Abstract
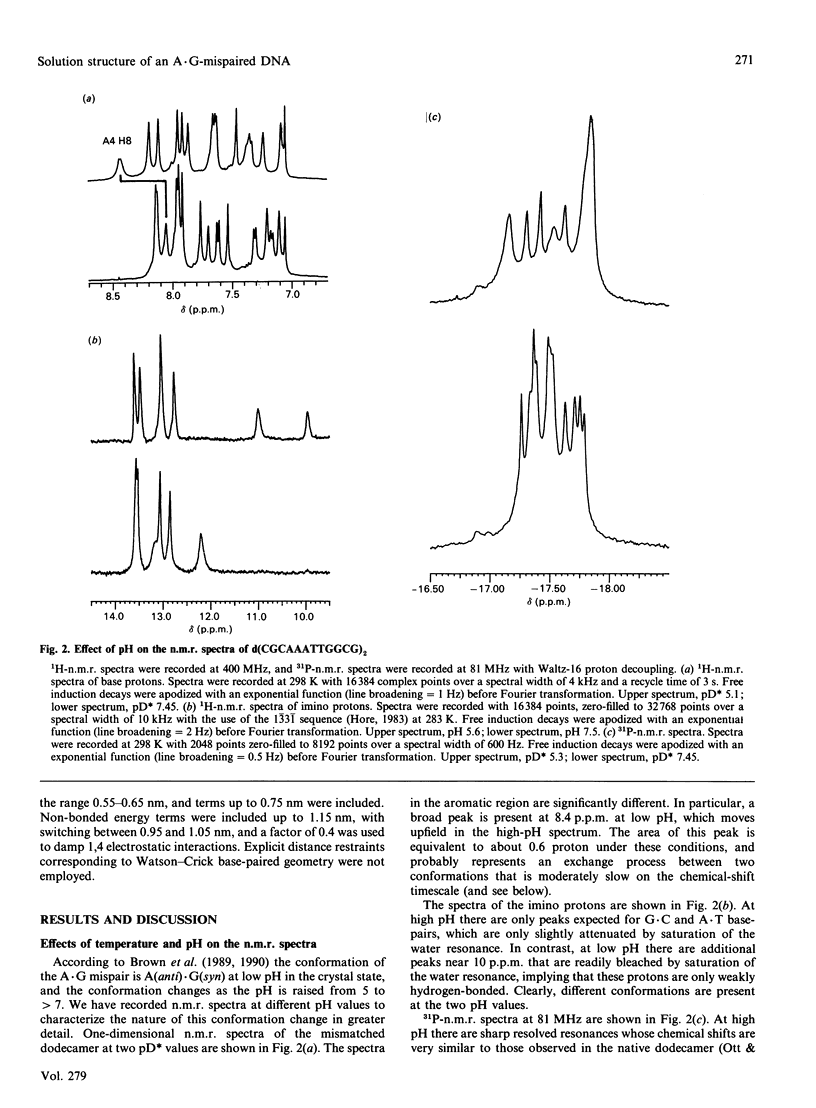
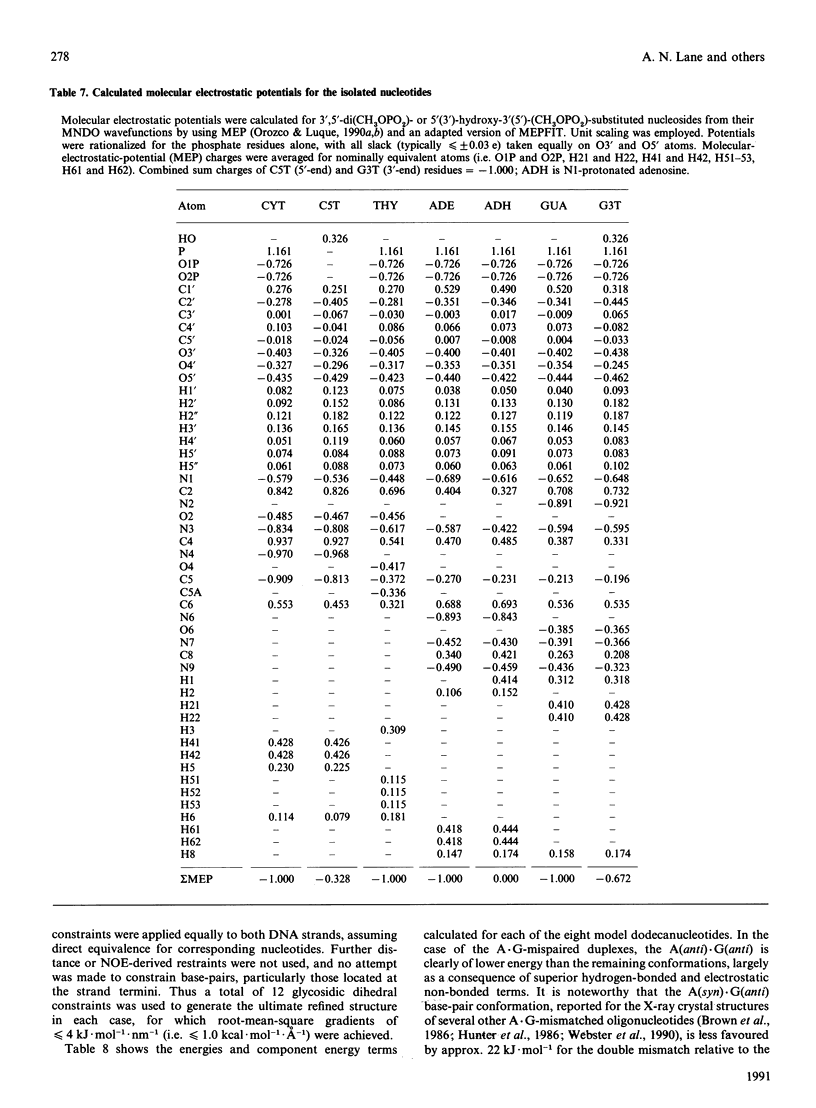
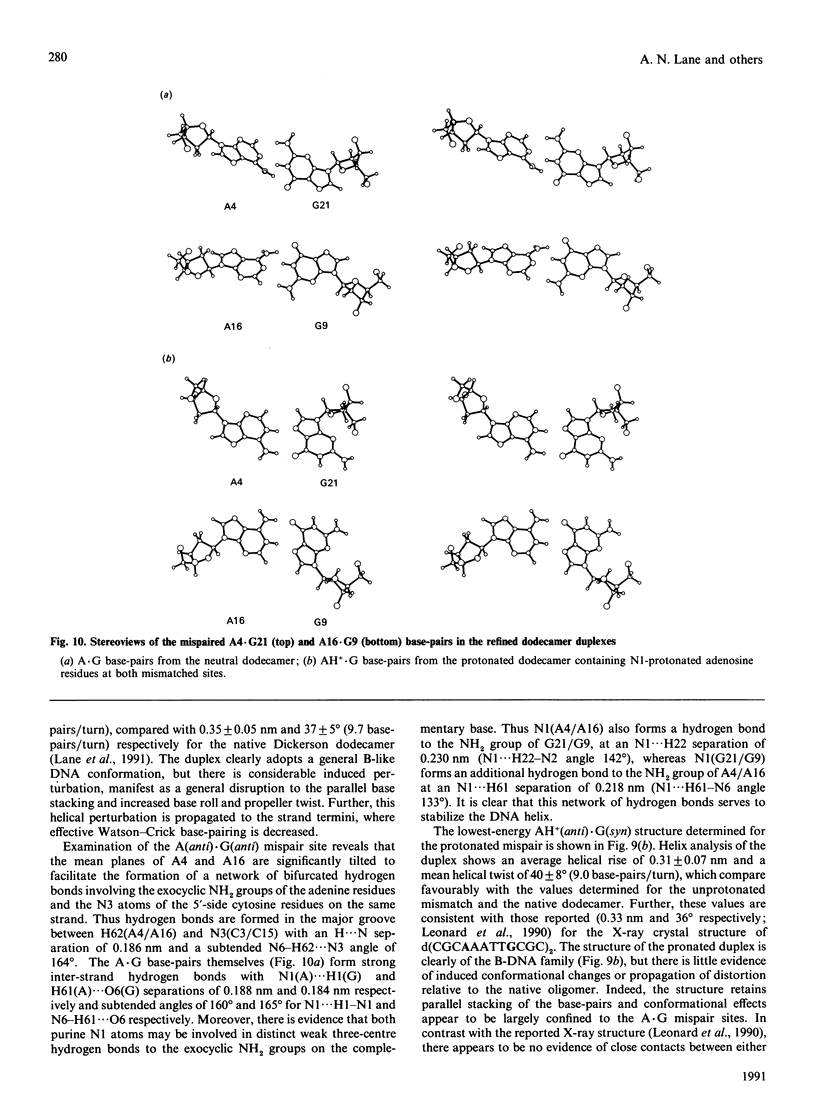
A.G base-paired mismatches that occur during replication are among the most difficult to detect by repair enzymes. Such purine.purine mispairs can exist in two conformations, one of which is stabilized by protons [Gao & Patel (1988) J. Am. Chem. Soc. 110, 5178-5182]. We have undertaken a 1H-n.m.r. and 31P-n.m.r. study of the mismatched dodecamer d(CGCAAATTGGCG)2 as a function of both temperature and pH to determine the conformational features of the A.G mismatch. At pH greater than 7 the mispaired bases are each in the anti conformation and are stacked in the B-like helix. As the pH is decreased, a second conformation becomes populated (apparent pKa approx. 5.9) with concomitant changes in the chemical shifts of protons of the mispaired bases and their nearest neighbours. Data from two-dimensional nuclear-Overhauser-enhancement spectroscopy show unequivocally that, at low pH, the dominant conformation is one in which the mismatched G residues are in the syn conformation and are hydrogen-bonded to the A residues that remain in the anti conformation. Residues not adjacent to the A.G sites are almost unaffected by the transition or the mispairing, suggesting considerable local flexibility of the unconstrained duplexes. Despite the bulging of the mispaired bases, the conformation of the A(anti).G(anti) duplex is very similar to the native dodecamer, whereas the AH+(anti).G(syn) duplex shows a greater variation in the backbone conformation at the mismatched site. According to the chemical shifts, the duplex retains twofold symmetry in solution. The equilibrium between the syn and anti conformations of G9/G21 is strongly dependent on pH, but only weakly dependent on temperature (delta H approx. 16 kJ.mol-1). The first-order rate constant for the transition is approx. 