcal infection over the left cerebral region was determined to be in a stable status from brain CT results after 6 months of antifungal therapy. A brain MRI conducted on June 2023 revealed a stable cryptococcoma, gradually decreasing in size, and perifocal edema had also subsided. The timeline for both treatment and follow-up is shown in Supplementary Fig. 1.
Discussion
Cryptococcosis was first identified in 1894 and later became a health threat during the acquired immune deficiency syndrome (AIDS) pandemic of the 1980s. It was initially classified as a single fungal species but was then divided into two separate species (C. gattii and C. neoformans) [6]. An acquired case of cryptococcosis would occur due to the inhalation of fungal cells from within the environment, subsequently causing pneumonia in immunosuppressed patients. In contrast, in immunocompetent cases, the fungal cells may develop into an asymptomatic latent infection and disseminate to other tissues, most commonly those found in the central nervous system, once the host has reached an immunosuppressant status. Upon establishing itself in the central nervous system, the cryptococcal infection often causes meningitis or encephalitis, along with increased intracranial pressure, possibly leading to mortality if no immediate and effective treatment is provided.
Cryptococcal meningitis (CM) is common in cases of HIV infection [7, 8]. Additionally, it can occur in other forms of both natural and iatrogenic immune suppression. In a study of over 300 HIV-negative patients with cryptococcal infection under iatrogenic immune suppression in the USA, 25% had received steroid therapy, 24% had chronic liver, kidney or lung disease, 16% had a malignancy, and 15% had received solid organ transplants [3, 9]. CM rarely occurs in previously healthy patients, while a number of primary immunologic defects or autoimmune diseases have been shown to be associated with cryptococcal meningitis. In particular, the presence of auto-antibodies against granulocyte–macrophage colony stimulating factor (GM-CSF) [10–13] is associated with intracellular infections, including cryptococcosis. As for our patient, a series of examinations to assess the risk factors for cryptococcal infection, including anti-HIV antibody, tumor marker, and autoimmunity titer (ANA, C3/4, SSA/B), all revealed normal results except for the patient’s prediabetic status. While he was not being treated with corticosteroids or any immune suppressants, the blood sampling of anti-GM CSF antibody revealed a positive result three times higher than normal.
As previously reported, along with the lungs and brain, cryptococcal infection involving the eyes, spleen, liver, bone marrow, kidneys, prostate, lymph nodes, peritoneum, and skin has also been reported. One of the possible explanations for this tropism to lipids is that large polysaccharide capsules, fungal metalloproteases, extracellular lipids, and phospholipids [14, 15] may act as virulent factors during an invasion of cryptococci in the patient’s central nervous system. Additionally, host sphingolipids aid in granuloma formation and can alter microbial uptake via macrophages, phagocytic cell response, and neutrophil’s killing ability during cryptococcal infection [16–19].
Nevertheless, Cryptococcus with pancreatic involvement is infrequently reported. A series of case reports in the 1990s showed that clinical symptoms (including those related to pancreatic involvement) and the severity of cryptococcal infection varied quite a lot [20–23].
Finally, the use of endoscopy ultrasound aids in the diagnosis of pancreas mass lesions, as previously reported [23]. In that case, an EUS-guided biopsy revealed a cryptococcoma for the peripancreatic mass, showing the differentiation of malignancy from the infectious process. An EUS-Fine needle aspiration (FNA) may significantly improve diagnostic accuracy, and thus patients’ outcomes as well. In addition, Kitano et al. [24] proposed implementing contrast-enhanced harmonic EUS to further assist a diagnosis more promptly. Therefore, when we encounter a pancreatic mass involving several other organs, we should be aware of possible cryptococcal infection even in immunocompetent patients, and use contrast-enhanced harmonic EUS and EUS-FNA to make a firm diagnosis, thus allowing for a proposal on appropriate treatment be made as early as possible.
Conclusion
The patient in our study is a case of disseminated cryptococcosis accompanied with brain, lung, and, most importantly, pancreatic involvement in a relatively immunocompetent individual, which can be lethal according to previous literature review. Aside from the traditional risk factor for Cryptococcus, a positive anti-GM-CSF result that highlights the nontraditional risk factors in relatively immunocompetent cases, patients still carry a higher risk of cryptococcal infection. Early prompt treatment, as undertaken in our case, could improve outcomes and avoid mortality.
Clinicians should be well aware of similar cases of pancreatic lesions being found upon using CEH-EUS and EUS-guided biopsies, thus allowing for a diagnosis to be made as early as possible. This will give clinicians the opportunity to then provide the correct treatment for improving patient outcomes.
Supplementary Information
Acknowledgements
We would like to thank Mr. Dean S. Dowers for English language editing.