9 s-1 at 283 K and approx. 60 s-1 at 298 K, with an activation enthalpy of approx. 100 kJ.mol-1. The stabilization of the A(anti).G(syn) conformation by protons is consistent with models invoking N1 protonation of adenine. Using the derived glycosidic torsion angles we have used restrained molecular dynamics to build models of the neutral and protonated d(CGCAAATTGGCG)2 oligomers. The results confirm that the A(anti).G(anti) and AH+(anti).G(syn) conformations are favoured at high pH and low pH respectively, in accord with n.m.r. and single-crystal X-ray data.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnott S., Chandrasekaran R., Hall I. H., Puigjaner L. C. Heteronomous DNA. Nucleic Acids Res. 1983 Jun 25;11(12):4141–4155. doi: 10.1093/nar/11.12.4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchall A. J., Lane A. N. Anisotropic rotation in nucleic acid fragments: significance for determination of structures from NMR data. Eur Biophys J. 1990;19(2):73–78. doi: 10.1007/BF00185089. [DOI] [PubMed] [Google Scholar]
- Brown T., Hunter W. N., Kneale G., Kennard O. Molecular structure of the G.A base pair in DNA and its implications for the mechanism of transversion mutations. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2402–2406. doi: 10.1073/pnas.83.8.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T., Leonard G. A., Booth E. D., Chambers J. Crystal structure and stability of a DNA duplex containing A(anti).G(syn) base-pairs. J Mol Biol. 1989 May 20;207(2):455–457. doi: 10.1016/0022-2836(89)90268-4. [DOI] [PubMed] [Google Scholar]
- Brown T., Leonard G. A., Booth E. D., Kneale G. Influence of pH on the conformation and stability of mismatch base-pairs in DNA. J Mol Biol. 1990 Apr 5;212(3):437–440. doi: 10.1016/0022-2836(90)90320-L. [DOI] [PubMed] [Google Scholar]
- Carbonnaux C., van der Marel G. A., van Boom J. H., Guschlbauer W., Fazakerley G. V. Solution structure of an oncogenic DNA duplex containing a G.A mismatch. Biochemistry. 1991 Jun 4;30(22):5449–5458. doi: 10.1021/bi00236a018. [DOI] [PubMed] [Google Scholar]
- Chuprina V. P., Poltev V. I. Possible conformations of double-helical polynucleotides containing incorrect base pairs. Nucleic Acids Res. 1983 Aug 11;11(15):5205–5222. doi: 10.1093/nar/11.15.5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fersht A. R., Knill-Jones J. W., Tsui W. C. Kinetic basis of spontaneous mutation. Misinsertion frequencies, proofreading specificities and cost of proofreading by DNA polymerases of Escherichia coli. J Mol Biol. 1982 Mar 25;156(1):37–51. doi: 10.1016/0022-2836(82)90457-0. [DOI] [PubMed] [Google Scholar]
- Gorenstein D. G., Schroeder S. A., Fu J. M., Metz J. T., Roongta V., Jones C. R. Assignments of 31P NMR resonances in oligodeoxyribonucleotides: origin of sequence-specific variations in the deoxyribose phosphate backbone conformation and the 31P chemical shifts of double-helical nucleic acids. Biochemistry. 1988 Sep 20;27(19):7223–7237. doi: 10.1021/bi00419a009. [DOI] [PubMed] [Google Scholar]
- Gronenborn A. M., Clore G. M. Analysis of the relative contributions of the nuclear Overhauser interproton distance restraints and the empirical energy function in the calculation of oligonucleotide structures using restrained molecular dynamics. Biochemistry. 1989 Jul 11;28(14):5978–5984. doi: 10.1021/bi00440a039. [DOI] [PubMed] [Google Scholar]
- Hare D. R., Wemmer D. E., Chou S. H., Drobny G., Reid B. R. Assignment of the non-exchangeable proton resonances of d(C-G-C-G-A-A-T-T-C-G-C-G) using two-dimensional nuclear magnetic resonance methods. J Mol Biol. 1983 Dec 15;171(3):319–336. doi: 10.1016/0022-2836(83)90096-7. [DOI] [PubMed] [Google Scholar]
- Hunter W. N., Brown T., Kennard O. Structural features and hydration of d(C-G-C-G-A-A-T-T-A-G-C-G); a double helix containing two G.A mispairs. J Biomol Struct Dyn. 1986 Oct;4(2):173–191. doi: 10.1080/07391102.1986.10506338. [DOI] [PubMed] [Google Scholar]