Author contributions
HEC drafted the manuscript, and HZY and SCL revised it. All authors read and approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and materials
The availability of data applies only in cases where no personal information is disclosed.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
No competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chowdhary A, Rhandhawa HS, Prakash A, Meis JF. Environmental prevalence of Cryptococcus neoformans and Cryptococcus gattii in India: an update. Crit Rev Microbiol. 2012;38(1):1–16. [DOI] [PubMed] [Google Scholar]
- 2.Litvintseva AP, Carbone I, Rossouw J, Thakur R, Govender NP, Mitchell TG. Evidence that the human pathogenic fungus Cryptococcus neoformans var. grubii may have evolved in Africa. PLoS ONE. 2011;6(5): e19688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pappas PG, et al. Cryptococcosis in human immunodeficiency virus-negative patients in the era of effective azole therapy. Clin Infect Dis. 2001;33:690–9. [DOI] [PubMed] [Google Scholar]
- 4.Byrnes EJ, Marr KA. The outbreak of Cryptococcus gattii in Western North America: epidemiology and clinical issues. Curr Infect Dis Rep. 2011;13:256–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee YC, Chew GT, Robinson BW. Pulmonary & meningeal cryptococcosis in pulmonary alveolar proteinosis. Aust N Z J Med. 1999;29:843–4. [DOI] [PubMed] [Google Scholar]
- 6.Hagen F, Khayhan K, Theelen B, Kolecka A, Polacheck I, Sionov E, Falk R, Parnmen S, Lumbsch HT, Boekhout T. Recognition of seven species in the Cryptococcus gattii/Cryptococcus neoformans species complex. Fungal Genet Biol. 2015;78:16–48. [DOI] [PubMed] [Google Scholar]
- 7.Rajasingham R, Rhein J, Klammer K, Musubire A, Nabeta H, Akampurira A, Mossel EC, Williams DA, Boxrud DJ, Crabtree MB, Miller BR, Rolfes MA, Tengsupakul S, Andama AO, Meya DB, Boulware DR. Epidemiology of meningitis in an HIV-infected Ugandan cohort. Am J Trop Med Hyg. 2015;92(2):274–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jarvis JN, et al. Adult meningitis in a setting of high. HIV and TB prevalence: findings from 4961 suspected cases. BMC Infect Dis. 2010;10:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beardsley J, Sorrell TC, Chen SC. Central nervous system cryptococcal infections in non-HIV infected patients. J Fungi. 2019;5(3):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmad DS, Esmadi M, Steinmann WC. Idiopathic CD4 lymphocytopenia: spectrum of opportunistic infections, malignancies, and autoimmune diseases. Avicenna J Med. 2013;3(2):37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gorska MM, Alam R. A mutation in the human uncoordinated 119 gene impairs TCR signaling and is associated with CD4 lymphopenia. Blood. 2012;119:1399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosen LB, Freeman AF, Yang LM, Jutivorakool K, Olivier KN, Angkasekwinai N, Suputtamongkol Y, Bennett JE, Pyrgos V, Williamson PR, Ding L, Holland SM, Browne SK. Anti-GM-CSF autoantibodies in patients with cryptococcal meningitis. J Immunol. 2013;190(8):3959–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saijo T, Chen J, Chen SC, Rosen LB, Yi J, Sorrell TC, Bennett JE, Holland SM, Browne SK, Kwon-Chung KJ. Anti-granulocyte-macrophage colony-stimulating factor autoantibodies are a risk factor for central nervous system infection by Cryptococcus gattii in otherwise immunocompetent patients. MBio. 2014;5(2):e00912-e914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Voelz K, May RC. Cryptococcal interactions with the host immune system. Eukaryot Cell. 2010;9:835–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vu K, Tham R, Uhrig JP, Thompson GR 3rd, Na Pombejra S, Jamklang M, Bautos JM, Gelli A. Invasion of the central nervous system by Cryptococcus neoformans requires a secreted fungal metalloprotease. MBio. 2014;5(3):e01101-e1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shea JM, Henry JL, Del Poeta M. Lipid metabolism in Cryptococcus neoformans. FEMS Yeast Res. 2006;6:469–79. [DOI] [PubMed] [Google Scholar]
- 17.Nolan SJ, Fu MS, Coppens I, Casadevall A. Lipids affect the Cryptococcus neoformans-macrophage interaction and promote nonlytic exocytosis. Infect Immun. 2017;85:e00564-e617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh A, MacKenzie A, Girnun G, Del Poeta M. Analysis of sphingolipids, sterols, and phospholipids in human pathogenic Cryptococcus strains. J Lipid Res. 2017;58:2017–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bryan AM, Del Poeta M, Luberto C. Sphingolipids as regulators of the phagocytic response to fungal infections. Mediat Inflamm. 2015;2015: 640540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonacini M, Nussbaum J, Ahluwalia C. Gastrointestinal, hepatic, and pancreatic involvement with Cryptococcus neoformans in AIDS. J Clin Gastroenterol. 1990;12(3):295–7. [DOI] [PubMed] [Google Scholar]
- 21.Alameri A, Museedi A, Al Qaisi A, Alshaikhli A, Kim A. S1379: cryptococcus can be a great mimicker in AIDS patients. Am J Gastroenterol. 2020;115:S694. [Google Scholar]
- 22.Wappler-Guzzetta EA, Gray AL, Dagostino J, Kerstetter JC. Diffuse adrenal gland and pancreas necrosis in a patient with disseminated cryptococcosis—case report. Life. 2022;12:1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dzwonkowski M, Shah N, Iqbal U, Confer B. Disseminated cryptococcosis masquerading as a large abdominal mass concerning for pancreatic malignancy in an HIV-positive patient. ACG Case Rep J. 2022;9(11): e00908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kitano M, Kamata K, Imai H, Miyata T, Yasukawa S, Yanagisawa A, Kudo M. Contrast-enhanced harmonic endoscopic ultrasonography for pancreatobiliary diseases. Dig Endosc. 2015;27(Suppl 1):60–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The availability of data applies only in cases where no personal information is disclosed.