- Keepers J. W., Schmidt P., James T. L., Kollman P. A. Molecular-mechanical studies of the mismatched base analogs of d(CGCGAATTCGCG)2:d(CGTGAATTCGCG)2, d(CGAGAATTCGCG)2, d(CGCGAATTCACG)2, d(CGCGAATTCTCG)2, and d(CGCAGAATTCGCG).d(CGCGAATTCGCG). Biopolymers. 1984 Dec;23(12):2901–2929. doi: 10.1002/bip.360231214. [DOI] [PubMed] [Google Scholar]
- Kochoyan M., Leroy J. L., Guéron M. Proton exchange and base-pair lifetimes in a deoxy-duplex containing a purine-pyrimidine step and in the duplex of inverse sequence. J Mol Biol. 1987 Aug 5;196(3):599–609. doi: 10.1016/0022-2836(87)90036-2. [DOI] [PubMed] [Google Scholar]
- Lane A. N., Jenkins T. C., Brown T., Neidle S. Interaction of berenil with the EcoRI dodecamer d(CGCGAATTCGCG)2 in solution studied by NMR. Biochemistry. 1991 Feb 5;30(5):1372–1385. doi: 10.1021/bi00219a030. [DOI] [PubMed] [Google Scholar]
- Lane A. N. The determination of the conformational properties of nucleic acids in solution from NMR data. Biochim Biophys Acta. 1990 Jun 21;1049(2):189–204. doi: 10.1016/0167-4781(90)90040-9. [DOI] [PubMed] [Google Scholar]
- Lane A. N. The solution conformations of a mutant trp operator determined by n.m.r. spectroscopy. Biochem J. 1991 Jan 15;273(Pt 2):383–391. doi: 10.1042/bj2730383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefèvre J. F., Lane A. N., Jardetzky O. Solution structure of the Trp operator of Escherichia coli determined by NMR. Biochemistry. 1987 Aug 11;26(16):5076–5090. doi: 10.1021/bi00390a029. [DOI] [PubMed] [Google Scholar]
- Leonard G. A., Booth E. D., Brown T. Structural and thermodynamic studies on the adenine.guanine mismatch in B-DNA. Nucleic Acids Res. 1990 Oct 11;18(19):5617–5623. doi: 10.1093/nar/18.19.5617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marion D., Lancelot G. Sequential assignment of the 1H and 31P resonances of the double stranded deoxynucleotide d (ATGCAT)2 by 2D-NMR correlation spectroscopy. Biochem Biophys Res Commun. 1984 Nov 14;124(3):774–783. doi: 10.1016/0006-291x(84)91025-8. [DOI] [PubMed] [Google Scholar]
- Nerdal W., Hare D. R., Reid B. R. Three-dimensional structure of the wild-type lac Pribnow promoter DNA in solution. Two-dimensional nuclear magnetic resonance studies and distance geometry calculations. J Mol Biol. 1988 Jun 20;201(4):717–739. doi: 10.1016/0022-2836(88)90469-x. [DOI] [PubMed] [Google Scholar]
- Orozco M., Laughton C. A., Herzyk P., Neidle S. Molecular-mechanics modelling of drug-DNA structures; the effects of differing dielectric treatment on helix parameters and comparison with a fully solvated structural model. J Biomol Struct Dyn. 1990 Oct;8(2):359–373. doi: 10.1080/07391102.1990.10507810. [DOI] [PubMed] [Google Scholar]
- Orozco M., Luque F. J. A practical procedure for the determination of electrostatic charges of large molecules. J Comput Aided Mol Des. 1990 Dec;4(4):411–426. doi: 10.1007/BF00117406. [DOI] [PubMed] [Google Scholar]
- Tidor B., Irikura K. K., Brooks B. R., Karplus M. Dynamics of DNA oligomers. J Biomol Struct Dyn. 1983 Oct;1(1):231–252. doi: 10.1080/07391102.1983.10507437. [DOI] [PubMed] [Google Scholar]
- Wagner G. Characterization of the distribution of internal motions in the basic pancreatic trypsin inhibitor using a large number of internal NMR probes. Q Rev Biophys. 1983 Feb;16(1):1–57. doi: 10.1017/s0033583500004911. [DOI] [PubMed] [Google Scholar]
- Webster G. D., Sanderson M. R., Skelly J. V., Neidle S., Swann P. F., Li B. F., Tickle I. J. Crystal structure and sequence-dependent conformation of the A.G mispaired oligonucleotide d(CGCAAGCTGGCG). Proc Natl Acad Sci U S A. 1990 Sep;87(17):6693–6697. doi: 10.1073/pnas.87.17.6693. [DOI] [PMC free article] [PubMed] [Google Scholar]


